Abstract
Rationale:
The International Classification of Cognitive Disorders in Epilepsy (IC-CoDE) was recently introduced as a consensus-based, empirically-driven taxonomy of cognitive disorders in epilepsy and has been effectively applied to patients with temporal lobe epilepsy (TLE). The purpose of this study was to apply the IC-CoDE to patients with frontal lobe epilepsy (FLE) using national multicenter data.
Methods:
Neuropsychological data of 455 patients with FLE aged 16 years or older were available across four US-based sites. First, we examined test-specific impairment rates across sites using two impairment thresholds (1.0 and 1.5 standard deviations below the normative mean). Following the proposed IC-CoDE guidelines, patterns of domain impairment were determined based on commonly used tests within five cognitive domains (language, memory, executive functioning, attention/processing speed, and visuospatial ability) to construct phenotypes. Impairment rates and distributions across phenotypes were then compared to those found in patients with TLE for which the IC-CoDE classification was initially validated.
Results:
The highest rates of impairment were found among tests of naming, verbal fluency, speeded sequencing and set-shifting, and complex figure copy. The following IC-CoDE phenotype distributions were observed using the two different threshold cutoffs: 23-40% cognitively intact, 24-29% single domain impairment, 13-20% bi-domain impairment, and 18-33% generalized impairment. Language was the most common single domain impairment (68% for both thresholds) followed by attention and processing speed (15-18%). Overall, patients with FLE demonstrated higher rates of cognitive impairment compared to patients with TLE.
Conclusions:
These results demonstrate the applicability of the IC-CoDE to epilepsy syndromes outside of TLE. Findings indicated generally stable and reproducible phenotypes across multiple epilepsy centers in the U.S. with diverse sample characteristics and varied neuropsychological test batteries. Findings also highlight opportunities for further refinement of the IC-CoDE guidelines as its application expands.
Keywords: epilepsy, phenotypes, neuropsychology, cognition, frontal lobe epilepsy
1. Introduction
Cognitive impairment is a significant comorbidity across epilepsy syndromes that can have direct impact on psychosocial functioning and quality of life1-4. However, until recently, there was no clear consensus on how to classify cognitive impairment in patients with epilepsy, which limited clinical care and research efforts. Thus, the International Classification of Cognitive Disorders in Epilepsy (IC-CoDE) was developed from collaborations between the International Neuropsychological Society (INS) and International League against Epilepsy (ILAE) Neuropsychology Task force5,6. One primary objective of the IC-CoDE is to develop and validate a harmonized cognitive taxonomy that can be applied globally to enhance collaborations and facilitate big data approaches in the field of neuropsychology.
The IC-CoDE was first validated among adult patients with temporal lobe epilepsy (TLE) using national, multicenter data5 and has since found reproducible phenotypes in Spanish-speaking patients with TLE7. These studies provide proof-of-principle that the IC-CoDE can be effectively applied across diverse TLE cohorts using varied neuropsychological tests. Additional studies are underway to apply the IC-CoDE to pediatric epilepsy populations8, as well as non-US sites to better understand cross-cultural considerations9, and has already been applied to other medical conditions, including multiple sclerosis10 and COVID11.
To further expand the utility of the IC-CoDE, the current study applies this taxonomy to patients with frontal lobe epilepsy (FLE) for the first time. FLE is the second most prevalent focal epilepsy, accounting for 20-30% of cases12, and has been associated with a variety of cognitive deficits. Unlike TLE, the cognitive deficits seen in FLE tend to be more widespread and typically involve alterations in confrontation naming, attention, working memory, processing speed, executive function, and inefficient learning and retrieval13-15. The wide array of cognitive deficits reflects the heterogeneity of FLE pathology and complex network involvement of the frontal lobe and has made understanding cognitive phenotypes in FLE quite challenging16.
Prior to the establishment of the IC-CoDE, we investigated cognitive phenotypes in FLE using independently established clinical criteria; however, the findings were limited by a relatively small, single-site study sample17. By using the IC-CoDE methodology, we have been able to combine data from multiple sites that use varied neuropsychological batteries to characterize cognitive profiles, ultimately creating one of the largest neuropsychological datasets for an FLE cohort. We investigated impairment rates across tests, as well as distributions across IC-CoDE phenotypes, to better understand the various cognitive deficits in FLE. We also compared these patterns of impairment to those published on the aforementioned TLE cohort5.
2. Methods
Neuropsychological data were retrospectively obtained from existing International Review Board (IRB)-approved studies or data registries from four epilepsy centers in the United States: Cleveland Clinic (CC; Cleveland, OH), NYU Langone Health (NYU; New York, NY), Columbia University Medical Center (CUMC; New York, NY) and Medical College of Wisconsin (MCW; Milwaukee, WI).
2.1. Participants
Participants included in this study were 16 years and older with pharmacoresistant FLE, without history of prior therapeutic neurosurgery, who underwent comprehensive neuropsychological evaluation. This resulted in a clinically representative sample of 455 patients.
2.2. Neuropsychological Measures
Neuropsychological test batteries varied across sites; however, all test batteries included measures across the five cognitive domains recommended by the IC-CoDE: attention/processing speed, language, memory, executive function, and visuospatial ability. Neuropsychological measures were separated into each of these cognitive domains according to published IC-CoDE guidelines5,6. Scores were standardized based on demographically corrected normative data per each site’s discretion based on standard clinical care; a list of neuropsychological measures, normative data used, and demographic corrections can be found in Supplemental Table 1.
2.3. Analyses
2.3.1. Sample Characteristics
ANOVAs and chi-square analyses were used to examine differences in sample characteristics across sites.
2.3.2. Impairment Rates Across Tests
We analyzed impairment rates for each individual neuropsychological test used to derive the IC-CoDE phenotypes to understand which tests were most sensitive to impairment in FLE and explain potential variability in IC-CoDE phenotypes across sites. Standard scores were classified as either impaired or not impaired using ≤ 1.0 and ≤ 1.5 standard deviation cutoffs. Rates of impairment were determined by dividing the number of patients with impaired scores on the measure by the total number of patients who completed the measure. Of note, tests that were administered to less than 10% of the total sample were excluded.
2.3.3. IC-CoDE Classification
The IC-CoDE was originally developed by consensus of core cognitive domains, as well as common tests used to assess these domains, to determine cognitive phenotype. Based on previously published IC-CoDE guidelines5, cognitive phenotypes were generated for all patients who had available data for at least two cognitive measures within at least four of the five following cognitive domains: language, memory, executive function, visuospatial abilities, and attention/processing speed. Thus far, both ≤ 1.0 and ≤ 1.5 standard deviation (SD) impairment thresholds have been assessed and, although early evidence suggests that ≤1.5 SD threshold may be most appropriate5, we opted to include both thresholds for thoroughness. A cognitive domain was considered impaired if two or more tests within that domain met threshold for impairment. Based on the number of cognitive domains impaired, patients were then classified into the following IC-CoDE phenotypes: cognitively intact (no domains impaired), single domain impairment (one cognitive domain impaired), bi-domain impairment (two cognitive domains impaired), or generalized impairment (three or more cognitive domains impaired). Then, the relative ranking of domain-specific impairments within the single and bi-domain categories were examined to understand the nature and frequency of abnormalities.
We examined IC-CoDE phenotype distributions in our FLE sample and differences in phenotype distributions across sites using chi-square goodness of fit tests and follow up pairwise comparisons with Bonferroni correction.
2.3.4. Comparisons between FLE and TLE
We conducted t-tests to compare rates of test-specific impairment between our sample of FLE patients and previously published multicenter TLE data5. We also compared the IC-CoDE phenotypes found in our FLE sample to those established in TLE.
3. Results
3.1. Sample Characteristics
Neuropsychological data of 455 patients across four epilepsy cohorts were collected. 54% of these patients were male and 76% self-identified as White. On average, patients were 33 years old with 13 years of education. Mean age at seizure onset was 16.5, and average duration of seizure disorder was 17 years. Demographic variables across sites are outlined in Table 1. Follow up analyses revealed differences in patient characteristics among sites for age (f(3,451) = 11.13; p < 0.01), education (f(3,451) = 8.09; p < 0.01), ethnicity (χ2 = 76.81, ; p < 0.01), age at seizure onset (f(3,451) = 10.39; p < 0.01), and seizure laterality (χ2 = 27.81, ; p < 0.01). Post-hoc multiple comparisons indicated that patients at CUMC were significantly older than patients at CC and NYU, had an older age at seizure onset than patients at CC, NYU, and MCW, and had a higher level of education than patients at CC. A higher proportion of patients at CC were White compared to CUCM and NYU, and CUMC and NYU had a higher proportion of Hispanic patients as compared to CC and MCW. CC also had a higher proportion of patients with right-sided seizures as compared to the other sites, while MCW had a higher proportion of patients with bilateral seizures as compared to CC.
Table 1.
Characteristics of Patient Cohorts in Base Rate Impairment Analyses
| Cleveland Clinic |
Columbia University |
New York University |
Medical College of Wisconsin |
All Sites | |
|---|---|---|---|---|---|
| N = 187 | N = 154 | N = 37 | N = 77 | N = 455 | |
| Demographics | |||||
| Age | 30.14 (11.33) | 38.07 (15.04) | 30.92 (11.81) | 34.22 (12.72) | 33.34 |
| Education | 12.71 (2) | 14.03 (3.01) | 13.8 (3.12) | 13.2 (2.39) | 13.44 |
| Sex | |||||
| Male | 104 (56%) | 81 (53%) | 18 (49%) | 44 (57%) | 247 (54%) |
| Female | 83 (44%) | 73 (47%) | 19 (51%) | 33 (43%) | 208 (46%) |
| Race | |||||
| White | 170 (91%) | 88 (57%) | 21 (57%) | 65 (84%) | 344 (76%) |
| Black | 11 (6%) | 17 (11%) | 3 (8%) | 10 (13%) | 41 (9%) |
| Asian | 1 (1%) | 4 (3%) | 5 (14%) | 1 (1%) | 11 (2%) |
| Native American | 0 | 0 | 0 | 0 | 0 |
| Hispanic | 4 (2%) | 28 (18%) | 7 (19%) | 1 (1%) | 40 (9%) |
| Bi/Multiracial | 0 | 1 (1%) | 0 | 0 | 1 (<1%) |
| Unknown | 1 (1%) | 16 (10%) | 1 (3%) | 0 | 18 (4%) |
| Seizure Variables | |||||
| Age at Seizure Onset | 13.92 (10.85) | 22.38 (18.92) | 14.03 (12.61) | 15.77 (13.63) | 16.53 |
| Disease Duration | 16.35 (19.21) | 15.53 (13.21) | 17.95 (12.28) | 19.57 (12.8) | 17.35 |
| Laterality | |||||
| Right | 97 (52%) | 56 (36%) | 11 (30%) | 30 (39%) | 194 (43%) |
| Left | 90 (48%) | 85 (55%) | 20 (54%) | 38 (49%) | 233 (51%) |
| Bilateral | 0 | 6 (4%) | 3 (8%) | 9 (12%) | 18 (4%) |
| Unknown | 0 | 7 (5%) | 3 (8%) | 0 | 10 (2%) |
3.2. Impairment Rates Across Tests
Test-specific impairment rates across sites were averaged together. Combined test impairment rates using varying threshold cutoffs across neuropsychological measures are depicted in Figure 1. The highest impairment rates were found with naming, with deficits observed in 48-72% of patients depending on the specific naming test and impairment threshold used. The Auditory Naming Test had the highest rate of impairment (59-72%) followed by the Boston Naming Test (51-62%) and Visual Naming Test (48-57%). Verbal fluencies also had high impairment rates, with phonemic fluency being impaired in 43-54% of patients and category fluency being impaired in 36-53% of patients.
Figure 1. Test-specific impairment rates by ≤ 1.0 and ≤ 1.5 SD cutoffs.
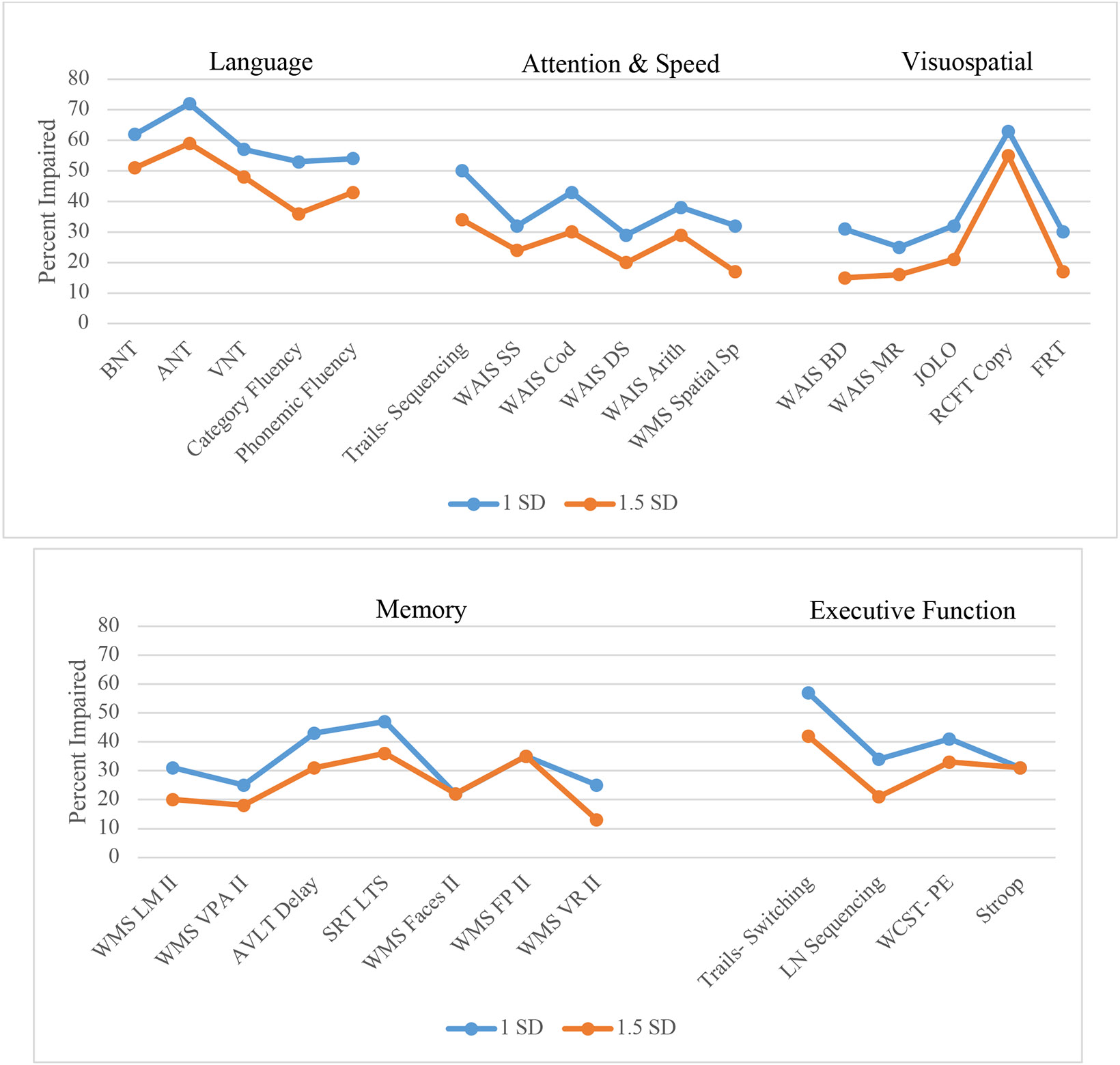
BNT= Boston Naming Test; ANT: Auditory Naming Test; VNT: Visual Naming Test; WAIS: Wechsler Adult Intelligence Scale; SS: Symbol Search; Cod: Coding; DS: Digit Span; Arith: Arithmetic: Sp= Span; BD: Block Design; MR: Matrix Reasoning; JOLO: Judgement of Line Orientation; RCFT: Rey Complex Figure Test; FRT: Facial Recognition Test; WMS: Wechsler Memory Scale; LM II: Logical Memory delay; VPA II: Verbal Paired Associates delay; AVLT: Rey Auditory Verbal Learning Test; SRT LTR: Buschke Selective Reminding Test Long Term Storage; FP: Family Pictures; VR: Visual Reproductions; LN: Letter Number; WCST-PE: Wisconsin Card Sorting Test- Perseverative Errors
The next most impaired tests involved metrics of memory, attention/processing speed, and executive function. Patients showed the greatest memory impairment on the Selective Reminding Test-Long Term Storage (SRT LTS; 36-47%) and word list recall test (Rey Auditory Verbal Learning Test; 31-43%) while other verbal memory tests showed impairment rates of 9-31%. Tests of visual memory had similar impairment rates to tests of verbal memory, with rates ranging from 13-35%. Across tests of processing speed, speeded sequencing (Trail Making Test-Part A) had the highest impairment rates (34-50%). Tests of attention and working memory were slightly less impacted, with rates of impairment ranging from 17-38%. Impairment rates across tests of executive function were variable; speeded mental flexibility (Trail Making Test-Part B) showed the highest rate of impairment within this domain (42-57%) followed by novel problem-solving (Wisconsin Card Sorting Test; 33-41%).
Visuospatial tests generally had the lowest rates of impairment. However, the exception was copy of a complex figure, which showed high rates of impairment (55-63%).
3.3. IC-CoDE Phenotypes
IC-CoDE phenotypes were constructed for all patients who had data for at least two cognitive measures within at least four cognitive domains, which resulted in a sample size of 336. The number of cognitive measures per domain across centers ranged from 3-5 tests for language and memory, 3 tests for executive function, 2-4 tests for visuospatial ability, and 3-6 tests for attention/processing speed. The demographic and clinical variables of this sample subset are summarized in Table 2. Follow up analyses indicated that patient characteristics differed among sites on education (f(3,334) = 8.51; p < 0.01), ethnicity (χ2 = 45.56, ; p < 0.01), age at seizure onset (f(3,334) = 3.244; p = 0.02), and seizure laterality (χ2 = 24.66, ; p < 0.01). Post-hoc multiple comparisons found that those patients at CUMC had an older age at seizure onset than patients at CC, NYU, and MCW. Patients at CUMC also had a higher level of education than patients at CC. CC had a higher proportion of White patients compared to CUMC and NYU, while CUMC had a higher proportion of Hispanic patients and NYU had a higher proportion of Asian patients as compared to the other sites. MCW had a higher proportion of patients with bilateral seizures as compared to CC.
Table 2.
Characteristics of Patient Cohorts Included in IC-CoDE Phenotyping Analyses
| Cleveland Clinic |
Columbia University |
New York University |
Medical College of Wisconsin |
All Sites | |
|---|---|---|---|---|---|
| N = 138 | N = 94 | N = 30 | N = 74 | N = 336 | |
| Demographics | |||||
| Age | 31.06 (21.19) | 35.7 (13.78) | 29.7 (10.46) | 34.32 (12.60) | 32.7 |
| Education | 12.75 (1.96) | 14.32 (2.8) | 13.97 (2.97) | 13.22 (2.32) | 13.57 |
| Sex | |||||
| Male | 78 (57%) | 45 (48%) | 16 (53%) | 42 (57%) | 181 (54%) |
| Female | 60 (44%) | 49 (52%) | 14 (47%) | 32 (43%) | 155 (46%) |
| Race | |||||
| White | 125 (91%) | 61 (65%) | 19 (63%) | 63 (85%) | 268 (79%) |
| Black | 8 (6%) | 10 (11%) | 3 (10%) | 10 (14%) | 31 (9%) |
| Asian | 1 (1%) | 3 (3%) | 4 (13%) | 0 | 8 (2%) |
| Native American | 0 | 0 | 0 | 0 | 0 |
| Hispanic | 4 (3%) | 13 (14%) | 3 (10%) | 1 (1%) | 21 (6%) |
| Bi/Multiracial | 0 | 1 (1%) | 0 | 0 | 1 (<1%) |
| Unknown | 0 | 6 (6%) | 1 (3%) | 0 | 7 (2%) |
| Seizure Variables | |||||
| Age at Seizure Onset | 13.84 (11.05) | 19.15 (15.51) | 14.17 (12.08) | 15.91 (13.78) | 15.77 |
| Disease Duration | 16.95 (11.24) | 16.43 (13) | 16.9 (11.86) | 19.59 (12.87) | 17.47 |
| Laterality | |||||
| Right | 73 (53%) | 34 (36%) | 10 (33%) | 30 (41%) | 147 (43%) |
| Left | 65 (47%) | 53 (56%) | 18 (60%) | 35 (47%) | 171 (50%) |
| Bilateral | 0 | 3 (3%) | 1 (3%) | 9 (12%) | 13 (4%) |
| Unknown | 0 | 4 (4%) | 1 (3%) | 0 | 5 (1%) |
Using the cutoff threshold of ≤ 1.0 SD, 23% of patients were cognitively intact, 24% fell into the single domain impairment phenotype, 20% in the bi-domain impairment phenotype, and 33% with generalized impairment. Using the cutoff threshold of ≤ 1.5 SD, 40% of patients were cognitively intact, 29% demonstrated single domain impairment, 13% bi-domain impairment, and 18% with generalized impairment. Across both impairment thresholds, language was the most common domain impairment (68% for both thresholds), followed by attention and processing speed (15-18%); memory (7-11%) and executive function (10% for both thresholds) were ranked third and fourth, while visuospatial skills (6% for both thresholds) had the lowest single domain impairment rates. The most common bi-domain impairment involved impairment in language and attention/processing speed domains (24-35%). Bi-domain impairments in language and memory (14-18%) and language and visuospatial skills (15-18%) were also relatively common.
Figure 2 displays the distributions of IC-CoDE phenotypes across sites. To understand the stability of phenotypes across the four sites, chi-square goodness of fit tests were conducted. Using the ≤ 1.0 SD cutoff, chi-square was significant (χ2 = 32.46, p < 0.001); however, follow-up pairwise comparisons did not indicate any significant differences in phenotype distributions across the four sites, rather the variability across the different phenotypes resulted in significant chi-square. Using ≤ 1.5 SD cutoff, chi-square was again significant (χ2 = 28.51, p < 0.001) and follow up pairwise comparisons indicated that two sites significantly differed between the number of patients with single domain impairment (38% CC patients versus 14% MCW patients). Upon further examination, the two sites had similar rates of cognitively intact patients, but MCW had higher rates of patients with bi-domain and generalized impairment as compared to CC. Looking across test-specific impairment rates, the two sites tended to differ the most on memory tests, partly because MCW had high rates of impairment on the Brief Visuospatial Memory Test (BVMT) and the SRT, two tests that are not routinely given at CC, as well as slightly higher rates of impairment on several processing speed and attention measures. MCW also had a slightly higher proportion of patients with bilateral seizures as compared to CC, but this represents a relatively small subset of MCW’s overall sample. There were no other significant differences in patient demographics or seizure characteristics between CC and MCW and the two sites included a similar number of tests in each cognitive domain to construct their respective phenotypes.
Figure 2. IC-CoDE Phenotypes using ≤ 1.0 and ≤ 1.5 SD cutoffs by site.
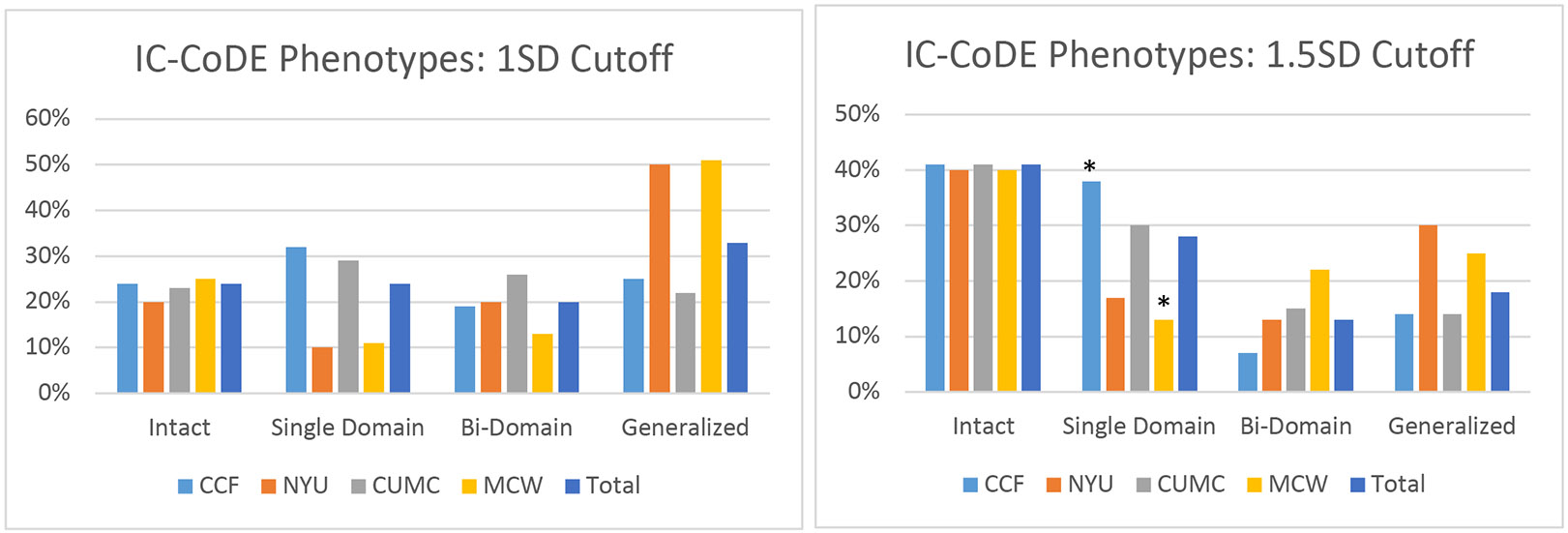
CCF= Cleveland Clinic Foundation; NYU= New York University, CUMC= Columbia University Medical Center, MCW= Medical College of Wisconsin
* corrected p < 0.05 with follow up pairwise comparisons
For thoroughness, analyses were repeated for patients who had available data on at least two measures in all five cognitive domains and results did not significantly differ. Those patients who had missing data were most commonly missing a second score in either the visuospatial or executive function domains.
3.4. Comparisons between FLE and TLE
Test impairment rates in our sample were compared to those published in TLE using a ≤ 1.5SD impairment threshold and are depicted in Figure 3. Post-hoc t-tests were conducted to compare mean impairment rates between patients with FLE and TLE. Results indicated that patients with FLE had significantly higher rates of impairment on most measures of attention and processing speed (Trail Making Test Part A: t(2098) = 11.44, p < 0.01; Symbol Search: t(1356) = 8.20, p < 0.01; Coding: t(1431) = 13.14, p < 0.01; Arithmetic: t(2032) = 10.30, p < 0.01; Spatial Span: t(1109) = 3.58, p < 0.01). Patients with FLE also had higher rates of impairment in aspects of language (Auditory Naming Test: t(197) = 5.21, p < 0.01; phonemic fluency: t(580) = 3.30, p < 0.01), visuospatial abilities (Block Design: t(2242) = 11.59 p < 0.01; Matrix Reasoning: t(1954) = 8.81, p < 0.01), and executive function (Trail Making Test Part B: t(2098) = 15.90, p < 0.01; Letter Number Sequencing: t(948) = 5.17, p < 0.01) compared to patients with TLE. Memory impairment rates between patients with TLE and FLE were generally similar, although patients with TLE had higher rates of impairment on a story delayed recall task (Logical Memory: t(2035) = 3.85, p < 0.01).
Figure 3. Comparison of test-specific impairment rates using < 1.5 SD cutoff threshold by epilepsy syndrome (FLE vs. TLE).
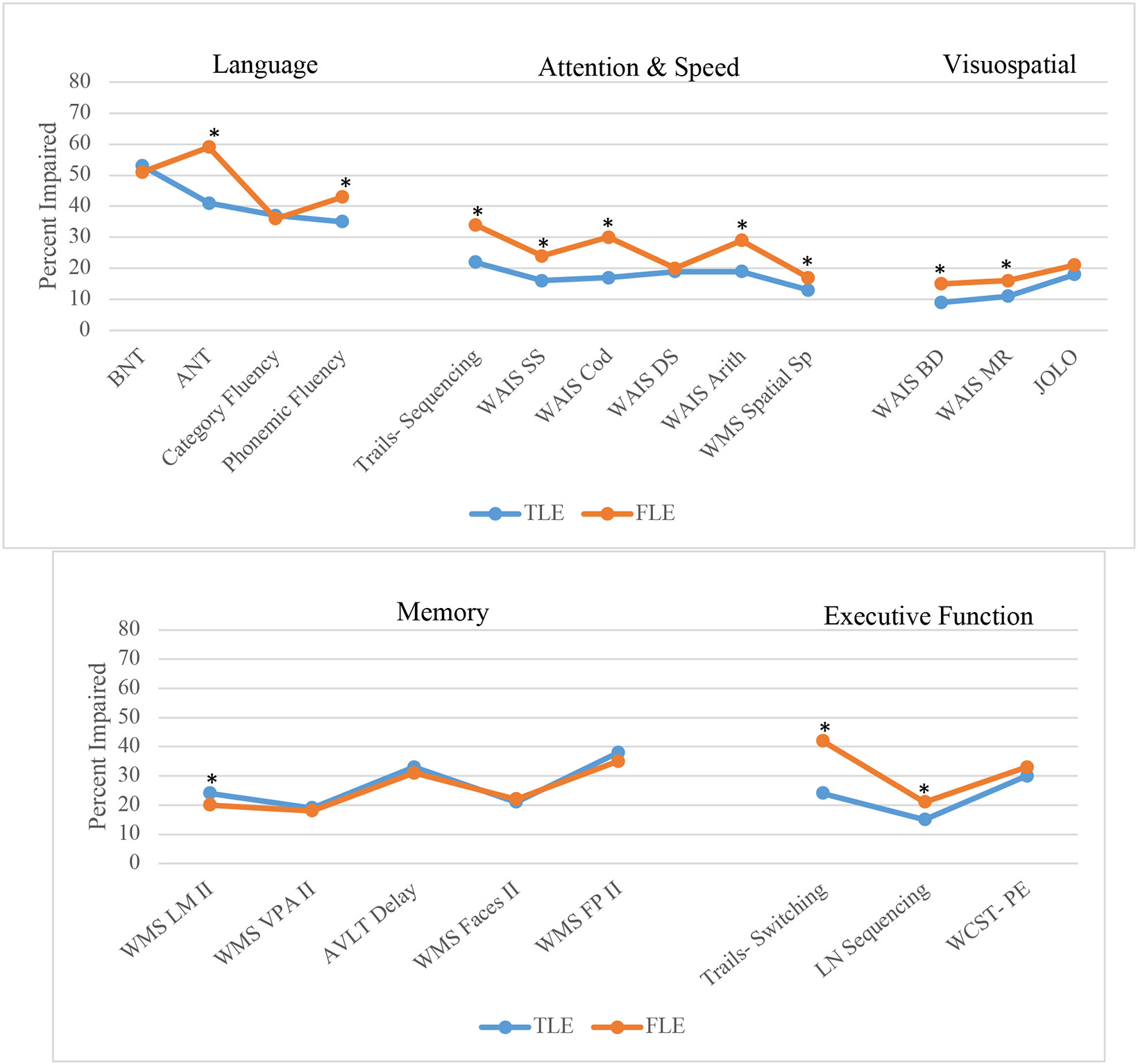
* p < 0.01
BNT= Boston Naming Test; ANT: Auditory Naming Test; WAIS: Wechsler Adult Intelligence Scale; SS: Symbol Search; Cod: Coding; DS: Digit Span; Arith: Arithmetic: Sp= Span; BD: Block Design; MR: Matrix Reasoning; JOLO: Judgement of Line Orientation; WMS: Wechsler Memory Scale; LM II: Logical Memory delay; VPA II: Verbal Paired Associates delay; AVLT: Rey Auditory Verbal Learning Test; FP: Family Pictures; LN: Letter Number; WCST-PE: Wisconsin Card Sorting Test- Perseverative Errors
Lastly, we compared our FLE IC-CoDE phenotype distributions to those published on TLE, depicted in Figure 4. In general, patients with FLE were more frequently classified in more severe phenotype categories compared to patients with TLE. Specifically, using the ≤ 1.0 SD impairment threshold resulted in 33% of patients with FLE falling in the generalized impairment phenotype compared to 22% of patients with TLE, and only 23% of patients with FLE were classified as cognitively intact compared to 30% of patients with TLE. A similar pattern was observed using the ≤ 1.5 SD impairment threshold; 18% of patients with FLE had generalized impairment compared to 10% in patients with TLE and 40% of patients with FLE were cognitively intact compared to 50% in patients with TLE.
Figure 4. Comparison of IC-CoDE phenotypes between patients with FLE and TLE.
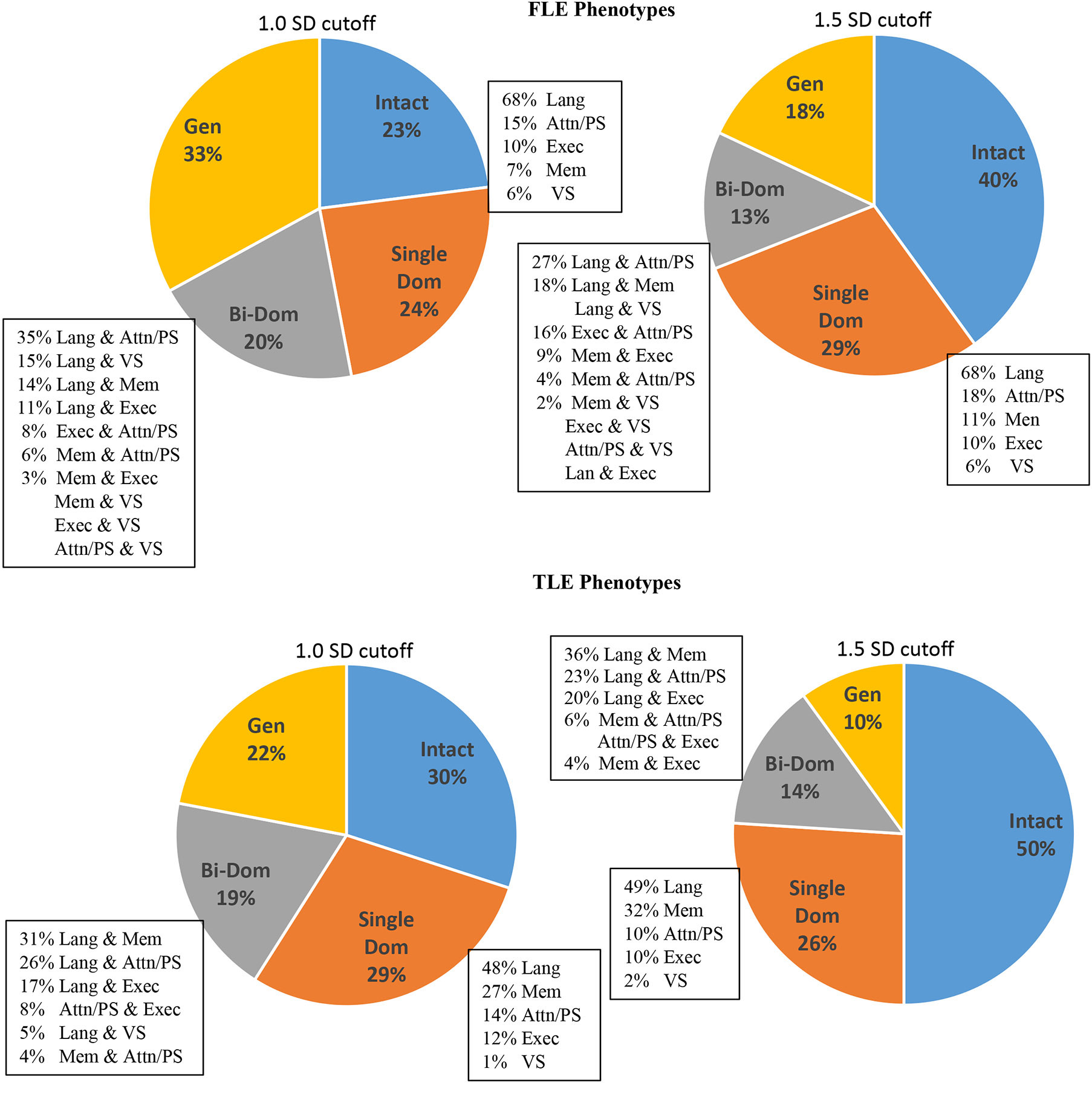
Lang: Language; Mem: Memory; Attn: Attention; PS: Processing Speed; Exec: Executive; VS: Visuospatial
Language domain impairment was the most common domain-specific impairment across both groups. However, memory was a clear second domain-specific impairment in TLE, while secondary domain-specific impairments in FLE were similar for the domains of memory, attention/processing speed, and executive function.
4. Discussion
This is the first study to apply the newly developed IC-CoDE to patients with FLE using multicenter data. The following phenotype distributions were found using ≤ 1.0 SD or ≤ 1.5 SD impairment thresholds: 23% or 40% cognitively intact, 24% or 29% single domain impairment, 20% or 13% bi-domain impairment, and 33% or 18% generalized impairment, respectively. We found that patients with FLE had the highest rates of impairment on measures of language, followed by memory, executive function, and attention/processing speed, all of which had similar rates of impairment. Visuospatial tests had the lowest rates of impairment. Importantly, phenotype distributions were mostly stable across sites, supporting the applicability of IC-CoDE in extratemporal epilepsy and the potential for expanding global research efforts using multicenter neuropsychological data.
4.1. Impairment Rates Across Tests
Although fronto-subcortical patterns of dysfunction (i.e., deficits in attention, processing speed, and executive function) are often associated with FLE14, our results provide interesting insight into the variability across tests. Most notably, language tests showed the highest rates of impairment, with both naming and verbal fluencies being less than 1 SD below the normative mean in over half of patients with FLE. The only other study published on cognitive phenotypes in FLE used a subset of patients included in this sample and found similar results17. It is noteworthy that this finding remained after adding data from multiple sites diverse in geographical location and patient characteristics, indicating that the initial finding was not a site-specific anomaly. Language is likely impacted in many patients with FLE because expressive language skills rely on a broad network of regions including the pars opercularis and pars triangularis of the left inferior frontal gyrus18 as well as association cortex within frontoparietal and frontotemporal regions, and white matter tracts interconnecting this heteromodal cortex19,20.
We found similar impairment rates across tests used to assess memory, executive function, and attention/processing speed. Impairment on these tests has been found in prior literature14, which is not surprising given that many of these tests are associated with frontal lobe functions. Of note, there did not appear to be a clear difference in modality-specific memory performance, but our sample included a relatively even split between patients with right- versus left-sided seizures; future studies would benefit from determining if side of seizures influence memory impairment patterns. Overall, the highest rates of memory impairment were observed on word list memory tasks, which is not surprising as these are considered more challenging memory tasks that are less process-pure than other memory measures, requiring lexical retrieval and tapping into a variety of frontally-mediated cognitive processes21. Post-hoc analyses confirmed that the word list tests were often correlated with working memory, processing speed, and executive function tests, in addition to other memory measures.
Across measures of attention/processing speed and executive function, Trail Making Test parts A and B both showed the highest rate of impairment in their respective domains. The Trail Making Test is corrected for age, education, sex, and race, while the other tests within the attention/processing speed and executive function domains are only corrected for age, and sometimes education, and this may be one reason for the higher rates of impairment. The Trail Making Test also heavily relies on scanning and eye-hand-motor coordination.
Rates of visuospatial impairment were relatively low, except for copy of a complex figure. This finding is likely due to two factors: 1) complex figure copy is thought to be dependent on aspects of executive function, such as planning and organization, and visuospatial skills22, which was supported by follow up post-hoc correlations, and 2) the normative data used to score this test is skewed, resulting in an impaired score when just a few points are deducted. As the IC-CoDE is further refined, identifying the complexities of individual test characteristics, such as those with the complex figure copy, will be important.
4.2. IC-CoDE Phenotypes in FLE
IC-CoDE phenotype distributions differed depending on the cutoff threshold used. Using a ≤ 1.0 SD cutoff produced an almost even distribution between the four IC-CoDE phenotypes (rates ranging from 20-33%), while using a ≤ 1.5 SD cutoff was more conservative, but still classified the majority (60%) of patients as having at least some level of cognitive impairment. These results are generally consistent with the only other published study on cognitive phenotypes in FLE that were constructed using clinical criteria and a ≤ 1.0 SD cutoff threshold, although the phenotype groups differed slightly in criteria17. Post-hoc analyses also indicated that phenotype distributions did not differ by side of seizures, and language continued to be the most prominent single domain impairment, although language impairment rates were slightly higher in those with left-sided FLE compared to those with right-sided FLE (77-82% vs. 51-55% depending on cutoff threshold, respectively).
Distribution of phenotypes across the four sites included in the study were fairly consistent, although two sites significantly differed in the proportion of patients falling into the single domain impairment phenotype using the ≤ 1.5 SD threshold. This difference appeared related to one site classifying a higher proportion of patients as impaired on measures related to memory and attention/processing speed compared to the other site, ultimately increasing the number of patients in their bi-domain and generalized impairment phenotypes, while the other site had a higher number of single domain impairment patients. A contributing factor appeared related to differences in memory tests administered between the two sites. Specifically, one site administered the SRT and, in some cases, the BVMT, and these tests had relatively high rates of impairment (36% and 67%, respectively, using the ≤ 1.5 SD threshold). The BVMT, a visual memory test in which participants are briefly shown simple designs that they then must replicate, was a particular anomaly. The slightly skewed normative data and relatively strict scoring criteria may have caused higher rates of impairment. The BVMT is also thought to rely on visuospatial skills and processing speed23, although, in this sample, the BVMT was weakly correlated (r < 0.4) with other cognitive measures, including memory. The BVMT was not included in the initial test impairment statistics because it was administered to so few patients; however, this finding highlights another outlier test that warrants further investigation. The site with lower rates of single domain impairment also had a higher proportion of patients with bilateral seizures, and this may have contributed to a small subset of patients with greater cognitive impairment. Otherwise, the two sites did not significantly differ on demographics, clinical variables, or general number of tests included in phenotyping that could further explain the difference in impairment rates, although other factors that were not examined in this study, such as extent of epileptogenic zone, could be playing a role.
4.3. Comparison of FLE and TLE
Finally, we compared test-specific impairment rates and IC-CoDE phenotype distributions in FLE to those previously published on TLE5. Patients with FLE showed higher rates of impairment across many of the tests as compared to TLE, particularly on measures of attention/processing speed and aspects of language and executive function. Memory impairment rates were quite similar between patients with FLE and TLE and previous literature has suggested that this is likely because of the rapid propagation between the frontal and temporal lobes24. Memory also requires multiple cognitive processes, including working memory, organization, and strategizing, that are linked to frontal lobe functions. However, memory scores used in our study may have primarily captured retrieval-based deficits (i.e., delayed recall), and there are likely multiple memory profiles in our patients (e.g., encoding deficits) that differ between FLE and TLE and are not captured with these data.
Consistent with the test-specific impairment rates, a higher portion of patients with FLE fell into more severe IC-CoDE phenotypes. Higher cognitive impairment in FLE is not surprising as we know that FLE is more heterogeneous than TLE, commonly characterized by complex and widespread epileptogenicity with varied etiologies25. Neuropsychological measures used to assess frontally-mediated cognitive abilities are also less process-pure, resulting in more overlap between cognitive constructs that may result in multidomain impairments. Amodal processing, top-down regulation, and delayed maturation of white matter tracts of the frontal lobe are other factors that have been associated with more diffuse cognitive dysfunction26-28.
4.4. Limitations and Future Directions
There are several limitations to the current study. We limited our sample to English-speaking patients; however, other studies are underway to apply the IC-CoDE to different languages and cultures7. The current study is also limited to US-based sites, and it will be important for future research to expand to international cohorts. We did not limit the number of tests included within a single domain, similar to the original IC-CoDE validation study; however, this could result in higher impairment rates among patients who were given more tests or sites that have larger test batteries. In general, the sites included in this study had a similar number of tests included in the domains used for phenotyping, but this may not be the case for other centers; thus, this is an area of future investigation for the IC-CoDE. Our findings also highlight how certain test-specific characteristics may impact IC-CoDE phenotyping between sites, and large-scale studies are underway to further refine IC-CoDE guidelines as more research is needed to understand how to best balance the flexibility of the application of the IC-CoDE, while also limiting source error when test batteries vary substantially. More research is also needed to understand the underlying mechanisms, such as clinical and etiological factors, behind these distinct cognitive phenotypes and investigate whether the underlying mechanisms differ across epilepsy syndromes. This study relied on group-level data from certain sites; therefore, additional analyses examining the relationship between reported demographic and clinical characteristics could not be conducted, but this is certainly an area for future study. Given that the frontal lobe can also be further subdivided into regions associated with distinct cognitive processes, future research will also benefit from examining more specific associations between IC-CoDE phenotypes and epileptogenic zone, including side of seizures, as well as structural, diffusion, and functional imaging correlates.
5. Conclusion
This study demonstrates that the IC-CoDE, a harmonized diagnostic approach to characterize cognitive disorders in epilepsy, can be applied to epilepsy syndromes outside of TLE, with generally stable and reproducible phenotypes. That said, this work also highlighted a few anomalies that warrant investigation and large-scale studies are underway to further refine the IC-CoDE guidelines. Nonetheless, the IC-CoDE allowed us to create a rich data source to examine the cognitive presentation of FLE when previous studies have been significantly limited by sample size, which was one of the primary goals of the IC-CoDE.
Supplementary Material
Funding Information:
Support for this study was provided by the Cleveland Clinic Epilepsy Center.
Footnotes
Conflict of Interest: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Dr. Hamberger receives support from NIH R01 NS35140. None of the other authors have any conflicts of interest to disclose.
References:
- 1.Giovagnoli AR, Parente A, Tarallo A, Casazza M, Franceschetti S, Avanzini G. Self-rated and assessed cognitive functions in epilepsy: impact on quality of life. Epilepsy Res. 2014;108(8):1461–1468. [DOI] [PubMed] [Google Scholar]
- 2.Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62(10):1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34 [DOI] [PubMed] [Google Scholar]
- 3.Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54(4):425–432. doi: 10.1002/ana.10692 [DOI] [PubMed] [Google Scholar]
- 4.McAuley JW, Elliott JO, Patankar S, et al. Comparing patients’ and practitioners’ views on epilepsy concerns: a call to address memory concerns. Epilepsy Behav EB. 2010;19(4):580–583. doi: 10.1016/j.yebeh.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 5.McDonald CR, Busch RM, Reyes A, et al. Development and application of the International Classification of Cognitive Disorders in Epilepsy (IC-CoDE): Initial results from a multi-center study of adults with temporal lobe epilepsy. Neuropsychology. Published online January 27, 2022. doi: 10.1037/neu0000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman M, Wilson SJ, Baxendale S, et al. Addressing neuropsychological diagnostics in adults with epilepsy: Introducing the International Classification of Cognitive Disorders in Epilepsy: The IC CODE Initiative. Epilepsia Open. 2021;6(2):266–275. doi: 10.1002/epi4.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyes A, Salinas L, Hermann BP, et al. Establishing the cross-cultural applicability of a harmonized approach to cognitive diagnostics in epilepsy: Initial results of the International Classification of Cognitive Disorders in Epilepsy in a Spanish-speaking sample. Epilepsia. 2023;64(3):728–741. doi: 10.1111/epi.17501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson L, Arenivas A, Arrotta K, et al. Application of the International Classification of Cognitive Disorders in Epilepsy (IC-CoDE) to children with pharmacoresistant epilepsy. In: ; 2023. [Google Scholar]
- 9.Shah U, Rajeshree S, Reyes A, et al. Cross Cultural Application of the International Classification of Cognitive Disorders in Epilepsy (IC CoDE) Cognitive Phenotypes in People with Temporal Lobe Epilepsy in India. In: ; 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock LM, Galioto R, Samsonov A, Busch RM, Hermann B, Matias-Guiu JA. A proposed new taxonomy of cognitive phenotypes in multiple sclerosis: The International Classification of Cognitive Disorders in MS (IC-CoDiMS). Mult Scler Houndmills Basingstoke Engl. 2023;29(4-5):615–627. doi: 10.1177/13524585221127941 [DOI] [PubMed] [Google Scholar]
- 11.Matias-Guiu JA, Herrera E, González-Nosti M, et al. Development of criteria for cognitive dysfunction in post-COVID syndrome: the IC-CoDi-COVID approach. Psychiatry Res. 2023;319:115006. doi: 10.1016/j.psychres.2022.115006 [DOI] [PubMed] [Google Scholar]
- 12.Manford MM, Hart YM, Sander JWAS, Shorvon SD, NGPSE. National General Practice Study of Epilepsy (NGPSE): Partial seizure patterns in a general population. Neurology. 1992;42(10):1911–1911. doi: 10.1212/WNL.42.10.1911 [DOI] [PubMed] [Google Scholar]
- 13.Patrikelis P, Angelakis E, Gatzonis S. Neurocognitive and behavioral functioning in frontal lobe epilepsy: A review. Epilepsy Behav. 2009;14(1):19–26. doi: 10.1016/j.yebeh.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 14.Verche E, San Luis C, Hernández S. Neuropsychology of frontal lobe epilepsy in children and adults: Systematic review and meta-analysis. Epilepsy Behav. 2018;88:15–20. doi: 10.1016/j.yebeh.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Centeno M, Thompson PJ, Koepp MJ, Helmstaedter C, Duncan JS. Memory in frontal lobe epilepsy. Epilepsy Res. 2010;91(2-3):123–132. doi: 10.1016/j.eplepsyres.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 16.Risse GL. Cognitive outcomes in patients with frontal lobe epilepsy. Epilepsia. 2006;47 Suppl 2:87–89. doi: 10.1111/j.1528-1167.2006.00699.x [DOI] [PubMed] [Google Scholar]
- 17.Arrotta K, Reyes A, Kaestner E, et al. Cognitive Phenotypes in Frontal Lobe Epilepsy. Epilepsia. Published online April 16, 2022. doi: 10.1111/epi.17260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakreida K, Lange I, Willmes K, et al. High-resolution language mapping of Broca’s region with transcranial magnetic stimulation. Brain Struct Funct. Published online November 7, 2017. doi: 10.1007/s00429-017-1550-8 [DOI] [PubMed] [Google Scholar]
- 19.Hamberger MJ, Cole J. Language organization and reorganization in epilepsy. Neuropsychol Rev. 2011;21(3):240–251. doi: 10.1007/s11065-011-9180-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smits M, Jiskoot LC, Papma JM. White Matter Tracts of Speech and Language. Semin Ultrasound CT MRI. 2014;35(5):504–516. doi: 10.1053/j.sult.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 21.Consonni M, Rossi S, Cerami C, et al. Executive dysfunction affects word list recall performance: Evidence from amyotrophic lateral sclerosis and other neurodegenerative diseases. J Neuropsychol. 2017;11(1):74–90. doi: 10.1111/jnp.12072 [DOI] [PubMed] [Google Scholar]
- 22.Salvadori E, Dieci F, Caffarra P, Pantoni L. Qualitative Evaluation of the Immediate Copy of the Rey–Osterrieth Complex Figure: Comparison Between Vascular and Degenerative MCI Patients. Arch Clin Neuropsychol. 2019;34(1):14–23. doi: 10.1093/arclin/acy010 [DOI] [PubMed] [Google Scholar]
- 23.Caneda MAGD, Cuervo DLM, Marinho NE, Vecino MCAD. The Reliability of the Brief Visuospatial Memory Test - Revised in Brazilian multiple sclerosis patients. Dement Neuropsychol. 2018;12(2):205–211. doi: 10.1590/1980-57642018dn12-020014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kibby MY, Cohen MJ, Stanford L, Park YD. Are frontal and temporal lobe epilepsy dissociable in their memory functioning? Epilepsy Behav. 2019;99:106487. doi: 10.1016/j.yebeh.2019.106487 [DOI] [PubMed] [Google Scholar]
- 25.Bonini F, McGonigal A, Trébuchon A, et al. Frontal lobe seizures: From clinical semiology to localization. Epilepsia. 2014;55(2):264–277. doi: 10.1111/epi.12490 [DOI] [PubMed] [Google Scholar]
- 26.Tamber-Rosenau BJ, Dux PE, Tombu MN, Asplund CL, Marois R. Amodal processing in human prefrontal cortex. J Neurosci Off J Soc Neurosci. 2013;33(28):11573–11587. doi: 10.1523/JNEUROSCI.4601-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9(2):141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buyanova IS, Arsalidou M. Cerebral White Matter Myelination and Relations to Age, Gender, and Cognition: A Selective Review. Front Hum Neurosci. 2021;15:662031. doi: 10.3389/fnhum.2021.662031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


