Abstract
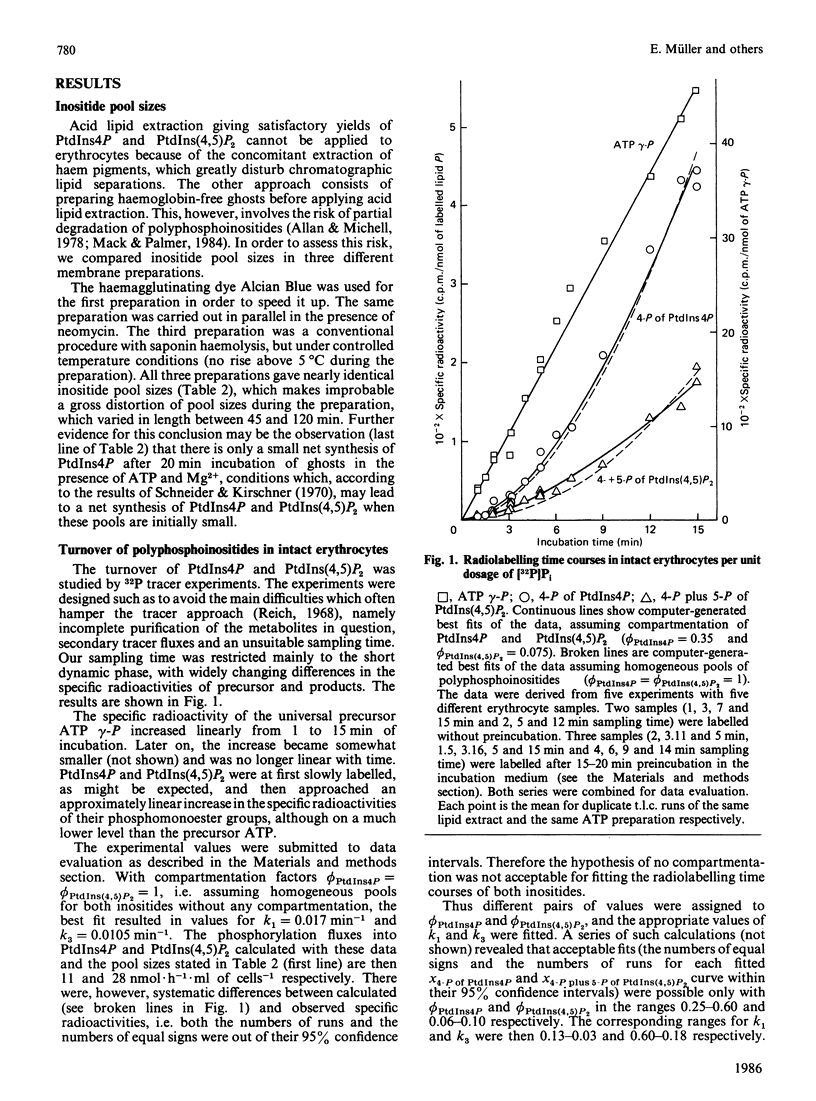
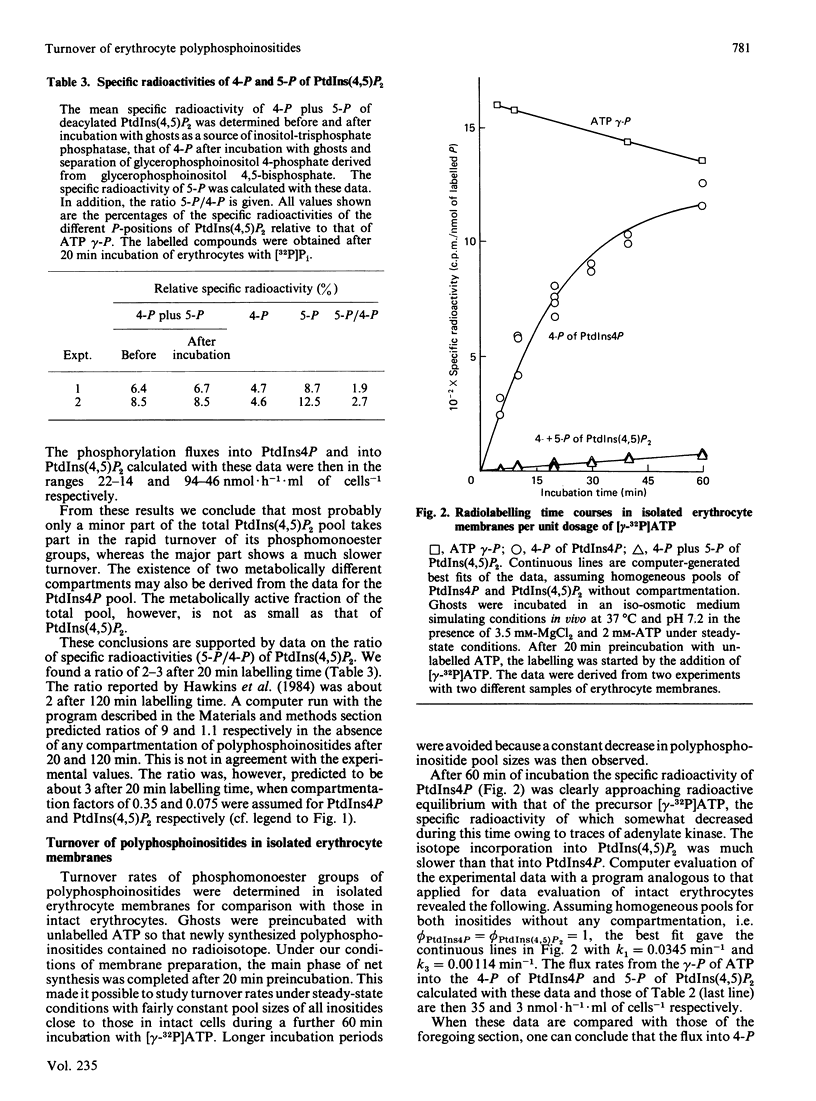
The turnover of phosphomonoester groups of phosphatidylinositol 4-phosphate (PtdIns4P) and phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] was investigated in human erythrocytes by short-term labelling with [32P]Pi. The procedure applied ensured a quantitative extraction of erythrocyte polyphosphoinositides as well as their reliable separation for the determinations of pool sizes and specific radioactivities. The pool sizes of phosphatidylinositol (PtdIns), PtdIns4P and PtdIns(4,5)P2 are 25, 11 and 44 nmol/ml of cells respectively. Under steady-state conditions, the phosphorylation fluxes from [gamma-32P]ATP into PtdIns4P and PtdIns(4,5)P2 are in the ranges 14-22 and 46-94 nmol X h-1 X ml of cells-1 respectively. Only 25-60% of total PtdIns4P and 6-10% of total PtdIns(4,5)P2 take part in the rapid tracer exchange, i.e. are compartmentalized. In isolated erythrocyte ghosts, the turnover of PtdIns4P approximately corresponds to that in intact erythrocytes, although any compartmentation can be excluded in this preparation. Under the conditions of incubation employed, the turnover of PtdIns(4,5)P2 is more than one order of magnitude smaller in isolated ghosts than that obtained for intact erythrocytes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D. Inositol lipids and membrane function in erythrocytes. Cell Calcium. 1982 Oct;3(4-5):451–465. doi: 10.1016/0143-4160(82)90030-6. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. A calcium-activated polyphosphoinositide phosphodiesterase in the plasma membrane of human and rabbit erythrocytes. Biochim Biophys Acta. 1978 Apr 4;508(2):277–286. doi: 10.1016/0005-2736(78)90330-9. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BROCKERHOFF H., BALLOU C. E. On the metabolism of the brain phosphoinositide complex. J Biol Chem. 1962 Jun;237:1764–1766. [PubMed] [Google Scholar]
- BROCKERHOFF H., BALLOU C. E. Phosphate incorporation in brain phosphionositides. J Biol Chem. 1962 Jan;237:49–52. [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- Busch D., Pelz K. Erythrocytenisolierung aus Blut mit Baumwolle. Klin Wochenschr. 1966 Aug 15;44(16):983–984. doi: 10.1007/BF01711475. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud F., M'Zali H., Chailley B., Mazet F. Changes in morphology and in polyphosphoinositide turnover of human erythrocytes after cholesterol depletion. Biochim Biophys Acta. 1984 Nov 21;778(1):191–200. doi: 10.1016/0005-2736(84)90462-0. [DOI] [PubMed] [Google Scholar]
- Halbhuber K. J., Geyer G., Feuerstein H. Agglutinationsverhalten roter Blutzellen nach experimenteller Glykokalyxalteration. Folia Haematol Int Mag Klin Morphol Blutforsch. 1977;104(1):85–97. [PubMed] [Google Scholar]
- Hawkins P. T., Michell R. H., Kirk C. J. Analysis of the metabolic turnover of the individual phosphate groups of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate. Validation of novel analytical techniques by using 32P-labelled lipids from erythrocytes. Biochem J. 1984 Mar 15;218(3):785–793. doi: 10.1042/bj2180785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., McKenna K., Bramhall J. Kinetic studies of human erythrocyte membrane resealing. Biochim Biophys Acta. 1985 Apr 26;815(1):128–134. doi: 10.1016/0005-2736(85)90482-1. [DOI] [PubMed] [Google Scholar]
- Litosch I., Lin S. H., Fain J. N. Rapid changes in hepatocyte phosphoinositides induced by vasopressin. J Biol Chem. 1983 Nov 25;258(22):13727–13732. [PubMed] [Google Scholar]
- Mack S. E., Palmer F. B. Evidence for a specific phosphatidylinositol 4-phosphate phosphatase in human erythrocyte membranes. J Lipid Res. 1984 Jan;25(1):75–85. [PubMed] [Google Scholar]
- Marche P., Koutouzov S., Meyer P. Metabolism of phosphoinositides in the rat erythrocyte membrane. A reappraisal of the effect of magnesium on the 32P incorporation into polyphosphoinositides. Biochim Biophys Acta. 1982 Mar 12;710(3):332–340. doi: 10.1016/0005-2760(82)90116-3. [DOI] [PubMed] [Google Scholar]
- Minenko A., Hajdu I., Oehme P. 32[-Einbau in Polyphosphoinositide von Erythrozyten essentieller Hypertoniker. ?k19H. Acta Biol Med Ger. 1981;40(3):341–344. [PubMed] [Google Scholar]
- Nash G. B., Meiselman H. J. Effects of preparative procedures on the volume and content of resealed red cell ghosts. Biochim Biophys Acta. 1985 May 28;815(3):477–485. doi: 10.1016/0005-2736(85)90376-1. [DOI] [PubMed] [Google Scholar]
- Peterson S. C., Kirschner L. B. Di- and triphosphoinositide metabolism in intact swine erythrocytes. Biochim Biophys Acta. 1970 Mar 10;202(2):295–304. doi: 10.1016/0005-2760(70)90191-8. [DOI] [PubMed] [Google Scholar]
- Quist E. E. Polyphosphoinositide synthesis in rabbit erythrocyte membranes. Arch Biochem Biophys. 1982 Nov;219(1):58–64. doi: 10.1016/0003-9861(82)90133-3. [DOI] [PubMed] [Google Scholar]
- RANDERATH K. A comparison between thin-layer chromatography and paper chromatography of nucleic acid derivatives. Biochem Biophys Res Commun. 1962 Jan 24;6:452–457. doi: 10.1016/0006-291x(62)90374-1. [DOI] [PubMed] [Google Scholar]
- Reich J. G. Analogue computer analysis of tracer flow patterns through the glycolytic and related pathway in erythrocytes and other intact metabolic systems. Eur J Biochem. 1968 Nov;6(3):395–403. doi: 10.1111/j.1432-1033.1968.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Roach P. D., Palmer F. B. Human erythrocyte cytosol phosphatidyl-inositol-bisphosphate phosphatase. Biochim Biophys Acta. 1981 Oct 13;661(2):323–333. doi: 10.1016/0005-2744(81)90021-8. [DOI] [PubMed] [Google Scholar]
- Schick P. K., Schick B. P., Foster K., Block A. Arachidonate synthesis and uptake in isolated guinea-pig megakaryocytes and platelets. Biochim Biophys Acta. 1984 Sep 12;795(2):341–347. doi: 10.1016/0005-2760(84)90084-5. [DOI] [PubMed] [Google Scholar]
- Schneider R. P., Kirscher L. B. Di- and triphosphoinositide metabolism in swine erythrocyte membranes. Biochim Biophys Acta. 1970 Mar 10;202(2):283–294. doi: 10.1016/0005-2760(70)90190-6. [DOI] [PubMed] [Google Scholar]
- Till U., Petermann H., Wenz I., Frunder H. Relations between ion shifting, ATP depletion and lactic acid formation in human red cells during moderate calcium loading using the ionophore A 23187. Acta Biol Med Ger. 1977;36(3-4):597–610. [PubMed] [Google Scholar]


