Abstract
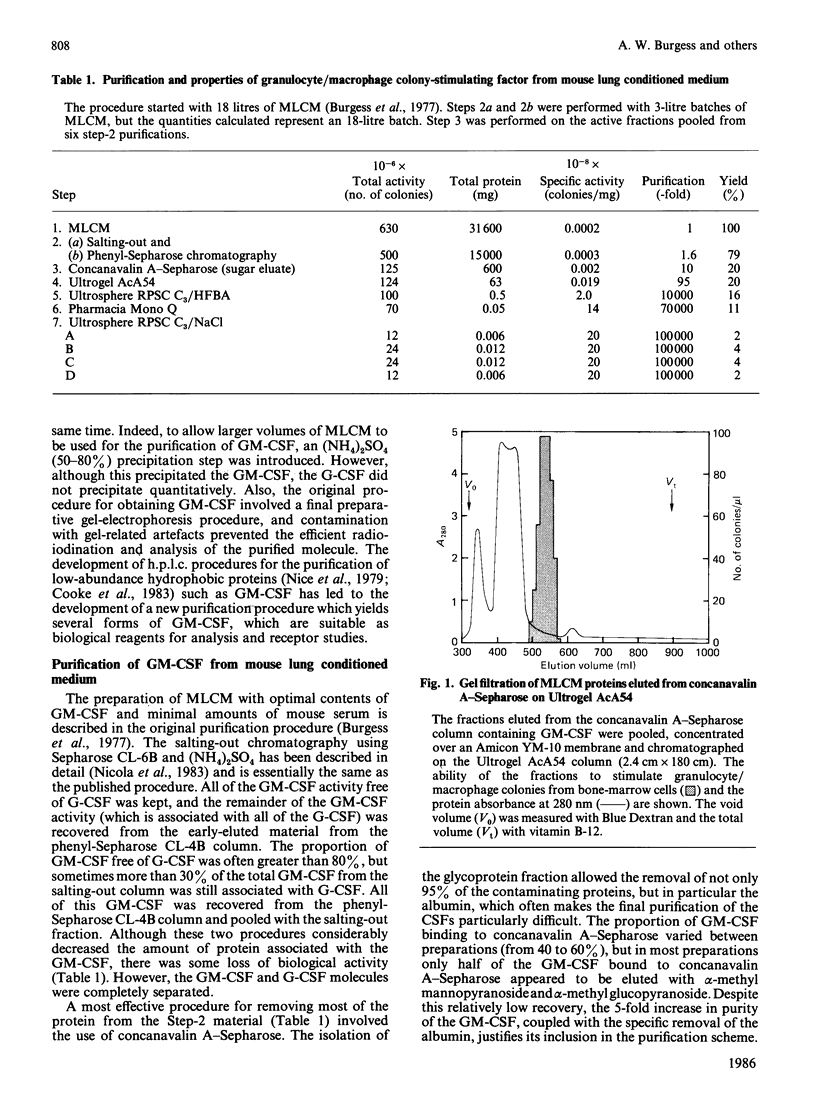
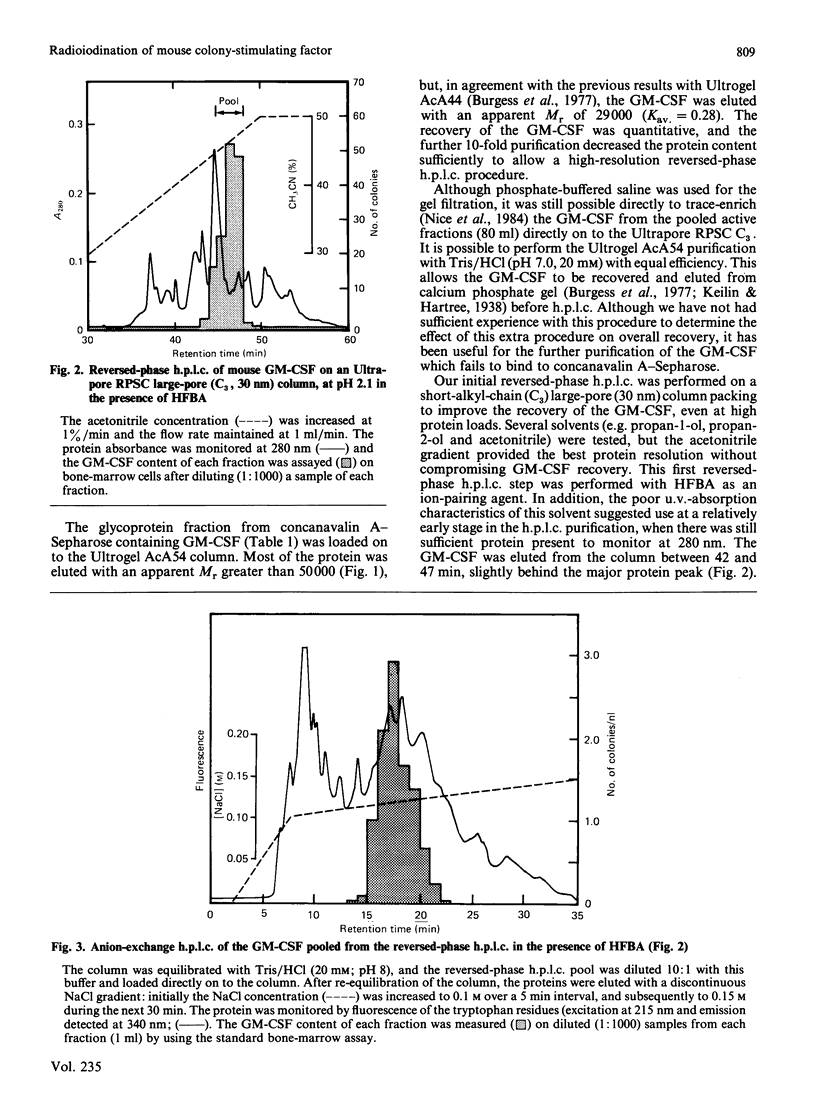
Four forms of mouse granulocyte/macrophage colony-stimulating factor (GM-CSF) were purified 100,000-fold from mouse lung conditioned medium. Each of the CSF species stimulated the formation of both granulocyte and macrophage colonies, and half-maximal stimulation in the semi-solid mouse bone-marrow colony assay occurred at 1 pm. The four GM-CSF species exhibited similar charge microheterogeneity, focusing between pH 4.2 and pH 5.2. On SDS/polyacrylamide gels two of the GM-CSF sub-species had apparent Mr values of 23,000, and the other two, 21, 000. Treatment with neuraminidase decreased the Mr values of these two sets to 21,000 and 19,000 respectively. Incubation with endoglucosidase F decreased the charge heterogeneity and the Mr of all species to 16,500. A gas-phase radioiodination procedure was used to incorporate 2-3 atoms of 125I/molecule into purified GM-CSF without any loss of biological activity. The 125I-labelled GM-CSF was analysed on a microbore reversed-phase h.p.l.c. column to determine its specific radioactivity directly. This 125I-labelled GM-CSF molecule is suitable for cell-surface receptor-binding studies.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Isaka K., Seno T., Tomida M., Yamamoto Y., Hozumi M., Takatsuki A., Tamura G. Effect of tunicamycin on molecular heterogeneity of colony stimulating factor in cultured mouse mammary carcinoma FM3A cells. Biochem Biophys Res Commun. 1979 Oct 12;90(3):783–787. doi: 10.1016/0006-291x(79)91896-5. [DOI] [PubMed] [Google Scholar]
- Bazill G. W., Haynes M., Garland J., Dexter T. M. Characterization and partial purification of a haemopoietic cell growth factor in WEHI-3 cell conditioned medium. Biochem J. 1983 Mar 15;210(3):747–759. doi: 10.1042/bj2100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Burgess A. W., Knesel J., Sparrow L. G., Nicola N. A., Nice E. C. Two forms of murine epidermal growth factor: rapid separation by using reverse-phase HPLC. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5753–5757. doi: 10.1073/pnas.79.19.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Lloyd C. J., Nice E. C. Murine epidermal growth factor: heterogeneity on high resolution ion-exchange chromatography. EMBO J. 1983;2(11):2065–2069. doi: 10.1002/j.1460-2075.1983.tb01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D. Characterization of a serum factor stimulating the differentiation of myelomonocytic leukemic cells. Int J Cancer. 1980 Nov 15;26(5):647–654. doi: 10.1002/ijc.2910260517. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Russell S. H., Nicola N. A. Granulocyte/macrophage-, megakaryocyte-, eosinophil- and erythroid-colony-stimulating factors produced by mouse spleen cells. Biochem J. 1980 Feb 1;185(2):301–314. doi: 10.1042/bj1850301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D. Serum half-life and organ distribution of radiolabeled colony stimulating factor in mice. Exp Hematol. 1977 Nov;5(6):456–464. [PubMed] [Google Scholar]
- Das S. K., Stanley E. R. Structure-function studies of a colony stimulating factor (CSF-1). J Biol Chem. 1982 Nov 25;257(22):13679–13684. [PubMed] [Google Scholar]
- Dexter T. M. Blood cell development. The message in the medium. 1984 Jun 28-Jul 4Nature. 309(5971):746–747. doi: 10.1038/309746a0. [DOI] [PubMed] [Google Scholar]
- Gough E. J., Gough N. M. Direct calculation of the sizes of DNA fragments separated by gel electrophoresis using programmes written for a pocket calculator. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):845–853. doi: 10.1093/nar/12.1part2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Gough J., Metcalf D., Kelso A., Grail D., Nicola N. A., Burgess A. W., Dunn A. R. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. 1984 Jun 28-Jul 4Nature. 309(5971):763–767. doi: 10.1038/309763a0. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980 Apr;85(1):153–159. doi: 10.1083/jcb.85.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lotem J., Lipton J. H., Sachs L. Separation of different molecular forms of macrophage- and granulocyte-inducing proteins for normal and leukemic myeloid cells. Int J Cancer. 1980 Jun 15;25(6):763–771. doi: 10.1002/ijc.2910250612. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Clonal extinction of myelomonocytic leukemic cells by serum from mice injected with endotoxin. Int J Cancer. 1980 Feb 15;25(2):225–233. doi: 10.1002/ijc.2910250210. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nice E. C., Capp M., O'Hare M. J. Use of hydrophobic interaction methods in the isolation of proteins from endocrine and paraendocrine tissues and cells by high-performance liquid chromatography. J Chromatogr. 1979 Dec 20;185:413–427. doi: 10.1016/s0021-9673(00)85618-6. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Metcalf D. Similar molecular properties of granulocyte-macrophage colony-stimulating factors produced by different mouse organs in vitro and in vivo. J Biol Chem. 1979 Jun 25;254(12):5290–5299. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Binding of the differentiation-inducer, granulocyte-colony-stimulating factor, to responsive but not unresponsive leukemic cell lines. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3765–3769. doi: 10.1073/pnas.81.12.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Biochemical properties of differentiation factors for murine myelomonocytic leukemic cells in organ conditioned media--separation from colony-stimulating factors. J Cell Physiol. 1981 Nov;109(2):253–264. doi: 10.1002/jcp.1041090208. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Nice E. C. In situ cyanogen bromide cleavage of N-terminally blocked proteins in a gas-phase sequencer. Biochem Int. 1984 Jun;8(6):787–791. [PubMed] [Google Scholar]
- Simpson R. J., Smith J. A., Moritz R. L., O'Hare M. J., Rudland P. S., Morrison J. R., Lloyd C. J., Grego B., Burgess A. W., Nice E. C. Rat epidermal growth factor: complete amino acid sequence. Homology with the corresponding murine and human proteins; isolation of a form truncated at both ends with full in vitro biological activity. Eur J Biochem. 1985 Dec 16;153(3):629–637. doi: 10.1111/j.1432-1033.1985.tb09346.x. [DOI] [PubMed] [Google Scholar]
- Sparrow L. G., Metcalf D., Hunkapiller M. W., Hood L. E., Burgess A. W. Purification and partial amino acid sequence of asialo murine granulocyte-macrophage colony stimulating factor. Proc Natl Acad Sci U S A. 1985 Jan;82(2):292–296. doi: 10.1073/pnas.82.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Tejedor F., Ballesta J. P. Iodination of biological samples without loss of functional activity. Anal Biochem. 1982 Nov 15;127(1):143–149. doi: 10.1016/0003-2697(82)90156-7. [DOI] [PubMed] [Google Scholar]
- Walker F., Burgess A. W. Specific binding of radioiodinated granulocyte-macrophage colony-stimulating factor to hemopoietic cells. EMBO J. 1985 Apr;4(4):933–939. doi: 10.1002/j.1460-2075.1985.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





