Abstract
Human immunodeficiency virus (HIV)-specific CD4 T-cell responses, particularly to the envelope glycoproteins of the virus, are weak or absent in most HIV-infected patients. Although these poor responses can be attributed simply to the destruction of the specific CD4 T cells by the virus, other factors also appear to contribute to the suppression of these virus-specific responses. We previously showed that human monoclonal antibodies (MAbs) specific for the CD4 binding domain of gp120 (gp120CD4BD), when complexed with gp120, inhibited the proliferative responses of gp120-specific CD4 T-cells. MAbs to other gp120 epitopes did not exhibit this activity. The present study investigated the inhibitory mechanisms of the anti-gp120CD4BD MAbs. The anti-gp120CD4BD MAbs complexed with gp120 suppressed gamma interferon production as well as proliferation of gp120-specific CD4 T cells. Notably, the T-cell responses to gp120 were inhibited only when the MAbs were added to antigen-presenting cells (APCs) during antigen pulse; the addition of the MAbs after pulsing caused no inhibition. However, the anti-gp120CD4BD MAbs by themselves, or as MAb/gp120 complexes, did not affect the presentation of gp120-derived peptides by the APCs to T cells. These MAb/gp120 complexes also did not inhibit the ability of APCs to process and present unrelated antigens. To test whether the suppressive effect of anti-gp120CD4BD antibodies is caused by the antibodies' ability to block gp120-CD4 interaction, APCs were treated during antigen pulse with anti-CD4 MAbs. These treated APCs remained capable of presenting gp120 to the T cells. These results suggest that anti-gp120CD4BD Abs inhibit gp120 presentation by altering the uptake and/or processing of gp120 by the APCs but their inhibitory activity is not due to blocking of gp120 attachment to CD4 on the surface of APCs.
The importance of CD4 Th cells in controlling chronic virus infections has been documented in the literature (15, 17, 23, 34). Among human immunodeficiency virus (HIV)-infected individuals, the presence of HIV-specific Th-cell responses correlated with high levels of HIV-specific cytotoxic T lymphocyte precursors and lower viral load (10). However, HIV-positive (HIV+) individuals who are capable of maintaining HIV-specific Th responses and successfully controlling HIV infection are clearly exceptions to the norm. In the majority of HIV+ individuals, CD4 T-cell responses to HIV antigens are poor or undetectable (2, 25, 33). Multiple factors have been implicated to account for the loss of these specific responses, with the simplest explanation being the suppression of these CD4 T cells due to direct infection and killing by the virus (5, 30). However, using flow-cytometric detection of antigen-induced intracellular cytokines, significant numbers of CD4 memory T cells were found in many HIV+ subjects with progressive disease, although many fewer envelope (Env)-specific CD4 T cells than Gag-specific CD4 T cells were detectable (24).
It was previously demonstrated that antibodies produced during HIV infection could contribute to the poor CD4 T-cell responses observed in infected individuals (7). The proliferative responses of gp120-specific CD4 T-cell lines were inhibited in the presence of purified antibodies (Abs) from the sera of HIV-infected subjects. By screening a panel of human monoclonal antibodies (MAbs) directed to different epitopes of gp120, this inhibitory activity was found to be mediated by MAbs to the CD4 binding domain of gp120 (gp120CD4BD). All six anti-gp120CD4BD MAbs tested, when complexed with gp120, inhibited the CD4 T-cell responses to gp120. This inhibitory effect was observed with all five gp120-specific CD4 T-cell lines examined. In contrast, other MAbs in the panel specific for gp120 epitopes C2, V2, V3, or C5 did not exhibit this activity. Notably, none of the antibody binding sites overlap with the epitopes recognized by the CD4 T-cell lines. We also observed that the anti-gp120CD4BD MAbs by themselves had no direct negative effect on the CD4 T cells, but the mechanism(s) by which these Abs exert their inhibitory effects are not yet known.
In the present study, we explored several possibilities that could explain the inhibitory activity of the anti-gp120CD4BD/gp120 complexes. These Abs, by themselves or as immune complexes, did not affect the ability of antigen-presenting cells (APCs) to ingest, process, or present unrelated antigens. The data indicate that anti-gp120CD4BD Abs, by forming complexes with gp120, alter the uptake and/or processing of gp120 by the APCs such that the presentation of this particular antigen to CD4 T cells is abolished. However, the inhibitory activity by the Abs cannot be attributed to the blocking of gp120 binding and uptake via CD4 on the APC surfaces.
MATERIALS AND METHODS
Antigens.
Recombinant gp120SF2, gp120IIIB, and gp160NL4.3 were used in the study and obtained as follows: gp120SF2 was secreted from CHO cells and obtained from Austral Biologicals (San Ramon, Calif.); gp120IIIB was produced in the baculovirus expression system and purchased from ImmunoDiagnostics (Woburn, Mass.); gp160NL4.3 was produced in baculovirus-infected cells by MicroGeneSys (this recombinant protein does not bind to CD4 or to any anti-gp120CD4BD MAbs used in this study). A synthetic peptide corresponding to gp120 residues 221 to 240 (p740.19) was provided by the Medical Research Council AIDS Reagent Project. Recombinant p24 protein and cytomegalovirus (CMV) antigens from strain AD169 were purchased from Protein Science (Meriden, Conn.) and BioWhittaker (Walkersville, Md.), respectively.
Antibodies.
Human MAbs specific for the CD4 binding domain (654-D and 559/64D) and C5 domain (450-D) of gp120 were used after protein A purification. The generation and specificities of these MAbs were reported previously (11, 12, 37). Mouse MAbs to human CD4 (SIM.4, RPA-T4, and OKT4) were tested in the study. MAbs SIM.4 and RPA-T4 are capable of blocking gp120-CD4 interaction, while MAb OKT4 has no blocking activity. These MAbs were purified by protein G affinity chromatography. SIM.4 and OKT4 were obtained from the National Institutes of Health AIDS Reagent Repository and the American Type Culture Collection, respectively. MAb RPA-T4 was purchased from BD PharMingen (San Diego, Calif.).
CD4 T-cell lines.
gp120-specific CD4 T-cell lines DMg26 and 027-563 were used in the study. The generation and maintenance of these T-cell lines were reported previously (7, 9, 26). DMg26 is specific for a DR1-restricted epitope in the C2 domain (within gp120 residues 221 to 240) and recognizes gp120 from various HIV-1 strains, including SF2, IIIB, NL4.3, and W61D. T-cell line 027-563 recognizes multiple peptides representing the V2 and V3 domains of gp120IIIB. A CD4 T-cell line specific for p24 (AC-25) was also studied; this line recognizes an epitope within p24 and is DR1 restricted (P. J. Norris et al., unpublished data).
T-cell proliferation assays.
T-cell proliferation was measured in [3H]thymidine incorporation assays. T cells (2 × 104 cells) were incubated with APCs (105 cells) pretreated with antigen or antigen-antibody mixtures at the designated concentrations in 96-well flat-bottom plates. APCs were prepared from either autologous B lymphoblastoid cell lines (BCLs) or HLA-DR-matched heterologous peripheral blood mononuclear cells (PBMCs). Immune complexes were formed by incubating gp120 and anti-gp120 MAbs at the designated Ab/gp120 ratios for up to 4 h at 37°C as described previously (7). For some experiments as designated, anti-gp120CD4BD MAb 654-D (10 μg/ml) was added to APCs either together with gp120 (0.1 to 3 μg/ml) during antigen pulse or after gp120 pulsing. For other experiments, the APCs were pretreated with anti-CD4 MAbs (10 μg/ml), pulsed with gp120, gp120/MAb complexes, or no antigen, and washed prior to incubation with the CD4 T cells. After 2 days, the cells were pulsed with [3H]thymidine (NEN Life Science, Boston, Mass.) and harvested 18 to 24 h later. Each culture condition was tested in triplicate and the mean counts per minute (cpm) and standard deviations were calculated. For background control, [3H]thymidine incorporation of T cells cultured with APCs with medium alone was measured in each experiment. All experiments were performed at least twice, and the data from one representative experiment are shown.
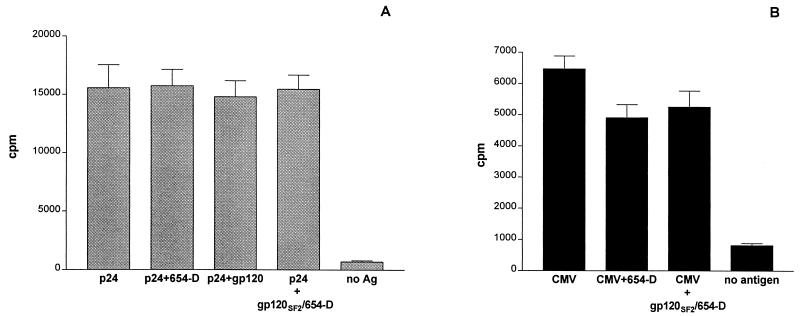
The T-cell response to p24 was examined using a p24-specific CD4 T-cell line, AC-25. Recombinant p24 was incubated with an autologous BCL, used as APCs, in the presence of anti-gp120CD4BD MAb 654-D, gp120, or gp120/654-D complex. After overnight incubation at 37°C, the AC-25 T cells were added and incubated for 2 days. T-cell proliferation was assessed by [3H]thymidine incorporation as described above.
The lymphoproliferative response to CMV was examined using PBMCs from CMV-seropositive HIV-seronegative individuals. CMV antigens were preincubated with gp120, anti-gp120CD4BD MAb, gp120/MAb complex, or medium for 4 h at 37°C. PBMCs were plated with the different antigen preparations in triplicate wells of flat-bottom 96-well plates. After 5 days, the cells were pulsed with [3H]thymidine and harvested 18 to 24 h later. [3H]thymidine incorporation by the cells cultured with phytohemagglutinin (5 μg/ml) and with medium alone was measured for positive and negative controls, respectively.
ELISPOT assays.
Enzyme-linked immunospot (ELISPOT) assays were performed to examine the number of T cells producing gamma interferon (IFN-γ) in response to gp120 or gp120 complexed with anti-gp120 MAbs. Briefly, autologous BCLs or HLA-DR-matched heterologous PBMCs used as APCs in these assays were pulsed with gp120 or gp120-MAb mixtures for 18 to 24 h at 37°C. For some experiments, the APCs were pretreated with anti-CD4 MAbs, as described above, and then pulsed with gp120. After washing, these APCs (5 × 104 cells/well) were incubated with T cells (102 to 105 cells/well) on MultiScreen 96-well plates (MAIP S45; Millipore Corp., Bedford, Mass.) precoated with anti-human IFN-γ MAb (clone 2G1; Endogen, Woburn, Mass.). After an overnight incubation, the plates were developed with biotinylated anti-human IFN-γ MAb (clone B133.5; Endogen) and the Bio-Rad alkaline-phosphotase streptavidin conjugate kit (Hercules, Calif.). The frequency of IFN-γ-secreting cells was calculated as the numbers of spots per 105 T cells plated. Each culture condition was tested in duplicate or triplicate wells.
RESULTS
The presence of anti-gp120CD4BD MAbs suppresses gp120-specific CD4 T-cell production of IFN-γ.
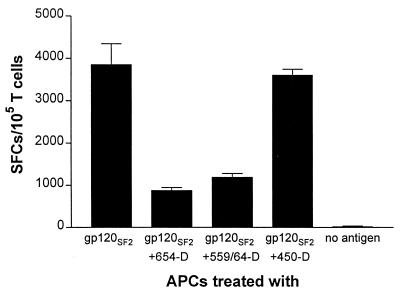
We previously showed that proliferative responses of human gp120-specific CD4 T-cell lines to gp120 were inhibited by the presence of human MAbs to gp120CD4BD but not by human MAbs to other gp120 epitopes (C2, V2, V3, and C5) (7). In order to examine whether anti-gp120CD4BD MAbs also affected IFN-γ production by these gp120-specific Th1 cell lines, an ELISPOT assay with anti-human IFN-γ MAbs was utilized. Cells which produced IFN-γ in response to APCs pulsed with gp120 in the presence of MAbs to gp120CD4BD (654-D and 559-64D) or C5 (450-D) were enumerated. The gp120-specific, HLA-DR1-restricted CD4 T-cell line DMg26, which had been tested in previous studies and shown to produce Th1 cytokines (7, 9), was used. Figure 1 shows that the DMg26 T cells produced IFN-γ in response to gp120 alone or gp120 mixed with the anti-C5 MAb 450-D; however, the presence of anti-gp120CD4BD MAbs dramatically decreased the number of IFN-γ-producing cells. Recombinant gp120SF2 generated in mammalian CHO cells was used here, but we obtained similar results when other preparations of gp120 and gp160 (e.g., recombinant proteins from baculovirus-infected insect cells, from vaccinia-infected mammalian cells, or native proteins from HIV-infected cells) were used (data not shown). The APCs alone and the T cells stimulated with APCs in the absence of antigen did not produce significant levels of IFN-γ. In the experiment shown in Fig. 1, an autologous EBV-transformed BCL was used as APCs, and the same results were observed using HLA-DR1+ heterologous PBMCs as APCs (data not shown). These results demonstrate that the presence of anti-gp120CD4BD MAbs suppressed the IFN-γ production of gp120-specific CD4 T cells, and this effect corresponded to that seen in the proliferative responses of these T cells (7) (see Fig. 2).
FIG. 1.
Effect of anti-gp120 MAbs on IFN-γ production by CD4 T-cell line DMg26 in response to gp120. An ELISPOT assay was performed to examine IFN-γ production by the gp120-specific T-cell line DMg26 following stimulation with gp120SF2, gp120SF2 complexed with anti-gp120CD4BD MAbs (654-D or 559/64D), gp120SF2 complexed with an anti-C5 MAb (450-D), or with no antigen. Recombinant gp120SF2 was tested at 5 μg/ml and mixed with medium alone or with MAbs used at 10 μg/ml. An autologous EBV-transformed BCL was used as APCs in this particular experiment. Each condition was tested in duplicate, and the results were confirmed in two independent experiments. The mean numbers of spot-forming cells (SFCs) per 105 T cells and the standard deviations are presented on the y axis.
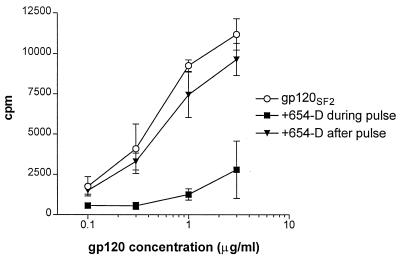
FIG. 2.
Inhibition of CD4 T-cell proliferative responses to gp120 is observed only when the anti-gp120CD4BD MAb is added together with gp120 to the APCs during antigen pulse. We examined the effects of anti-gp120CD4BD MAb 654-D added to gp120SF2 at different times on proliferation of T-cell line DMg26. MAb 654-D was added either together with gp120SF2 to APCs during antigen pulse (■) or after the APCs were pulsed with gp120 (▾). The response to gp120SF2 in the absence of MAb 654-D (○) was also measured for comparison. Recombinant gp120SF2 was used at various concentrations of 0.1 to 3 μg/ml, while MAb 654-D was tested at a constant amount of 10 μg/ml. HLA-DR1+ heterologous PBMCs were used as APCs, and the background response to APCs alone (no antigen) was 406 cpm.
Inhibition of gp120-specific CD4 T-cell responses is observed when the anti-gp120CD4BD MAbs are added together with gp120 to APCs during antigen pulsing.
The mechanism(s) by which anti-gp120CD4BD Abs suppress the CD4 T-cell responses to gp120 are still unknown. To address this issue, we compared the effects of the anti-gp120CD4BD MAbs when added at different times: (i) when the MAb and gp120 were added together to the APCs and were both present during antigen pulsing, and (ii) when gp120 was added first to the APCs for 24 h of antigen pulsing and the anti-gp120CD4BD MAb was added later after gp120 pulsing (Fig. 2). The presence of anti-gp120CD4BD MAb during gp120 antigen pulsing was necessary to inhibit the T-cell response; the addition of the anti-gp120CD4BD MAb after antigen pulsing caused no inhibition. Notably, previous findings showed that a molar Ab/gp120 ratio of >1 was required for the inhibition (7). Taken together, these data suggest that anti-gp120CD4BD MAbs, by forming complexes with gp120, interfere with gp120 uptake, processing, and/or presentation by APCs.
Anti-gp120CD4BD /gp120 complexes do not affect T-cell recognition of gp120 peptides
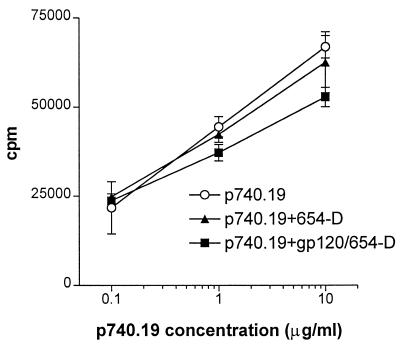
To test whether anti-gp120CD4BD Abs complexed with gp120 interfered in some way with the recognition of processed antigen by gp120-specific T cells, we measured the proliferative response of T-cell line DMg26 to its peptide epitope p740.19 in the presence or absence of the anti-gp120CD4BD/gp120 complexes (Fig. 3). The anti-gp120CD4BD MAb 654-D (10 μg/ml) was preincubated with gp120 (3 μg/ml) to form the complexes and then was added with peptide p740.19 to the APCs. MAb 654-D recognizes a conformational epitope in gp120 and does not bind to peptide p740.19. The DMg26 cells proliferated equally well in response to p740.19 alone or p740.19 in the presence of the anti-gp120CD4BD/gp120 complexes. In agreement with previous findings (7), the addition of anti-gp120CD4BD MAb 654-D by itself also had no inhibitory effect on the T-cell response to p740.19. Hence, the anti-gp120CD4BD MAb, either alone or when complexed with gp120, did not alter the ability of the APCs to present gp120 epitopes and to stimulate the specific CD4 T cells. Moreover, these data showed that the anti-gp120CD4BD MAb and the MAb/gp120 complexes did not cause T-cell death and did not inhibit antigen recognition by the CD4 T cells.
FIG. 3.
Anti-gp120CD4BD MAb 654-D, either by itself or complexed with gp120, does not inhibit CD4 T-cell recognition of gp120-derived peptides. Proliferative responses of the gp120-specific CD4 T-cell line DMg26 to its peptide epitope p740.19 (gp120 residues 221 to 240) was measured in the presence of MAb 654-D/gp120 complexes or MAb 654-D. To form the complexes, recombinant gp120IIIB (3 μg/ml) was mixed with 654-D (10 μg/ml). The complexes or MAb 654-D alone (10 μg/ml) were then added to APCs along with p740.19 (0.1 to 10 μg/ml). Heterologous HLA-DR1+ PBMCs were used as APCs in this experiment. The T-cell response to APCs with no peptide was 2,674 cpm.
Anti-gp120CD4BD/gp120 complexes do not generically disrupt antigen uptake or processing functions of the APCs
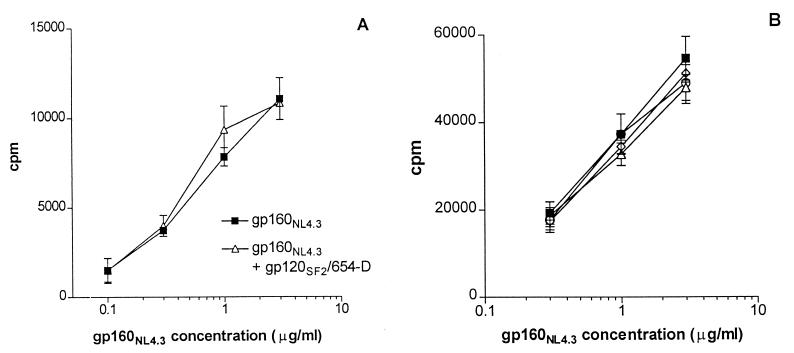
Another possible scenario for the inhibitory mechanism of the anti-gp120CD4BD/gp120 complexes is that these complexes affect the capacity of APCs to ingest and process antigens, such that presentation of antigens is suppressed. To examine this idea, we first tested the effect of anti-gp120CD4BD/gp120 complexes on the proliferation of gp120-specific CD4 T-cell line 027-563 in response to APCs pulsed with gp160NL4.3. This recombinant gp160 protein lacks CD4 binding activity and does not bind to anti-gp120CD4BD MAbs (14, 32) (data not shown). As shown in Fig. 4A, the presence of gp120SF2 (2 μg/ml) complexed with anti-gp120CD4BD MAb 654-D (6 μg/ml) did not affect the presentation of gp160NL4.3 by the HLA-DR1+ PBMCs used in this experiment as APCs.
FIG. 4.
Anti-gp120CD4BD/gp120 complexes do not affect the capacity of APCs to take up, process, and present gp160NL4.3 to the CD4 T cells. (A) Proliferation of gp120-specific CD4 T-cell line 027-563 was measured in response to APCs pulsed with gp160NL4.3 in the presence or absence of anti-gp120CD4BD/gp120 complex (6 μg of 654-D per ml + 2 μg of gp120SF2 per ml). Recombinant gp160NL4.3 has no CD4 binding activity and does not react with anti-gp120CD4BD MAbs. HLA-DR-matched heterologous PBMCs were used as APCs. The background response to APCs alone (no antigen) was 500 cpm. (B) Presentation of gp160NL4.3 was not affected by the addition of increasing concentrations of anti-gp120CD4BD/gp120 complex. T-cell response to gp160NL4.3 was assessed in the presence of different concentrations of the immune complex: 30 μg of 654-D per ml + 10 μg of gp120SF2 per ml (open triangles), 10 μg of 654-D per ml + 3 μg of gp120SF2 per ml (open diamonds), 3 μg of 654-D per ml + 1 μg of gp120SF2 per ml (open circles), or no 654-D/gp120 complex (filled squares). To test a more homogenous population of APCs, an autologous BCL was used in this experiment. T-cell response to APCs with no antigen was 759 cpm.
Due to the heterogeneity of PBMC populations, the anti-gp120CD4BD/gp120 complex may have interacted with and affected only some APCs, while other cells were not affected and remained capable of presenting gp160NL4.3 antigen. To rule out this possibility, we examined the effect of anti-gp120CD4BD/gp120 complexes on a more homogeneous population of APCs, i.e., an autologous BCL. The effects of increasing concentrations of the immune complex were also examined. The results in Fig. 4B show that the capacity of BCLs to present gp160NL4.3 antigen was not suppressed by the immune complexes even at the highest concentration tested, i.e., 10 μg of gp120SF2 per ml complexed with 30 μg of MAb 654-D per ml. This indicates that upon exposure to immune complexes, the APCs remained able to take up, process, and present this uncomplexed gp160NL4.3 antigen to the T cells. The presence of anti-gp120CD4BD MAb 654-D by itself also had no effect (data not shown). We further observed that neither the anti-gp120CD4BD MAbs (654-D and 559/64D) nor the MAb/gp120 complexes inhibited CD4 T-cell responses to HIV p24 (Fig. 5A), cytomegalovirus (Fig. 5B), or Mycobacterium tuberculosis antigens (7). Taken together, these data demonstrate that the anti-gp120CD4BD Abs, either by themselves or complexed with gp120, did not obstruct the uptake, processing, and presentation of antigens in general. Instead, the anti-gp120CD4BD Abs, when forming complexes with gp120, affect only the uptake or processing of the complexed gp120 molecule, such that the CD4 T-cell response to this complexed antigen is specifically inhibited.
FIG. 5.
Anti-gp120CD4BD/gp120 complexes do not inhibit the presentation of HIV-1 p24 or CMV antigens to CD4 T cells. (A) Proliferative response of p24-specific CD4 T-cell line AC-25 was assessed in the presence of p24 alone or p24 mixed with 654-D, gp120, or gp120/654-D complexes, or in the absence of any antigen. An autologous B-cell line was used as APCs in this experiment. (B) Lymphoproliferative responses of PBMCs from a CMV-seropositive individual were measured to CMV antigens alone, CMV + gp120/654-D complexes, CMV + 654-D, or no antigen. In each of these experiments, anti-gp120CD4BD MAb 654-D (5 μg/ml) was preincubated with gp120SF2 (2 μg/ml) and then mixed with p24 (1 μg/ml) or CMV antigen (diluted 1:10) (A and B, respectively). T-cell responses were measured in [3H]thymidine incorporation assays. The responses to p24 and CMV in the absence or presence of 654-D and the gp120/654-D complex were not significantly different (P ≥ 0.1 by the Mann-Whitney test).
Anti-CD4 MAbs, unlike anti-gp120CD4BD MAbs, did not affect gp120 uptake and processing by APCs.
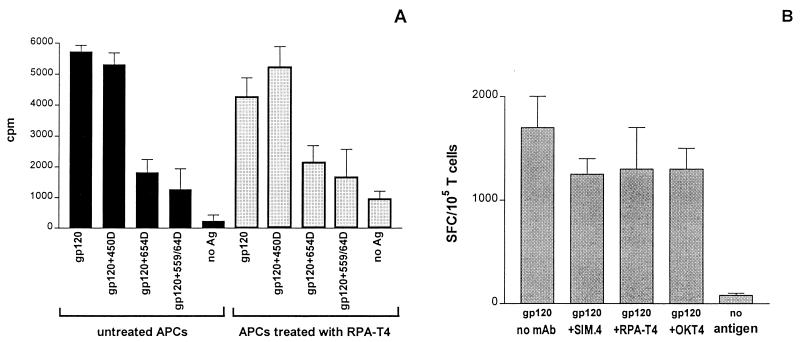
Since anti-gp120CD4BD MAbs are capable of blocking gp120-CD4 interaction, it is possible that these MAbs inhibit gp120 presentation to T cells by preventing gp120 binding to CD4 on the surfaces of APCs and thereby inhibiting its internalization. This rationale presumes that the uptake of gp120 by APCs is dependent upon its initial binding to CD4 on the APC surfaces. To examine whether gp120 binding to CD4 on APCs is crucial to its uptake and processing, we compared the ability of APCs to present gp120 to the CD4 T-cell line DMg26 when the APCs were pretreated with an anti-CD4 MAb that is known to block gp120-CD4 binding (RPA-T4) and their ability to do so when they were not thus pretreated. An autologous BCL that expresses a low level of CD4 (typically ≈1% of the cells express surface CD4 levels above background control) was used as APCs; this line was chosen because its low level of CD4 expression would make it most sensitive to the blocking effects by anti-CD4 MAbs. These APCs were incubated first with RPA-T4 (10 μg/ml) for 30 min, and then, without removing RPA-T4, the cells were treated with gp120 or gp120 complexed with either anti-gp120CD4BD MAbs (654-D or 559/64D) or an anti-C5 MAb (450-D). After 18 h, the APCs were washed extensively to remove free RPA-T4 that would interfere with the CD4 T cells. These APCs were then used to stimulate the DMg26 T cells, and the T-cell responses were measured by [3H]thymidine incorporation and IFN-γ production. Figure 6A shows that APCs pretreated with the anti-CD4 MAb RPA-T4, similar to the untreated APCs, were capable of presenting gp120 to DMg26 T cells. Treatment with RPA-T4 also did not inhibit the T-cell response to gp120 complexed with the anti-C5 MAb 450-D. Moreover, similar levels of inhibition were seen with T-cell responses to gp120 complexed with anti-gp120CD4BD MAbs (654-D or 559/64D), whether the APCs were treated or not treated with RPA-T4. These data show that the anti-CD4 MAb RPA-T4 had no effect on the uptake and processing of gp120 by APCs and did not contribute to the inhibition observed with anti-gp120CD4BD MAbs.
FIG. 6.
Unlike anti-gp120CD4BD MAbs, anti-CD4 MAbs have no effect on gp120 uptake and processing by APCs. (A) APCs were treated (dotted bars) or not treated (solid bars) with MAb RPA-T4 (10 μg/ml), an anti-CD4 MAb that can block gp120-CD4 interaction, and then were pulsed with gp120, with gp120 complexed with anti-C5 (450-D) or anti-gp120CD4BD MAbs (654-D and 559/64D), or with no antigen. These APCs were used to stimulate the gp120-specific CD4 T-cell line DMg26. T-cell proliferation was measured by [3H]thymidine incorporation. Recombinant gp120SF2 (3 μg/ml) was used either alone or mixed with anti-gp120 MAbs (10 μg/ml). (B) APCs were preincubated either with anti-CD4 MAbs capable of blocking gp120 binding to CD4 (SIM.4 and RPA-T4) or with a nonblocking anti-CD4 MAb (OKT4) and then pulsed with gp120. Each anti-CD4 MAb was used at 10 μg/ml. These APCs were tested in ELISPOT assays for the capacity to present gp120 antigen and induce IFN-γ production in DMg26 cells. In each of these experiments, autologous BCL was used as APCs.
To examine this issue with an additional assay, we used the IFN-γ ELISPOT assay. The data in Fig. 6B show that neither anti-CD4 MAbs that have gp120-CD4 blocking activity (RPA-T4 and SIM.4) nor a nonblocking anti-CD4 MAb, OKT-4, inhibited the ability of APCs in taking up, processing, or presenting gp120 antigen to gp120-specific CD4 T cells. These results demonstrate that anti-CD4 MAbs that could block gp120-CD4 interaction did not interfere with gp120 uptake by the APCs, indicating that this process is CD4 independent. In contrast, anti-gp120CD4BD MAbs complexed with gp120 consistently inhibit gp120 presentation to CD4 T cells. Hence, anti-gp120CD4BD Abs do not inhibit gp120 presentation by blocking gp120 binding to CD4 on the APCs. This conclusion is strengthened by the data in Fig. 4 showing that gp160NL4.3, which does not bind CD4, can be presented efficiently to T cells, indicating that this antigen can be taken up and processed by the APCs without CD4 involvement.
DISCUSSION
We have shown that, similar to the inhibitory effect previously observed on proliferative responses (7), IFN-γ production by CD4 T cells in response to gp120 is also suppressed in the presence of gp120 complexed with human MAbs specific for the CD4 binding domain of gp120. In contrast, MAbs to other gp120 epitopes did not exhibit such an inhibitory effect. Inhibition was also observed regardless of the types of APCs used to stimulate the T cells (7). In this study, we explored the possible mechanisms for the inhibitory activity of the anti-gp120CD4BD Abs. We observed that anti-gp120CD4BD MAbs exhibited the inhibitory activity only when added together with gp120 to the APCs during antigen pulse. No inhibition was seen when these MAbs alone were added to the T cells and APCs after antigen pulse, demonstrating that the anti-gp120CD4BD Abs did not act on the T cells or on the APCs per se. It should be noted, however, that a molar ratio of Ab to gp120 of >1 was necessary for the inhibitory activity of the anti-gp120CD4BD Abs (7). Hence, it appears that the inhibition observed was actually mediated by anti-gp120CD4BD Abs complexed with gp120, and not by the Abs themselves. However, the present study demonstrates that the anti-gp120CD4BD/gp120 complexes did not cause T-cell death or apoptosis; the CD4 T cells responded to the peptides for which they were specific in the presence or absence of the Ab/gp120 complexes (Fig. 3 and 4). We further demonstrated that these complexes did not affect the capacity of APCs to ingest, process, and present antigens in general (Fig. 4 and 5). Notably, the complexes had no effect on presentation of other antigens, such as HIV p24 (Fig. 5A) and CMV (Fig. 5B). On the basis of these findings, we postulate that the inhibitory activity of anti-gp120CD4BD Abs is specific for gp120 presentation to the CD4 T cells and that they do not generically affect the T-cell or the APC functions; instead, the anti-gp120CD4BD Abs, by binding to gp120, alter the uptake or processing of the gp120 by APCs, such that the presentation of this particular antigen to the CD4 T cells is blocked.
Inhibition of gp120-specific T-cell responses was mediated specifically by both Abs and CD4 constructs (CD4-immunoglobulin [Ig] and soluble CD4) capable of binding the gp120 region that forms the CD4 binding domain (7; C. E. Hioe et al., unpublished data). The capacity of soluble CD4 to mediate this effect rules out the possibility that the inhibitory mechanism is dependent on the Fc region of Ig via the Fc receptors. It is also unlikely that the anti-gp120CD4bd MAbs interfere with T-cell recognition of gp120 by directly masking the T-cell epitopes, since these MAbs affect the recognition of many T-cell epitopes located at distant domains of gp120 that are not parts of the CD4 binding site (7). Another unlikely explanation for our observations is that gp120 uptake by APCs mainly occurs following gp120 binding to CD4 on the surfaces of APCs and that the anti-gp120CD4BD Abs inhibit gp120 presentation to the T cells by blocking gp120 binding to CD4 on the APCs. In this study we examined this idea based on data from two experimental approaches. (i) Recombinant gp160NL4.3 that has no CD4 binding activity was used to pulse HLA-DR-matched heterologous PBMCs or autologous BCLs, used as APCs. These pulsed APCs were shown to stimulate gp120-specific CD4 T cells (Fig. 4A and B). Hence, this antigen could be taken up and processed by APCs independent of CD4. (ii) We also evaluated the effect of anti-CD4 MAbs that block gp120-CD4 binding on gp120 presentation. Unlike anti-gp120CD4BD MAbs, the presence of anti-CD4 MAbs did not affect gp120 presentation to CD4 T cells (Fig. 6A and B). In this study, autologous BCLs that express low levels of surface CD4 were used, such that treatment of the cells with 10 μg of anti-CD4 MAb per ml should effectively block all surface CD4 molecules from interacting with gp120. Nevertheless, the uptake, processing, and presentation of gp120 by BCLs was unaffected by the anti-CD4 MAbs, while the anti-gp120CD4BD MAbs readily inhibited the presentation of this antigen by BCLs, indicating that the anti-gp120CD4BD Abs affect a gp120 presentation pathway that is independent of CD4. These results are also consistent with previous studies by Siliciano et al. (27, 28), showing that anti-CD4 Abs had no effect on gp120 uptake and presentation by BCLs. Altogether, the data presented here demonstrate that anti-gp120CD4BD MAbs suppress gp120 presentation to the CD4 T cells by binding to gp120 and inhibiting the uptake and/or processing of this antigen by APCs, but this activity is not due to the blocking of gp120 binding to CD4 on the surface of APCs.
At this point, the exact step(s) in gp120 uptake or processing that is affected by the anti-gp120CD4BD Abs is not yet known. It is possible that the anti-gp120CD4BD Abs prevent gp120 presentation at the level of uptake, but receptors other than CD4 may be involved. Inhibition of gp120 uptake would globally affect the presentation of all gp120 T-cell epitopes. In support of this, the inhibitory effect of anti-gp120CD4BD MAbs was observed with all five CD4 T-cell lines that we have tested; these T-cell lines recognize distinct epitopes located in various domains of gp120, and none of these epitopes overlaps directly with the CD4 binding site (7). Alternatively, the complexes may be taken up by the APCs but may not reach the endocytic compartments required for antigen processing and presentation. This process could occur if, in binding to gp120, anti-gp120CD4BD Abs trigger conformational changes that allow gp120 to bind to the chemokine receptors. The effect of anti-gp120CD4BD Abs was noted with gp120 from X4 or R5 strains (gp120SF2 and gp120W61D, respectively) (7); hence both CXCR4 and CCR5 receptors may be involved. In support of this mechanism, Misse et al. (20) have shown that gp120 interaction with the chemokine receptor CXCR4 caused endocytosis of gp120 and the coreceptors but that these molecules appeared to reach only the early endosomes and not beyond. Since entry into the early endosomes has been shown to be insufficient for antigen presentation by class II major histocompatibility complex (MHC) (22), gp120 endocytosis via this process presumably would prevent efficient antigen presentation to the CD4 T cells.
The data reported here pose yet another possibility, i.e., that the anti-gp120CD4BD MAbs interfere with intracellular processing of gp120. The CD4 binding domain includes more than 10 amino acid residues scattered over the five constant domains of gp120 (13, 19; S. Zolla-Pazner et al., unpublished data); thus, the anti-gp120CD4BD Abs may protect multiple large fragments of gp120 from proteolytic digestion and prevent the generation of peptides that bind to MHC class II molecules. The capacity of Abs to alter this type of antigen processing has been studied with various antigens, including apo-cytochrome c (4), tetanus toxoid (29), and β-galactosidase (16). Thus, anti-cytochrome c MAbs were reported to protect antigen sites from proteolytic digestion (4). Fragmentation of tetanus toxoid taken up by APCs was altered when this antigen was complexed with antitetanus Abs, and the fragmentation patterns varied depending on the fine specificity of the Abs (6). Earlier studies also reported that some MAbs specific for β-galactosidase, cytochrome c, or tetanus toxoid could alter the processing of the respective antigens, resulting in the suppression of the CD4 T-cell responses to the specific antigens (4, 16, 29, 35). More recently, Antoniou et al. (1) revealed that the initial cleavage of tetanus toxoid antigen at a single proteolytic site was crucial for the subsequent processing and effective presentation of different epitopes in that particular antigen. In view of the heavy glycosylation of gp120, and since the CD4 binding domain appears to be the only sizable surface on gp120 devoid of any known N-linked glycosylation sites (36), this domain may be the key cleavage site accessible to the endosomal proteases. Thus, the binding of anti-gp120CD4BD Abs to gp120 may prevent the initial proteolytic cleavage necessary for efficient processing of gp120. Further studies are under way to investigate this and the other potential effects of anti-gp120CD4BD Abs.
Anti-gp120CD4BD Abs are present in high levels in the sera of most HIV-seropositive subjects (31), and we observed that purified serum IgG from HIV+ subjects also caused significant inhibition of the gp120-specific CD4 T-cell responses (7; Hioe et al., unpublished). Moreover, most of anti-gp120CD4BD MAbs derived from cells of HIV+ subjects have poor or no neutralizing activity against HIV-1 primary isolates, and patients' sera with high titers of these Abs do not exhibit broadly neutralizing activity (3, 8; Chien et al., unpublished data). Thus, one may presume that the generation of high levels of such inhibitory Abs during HIV infection would not be protective and could actually diminish the ability of the infected hosts to induce and maintain strong gp120-specific CD4 T-cell responses. Indeed, the vast majority of HIV-infected individuals exhibit very low or undetectable levels of Env-specific CD4 T-cell responses (2, 25, 33), and impairment of these responses can be observed within 3 months postinfection (21). While the loss of these CD4 T-cell responses is most likely caused by multiple factors, including direct killing by the virus, hyperactivation, and apoptosis, the data presented here suggest that the contribution of Abs such as anti-gp120CD4BD Abs in suppressing gp120 presentation to the CD4 T cells should not be overlooked. It is of interest to point out that the CD4 T-cell response to HIV p24 was also suppressed in the presence of some HIV+ polyclonal Ab samples and certain MAbs to p24 (Hioe et al., unpublished), suggesting that this phenomenon may be pertinent not only to gp120 but also to other HIV antigens.
It is intriguing that, in an earlier study by Mazzoli et al. (18), which compared HIV-seropositive subjects and their HIV-exposed seronegative partners, higher levels of Env-specific Th responses were detected more often among the seronegative than among the seropositive partners. A preliminary study in our lab also found that sera from rare HIV-infected subjects who have remained healthy for more than 10 years postinfection and who consistently exhibit Env-specific lymphoproliferative responses had undetectable or very low titers of anti-gp120CD4BD Abs and lower titers of anti-gp120 Abs in general than those seen in most HIV+ patients (Hioe et al., unpublished). These observations are consistent with the idea that in the absence or at relatively low levels of Abs to gp120, especially the anti-gp120CD4BD Abs, Env-specific CD4 T-cell responses may be retained, leading to more effective control of viremia and disease progression.
Our findings also have significant implications for vaccine design, since induction of suppressive Abs, such as anti-gp120CD4BD Abs, by Env-based AIDS vaccines could down-regulate the Env-specific CD4 T-cell responses that are required to maintain both the long-term memory response and the effector immune functions which are thought to be essential for protection against HIV challenge. While the in vivo significance of the anti-gp120CD4BD Abs needs to be further documented, these studies provide the initial evidence for the role these Abs may play in modulating the CD4 Th responses to HIV in vivo.
ACKNOWLEDGMENTS
We are grateful to Constance Williams for preparing the human MAbs used in the study under the aegis of the NYU Center for AIDS Research Immunology Core (AI 27742) and to Norman Letvin for intellectual contributions to this project.
This work was supported by a Merit Review Entry Program Award from the Department of Veteran Affairs, a Pilot Project Grant from the NYU Center for AIDS Research (C.E.H.), by the Research Center for AIDS and HIV Infection (RCAHI) of the Department of Veteran Affairs, and by NIH grants HL59725 and AI43224 (S.Z.-P.).
REFERENCES
- 1.Antoniou A N, Blackwood S L, Mazzeo D, Watts C. Control of antigen presentation by a single protease cleavage site. Immunity. 2000;12:391–398. doi: 10.1016/s1074-7613(00)80191-0. [DOI] [PubMed] [Google Scholar]
- 2.Borkowsky W, Krasinski K, Moore T, Papaevangelou V. Lymphocyte proliferative responses to HIV-1 envelope and core antigens by infected and uninfected adults and children. AIDS Res Hum Retrovir. 1990;6:673–678. doi: 10.1089/aid.1990.6.673. [DOI] [PubMed] [Google Scholar]
- 3.Cecilia D, Kleeberger C, Munoz A, Giorgi J V, Zolla-Pazner S. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J Infect Dis. 1999;179:1365–1374. doi: 10.1086/314773. [DOI] [PubMed] [Google Scholar]
- 4.Corradin G, Engers H D. Inhibition of antigen-induced T-cell clone proliferation by antigen-specific antibodies. Nature. 1984;308:547–548. doi: 10.1038/308547a0. [DOI] [PubMed] [Google Scholar]
- 5.Cottrez F, Manca F, Dalgleish A G, Arenzana-Seisdedos F, Capron A, Groux H. Priming of human CD4+ antigen-specific T cells to undergo apoptosis by HIV-infected monocytes. A two-step mechanism involving the gp120 molecule. J Clin Investig. 1997;99:257–266. doi: 10.1172/JCI119154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson H W, Watts C. Epitope-directed processing of specific antigen by B lymphocytes. J Cell Biol. 1989;109:85–92. doi: 10.1083/jcb.109.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hioe C E, Jones G J, Rees A D, Ratto-Kim S, Birx D, Munz C, Gorny M K, Tuen M, Zolla-Pazner S. Anti-CD4 binding domain antibodies complexed with HIV-1 gp120 inhibit CD4+ T cell proliferative responses to gp120. AIDS Res Hum Retrovir. 2000;16:893–905. doi: 10.1089/08892220050042837. [DOI] [PubMed] [Google Scholar]
- 8.Hioe C E, Xu S, Chigurupati P, Burda S, Williams C, Gorny M K, Zolla-Pazner S. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int Immunol. 1997;9:1281–1290. doi: 10.1093/intimm/9.9.1281. [DOI] [PubMed] [Google Scholar]
- 9.Jones G J, von Hoegen P, Weber J, Rees A D. Immunization with human immunodeficiency virus type 1 rgp120W61D in QS21/MPL adjuvant primes T cell proliferation and C-C chemokine production to multiple epitopes within variable and conserved domains of gp120W61D. J Infect Dis. 1999;179:558–566. doi: 10.1086/314626. [DOI] [PubMed] [Google Scholar]
- 10.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karwowska S, Gorny M K, Buchbinder A, Gianakakos V, Williams C, Fuerst T, Zolla-Pazner S. Production of human monoclonal antibodies specific for conformational and linear non-V3 epitopes of gp120. AIDS Res Hum Retrovir. 1992;8:1099–1106. doi: 10.1089/aid.1992.8.1099. [DOI] [PubMed] [Google Scholar]
- 12.Karwowska S, Gorny M K, Culpepper S, Burda S, Laal S, Samanich K, Zolla-Pazner S. Similarities and diversity among human monoclonal antibodies to the CD4-binding domain of HIV-1. Vaccines (Cold Spring Harbor) 1993;93:229. [Google Scholar]
- 13.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leandersson A C, Bratt G, Hinkula J, Gilljam G, Cochaux P, Samson M, Sandstrom E, Wahren B. Induction of specific T-cell responses in HIV infection. AIDS. 1998;12:157–166. doi: 10.1097/00002030-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Li C R, Greenberg P D, Gilbert M J, Goodrich J M, Riddell S R. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–1979. [PubMed] [Google Scholar]
- 16.Manca F, Fenoglio D, Kunkl A, Cambiaggi C, Sasso M, Celada F. Differential activation of T cell clones stimulated by macrophages exposed to antigen complexed with monoclonal antibodies. A possible influence of paratope specificity on the mode of antigen processing. J Immunol. 1988;140:2893–2898. [PubMed] [Google Scholar]
- 17.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzoli S, Trabattoni D, Lo Caputo S, Piconi S, Ble C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, Fusi M L, Tofani N, Biasin M, Villa M L, Mazzotta F, Clerici M. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 19.McKeating J A, Thali M, Furman C, Karwowska S, Gorny M K, Cordell J, Zolla-Pazner S, Sodroski J, Weiss R A. Amino acid residues of the human immunodeficiency virus type I gp120 critical for the binding of rat and human neutralizing antibodies that block the gp120-sCD4 interaction. Virology. 1992;190:134–142. doi: 10.1016/0042-6822(92)91199-5. [DOI] [PubMed] [Google Scholar]
- 20.Misse D, Cerutti M, Noraz N, Jourdan P, Favero J, Devauchelle G, Yssel H, Taylor N, Veas F. A CD4-independent interaction of human immunodeficiency virus-1 gp120 with CXCR4 induces their cointernalization, cell signaling, and T-cell chemotaxis. Blood. 1999;93:2454–2462. [PubMed] [Google Scholar]
- 21.Musey L K, Krieger J N, Hughes J P, Schacker T W, Corey L, McElrath M J. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J Infect Dis. 1999;180:278–284. doi: 10.1086/314868. [DOI] [PubMed] [Google Scholar]
- 22.Niebling W L, Pierce S K. Antigen entry into early endosomes is insufficient for MHC class II processing. J Immunol. 1993;150:2687–2697. [PubMed] [Google Scholar]
- 23.Pape G R, Gerlach T J, Diepolder H M, Gruner N, Jung M, Santantonio T. Role of the specific T-cell response for clearance and control of hepatitis C virus. J Viral Hepat. 1999;6(Suppl. 1:36–40. doi: 10.1046/j.1365-2893.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 25.Pontesilli O, Carlesimo M, Varani A R, Ferrara R, Guerra E C, Bernardi M L, Ricci G, Mazzone A M, D'Offizi G, Aiuti F. HIV-specific lymphoproliferative responses in asymptomatic HIV-infected individuals. Clin Exp Immunol. 1995;100:419–424. doi: 10.1111/j.1365-2249.1995.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratto S, Sitz K V, Scherer A M, Manca F, Loomis L D, Cox J H, Redfield R R, Birx D L. Establishment and characterization of human immunodeficiency virus type 1 (HIV-1) envelope-specific CD4+ T lymphocyte lines from HIV-1-seropositive patients. J Infect Dis. 1995;171:1420–1430. doi: 10.1093/infdis/171.6.1420. [DOI] [PubMed] [Google Scholar]
- 27.Siliciano R F, Knall C, Lawton T, Berman P, Gregory T, Reinherz E L. Recognition of HIV glycoprotein gp120 by T cells. Role of monocyte CD4 in the presentation of gp120. J Immunol. 1989;142:1506–1511. [PubMed] [Google Scholar]
- 28.Siliciano R F, Lawton T, Knall C, Karr R W, Berman P, Gregory T, Reinherz E L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988;54:561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- 29.Simitsek P D, Campbell D G, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–1963. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sylwester A W, Grivel J C, Fitzgerald W, Rossio J L, Lifson J D, Margolis L B. CD4(+) T-lymphocyte depletion in human lymphoid tissue ex vivo is not induced by noninfectious human immunodeficiency virus type 1 virions. J Virol. 1998;72:9345–9347. doi: 10.1128/jvi.72.11.9345-9347.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turbica I, Posner M, Bruck C, Barin F. Simple enzyme immunoassay for titration of antibodies to the CD4-binding site of human immunodeficiency virus type 1 gp120. J Clin Microbiol. 1995;33:3319–3323. doi: 10.1128/jcm.33.12.3319-3323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wahren B, Bratt G, Persson C, Leven B, Hinkula J, Gilljam G, Nordlund S, Eriksson L, Volvovitz. Broliden F P A, et al. Improved cell-mediated immune responses in HIV-1-infected asymptomatic individuals after immunization with envelope glycoprotein gp160. J Acquir Immune Defic Syndr. 1994;7:220–229. [PubMed] [Google Scholar]
- 33.Wahren B, Morfeldt-Mansson L, Biberfeld G, Moberg L, Sonnerborg A, Ljungman P, Werner A, Kurth R, Gallo R, Bolognesi D. Characteristics of the specific cell-mediated immune response in human immunodeficiency virus infection. J Virol. 1987;61:2017–2023. doi: 10.1128/jvi.61.6.2017-2023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 35.Watts C, Lanzavecchia A. Suppressive effect of antibody on processing of T cell epitopes. J Exp Med. 1993;178:1459–1463. doi: 10.1084/jem.178.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 37.Zolla-Pazner S, O'Leary J, Burda S, Gorny M K, Kim M, Mascola J, McCutchan F. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J Virol. 1995;69:3807–3815. doi: 10.1128/jvi.69.6.3807-3815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]