Abstract
Complement receptor type 1 (CR1) is a glycoprotein of Mr about 250 000 present on erythrocytes and other cell types. CR1 acts as a cofactor in the factor I-mediated breakdown of complement fragment C3b to form iC3b. Using an assay of cofactor activity, a wide variation in mean CR1 levels between erythrocytes from individual donors is observed. CR1 levels also decrease on ageing of erythrocytes in vivo, and again the rate of loss is widely variable between individuals. However, variable loss of CR1 during ageing of erythrocytes is likely to make only a minor contribution to the observed variation in mean CR1 levels. CR1 is very sensitive to proteolysis, and random proteolytic removal of CR1 from erythrocytes is likely to be an important factor in loss of CR1 on ageing of red cells in vivo. In vitro, mild trypsin treatment, plasmin or thrombin digestion of erythrocytes results in the loss of the factor I cofactor activity from the cell surface, and appearance of this activity in the supernatant. We conclude that an active fragment of CR1 is released from the cell surface on proteolysis. Subsequent prolonged trypsin treatment destroys most of the activity of this fragment. Proteolytic removal of CR1 from red cells may account not only for loss on ageing of cells, but also for the acquired CR1 deficiencies observed by others in systemic lupus erythematosus.
Full text
PDF

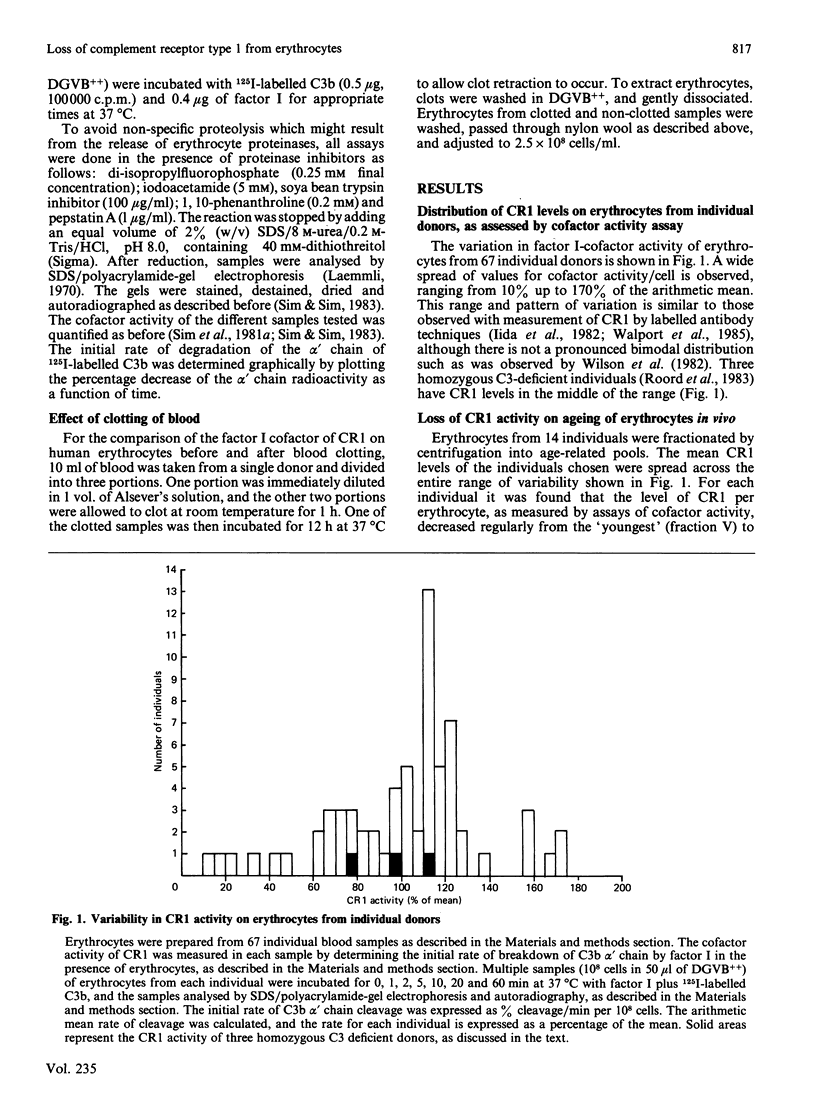
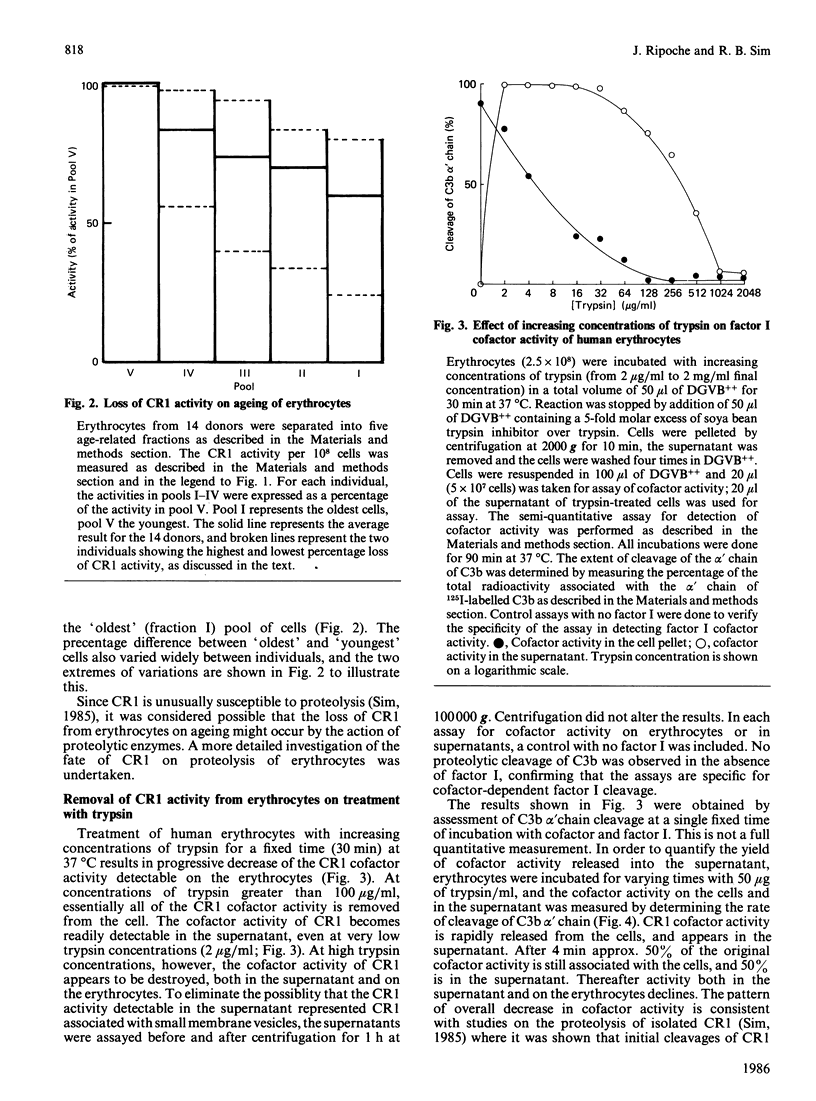
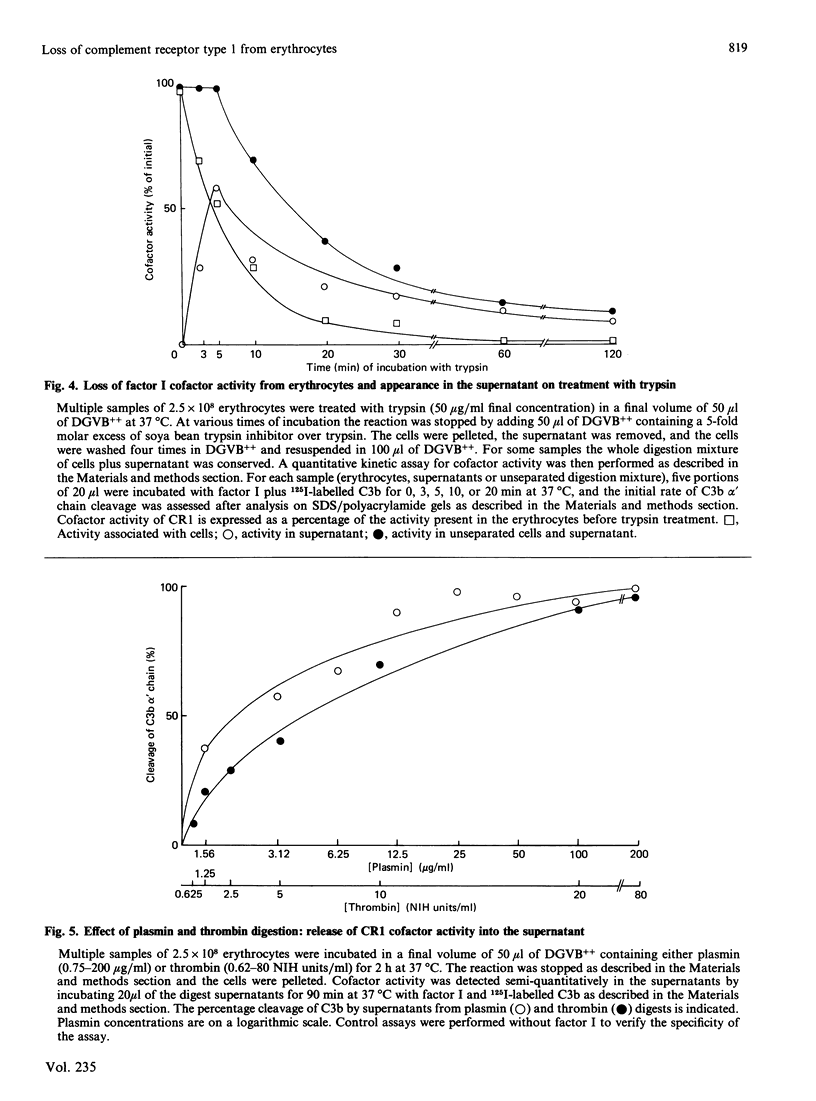


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Melamed J., Tack B. F., Colten H. R. Characterization of the human complement (c3b) receptor with a fluid phase C3b dimer. J Immunol. 1981 Oct;127(4):1348–1354. [PubMed] [Google Scholar]
- Cornacoff J. B., Hebert L. A., Smead W. L., VanAman M. E., Birmingham D. J., Waxman F. J. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983 Feb;71(2):236–247. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Harrison R. A. Structural characterization of factor I mediated cleavage of the third component of complement. Biochemistry. 1982 Nov 9;21(23):5745–5749. doi: 10.1021/bi00266a003. [DOI] [PubMed] [Google Scholar]
- Dykman T. R., Hatch J. A., Aqua M. S., Atkinson J. P. Polymorphism of the C3b/C4b receptor (CR1): characterization of a fourth allele. J Immunol. 1985 Mar;134(3):1787–1789. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. The physiological breakdown of the third component of human complement. Mol Immunol. 1980 Jan;17(1):9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Hsiung L., Barclay A. N., Brandon M. R., Sim E., Porter R. R. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982 Apr 1;203(1):293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Mornaghi R., Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982 May 1;155(5):1427–1438. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOPSTOCK A., SCHWARTZ J., BLEIBERG Y., ADAM A., SZEINBERG A. HEREDITARY NATURE OF THE BEHAVIOUR OF ERYTHROCYTES IN THE IMMUNE ADHERENCE HAEMAGGLUTINATION PHENOMENON. Vox Sang. 1965 Mar-Apr;10:177–187. doi: 10.1111/j.1423-0410.1965.tb04335.x. [DOI] [PubMed] [Google Scholar]
- Kadlubowski M., Agutter P. S. Changes in the activities of some membrane-associated enzymes during in vivo ageing of the normal human erythrocyte. Br J Haematol. 1977 Sep;37(1):111–125. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Yamada A., Kosaka K., Tsuda F., Kosugi E., Mayumi M. Defective immune-adherence (C3b) receptor on erythrocytes from patients with systemic lupus erythematosus. Lancet. 1981 Sep 5;2(8245):493–497. doi: 10.1016/s0140-6736(81)90882-5. [DOI] [PubMed] [Google Scholar]
- Murphy J. R. Influence of temperature and method of centrifugation on the separation of erythrocytes. J Lab Clin Med. 1973 Aug;82(2):334–341. [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITMAN S., FRANKEL S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957 Jul;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Reid K. B. Proteins involved in the activation and control of the two pathways of human complement. Biochem Soc Trans. 1983 Jan;11(1):1–12. doi: 10.1042/bst0110001. [DOI] [PubMed] [Google Scholar]
- Roord J. J., Daha M., Kuis W., Verbrugh H. A., Verhoef J., Zegers B. J., Stoop J. W. Inherited deficiency of the third component of complement associated with recurrent pyogenic infections, circulating immune complexes, and vasculitis in a Dutch family. Pediatrics. 1983 Jan;71(1):81–87. [PubMed] [Google Scholar]
- Ross G. D., Yount W. J., Walport M. J., Winfield J. B., Parker C. J., Fuller C. R., Taylor R. P., Myones B. L., Lachmann P. J. Disease-associated loss of erythrocyte complement receptors (CR1, C3b receptors) in patients with systemic lupus erythematosus and other diseases involving autoantibodies and/or complement activation. J Immunol. 1985 Sep;135(3):2005–2014. [PubMed] [Google Scholar]
- Sim E., Sim R. B. Binding of fluid-phase complement components C3 and C3b to human lymphocytes. Biochem J. 1981 Sep 15;198(3):509–518. doi: 10.1042/bj1980509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E., Sim R. B. Enzymic assay of C3b receptor on intact cells and solubilized cells. Biochem J. 1983 Feb 15;210(2):567–576. doi: 10.1042/bj2100567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E., Wood A. B., Hsiung L. M., Sim R. B. Pattern of degradation of human complement fragment, C3b. FEBS Lett. 1981 Sep 14;132(1):55–60. doi: 10.1016/0014-5793(81)80426-7. [DOI] [PubMed] [Google Scholar]
- Sim R. B. Large-scale isolation of complement receptor type 1 (CR1) from human erythrocytes. Proteolytic fragmentation studies. Biochem J. 1985 Dec 15;232(3):883–889. doi: 10.1042/bj2320883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Twose T. M., Paterson D. S., Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981 Jan 1;193(1):115–127. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walport M. J., Ross G. D., Mackworth-Young C., Watson J. V., Hogg N., Lachmann P. J. Family studies of erythrocyte complement receptor type 1 levels: reduced levels in patients with SLE are acquired, not inherited. Clin Exp Immunol. 1985 Mar;59(3):547–554. [PMC free article] [PubMed] [Google Scholar]
- Wenzel H. R., Engelbrecht S., Reich H., Mondry W., Tschesche H. Synthesis and analytical use of 3-carboxypropionyl-alanyl-alanyl-valine-4-nitroanilide: a specific substrate for human leukocyte elastase. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1413–1416. doi: 10.1515/bchm2.1980.361.2.1413. [DOI] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. G., Jack R. M., Wong W. W., Schur P. H., Fearon D. T. Autoantibody to the C3b/C4b receptor and absence of this receptor from erythrocytes of a patient with systemic lupus erythematosus. J Clin Invest. 1985 Jul;76(1):182–190. doi: 10.1172/JCI111944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. G., Wong W. W., Schur P. H., Fearon D. T. Mode of inheritance of decreased C3b receptors on erythrocytes of patients with systemic lupus erythematosus. N Engl J Med. 1982 Oct 14;307(16):981–986. doi: 10.1056/NEJM198210143071604. [DOI] [PubMed] [Google Scholar]
- Wong W. W., Wilson J. G., Fearon D. T. Genetic regulation of a structural polymorphism of human C3b receptor. J Clin Invest. 1983 Aug;72(2):685–693. doi: 10.1172/JCI111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. H., Fearon D. T. Characterization of a soluble form of the C3b/C4b receptor (CR1) in human plasma. J Immunol. 1985 May;134(5):3332–3338. [PubMed] [Google Scholar]


