Abstract
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a thermal breakdown product of a meperidine-like narcotic used by drug abusers as a heroin substitute, produces Parkinsonian symptoms in humans and primates. The nigrostriatal toxicity is not due to MPTP itself but to one or more oxidation products resulting from the action of monoamine oxidase (MAO) on this tertiary allylamine. Both MAO A and B catalyse the oxidation of MPTP to the 1-methyl-4-phenyl-2,3-dihydropyridinium species (MPDP+), which undergoes further oxidation to the fully aromatic 1-methyl-4-phenylpyridinium species (MPP+). These bio-oxidations are blocked by selective inhibitors of MAO A and B. Additionally, MPTP, MPDP+ and MPP+ are competitive inhibitors of MAO A and B. The A form of the enzyme is particularly sensitive to this type of reversible inhibition. Both MAO A and B also are irreversibly inactivated by MPTP and MPDP+, but not by MPP+. This inactivation obeys the characteristics of a mechanism-based or 'suicide' process. The inactivation, which is accompanied by the incorporation of radioactivity from methyl-labelled MPTP, is likely to result from covalent modification of the enzyme.
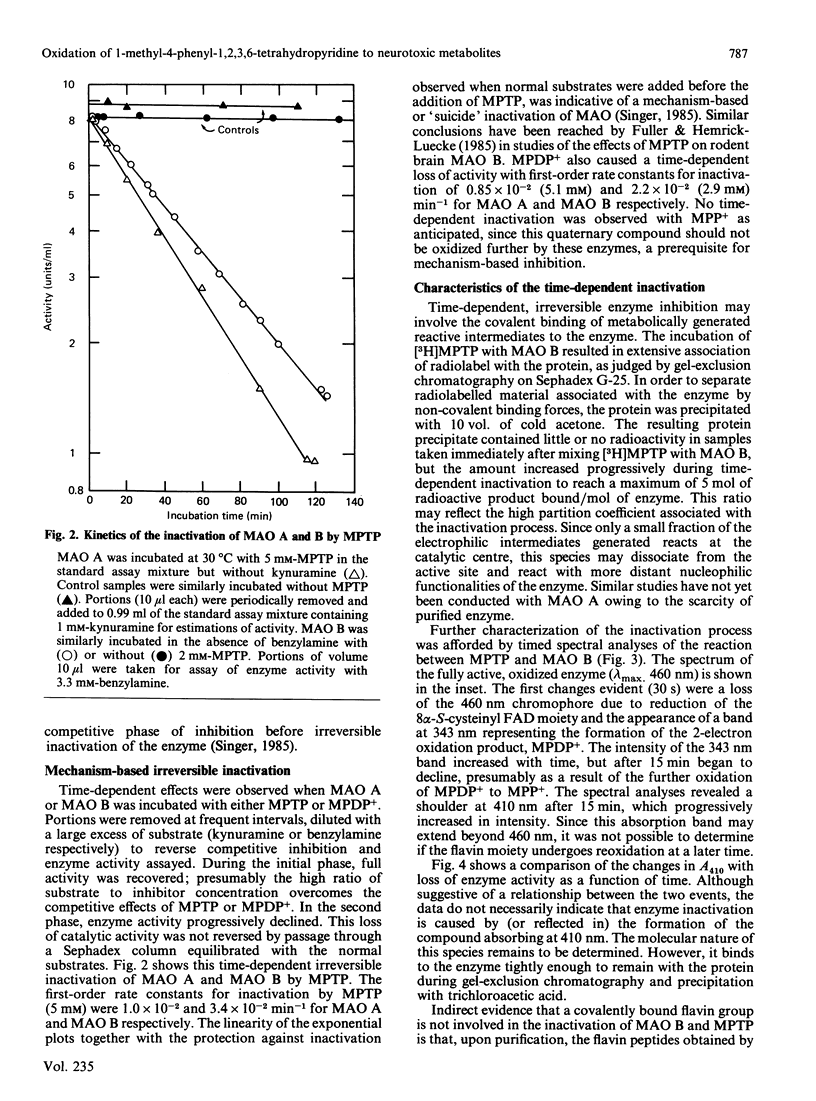
Full text
PDF

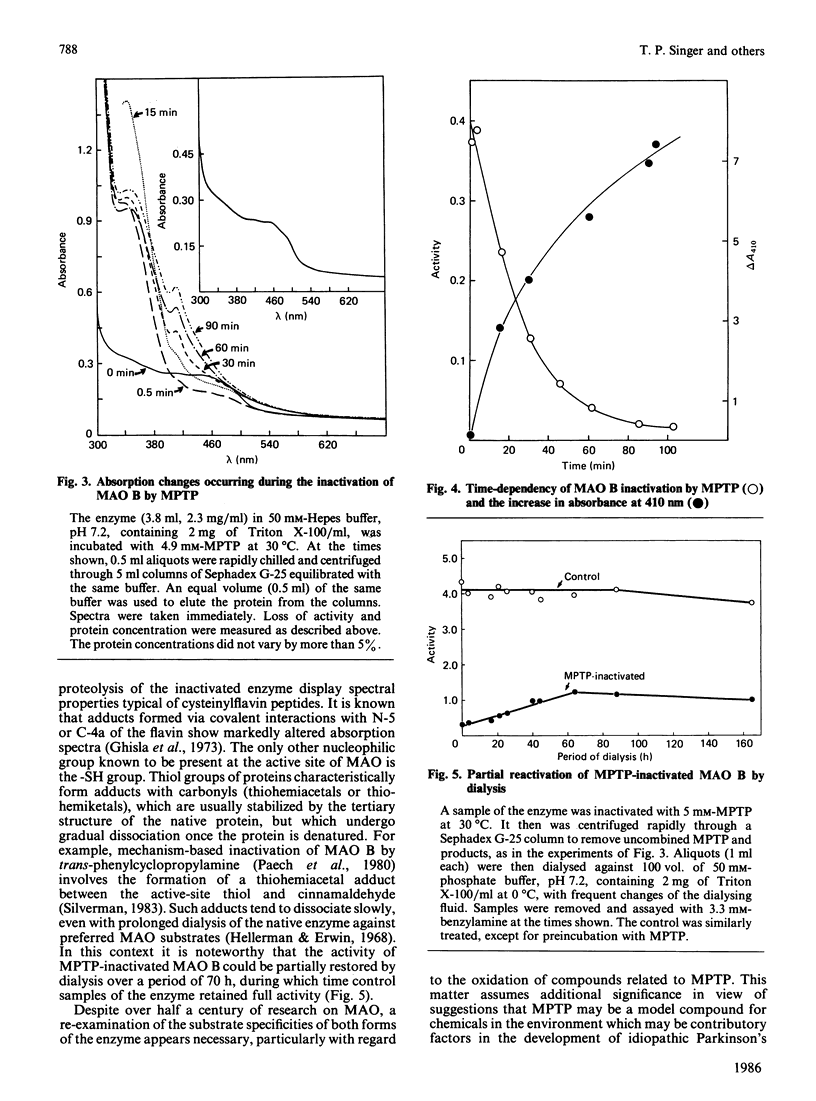

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns R. S., Chiueh C. C., Markey S. P., Ebert M. H., Jacobowitz D. M., Kopin I. J. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne D. B., Langston J. W. Aetiology of Parkinson's disease. Lancet. 1983 Dec 24;2(8365-66):1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Castagnoli N., Jr, Chiba K., Trevor A. J. Potential bioactivation pathways for the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Life Sci. 1985 Jan 21;36(3):225–230. doi: 10.1016/0024-3205(85)90063-3. [DOI] [PubMed] [Google Scholar]
- Chiba K., Peterson L. A., Castagnoli K. P., Trevor A. J., Castagnoli N., Jr Studies on the molecular mechanism of bioactivation of the selective nigrostriatal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Drug Metab Dispos. 1985 May-Jun;13(3):342–347. [PubMed] [Google Scholar]
- Chiba K., Trevor A., Castagnoli N., Jr Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984 Apr 30;120(2):574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Hemrick-Luecke S. K. Inhibition of types A and B monoamine oxidase by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Pharmacol Exp Ther. 1985 Mar;232(3):696–701. [PubMed] [Google Scholar]
- Heikkila R. E., Manzino L., Cabbat F. S., Duvoisin R. C. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984 Oct 4;311(5985):467–469. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- Hellerman L., Erwin V. G. Mitochondrial monoamine oxidase. II. Action of various inhibitors for the bovine kidney enzyme. Catalytic mechanism. J Biol Chem. 1968 Oct 25;243(20):5234–5243. [PubMed] [Google Scholar]
- Javitch J. A., D'Amato R. J., Strittmatter S. M., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata G. Monkey model of Parkinson's disease. Science. 1983 May 13;220(4598):705–705. doi: 10.1126/science.6403987. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Irwin I., Langston E. B., Forno L. S. Pargyline prevents MPTP-induced parkinsonism in primates. Science. 1984 Sep 28;225(4669):1480–1482. doi: 10.1126/science.6332378. [DOI] [PubMed] [Google Scholar]
- Lewin R. Parkinson's disease: an environmental cause? Science. 1985 Jul 19;229(4710):257–258. doi: 10.1126/science.3925554. [DOI] [PubMed] [Google Scholar]
- Nakajima R., Yamazaki I., Griffin B. W. Spectra of chloroperoxidase compounds II and III. Biochem Biophys Res Commun. 1985 Apr 16;128(1):1–6. doi: 10.1016/0006-291x(85)91635-3. [DOI] [PubMed] [Google Scholar]
- Paech C., Salach J. I., Singer T. P. Suicide inactivation of monoamine oxidase by trans-phenylcyclopropylamine. J Biol Chem. 1980 Apr 10;255(7):2700–2704. [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Peterson L. A., Caldera P. S., Trevor A., Chiba K., Castagnoli N., Jr Studies on the 1-methyl-4-phenyl-2,3-dihydropyridinium species 2,3-MPDP+, the monoamine oxidase catalyzed oxidation product of the nigrostriatal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). J Med Chem. 1985 Oct;28(10):1432–1436. doi: 10.1021/jm00148a010. [DOI] [PubMed] [Google Scholar]
- Salach J. I. Monoamine oxidase from beef liver mitochondria: simplified isolation procedure, properties, and determination of its cysteinyl flavin content. Arch Biochem Biophys. 1979 Jan;192(1):128–137. doi: 10.1016/0003-9861(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Salach J. I., Singer T. P., Castagnoli N., Jr, Trevor A. Oxidation of the neurotoxic amine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) by monoamine oxidases A and B and suicide inactivation of the enzymes by MPTP. Biochem Biophys Res Commun. 1984 Dec 14;125(2):831–835. doi: 10.1016/0006-291x(84)90614-4. [DOI] [PubMed] [Google Scholar]
- Salach J. I., Weyler W. Iron content and spectral properties of highly purified bovine liver monoamine oxidase. Arch Biochem Biophys. 1981 Nov;212(1):147–153. doi: 10.1016/0003-9861(81)90353-2. [DOI] [PubMed] [Google Scholar]
- Silverman R. B. Mechanism of inactivation of monoamine oxidase by trans-2-phenylcyclopropylamine and the structure of the enzyme-inactivator adduct. J Biol Chem. 1983 Dec 25;258(24):14766–14769. [PubMed] [Google Scholar]
- Singer T. P., Salach J. I., Crabtree D. Reversible inhibition and mechanism-based irreversible inactivation of monoamine oxidases by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Biochem Biophys Res Commun. 1985 Mar 15;127(2):707–712. doi: 10.1016/s0006-291x(85)80219-9. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., D'Amato R. J. Predicting Parkinson's disease. Nature. 1985 Sep 19;317(6034):198–199. doi: 10.1038/317198a0. [DOI] [PubMed] [Google Scholar]
- Weyler W., Salach J. I. Purification and properties of mitochondrial monoamine oxidase type A from human placenta. J Biol Chem. 1985 Oct 25;260(24):13199–13207. [PubMed] [Google Scholar]


