Abstract
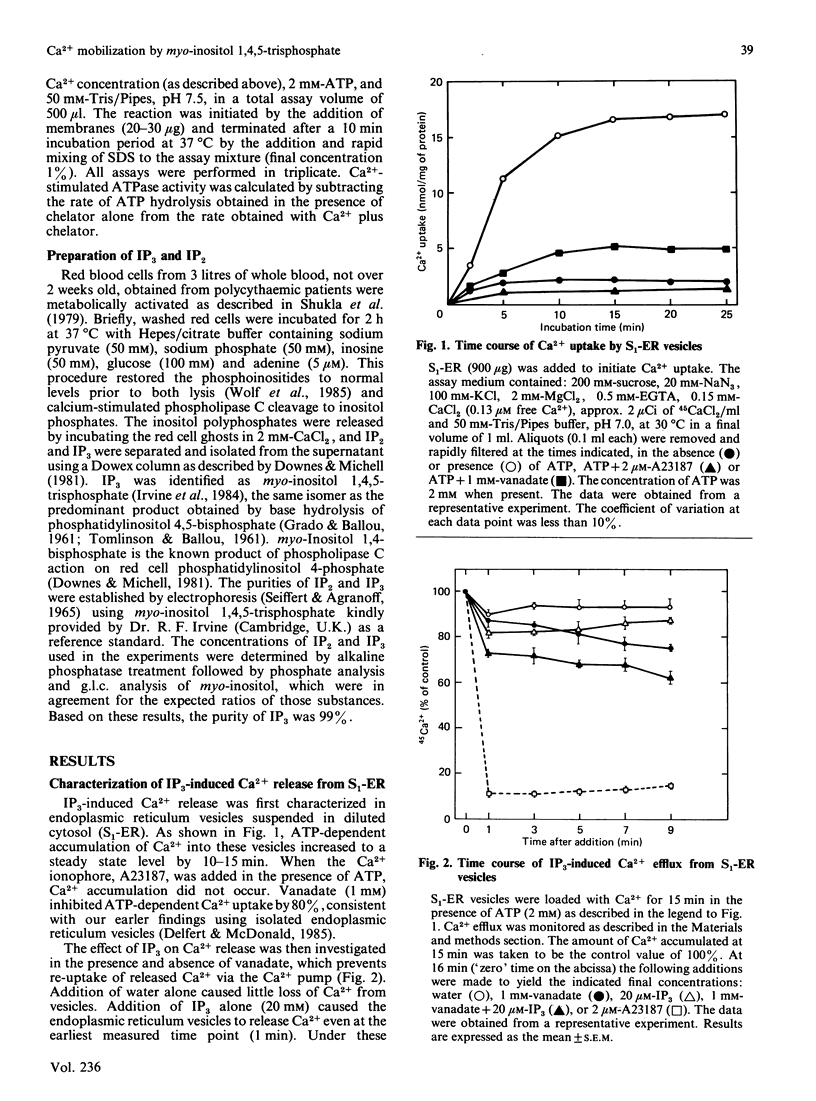
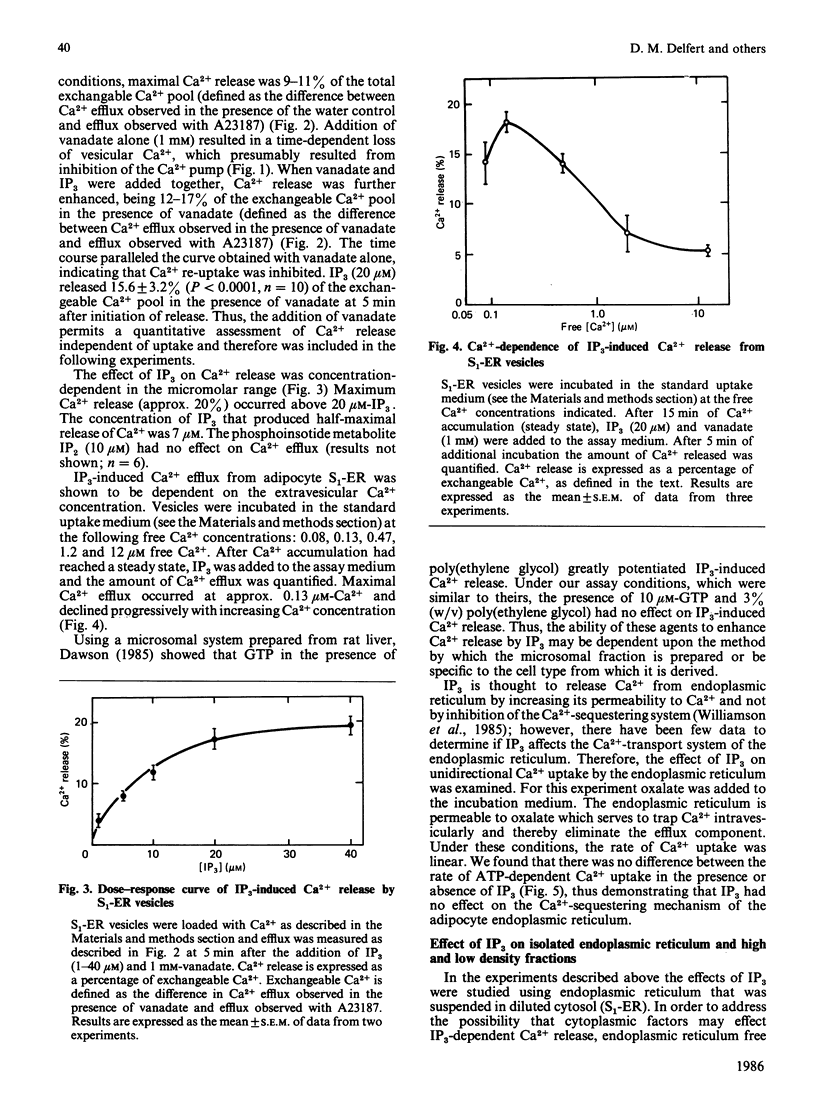
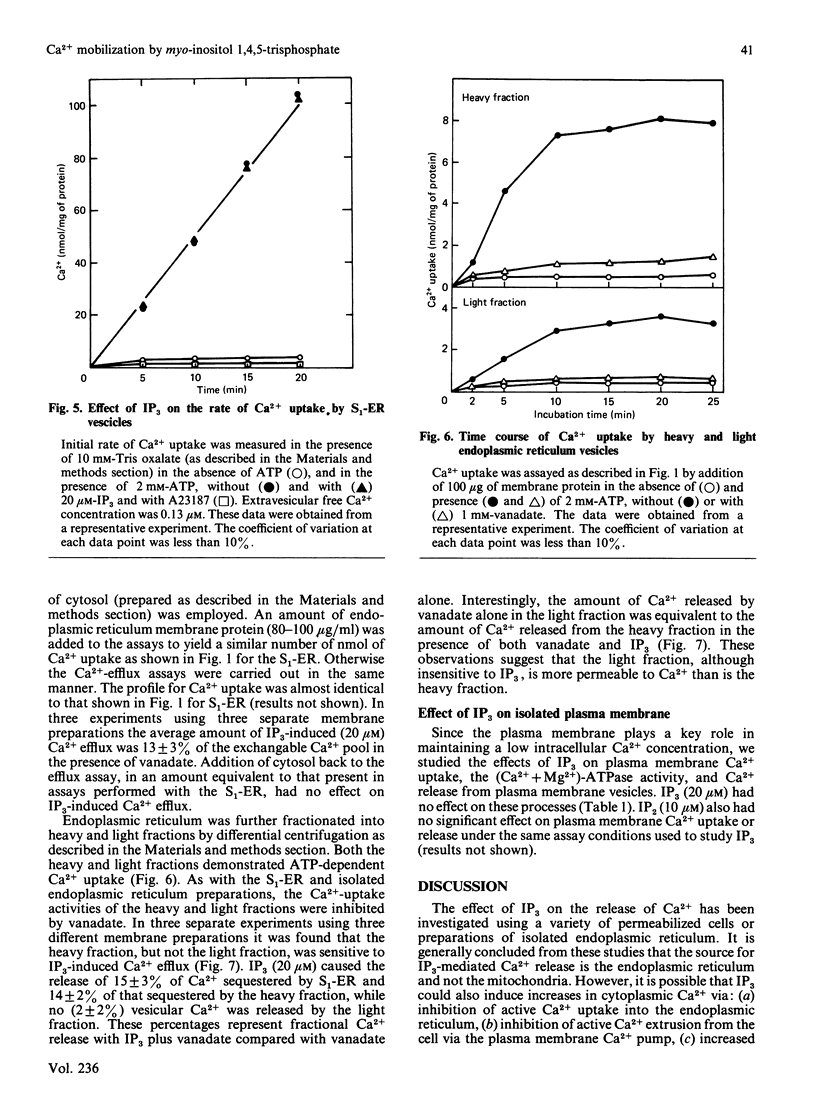
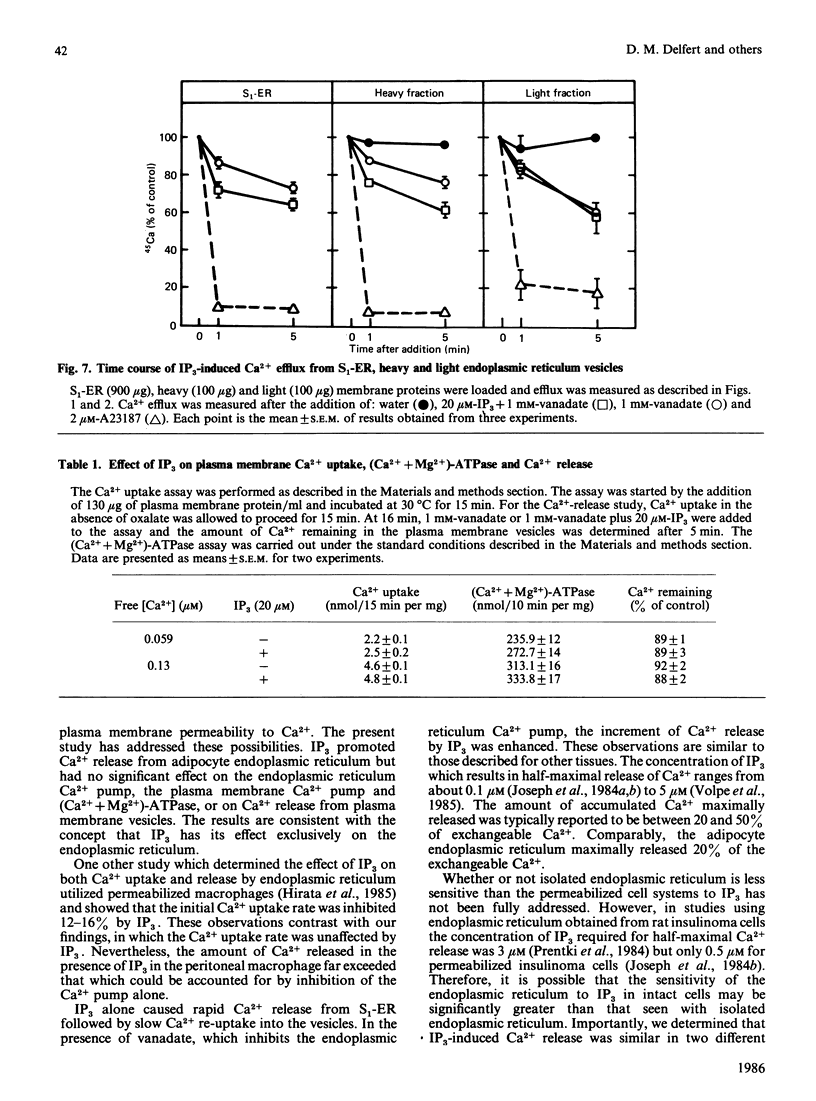
The effects of myo-inositol 1,4,5-trisphosphate (IP3) on Ca2+ uptake and release from isolated adipocyte endoplasmic reticulum and plasma membrane vesicles were investigated. Effects of IP3 were initially characterized using an endoplasmic reticulum preparation with cytosol present (S1-ER). Maximal and half-maximal effects of IP3 on Ca2+ release from S1-ER vesicles occurred at 20 microM- and 7 microM-IP3, respectively, in the presence of vanadate which prevents the re-uptake of released Ca2+ via the endoplasmic reticulum Ca2+ pump. At saturating IP3 concentrations, Ca2+ release in the presence of vanadate was 20% of the exchangeable Ca2+ pool. IP3-induced release of Ca2+ from S1-ER was dependent on extravesicular free Ca2+ concentration with maximal release occurring at 0.13 microM free Ca2+. At 20 microM-IP3 there was no effect on the initial rate of Ca2+ uptake by S1-ER. IP3 promoted Ca2+ release from isolated endoplasmic reticulum vesicles (cytosol not present) to a similar level as compared with S1-ER. Addition of cytosol to isolated endoplasmic reticulum vesicles did not affect IP3-induced Ca2+ release. The endoplasmic reticulum preparation was further fractionated into heavy and light vesicles by differential centrifugation. Interestingly, the heavy fraction, but not the light fraction, released Ca2+ when challenged with IP3. IP3 (20 microM) did not promote Ca2+ release from plasma membrane vesicles and had no effect on the (Ca2+ + Mg2+)-ATPase activity or on the initial rate of ATP-dependent Ca2+ uptake by these vesicles. These results support the concept that IP3 acts exclusively at the endoplasmic reticulum to promote Ca2+ release.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black B. L., Jarett L., McDonald J. M. Relationship between calcium ion transport and (Ca2+ + Mg2+)-atpase activity in adipocyte endoplasmic reticulum. Biochim Biophys Acta. 1980 Mar 13;596(3):359–371. doi: 10.1016/0005-2736(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Snowdowne K. W. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982 Jul 16;217(4556):252–254. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- Bruns D. E., McDonald J. M., Jarett L. Energy-dependent calcium transport in endoplasmic reticulum of adipocytes. J Biol Chem. 1976 Nov 25;251(22):7191–7197. [PubMed] [Google Scholar]
- Burgess G. M., Irvine R. F., Berridge M. J., McKinney J. S., Putney J. W., Jr Actions of inositol phosphates on Ca2+ pools in guinea-pig hepatocytes. Biochem J. 1984 Dec 15;224(3):741–746. doi: 10.1042/bj2240741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K., Larner J. Unidirectional actions of insulin and Ca2+-dependent hormones on adipocyte pyruvate dehydrogenase. J Biol Chem. 1985 May 10;260(9):5279–5285. [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P. GTP enhances inositol trisphosphate-stimulated Ca2+ release from rat liver microsomes. FEBS Lett. 1985 Jun 3;185(1):147–150. doi: 10.1016/0014-5793(85)80759-6. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Delfert D. M., McDonald J. M. Vanadyl and vanadate inhibit Ca2+ transport systems of the adipocyte plasma membrane and endoplasmic reticulum. Arch Biochem Biophys. 1985 Sep;241(2):665–672. doi: 10.1016/0003-9861(85)90593-4. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Hirata M., Kukita M., Sasaguri T., Suematsu E., Hashimoto T., Koga T. Increase in Ca2+ permeability of intracellular Ca2+ store membrane of saponin-treated guinea pig peritoneal macrophages by inositol 1,4,5-trisphosphate. J Biochem. 1985 Jun;97(6):1575–1582. doi: 10.1093/oxfordjournals.jbchem.a135214. [DOI] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarett L. Subcellular fractionation of adipocytes. Methods Enzymol. 1974;31:60–71. doi: 10.1016/0076-6879(74)31007-5. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J., Corkey B. E., Matschinsky F. M., Williamson J. R. The effect of inositol trisphosphate on Ca2+ fluxes in insulin-secreting tumor cells. J Biol Chem. 1984 Nov 10;259(21):12952–12955. [PubMed] [Google Scholar]
- Koepfer-Hobelsberger B., Wieland O. H. Insulin activates phospholipase C in fat cells: similarity with the activation of pyruvate dehydrogenase. Mol Cell Endocrinol. 1984 Jun;36(1-2):123–129. doi: 10.1016/0303-7207(84)90091-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin S. H. Novel ATP-dependent calcium transport component from rat liver plasma membranes. The transporter and the previously reported (Ca2+-Mg2+)-ATPase are different proteins. J Biol Chem. 1985 Jul 5;260(13):7850–7856. [PubMed] [Google Scholar]
- Machicao E., Wieland O. H. Evidence that the insulin receptor-associated protein kinase acts as a phosphatidylinositol kinase. FEBS Lett. 1984 Sep 17;175(1):113–116. doi: 10.1016/0014-5793(84)80581-5. [DOI] [PubMed] [Google Scholar]
- McDonald J. M., Bruns D. E., Jarett L. The ability of insulin to alter the stable calcium pools of isolated adipocyte subcellular fractions. Biochem Biophys Res Commun. 1976 Jul 12;71(1):114–121. doi: 10.1016/0006-291x(76)90256-4. [DOI] [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Pershadsingh H. A., Landt M., McDonald J. M. Calmodulin-sensitive ATP-dependent Ca2+ transport across adipocyte plasma membranes. J Biol Chem. 1980 Oct 10;255(19):8983–8986. [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. A high affinity calcium-stimulated magnesium-dependent adenosine triphosphatase in rat adipocyte plasma membranes. J Biol Chem. 1980 May 10;255(9):4087–4093. [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. Hormone-receptor coupling and the molecular mechanism of insulin action in the adipocyte: a paradigm for Ca2+ homeostasis in the initiation of the insulin-induced metabolic cascade. Cell Calcium. 1984 Apr;5(2):111–130. doi: 10.1016/0143-4160(84)90011-3. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Bruns D., Jarett L. A sensitive and precise isotopic assay of ATPase activity. Anal Biochem. 1978 Oct 15;90(2):785–795. doi: 10.1016/0003-2697(78)90169-0. [DOI] [PubMed] [Google Scholar]
- Seiffert U. B., Agranoff B. W. Isolation and separation of inositol phosphates from hydrolysates of rat tissues. Biochim Biophys Acta. 1965 Jun 1;98(3):574–581. doi: 10.1016/0005-2760(65)90154-2. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Coleman R., Finean J. B., Michell R. H. Are polyphosphoinositides associated with glycophorin in human erythrocyte membranes? Biochem J. 1979 May 1;179(2):441–444. doi: 10.1042/bj1790441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- TOMLINSON R. V., BALLOU C. E. Complete characterization of the myo-inositol polyphosphates from beef brain phosphoinositide. J Biol Chem. 1961 Jul;236:1902–1906. [PubMed] [Google Scholar]
- Taylor D., Uhing R. J., Blackmore P. F., Prpić V., Exton J. H. Insulin and epidermal growth factor do not affect phosphoinositide metabolism in rat liver plasma membranes and hepatocytes. J Biol Chem. 1985 Feb 25;260(4):2011–2014. [PubMed] [Google Scholar]
- Volpe P., Salviati G., Di Virgilio F., Pozzan T. Inositol 1,4,5-trisphosphate induces calcium release from sarcoplasmic reticulum of skeletal muscle. Nature. 1985 Jul 25;316(6026):347–349. doi: 10.1038/316347a0. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Comens P. G., Ackermann K. E., Sherman W. R., McDaniel M. L. The digitonin-permeabilized pancreatic islet model. Effect of myo-inositol 1,4,5-trisphosphate on Ca2+ mobilization. Biochem J. 1985 May 1;227(3):965–969. doi: 10.1042/bj2270965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]


