Abstract
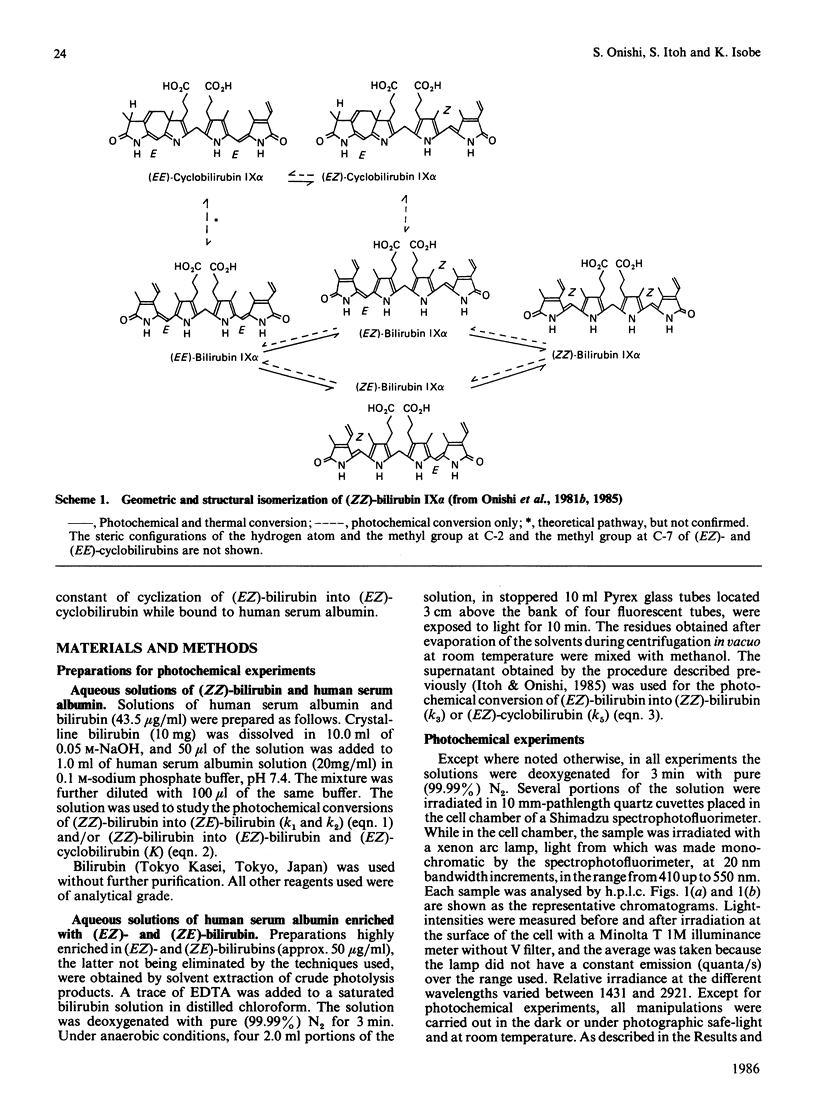
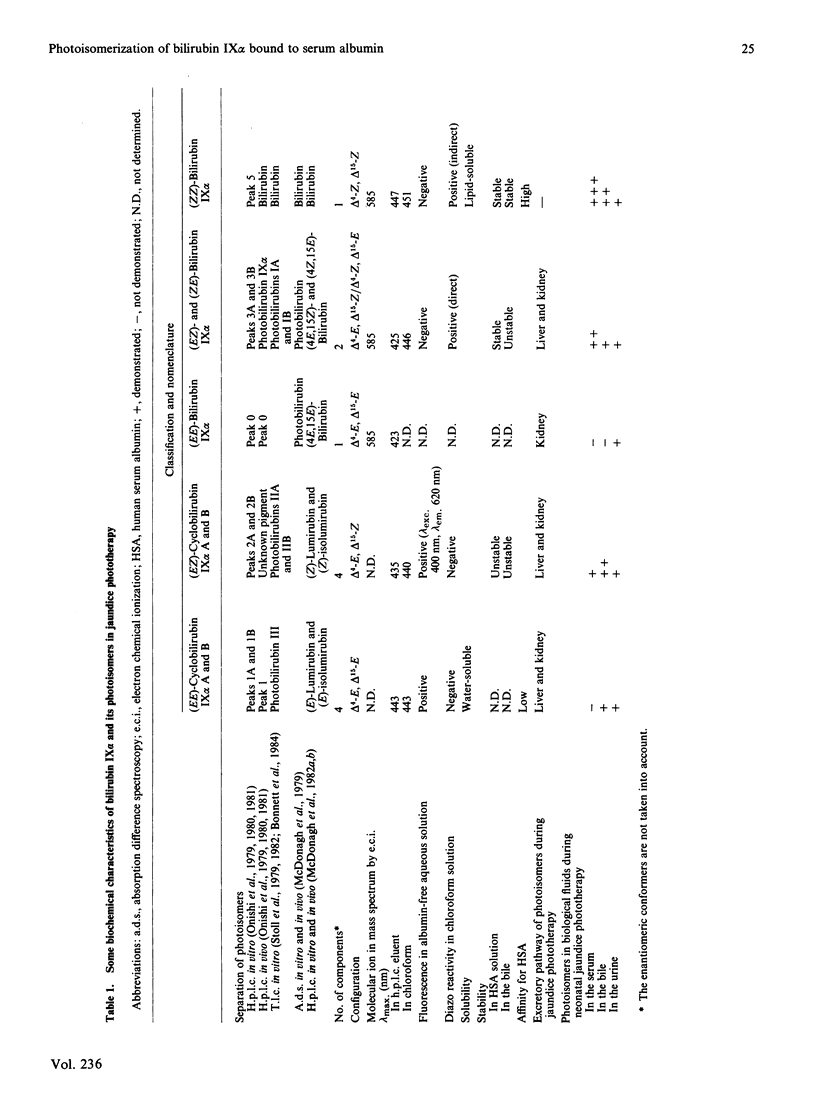
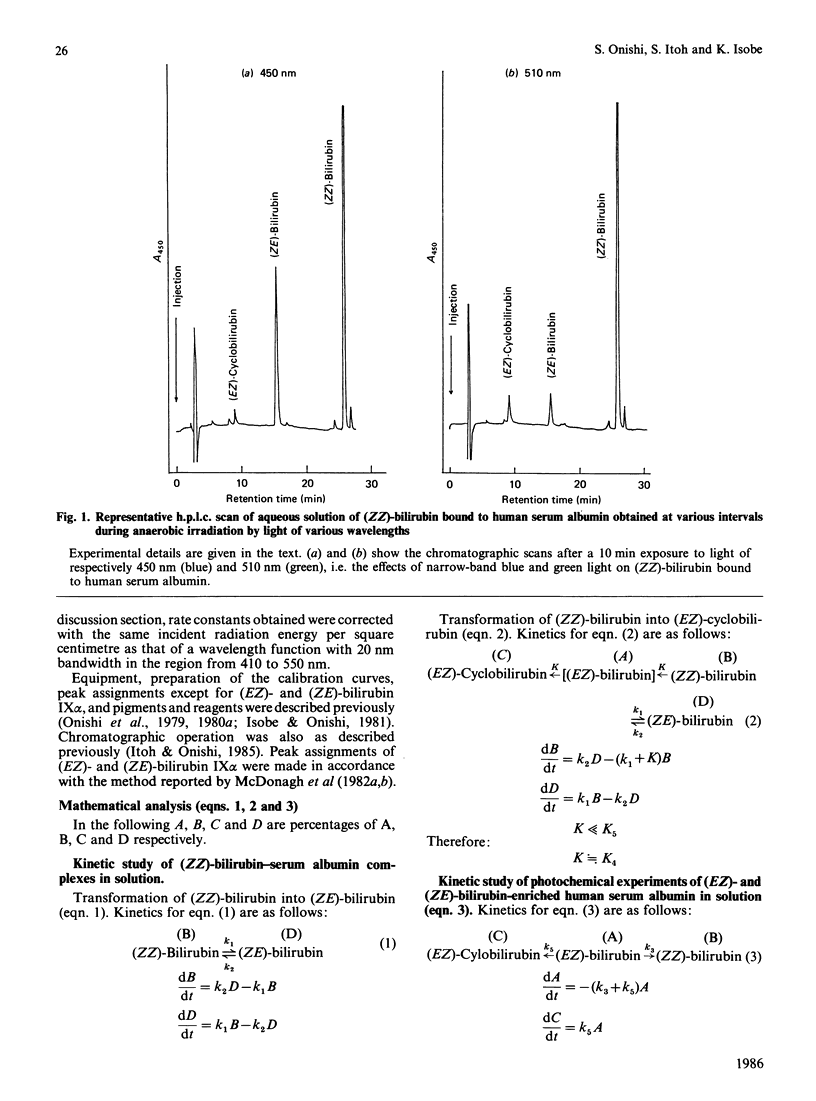
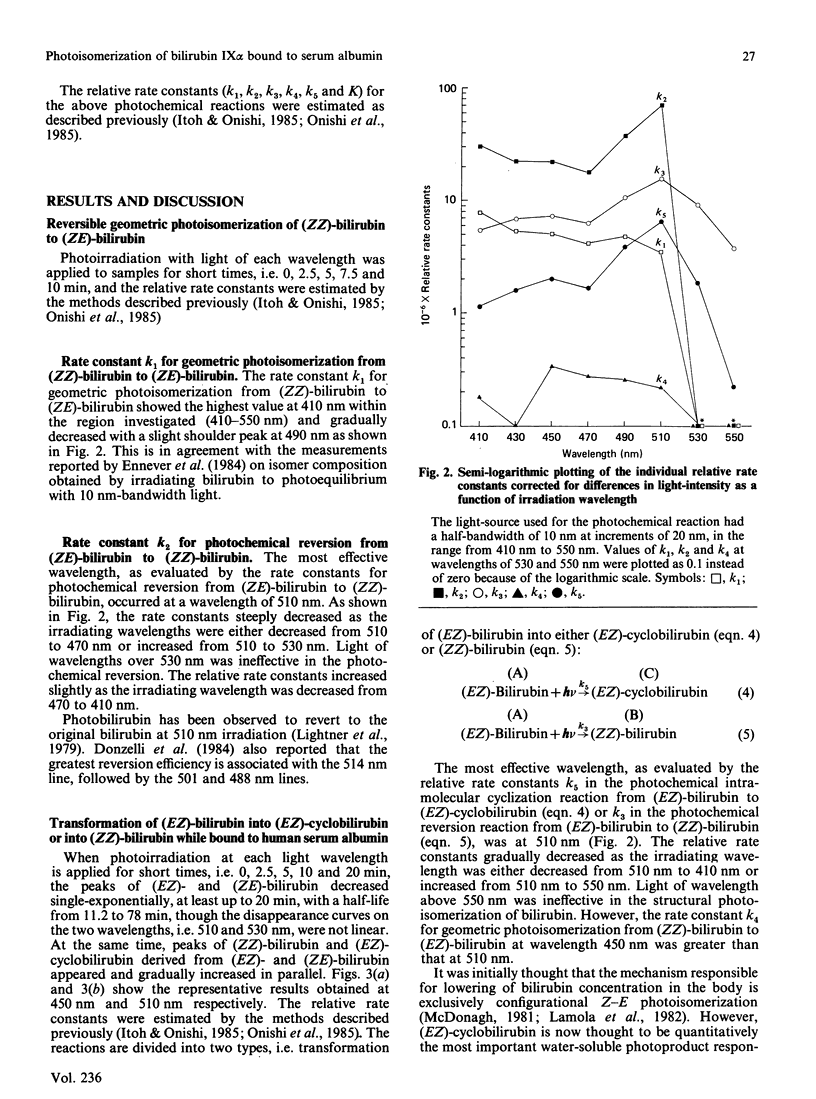
The kinetics for the quantitatively important reaction: (Formula: see text) that is, the photochemical interconversion between bilirubin and its geometric and structural photoisomers bound to human serum albumin in aqueous solution when various wavelengths of monochromatic light were used, were assayed by h.p.l.c. In order to clarify the wavelength-dependence of the relative rate constants in the individual steps, a light-source with a half-bandwidth of 10 nm was used at increments of 20 nm, in the range from 410 nm to 550 nm. We describe for the first time studies on the wavelength-dependence of rate constants in geometric and structural photoisomerization reactions in vitro of (ZZ)-bilirubin or (EZ)-bilirubin bound to human serum albumin, especially the relative rate constants of cyclization of (EZ)-bilirubin into (EZ)-cyclobilirubin. Because studies in vitro have demonstrated that the wavelengths from 350 to 450 nm are mutagenic, the results obtained indicated that the safest and ideal light-source for phototherapy is green light of 510 nm, which keeps (ZE)-bilirubin concentrations as low as possible, as shown by a maximal value of k2 at 510 nm and a relatively low value of k1 at 510 nm. This light-source still ensures the substantial absorption of (ZZ)-bilirubin, which is the precursor of (EZ)-bilirubin, the intermediate in (EZ)-cyclobilirubin formation and, furthermore, as shown by the maximal value of k5 and a considerable value of k4 at 510 nm, promotes the cyclization of (EZ)-bilirubin derived from (ZZ)-bilirubin even though k3 at 510 nm also shows a peak value.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnett R., Buckley D. G., Hamzetash D., Hawkes G. E., Ioannou S., Stoll M. S. Photobilirubin II. Biochem J. 1984 May 1;219(3):1053–1056. doi: 10.1042/bj2191053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Erickson L. C., Kohn K. W. Non-enzymatic DNA strand breaks induced in mammalian cells by fluorescent light. Biochim Biophys Acta. 1978 Aug 23;520(1):11–20. doi: 10.1016/0005-2787(78)90003-5. [DOI] [PubMed] [Google Scholar]
- Brown A. K., McDonagh A. F. Phototherapy for neonatal hyperbilirubinemia: efficacy, mechanism and toxicity. Adv Pediatr. 1980;27:341–389. [PubMed] [Google Scholar]
- Burki H. J., Lam C. K. Comparison of the lethal and mutagenic effects of gold and white fluorescent lights on cultured mammalian cells. Mutat Res. 1978 Dec;54(3):373–377. doi: 10.1016/0165-1161(78)90027-4. [DOI] [PubMed] [Google Scholar]
- Cohen A. N., Ostrow J. D. New concepts in phototherapy: photoisomerization of bilirubin IX alpha and potential toxic effects of light. Pediatrics. 1980 Apr;65(4):740–750. [PubMed] [Google Scholar]
- Costarino A. T., Ennever J. F., Baumgart S., Speck W. T., Paul M., Polin R. A. Bilirubin photoisomerization in premature neonates under low- and high-dose phototherapy. Pediatrics. 1985 Mar;75(3):519–522. [PubMed] [Google Scholar]
- Ennever J. F., Sobel M., McDonagh A. F., Speck W. T. Phototherapy for neonatal jaundice: in vitro comparison of light sources. Pediatr Res. 1984 Jul;18(7):667–670. doi: 10.1203/00006450-198407000-00021. [DOI] [PubMed] [Google Scholar]
- Isobe K., Onishi S. Kinetics of the photochemical interconversion among geometric photoisomers of bilirubin. Biochem J. 1981 Mar 1;193(3):1029–1031. doi: 10.1042/bj1931029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S., Onishi S. Kinetic study of the photochemical changes of (ZZ)-bilirubin IX alpha bound to human serum albumin. Demonstration of (EZ)-bilirubin IX alpha as an intermediate in photochemical changes from (ZZ)-bilirubin IX alpha to (EZ)-cyclobilirubin IX alpha. Biochem J. 1985 Feb 15;226(1):251–258. doi: 10.1042/bj2260251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox I., Ennever J. F., Speck W. T. Urinary excretion of an isomer of bilirubin during phototherapy. Pediatr Res. 1985 Feb;19(2):198–201. doi: 10.1203/00006450-198502000-00010. [DOI] [PubMed] [Google Scholar]
- Lamola A. A., Blumberg W. E., McClead R., Fanaroff A. Photoisomerized bilirubin in blood from infants receiving phototherapy. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1882–1886. doi: 10.1073/pnas.78.3.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamola A. A., Flores J., Doleiden F. H. Quantum yield and equilibrium position of the configurational photoisomerization of bilirubin bound to human serum albumin. Photochem Photobiol. 1982 May;35(5):649–654. doi: 10.1111/j.1751-1097.1982.tb02624.x. [DOI] [PubMed] [Google Scholar]
- Lewis H. M., Campbell R. H., Hambleton G. Use or abuse of phototherapy for physiological jaundice of newborn infants. Lancet. 1982 Aug 21;2(8295):408–410. doi: 10.1016/s0140-6736(82)90440-8. [DOI] [PubMed] [Google Scholar]
- McDonagh A. F., Lightner D. A. 'Like a shrivelled blood orange'--bilirubin, jaundice, and phototherapy. Pediatrics. 1985 Mar;75(3):443–455. [PubMed] [Google Scholar]
- McDonagh A. F. Phototherapy: a new twist to bilirubin. J Pediatr. 1981 Dec;99(6):909–911. doi: 10.1016/s0022-3476(81)80016-9. [DOI] [PubMed] [Google Scholar]
- Onishi S., Isobe K., Itoh S., Kawade N., Sugiyama S. Demonstration of a geometric isomer of bilirubin-IX alpha in the serum of a hyperbilirubinaemic newborn infant and the mechanism of jaundice phototherapy. Biochem J. 1980 Sep 15;190(3):533–536. doi: 10.1042/bj1900533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S., Itoh S., Kawade N., Isobe K., Sugiyama S. The separation of configurational isomers of bilirubin by high pressure liquid chromatography and the mechanism of jaundice phototherapy. Biochem Biophys Res Commun. 1979 Oct 12;90(3):890–896. doi: 10.1016/0006-291x(79)91911-9. [DOI] [PubMed] [Google Scholar]
- Onishi S., Itoh S., Yamakawa T., Isobe K., Manabe M., Toyota S., Imai T. Comparison of kinetic study of the photochemical changes of (ZZ)-bilirubin IX alpha bound to human serum albumin with that bound to rat serum albumin. Biochem J. 1985 Sep 15;230(3):561–567. doi: 10.1042/bj2300561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S., Kawade N., Itoh S., Isobe K., Sugiyama S. High-pressure liquid chromatographic analysis of anaerobic photoproducts of bilirubin-IX alpha in vitro and its comparison with photoproducts in vivo. Biochem J. 1980 Sep 15;190(3):527–532. doi: 10.1042/bj1900527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S., Miura I., Isobe K., Itoh S., Ogino T., Yokoyama T., Yamakawa T. Structure and thermal interconversion of cyclobilirubin IX alpha. Biochem J. 1984 Mar 15;218(3):667–676. doi: 10.1042/bj2180667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S., Ogino T., Yokoyama T., Isobe K., Itoh S., Yamakawa T., Hashimoto T. Biliary and urinary excretion rates and serum concentration changes of four bilirubin photoproducts in Gunn rats during total darkness and low or high illumination. Biochem J. 1984 Aug 1;221(3):717–721. doi: 10.1042/bj2210717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parshad R., Gantt R., Sanford K. K., Jones G. M., Camalier R. F. Light-induced chromatid damage in human skin fibroblasts in culture in relation to their neoplastic potential. Int J Cancer. 1981 Sep 15;28(3):335–340. doi: 10.1002/ijc.2910280313. [DOI] [PubMed] [Google Scholar]
- Parshad R., Sanford K. K., Jones G. M., Tarone R. E. Fluorescent light-induced chromosome damage and its prevention in mouse cells in culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1830–1833. doi: 10.1073/pnas.75.4.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratesi R., Agati G., Fusi F., Sbrana G., Migliorini M. G., Vecchi C., Donzelli G. Laser investigation of bilirubin-photobilirubin photoconversion. Pediatr Res. 1985 Feb;19(2):166–171. doi: 10.1203/00006450-198502000-00003. [DOI] [PubMed] [Google Scholar]
- Pratesi R. Two lights for phototherapy. Lancet. 1983 Oct 8;2(8354):859–859. doi: 10.1016/s0140-6736(83)90784-5. [DOI] [PubMed] [Google Scholar]
- Sideris E. G., Papageorgiou G. C., Charalampous S. C., Vitsa E. M. A spectrum response study on single strand DNA breaks, sister chromatid exchanges, and lethality induced by phototherapy lights. Pediatr Res. 1981 Jul;15(7):1019–1023. doi: 10.1203/00006450-198107000-00008. [DOI] [PubMed] [Google Scholar]
- Speck W. T., Rosenkranz H. S. Base substitution mutations induced in Salmonella strains by visible light (450 nm). Photochem Photobiol. 1975 May;21(5):369–371. doi: 10.1111/j.1751-1097.1975.tb06687.x. [DOI] [PubMed] [Google Scholar]
- Stoll M. S., Vicker N., Gray C. H., Bonnett R. Concerning the structure of photobilirubin II. Biochem J. 1982 Jan 1;201(1):179–188. doi: 10.1042/bj2010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll M. S., Zenone E. A., Ostrow J. D., Zarembo J. E. Preparation and properties of bilirubin photoisomers. Biochem J. 1979 Oct 1;183(1):139–146. doi: 10.1042/bj1830139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi C., Donzelli G. P., Migliorini M. G., Sbrana G. Green light in phototherapy. Pediatr Res. 1983 Jun;17(6):461–463. doi: 10.1203/00006450-198306000-00006. [DOI] [PubMed] [Google Scholar]
- Vecchi C., Donzelli G. P., Migliorini M. G., Sbrana G., Pratesi R. New light in phototherapy. Lancet. 1982 Aug 14;2(8294):390–390. doi: 10.1016/s0140-6736(82)90584-0. [DOI] [PubMed] [Google Scholar]


