Abstract
Background
Previous research has shown that combining tyrosine kinase inhibitors (TKIs) with immunotherapy results in synergistic clinical efficacy. Cadonilimab, the first approved bi-specific antibody targeting PD-1 and CTLA-4, was studied to evaluate its efficacy and safety in combination with Lenvatinib as a first-line treatment for patients with unresectable hepatocellular carcinoma (uHCC).
Methods
A retrospective study was conducted on 29 uHCC patients diagnosed at Nanfang Hospital, Southern Medical University, between July 7, 2022, and March 3, 2023. Patients received Cadonilimab (10 mg/kg, IV, every 3 weeks) combined with Lenvatinib (8 mg, orally, daily). The primary endpoint was the objective response rate (ORR), with secondary endpoints including disease control rate (DCR), median progression-free survival (mPFS), median overall survival (mOS), median time to progression (mTTP), and safety.
Results
By April 2023, 29 patients had been enrolled in the study. The ORR was 37.9 %, DCR was 82.8 %, mPFS was 8.1 months, mTTP was 8.2 months, and mOS was not reached. A total of 93.1 % of patients experienced at least one treatment-related adverse event (TRAE). The most common adverse events were weight loss (51.7 %), increased aspartate aminotransferase (48.3 %), leukocytopenia (48.3 %), and neutropenia (48.3 %). TRAEs of grade 3 or higher occurred in 51.7 % of patients, with no grade 4 TRAEs observed.
Conclusion
This study demonstrated the efficacy and safety of this combination, potentially improving outcomes as a first-line therapy, and offering a novel therapeutic approach for advanced HCC.
Keywords: Hepatocellular carcinoma, Cadonilimab, Lenvatinib, Bi-specific antibody
1. Introduction
Hepatocellular carcinoma (HCC), ranking as the fourth most prevalent malignancy and the second leading cause of cancer-related mortality in China, posing significant global health challenges. In China, HCC is often diagnosed at an advanced stage due to prevalent risk factors like chronic hepatitis B and C infections, liver cirrhosis, and aflatoxin exposure. In recent years, systemic treatments including targeted therapy, immune checkpoint inhibitors (ICIs), and combination regimens have shown promising outcomes in extending survival and delaying disease progression in patients with unresectable HCC (uHCC) [1]. The combination of ICIs and targeted therapy is commonly used in uHCC. Currently, first-line combination regimens in China include atezolizumab plus bevacizumab, camrelizumab plus rivoceranib, and sintilimab plus a bevacizumab biosimilar. Based on current clinical data, the ORR is 20%–30 %, mPFS is approximately 4.6–8.2 months, and mOS is up to 22.1 months [[2], [3], [4]]. However, the efficacy remains modest, necessitating the exploration of better treatment options.
I In addition to combining immunotherapy with targeted therapy, dual immunotherapy options are also available. Dual immune checkpoint inhibitors, which work by simultaneously targeting two immune checkpoint proteins like programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), are an exciting new development in the field of cancer immunotherapy due to the synergistic effect of the two targets [5]. Multiple clinical trials have examined the efficacy and safety of combination immunotherapy using dual checkpoint inhibitors in HCC. The HIMALAYA study has demonstrated promising outcomes, the ORR was 20.1 %, mPFS was 3.8 months, and mOS was 16.4 months [6]. Although dual combination regimens improve efficacy, they also raise safety concerns. The dose-limiting toxicity associated with CTLA-4 inhibitors presents limitations in combination treatment protocols [[6], [7], [8]].
Cadonilimab, a bi-specific antibody targeting dual inhibitory checkpoints (PD-1 and CTLA-4), has been approved in China for the treatment of patients with relapsed or metastatic cervical cancer (r/mCC) who have progressed during or after platinum-based chemotherapy [9]. Cadonilimab has already been shown to be well tolerated and have a promise activity in a phase 1a/1b first-in-human study (COMPASSION-01) [10]. A phase 1b/2 trial (COMPASSION-01) further investigated the dose and efficacy in solid tumors, including patients with HCC [11]. Meanwhile, as a medication with two targets, cadonilimab demonstrated expanded potential combination therapy possibilities. In the Phase II clinical trial (AK104-206) of Cadonilimab combined with Lenvatinib for first-line HCC treatment, superior efficacy and safety were observed, with an ORR of 35.5%–35.7 % and mPFS of 8.6–9.8 months [12].
In this study, we performed a retrospective cohort analysis of this regimen for HCC patients in China to reflect therapeutic reality through real-world data and share our experience treating advanced HCC using the strategy mentioned above.
2. Patients and methods
2.1. Study design and patients
All data, including medical records and imaging data, were collected retrospectively from 29 patients with uHCC who underwent Cadonilimab (10 mg/kg, IV, Q3W) in combination with Lenvatinib (8 mg, PO, QD) as first-line therapy at Nanfang Hospital, Southern Medical University between July 7th, 2022 and March 3rd, 2023. Key inclusion criteria [1]: age between 18 and 75 years [2]; no prior systemic therapy [3]; Barcelona Clinical Liver Cancer (BCLC) stages B/C [4]; Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0–2 [5]; Child-Pugh class A/B [6]; at least one evaluable lesion [7]; not received anti-PD-L1/PD-1 and anti-CTLA-4 therapy. The approval for this retrospective study was granted by the Nanfang Hospital, Southern Medical University Ethical Committee (NFEC-2021-044).
2.2. Endpoints and assessments
The primary endpoint was objective response rate (ORR), and secondary endpoints included disease control rate (DCR), median progression-free survival (m.
PFS), median overall survival (mOS), and safety. ORR and DCR were assessed using RECIST 1.1 [13]. The ORR was defined as the proportion of participants with complete response (CR) or partial response (PR), while the DCR as CR, PR, or stable disease (SD). PFS was defined as the time from the date of the first administration until radiological disease progression or death, whichever came first. OS was defined as the period from the start of this therapy until the date of death. TTP was defined as the time from the date of the first administration to radiological disease progression. Meanwhile, the incidence and severity of adverse events during the antitumor procedure were assessed using version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE).
2.3. Statistical analysis
This study was designed as a retrospective study, in which patients were followed until death or last contact. Data on baseline characteristics, radiological tumor response, and AEs were summarized using descriptive statistics. Continuous variables were compared by Student's t-test or Mann-Whitney U test. For categorical variables, the frequencies (%) are shown, and the chi-square test or Fisher's exact was used to compare them. Survival curves were determined using the Kaplan-Meier method. Statistical analyses were performed using IBM SPSS Statistics version 25.0. P < 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
In this study, 29 cases were collected in total. Baseline characteristics are summarized in Table 1. With a mean age of 51, the majority of them were male (93.1 %). 23 patients (79.3 %) had three or more lesions, whereas 44.8 % of the patients had alpha-fetoprotein (AFP) values equal to or exceeding 400 ng/ml. Furthermore, the majority of patients (96.6 %) were in Barcelona Clinical Liver Cancer (BCLC) Stage C and had Child-Pugh class A liver function reserve (62.1 %).
Table 1.
Baseline patient characteristics.
| Characteristics | All (n = 29) |
|---|---|
| Age (y) | 51 ± 11.8 |
| Gender | |
| Men, n (%) | 27 (93.1) |
| Family history of HCC | |
| Present, n (%) | 3 (10.3) |
| Etiology | |
| HBV infection, n (%) | 26 (89.7) |
| HCV infection, n (%) | 1 (3.4) |
| Non-viral hepatitis, n (%) | 2 (6.9) |
| Diabetes mellitus | |
| Present, n (%) | 5 (17.2) |
| ECOG performance | |
| 0, n (%) | 19 (65.5) |
| 1, n (%) | 7 (24.1) |
| 2, n (%) | 3 (10.3) |
| BCLC | |
| B, n (%) | 1 (3.4) |
| C, n (%) | 28 (96.6) |
| Child-Pugh grade | |
| A, n (%) | 18 (62.1) |
| B, n (%) | 11 (37.9) |
| Tumor number | |
| <3 nodules, n (%) | 6 (20.7) |
| ≥3 nodules, n (%) | 23 (79.3) |
| Maximum Tumor diameter (mm) | 67.2 ± 44.1 |
| Extrahepatic metastasis | |
| Present, n (%) | 21 (72.4) |
| PVTT | |
| Present, n (%) | 17 (58.6) |
| AFP (ng/ml) | |
| <400 | 16 (55.2) |
| ≥400 | 13 (44.8) |
Note.—BCLC, Barcelona-Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; AFP, α-fetoprotein; PVTT, Portal Vein Tumor Thrombosis.
3.2. Efficacy
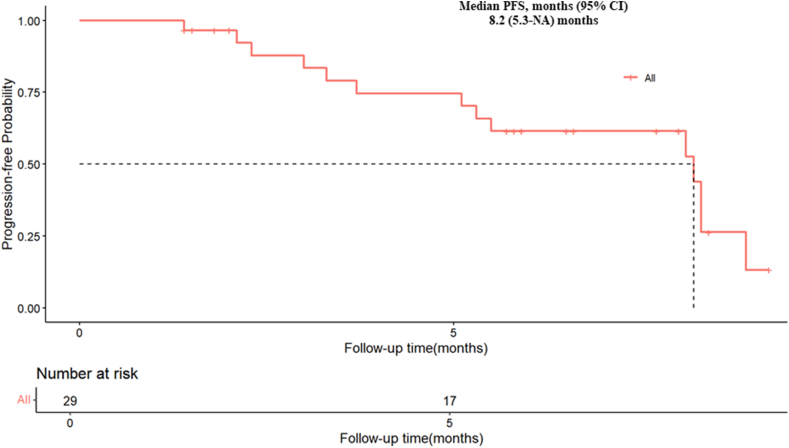
As of the data cutoff in April 2023, a total of 29 patients were enrolled in this study. The ORR was 37.9 % (11/29). The DCR was 82.8 % (24/29), as shown in Table 2. The percentage of best response for target lesion from baseline was presented in Fig. 1 and the efficacy assessment of 29 patients during their whole treatment was shown in Fig. 2. In the whole study population, the mPFS was 8.1 months (95 % CI 5.3-NA) and the mOS was not reached (95 % CI 7.97-NA), and the mTTP was 8.2 months (95 % CI 5.3-NA). The survival analysis data were presented in Fig. 3, Fig. 4, Fig. 5.
Table 2.
Tumor responses.
| Tumor response, n (%) | All (n = 29) |
|---|---|
| Partial response (PR) | 11 (37.9) |
| Stable disease (SD) | 13 (44.8) |
| Progressive disease (PD) | 5 (17.2) |
| ORR (CR + PR) | 11 (37.9) |
| DCR (CR + PR + SD) | 24 (82.8) |
DCR, disease control rate; ORR, objective response rate.
Fig. 1.
Percentage of best response for target lesion from baseline.
Fig. 2.
Spider Plot of change for target lesion from baseline.
Fig. 3.
Kaplan–Meier plots of PFS time.
Fig. 4.
Kaplan–Meier plots of OS time.
Fig. 5.
Kaplan–Meier plots of TTP.
3.3. Safety
As shown in Table 3, 27 (93.1 %) patients reported treatment-related adverse events (TRAEs), and 15 patients (51.7 %) had at least one grade-3 TRAE. The following TRAEs with the highest frequency of any grade were weight loss (51.7 %), aspartate aminotransferase increased (48.3 %), leukocytopenia (48.3 %), neutropenia (48.3 %), thrombocytopenia (41.4 %), hypertension (41.4 %) and proteinuria (41.4 %). The most prevalent TRAEs of grade 3 were neutropenia (10.3 %) and proteinuria (10.3 %). None of the patients observed exhibited grade 4 TRAEs.
Table 3.
Treatment related adverse events (TRAE) ≥10 %.
| Effects | All grades | Grade ≥3 |
|---|---|---|
| At least one TRAE | 27 (93.1) | 15 (51.7) |
| Alanine aminotransferase increased | 8 (27.6) | 2 (6.7) |
| Aspartate aminotransferase increased | 14 (48.3) | 2 (6.7) |
| Thrombocytopenia | 12 (41.4) | 1 (3.4) |
| Leukocytopenia | 14 (48.3) | 2 (6.7) |
| Neutropenia | 14 (48.3) | 3 (10.3) |
| Diarrhea | 6 (20.7) | 0 (0.0) |
| Fatigue | 11 (37.9) | 0 (0.0) |
| Fever | 5 (17.2) | 2 (6.7) |
| Hyperbilirubinemia | 9 (31.0) | 1 (3.4) |
| Hypertension | 12 (41.4) | 0 (0.0) |
| Hypothyroidism | 8 (27.6) | 0 (0.0) |
| Nausea | 9 (31.0) | 1 (3.4) |
| Proteinuria | 12 (41.4) | 3 (10.3) |
| Rash | 9 (31.0) | 0 (0.0) |
| Weight loss | 15 (51.7) | 0 (0.0) |
4. Discussion
In this study, the ORR was 37.9 %. The DCR was 82.8 %, the mPFS and mOS were 8.1 months and not reached, respectively. A total of 27 patients (93.1 %) reported TRAEs, with 15 patients (51.7 %) experiencing at least one grade-3 TRAE. None of the patients observed exhibited grade 4 TRAEs. To our knowledge, this was the first study to report the PD-1/CTLA-4 bispecific antibody in a real-world setting for uHCC. We retrospectively evaluated the efficacy and safety of cadonilimab in combination with lenvatinib in patients with uHCC as first-line treatment.
Currently, the combination of PD-1 or PD-L1 antibody immunotherapy with vascular endothelial growth factor (VEGF) inhibitors is the primary first-line treatment for uHCC [[2], [3], [4]]. However, the ORR in previous studies was 20%–30 %, whereas in this study, it was 37.9 %. Additionally, dual checkpoint inhibitor combination therapy is another primary option. Combinations of CTLA-4 and PD-(L)1 inhibitors have demonstrated additive antitumor activity along with complementary immunostimulatory effects in HIMALAYA and Checkmate-040 studies [7,8]. In the HIMALAYA (NCT03298451) study, the ORR was 20.1 %, the mPFS was 3.8 months, and the mOS was 16.4 months. Our study demonstrates more inspiring outcomes, with an ORR of 37.9 % and an mPFS of 8.1 months. As the CTLA-4 inhibitor is dose-dependent, the T75 + D regimen did not achieve the expected efficacy in the previous Phase 2 trial [6]. The STRIDE regimen (T300 + D), as a single, high priming dose of tremelimumab plus durvalumab, demonstrated encouraging clinical activity and safety [7]. However, this regimen still faces constraints in the efficacy due to CTLA-4 dose safety restrictions and combination limitations. Grade 3/4 treatment-emergent adverse events occurred for 50.5 % of patients with combination therapy in the HIMALAYA, which was similar to the result of this study. This indicates that the combination of cadonilimab and lenvatinib did not increase safety risks.
Cadonilimab, unlike other immune checkpoint inhibitors, offers a significant advantage as a humanized IgG1 bispecific antibody, binding simultaneously to both PD-1 and CTLA-4. Approved by the NMPA in June 2022 for recurrent or metastatic cervical cancer, cadonilimab paves the way for more combination possibilities with angiogenesis inhibitors [9]. A multicenter, multi-cohort phase 1b/2 trial (COMPASSION-03) of cadonilimab in patients with advanced solid tumors, including 24 HCC patients in phase 2, showed encouraging results. With a median follow-up of 19.6 months, the mPFS was 3.7 months, and the mOS was not reached [11]. G Based on the Leap002 study results, lenvatinib is an optimal anti-angiogenic combination option [14]. The safety and efficacy of cadonilimab combined with lenvatinib as a first-line therapy for HCC have been confirmed in a prospective phase II trial (AK104-206, NCT04444167). The ORR was 35.5 %, and the mPFS was 8.6 months in cohort A (6 mg/kg Q2W), while cohort B (15 mg/kg Q3W) had an ORR of 35.7 % and an mPFS of 9.8 months. The mOS was 27.1 months (95 % CI: 15.7, NE) for cohort A, while it was not reached in cohort B (12). Notably, these findings indicate that cadonilimab demonstrates a long-term survival advantage as either monotherapy or in combination with lenvatinib. The 12-month OS rate exceeded 80 % in both the COMPASSION-03 and AK104-206 trials, consistent with the findings of this study. A Although indirect comparisons should be made with caution, the combination of cadonilimab and lenvatinib shows a promising efficacy trend. The novel tetravalent form's higher avidity may enhance drug retention. Previous studies have shown that immune escape is a major mechanism of cancer malignancy, primarily due to the exhaustion of CD8+ T cells that recognize tumor antigens, with the PD-1 signaling pathway being a key regulator of this exhaustion [15,16]. Evidence suggests that PD-1/CTLA-4 blockade promotes the expansion of newly primed CD8+ T cells while preventing their progression to terminally exhausted CD8+ TILs in the tumor microenvironment (TME), leading to tumor remission [17]. Lenvatinib modulates cancer immunity in the tumor microenvironment by reducing tumor-associated macrophages (TAMs). When combined with PD-1 blockade, its antitumor activity is enhanced via the IFN signaling pathway [18]. Furthermore, cadonilimab's Fc-null design eliminates Fc receptor-mediated effector functions and reduces proinflammatory cytokine release, which could otherwise deplete PD-1 expressing T cells and compromise anti-tumor efficacy [[19], [20], [21]]. These mechanisms may explain the inspiring efficacy observed with the combination of cadonilimab and lenvatinib.
In this study, Grade 3–4 TRAEs occurred in 51.7 % of all patients, compared to 61.5 % in the Leap002 study [14]. Consistent with previous clinical studies, real-world data suggest that the combination therapy did not raise additional safety concerns. Without Fc receptor binding, cadonilimab exhibits minimal antibody-dependent cellular cytotoxicity, cellular phagocytosis, and release of interleukin-6 (IL-6) and interleukin-8 (IL-8) [20]. These features likely contribute to the observed reduction in toxicities associated with cadonilimab in clinical settings. TRAEs observed in this study due to the combination therapy included hypertension, weight loss, fatigue, elevated blood bilirubin, and proteinuria [22].
Despite these findings, our research has several limitations. Given the real-world nature of this study and the small sample size, patient selection lacked randomization, introducing potential selection bias. Additionally, as a single-arm study, the absence of control groups limits the robustness of the data and results. Moreover, as a real-world study, patient follow-up and management were more challenging, potentially resulting in incomplete follow-up data for some patients.
In conclusion, the results suggest that the combination of cadonilimab and lenvatinib demonstrates promising efficacy and a favorable safety profile in real-world clinical settings, offering a new option for first-line treatment of HCC. Given the limited real-world data on bispecific antibodies combined with TKIs, this study offers valuable insights and evidence for further exploration as a supplementary treatment for HCC. A large-scale, multicenter, prospective, randomized, controlled trial is still needed to assess the efficacy and safety of this combination. Additionally, exploring the efficacy of this combination with local treatments in patients with unresectable hepatocellular carcinoma is crucial for enhancing therapeutic outcomes. Moreover, further studies on biomarkers are necessary to identify the dominant patient population. The follow-up period for overall survival will be extended. Further investigation is needed to determine the safety and efficacy of bispecific antibodies as a viable option for patients undergoing ICI re-challenge.
Funding
This study was supported by grants from the National Key Research and Development Program of China (No.2022YFC2304800), the National Natural Science Foundation of China (82273429, 82102879), the Natural Science Foundation of Guangdong Province (2022A1515010526, 2021A1515012518) and Guangdong Medical Research Fund project (A2022340). The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was designed and performed by the Declaration of Helsinki and was approved by the Medical Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-2021-044) on February 10, 2021, and written, informed consent was obtained from each patient to retrospectively review and report on their medical records.
CRediT authorship contribution statement
Guosheng Yuan: Writing – original draft, Supervision, Software, Methodology, Investigation, Conceptualization. Yongru Chen: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Data curation. Peilin Zhu: Visualization, Software, Data curation. Qiong Deng: Validation, Supervision, Methodology, Data curation. Kaiyan Su: Visualization, Software, Data curation. Jie Liu: Investigation, Funding acquisition. Yan Wang: Writing – review & editing. Rong Li: Data curation. Wenli Li: Data curation. Mengya Zang: Supervision, Conceptualization. Xiaoyun Hu: Supervision, Methodology, Conceptualization. Jun-Jie Wang: Supervision, Conceptualization. Qi Li: Supervision, Methodology, Conceptualization. Yangfeng Du: Conceptualization. Jinzhang Chen: Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
All authors have completed the ICMJE uniform disclosure form. YW is employees of Akeso Biopharma. The other authors have no conflicts of interest to declare.
Contributor Information
Qi Li, Email: nfdoctorlee@126.com.
Yangfeng Du, Email: dyfer1982@126.com.
Jinzhang Chen, Email: chenjinzhang@smu.edu.cn.
References
- 1.Yang C., Zhang H., Zhang L., Zhu A.X., Bernards R., Qin W., Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023;20(4):203–222. doi: 10.1038/s41575-022-00704-9. [DOI] [PubMed] [Google Scholar]
- 2.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., Li D., Verret W., Xu D.Z., Hernandez S., Liu J., Huang C., Mulla S., Wang Y., Lim H.Y., Zhu A.X., Cheng A.L. IMbrave150 investigators, atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 3.Ren Z., Xu J., Bai Y., Xu A., Cang S., Du C., Li Q., Lu Y., Chen Y., Guo Y., Chen Z., Liu B., Jia W., Wu J., Wang J., Shao G., Zhang B., Shan Y., Meng Z., Wu J., Gu S., Yang W., Liu C., Shi X., Gao Z., Yin T., Cui J., Huang M., Xing B., Mao Y., Teng G., Qin Y., Wang J., Xia F., Yin G., Yang Y., Chen M., Wang Y., Zhou H., Fan J., ORIENT-32 study group Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 4.Qin S., Chan S.L., Gu S., Bai Y., Ren Z., Lin X., Chen Z., Jia W., Jin Y., Guo Y., Hu X., Meng Z., Liang J., Cheng Y., Xiong J., Ren H., Yang F., Li W., Chen Y., Zeng Y., Sultanbaev A., Pazgan-Simon M., Pisetska M., Melisi D., Ponomarenko D., Osypchuk Y., Sinielnikov I., Yang T.S., Liang X., Chen C., Wang L., Cheng A.L., Kaseb A., Vogel A., CARES-310 Study Group Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023 Sep 30;402(10408):1133–1146. doi: 10.1016/S0140-6736(23)00961-3. [DOI] [PubMed] [Google Scholar]
- 5.Giraud J., Chalopin D., Blanc J.F., Saleh M. Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front. Immunol. 2021 Mar 18;12 doi: 10.3389/fimmu.2021.655697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley R.K., Sangro B., Harris W., Ikeda M., Okusaka T., Kang Y.-K., et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J. Clin. Oncol. 2021;39(27):2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou-Alfa G.K., Lau G., Kudo M., Chan S.L., Kelley R.K., Furuse J., Sukeepaisarnjaroen W., Kang Y.K., Van Dao T., De Toni E.N., Rimassa L., Breder V., Vasilyev A., Heurgué A., Tam V.C., Mody K., Thungappa S.C., Ostapenko Y., Yau T., Azevedo S., Varela M., Cheng A.L., Qin S., Galle P.R., Ali S., Marcovitz M., Makowsky M., He P., Kurland J.F., Negro A., Sangro B. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence. 2022;1(8) doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 8.Yau T., Kang Y.K., Kim T.Y., El-Khoueiry A.B., Santoro A., Sangro B., Melero I., Kudo M., Hou M.M., Matilla A., Tovoli F., Knox J.J., Ruth He A., El-Rayes B.F., Acosta-Rivera M., Lim H.Y., Neely J., Shen Y., Wisniewski T., Anderson J., Hsu C. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11) doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keam S.J. Cadonilimab: first approval. Drugs. 2022;82(12):1333–1339. doi: 10.1007/s40265-022-01761-9. [DOI] [PubMed] [Google Scholar]
- 10.Frentzas S., Gan H.K., Cosman R., Coward J., Tran B., Millward M., Zhou Y., Wang W., Xia D., Wang Z.M., Li B., Xia M., Desai J. A phase 1a/1b first-in-human study (COMPASSION-01) evaluating cadonilimab in patients with advanced solid tumors. Cell Rep Med. 2023 doi: 10.1016/j.xcrm.2023.101242. undefined(undefined) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X., Xu N., Li Z., Shen L., Ji K., Zheng Z., Liu D., Lou H., Bai L., Liu T., Li Y., Li Y., Fan Q., Feng M., Zhong H., Huang Y., Lou G., Wang J., Lin X., Chen Y., An R., Li C., Zhou Q., Huang X., Guo Z., Wang S., Li G., Fei J., Zhu L., Zhu H., Li X., Li F., Liao S., Min Q., Tang L., Shan F., Gong J., Gao Y., Zhou J., Lu Z., Li X., Li J., Ren H., Liu X., Yang H., Li W., Song W., Wang Z.M., Li B., Xia M., Wu X., Ji J. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. 2023;24(10):1134–1146. doi: 10.1016/S1470-2045(23)00411-4. [DOI] [PubMed] [Google Scholar]
- 12.Qiao Q., Han C., Ye S., Li J., Shao G., Bai Y., Xu A., Sun M., Wang W., Wu J., Huang M., Song L., Huang L., Liu T., Liu W., Wang Z.M., Li B., Xia M., Bai L. The efficacy and safety of cadonilimab combined with lenvatinib for first-line treatment of advanced hepatocellular carcinoma (COMPASSION-08): a phase Ib/II single-arm clinical trial. Front. Immunol. 2023 Oct 24;14 doi: 10.3389/fimmu.2023.1238667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., Rubinstein L., Shankar L., Dodd L., Kaplan R., Lacombe D., Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009 Jan;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Finn R., Kudo M., Merle P., Meyer T., Qin S., Ikeda M. LBA34 Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC) Ann. Oncol. 2022;33 [Google Scholar]
- 15.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer sup-pression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. Epub 2011/03/26. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki T., Chikuma S., Iwai Y., Fagarasan S., Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013;14(12):1212–1218. doi: 10.1038/ni.2762. Epub 2013/11/19. [DOI] [PubMed] [Google Scholar]
- 17.Bufe S., Zimmermann A., Ravens S., Prinz I., Buitrago-Molina L.E., Geffers R., Woller N., Kühnel F., Talbot S.R., Noyan F., Manns M.P., Wedemeyer H., Hardtke-Wolenski M., Jaeckel E., Davalos-Misslitz A.C. PD-1/CTLA-4 blockade leads to expansion of cd8+pd-1 int TILs and results in tumor remission in experimental liver cancer. Liver Cancer. 2022 Oct 7;12(2):129–144. doi: 10.1159/000526899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato Y., Tabata K., Kimura T., Yachie-Kinoshita A., Ozawa Y., Yamada K., Ito J., Tachino S., Hori Y., Matsuki M., Matsuoka Y., Ghosh S., Kitano H., Nomoto K., Matsui J., Funahashi Y. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019 Feb 27;14(2) doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang X., Huang Z., Zhong T., Zhang P., Wang Z.M., Xia M. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. mAbs. 2023;15(1) doi: 10.1080/19420862.2023.2180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arlauckas S.P., Garris C.S., Kohler R.H., Kitaoka M., Cuccarese M.F., Yang K.S. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci. Transl. Med. 2017;9(389) doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Mathieu M., Brezski R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9(1):63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo M., Finn R.S., Qin S., Han K.-H., Ikeda K., Piscaglia F. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]