Abstract
We have analyzed the influence of codon usage modifications on the expression levels and immunogenicity of DNA vaccines, encoding the human immunodeficiency virus type 1 (HIV-1) group-specific antigen (Gag). In the presence of Rev, an expression vector containing the wild-type (wt) gag gene flanked by essential cis-acting sites such as the 5′-untranslated region and 3′-Rev response element supported substantial Gag protein expression and secretion in human H1299 and monkey COS-7 cells. However, only weak Gag production was observed from the murine muscle cell line C2C12. In contrast, optimization of the Gag coding sequence to that of highly expressed mammalian genes (syngag) resulted in an obvious increase in the G+C content and a Rev-independent expression and secretion of Gag in all tested mammalian cell lines, including murine C2C12 muscle cells. Mice immunized intramuscularly with the syngag plasmid showed Th1-driven humoral and cellular responses that were substantially higher than those obtained after injection of the Rev-dependent wild-type (wt) gag vector system. In contrast, intradermal immunization of both wt gag and syngag vector systems with the particle gun induced a Th2-biased antibody response and no cytotoxic T lymphocytes. Deletion analysis demonstrated that the CpG motifs generated within syngag by codon optimization do not contribute significantly to the high immunogenicity of the syngag plasmid. Moreover, low doses of coadministered stimulatory phosphorothioate oligodeoxynucleotides (ODNs) had only a weak effect on antibody production, whereas at higher doses immunostimulatory and nonstimulatory ODNs showed a dose-dependent suppression of humoral responses. These results suggest that increased Gag expression, rather than modulation of CpG-driven vector immunity, is responsible for the enhanced immunogenicity of the syngag DNA vaccine.
The development of a prophylactic and therapeutic human immunodeficiency virus type 1 (HIV-1) vaccine remains one of the most desirable objectives of research aimed at controlling the current AIDS epidemic. Abundant clinical evidence suggests that, besides neutralizing antibodies, cytotoxic T lymphocytes (CTL) may be a key protective immune parameter in HIV-1 infection (4, 18, 31).
A strong antiviral cytotoxic activity has been shown to correlate temporally with the clearance of viremia in primary infection (4, 11, 14, 32) and a long-lasting control of virus replication within certain populations of long-term nonprogressing individuals (15, 38). The usefulness of Gag immunogens for vaccine development and immunotherapeutic interventions is supported by the fact that the protein is relatively conserved among diverse HIV-1 subtypes, and broad cross-clade CTL recognition directed against Gag-specific targets has been well documented (2, 9, 22). Furthermore, in persons with chronic infection, a decline of Gag-specific CTL precursors was shown to coincide with a CD4 drop, increasing virus load, and disease progression (16).
Recently, direct injection of naked DNA into animals has been evaluated to be a promising approach for the induction of humoral and cellular immune responses (8, 33). Plasmid DNA immunization reveals some potential advantages compared to traditional protein vaccination due to the induction of strong T helper 1 (Th1) and CTL responses, the prolonged antigen expression, and the long-lived effector activity (35, 42). Plasmids expressing nonoptimized HIV-1-derived genes have been recently shown to induce humoral and cellular immune responses in rodents (8, 36), in nonhuman primates (6, 21, 25), and in phase I studies in humans (5, 23). However, in most of these initial studies both the titers of circulating antibodies and the titers of the specific CTL were transient and low.
Two factors have been suggested to be essential for the efficacy of a DNA expression vector: (i) the quality of foreign gene expression unit and (ii) the intrinsic adjuvant properties of the DNA, which are determined by the complex interaction of immunostimulatory and inhibitory sequence motifs (13). Furthermore, it was shown that the route and method of immunization are important modulators of DNA vaccination. The most widely used strategies for the application of DNA vaccine vectors are intramuscular (i.m.) needle injection and intradermal (i.d.) inoculation using a gene gun (35). Both immunization strategies strongly differ in the efficiency of DNA delivery. In general, i.m. immunization by saline needle injection requires 100- to 1,000-fold more DNA than gene gun immunization in order to generate an equivalent antibody response (27). However, the latter approach seems to favor Th2-mediated immune responses (10), which are considered to be less effective for the prevention or control of an HIV infection.
In the present study, we have constructed two HIV-1 Gag DNA expression vector systems and investigated the influence of codon optimization of HIV gag on Gag expression in different mammalian cells. Furthermore, we determined the impact of an increased CpG content on the immunogenicity of the gag DNA vaccine construct. Our data strongly indicate that syngag is more efficient in the priming of humoral and cellular immune responses than the Rev-dependent wild-type (wt) gag construct. Furthermore, our results clearly indicate that the increased immunogenicity of syngag is due to an enhanced Gag expression rather than to intragenic accumulation of CpG motifs as a consequence of codon usage modification.
MATERIALS AND METHODS
Plasmid constructs.
The construction and cloning of gagRRE, UTRgagRRE, and the p-syngag plasmid has been described previously in detail (12). The four typical CpG motifs (RRCGYY) located within the syngag reading frame and one CpG motif located 3′ to syngag were inactivated by replacing the central cytosine with another nucleotide, without alteration of the corresponding amino acid sequence. The mutations were generated by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.), according to the manufacturer's instructions, by using primer pairs, sense and antisense, respectively. Oligonucleotides A1sense (5′-CGC CAG CAT GGG AGC CAG GGC CAG CG-3′) and A1antisense (5′-CGC TGG CCC TGG CTC CCA TGC TGG CG-3′), corresponding to bp −6 to 19 within the syngag, were used to create a C-to-A transfersion at position 6. Oligonucleotides B1sense (5′-CAG GAC CCT GAA TGC CTG GGT GAA GG-3′) and B1antisense (5′-CCT TCA CCC AGG CAT TCA GGG TCC TG-3′), corresponding to bp 447 to 472, were utilized to create a C-to-T transition at position 459. Oligonucleotides C1sense (5′-CCC CAT GTT CAG TGC CCT GAG CGA GG-3′) and C1antisense (5′-CCT CGC TCA GGG CAC TGA ACA TGG GG′), corresponding to bp 507 to 532, were utilized to introduce a C-to-T transition at position 519. Oligonucleotides D1sense (5′-GCT GGT GCA GAA TGC CAA CCC CGA CTG C′) and D1antisense (5′-GCA GTC GGG GTT GGC ATT CTG CAC CAG C-3′), corresponding to bp 962 to 990, were used to introduce a C-to-T transition at position 915 within syngag. Oligonucleotides E1sense (5′-GAT CCG GGA GCG GAG TTC TCG AGC ATG-3′) and E1antisense (5′-CAT GCT CGA GAA CTC CGC TCC CGG ATC-3′), corresponding to bp 2 to 27 downstream of the stop codon of the syngag reading frame, were used to introduce a C-to-A transition at position +13. The CpG motifs are in boldface, and the mutated nucleic acids within the CpG motifs are in italics. For transient expression of the Gag polyprotein in mammalian cells and immunization studies, the syngagΔCpG sequence was placed into the KpnI and XhoI restriction sites of the pcDNA3.1(+) expression vector under the transcriptional control of the cytomegalovirus immediate-early promoter-enhancer, resulting in the plasmid p-syngagΔCpG (Fig. 1). The plasmid pCsRevsg25-GFP (termed pRev here) expressing Rev fused to the green fluorescent protein (GFP) was kindly provided by Marcus Neumann (GSF, Munich, Germany). The reading frames of all mutants were verified by DNA sequence analysis using the dye terminator cycle sequencing technique and a PE Biosystems ABI Prism 377 DNA sequencer (PE Biosystems, Weiterstadt, Germany).
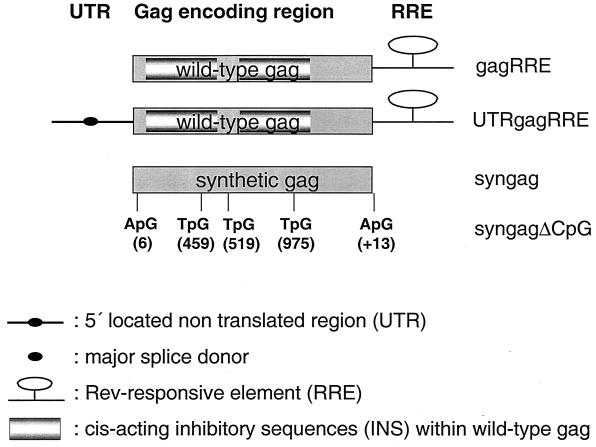
FIG. 1.
Schematic representation of different wt and synthetic HIV-1 gag gene constructs. Variations in cis-acting elements comprise (i) the presence or absence of the RRE, (ii) the 5′ UTR containing the major splice donor site (UTRgagRRE), (iii) extensive codon modification within the Gag encoding region (syngag), and (iv) the lack of five functional CpG motifs within the syngag gene (syngagΔCpG). The positions of the mutated cytosines within the gag gene within the CpG motifs are indicated.
Synthetic peptides and ODNs.
A 9-mer peptide (AMQMLKETI), which represents a defined Dd-restricted CTL epitope of HIV-1 p24(CA) in BALB/c mice, was purchased from Toplab (Martinsried, Germany). Nuclease-resistant phosphorothioate oligodeoxynucleotides (ODNs) were provided by Metabion (Munich, Germany) and used in single-stranded form. An immunostimulatory CpG ODN (TCATTGGAAAACGTTCTTCGGGGCG) and a control GpG ODN (TCATTGGAAAAGGTTCTTGGGGGGG) with inactivated CpG motifs were synthesized according to previously published sequences (34). In a second control oligodeoxynucleotide (CpG-M ODN) cytosines were substituted by methyl cytosines in all CpG dinucleotides. The lyophilized ODNs were resuspended in phosphate-buffered saline (PBS) prior to injection. The lipopolysaccharide content in all ODNs and plasmid DNA preparations was <10 ng/mg as determined by a Limulus amebocyte lysate QLC-1000 kit (Whittaker Bioproducts, Walkersville, Md.).
Determination of the stimulatory properties of plasmid DNA.
Spleens were recovered under sterile conditions from nonimmunized mice at an age of 50 to 70 days, and the obtained splenic single cell suspensions were seeded at 2 × 106 cells per ml in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS) and 1% penicillin-streptomycin (Gibco-BRL) in the presence or absence of 50 μg of different plasmid constructs. As a positive control, splenic cells were stimulated with 50 μg of CpG ODNs. After 36 h of culture, cytokine levels were determined from the precleared supernatant by using a commercial enzyme-linked immunosorbent assay (ELISA) assay according to the manufacturer's instructions (Becton Dickinson).
Cell lines and transfections.
The H-2d mastocytoma cell line P815 (TIB 64) and the H-2d B-lymphoma cell line A20 (TIB 208) were obtained from the American Type Culture Collection, Rockville, Md.). P815 and A20 cells were propagated in RPMI medium supplemented with 5% (vol/vol) heat-inactivated FCS, 50 μM 2-mercaptoethanol, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml. COS-7 (African green monkey kidney cells), H1299 (human lung carcinoma cells), and C2C12 (mouse muscle cells) were maintained in Dulbecco modified Eagle medium (Gibco-BRL, Eggenstein, Germany) supplemented with 10% FCS, 2 mM l-glutamine, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml. All mammalian cell lines were maintained in a humidified atmosphere with 7% CO2 at 37°C.
The cells were transfected by the calcium coprecipitation technique as described previously (12). Briefly, 1.5 × 106 C2C12, 3 × 106 H1299, or 5 × 106 COS-7 cells were seeded on 100-mm-diameter culture dishes, incubated for 24 h, and then transfected with 45 μg of different Nucleobond AX (Macherey-Nagel, Düren, Germany) purified plasmid constructs. At 16 h posttransfection, the cell culture supernatant was replaced with fresh medium.
Immunoblotting and p24 capture assay.
Total cell lysates were prepared 48 h posttransfection by using a triple-detergent buffer system (radioimmunoprecipitation assay), which was supplemented with a cocktail of protease inhibitors (Boehringer Complete Mini Kit; Mannheim GmbH, Mannheim, Germany). The total protein concentration was determined by a Bradford protein assay (Bio-Rad Laboratories, Munich, Germany). Equal amounts (100 μg of total protein) of lysates were separated by electrophoresis on a 12.5% denaturing sodium dodecyl sulfate (SDS)-polyacrylamide gel. Proteins were then transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), which were probed sequentially with a 1:2,000 dilution of the HIV-1 p24-specific monoclonal antibody 16-4-2 (41) and a 1:2,000 dilution of an alkaline phosphatase-labeled goat anti-mouse immunoglobulin G (IgG; Bio-Rad Laboratories, Munich, Germany). Proteins were visualized with NBT and BCIP solutions (0.3% 4-nitroblue tetrazolium chloride [NBT], 0.3% 5-bromo-4-chloro-3-indolylphosphate [BCIP], 100 mM Tris, 100 mM NaCl, 50 mM MgCl2; pH 9.5 [Boehringer]). Cell culture supernatants were collected 2 days after transfection and clarified by centrifugation at 3,000 × g for 10 min. The Gag content was determined by using a p24 capture ELISA as described earlier in detail (26). The p24-specific monoclonal antibodies 11-G-7 and 10-E-7, used in this assay, were kindly provided by Matthias Niedrig (RKI, Berlin, Germany). The Gag concentration was determined from a calibration curve by using different concentrations of purified Gag polyprotein, which was produced in insect cells by using the baculovirus expression system (37).
DNA vaccination of mice.
Female BALB/c mice (Charles River, Sulzfeld, Germany) were housed under specific-pathogen-free conditions and injected at the age of 6 to 12 weeks. Mice were immunized with the indicated plasmid concentrations by i.m. saline injection with 50 μl of plasmid DNA in separate sites in both tibialis anterior muscles, followed by i.m. booster immunizations with the same doses of plasmid DNA. For immunization with Gag expression vectors plus synthetic ODNs, mice received two i.m. inoculations with 80 μg of DNA plus various ODN concentrations at weeks 0 and 3. Alternatively, mice were gene gun inoculated on shaved abdominal skin with plasmid DNA-coated gold particles (0.95-μm particles, 2 μg of DNA/mg of gold, 0.5 mg of gold/shot) and a hand-held Accel gene gun device (Bio-Rad Laboratories, Hercules, Calif.) employing compressed helium (400 lb/in2) as the particle motive force.
Evaluation of antibody responses.
Serum was recovered from mice by tail bleeding at the indicated time points after the booster injection. Anti-Gag antibodies were quantified by an end-point dilution ELISA assay (in duplicate) on samples from individual animals.
In brief, the wells of F-bottom microtiter plates (Greiner, Frickenhausen, Germany) were coated with purified Gag protein (7) in 0.2 M carbonate buffer (pH 9.5 at 1 μg/ml; 100 μl/per well) and incubated overnight at 4°C. The plates were washed five times with wash buffer (PBS, 0.05% Tween 20) and blocked at 37°C for 1 h with 200 μl of blocking buffer (PBS, 3% FCS, 2% Tween 20)/well. Test sera were diluted 1/40, followed by serial twofold dilutions in blocking buffer. The block solution was aspirated; the plates were then incubated at 37°C for 2 h with 100 μl of each serum dilution/well. After five washings, the plates were incubated for 1 h at 37°C with 100 μl of horseradish peroxidase-conjugated goat anti-mouse IgG1 and IgG2a isotypes (1:1,000 in PBS–2% Tween 20–3% FCS; 100 μl/well [Becton Dickinson Biosciences, Heidelberg, Germany])/well, as well as a goat anti-mouse immunoglobulin antibody (Dako, Hamburg, Germany), each diluted 1:2,000 in blocking puffer. After final seven washes, the plates were developed with an OPD solution (Abbott, Wiesbaden, Germany) for 30 min. The reaction was stopped with 50 μl of 1 N H2SO4/well, and the optical density (OD) was measured at dual wavelengths of 492 to 690 nm. The reported titers correspond to the reciprocal of the highest serum dilution that gave a threefold-higher OD value than the corresponding dilution of a nonimmune serum. The serum of each mouse was assayed, and these values were used to calculate the mean and standard deviation (SD) for each group of mice.
Determination of cytokines.
Spleens were recovered under sterile conditions from mice 5 days after the booster injection, and the obtained splenic single cell suspensions were seeded at 2 × 106 cells per ml in RPMI 1640 medium containing 10% heat-inactivated FCS and 1% penicillin-streptomycin (Gibco-BRL) in the presence or absence of Gag protein (10 μg/ml). After 36 h of culture, cytokine levels were determined from the precleared supernatant by using a commercial ELISA assay according to the manufacturer's instructions (Becton Dickinson).
CTL assay.
Single cell suspensions were prepared aseptically from spleens of mice 5 days after the booster immunization. Cells were suspended in α-MEM medium (Gibco-BRL) supplemented with 10 mM HEPES buffer, 50 μM β-mercaptoethanol, and 10% FCS. Then, 10% of a selected batch of concanavalin A-stimulated rat spleen cell supernatants (30) were added to the culture medium as a source of growth factors. Responder cells (3 × 107) were cocultured with 1.5 × 106 syngeneic, 9-mer p24(CA) peptide-pulsed A20 cells (gamma irradiated with 20,000 rads) in 10 ml of tissue culture medium in upright 25-cm3 tissue culture flasks in a humidified atmosphere with 7% CO2 at 37°C. Cytotoxic effector populations were harvested after 6 days of in vitro culture. Serial dilutions of effector cells were cultured with 104 target cells in 200-μl round-bottom wells. Targets were autologous P815 cells incubated for 1 h at 37°C with 10 μM of the p24(CA)-derived peptide. Non-peptide-pulsed cells were used as a negative control. Target and control P815 cells were labeled with 51Cr (100 μCi/107 cells) for 1.5 h at 37°C and washed several times prior to being added to the effector cells. After a 3.5 h of incubation at 37°C, cells were sedimented by low-speed centrifugation (300 × g, 5 min), and 50 μl of supernatant was collected for gamma counting. The percentage of specific release was calculated as [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100. Total counts were measured after the addition of 1% Triton X-100 to the labeled target cells.
RESULTS
HIV-1 Gag expression in various mammalian cell lines.
The construction of the syngag expression vector p-syngag and the wt gag expression vectors gagRRE, UTRgagRRE has been previously described in detail (12) (Fig. 1).
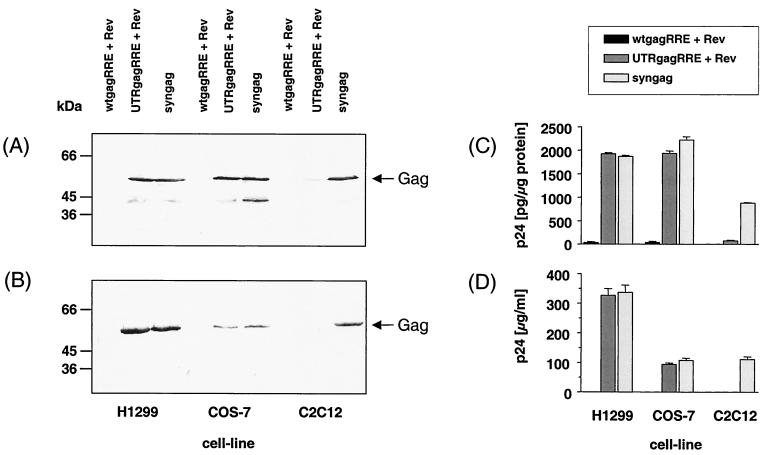
In order to assess the relative potency of wt and synthetic gag genes to express the Gag protein in mammalian cells, human H1299, monkey COS-7, and murine C2C12 muscle cells were transiently transfected with different wt gag and syngag expression vectors. Consistent with our previous results, induction of Gag protein expression and secretion from the gagRRE plasmid was extremely low or undetectable in all tested mammalian cell lines, even in presence of the Rev protein (Fig. 2). The addition of the authentic untranslated region (UTR) localized 5′ of the HIV-1 gag gene rendered Gag-expression Rev dependent and drastically increased Gag production in H1299 and COS-7 cells by several orders of magnitude, when cotransfected with the Rev expression vector pRev (Fig. 2A and C). Furthermore, significant concentrations of Gag protein were secreted into the supernatants of H1299 and COS-7 cell cultures (Fig. 2B and D). However, only a weak Gag expression (Fig. 2A and C) and no detectable Gag secretion (Fig. 2B and D) was observed after cotransfection of the murine C2C12 muscle cells with UTRgagRRE and pRev, suggesting that the Rev-Rev response element (RRE) system is not efficiently working in this cell type.
FIG. 2.
Increased expression of syngag in various mammalian cell lines. H1299, COS-7, and C2C12 cells were transiently transfected by calcium phosphate precipitation with the indicated plasmids. Nontransfected cells were used as controls. Cells (A and C) and cell culture supernatants (B and D) were harvested at 48 h posttransfection. (A and B) Cell lysates (50 μg of total protein) and sucrose-sedimented proteins from the culture supernatant were separated by SDS–10% polyacrylamide gel electrophoresis (PAGE) and analyzed by immunoblotting with a p24-specific monoclonal antibody. Arrows at the right indicate the position of the Gag polyprotein. Sizes are indicated in kilodaltons. (C and D) Yields of Gag protein were measured from the cell culture supernatant by a commercial p24 capture ELISA by using purified Gag for standardization. Bars represent Gag protein levels and are the mean of triplicate determinations.
In contrast, an efficient and Rev/RRE-independent Gag polyprotein expression and secretion was observed in all tested mammalian cells, including murine C2C12 muscle cells, after transfection with p-syngag. In our previous studies, we observed no obvious positive effect of the Rev export system on the Gag expression rates from p-syngag (12). p-syngag-mediated Gag expression levels in H1299 and COS-7 cells were similar to those induced by the UTRgagRRE and pRev vector system (Fig. 2). However, despite an almost equivalent Gag protein production, Gag release was about three times more efficient in H1299 cells, compared to COS-7 cells, independent from whether UTRgagRRE/pRev or p-syngag constructs were used in the transfection experiment. Gag expression in C2C12 cells by p-syngag was ca. 50% less efficient than that observed in H1299 and COS-7 cells, coinciding with a reduced transfection efficacy of the C2C12 cell line (data not shown). Furthermore, expression of Gag in C2C12 cells from the codon optimized p-syngag vector exceeded that of the UTRgagRRE/Rev system by more than 10-fold. These results suggest that cell-type specific factors may contribute to the observed Rev-responsiveness of UTRgagRRE. Furthermore, adaptation of the gag codon usage to that of highly expressed mammalian genes allows Rev-independent in vitro expression in absence of any cis-acting regulatory elements, resulting in high yields of Gag polyprotein.
Anti-Gag antibody responses in mice immunized with DNA vaccine vectors.
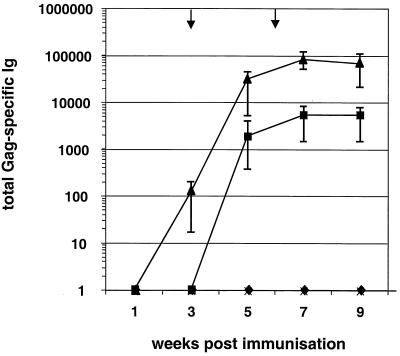
The capacity of the generated Gag expression vector systems to induce Gag-specific antibodies was investigated in female BALB/c mice. Three groups of five animals each received an i.m. primary immunization of plasmid DNA (100 μg/dose), followed by two i.m. boosts at weeks 3 and 6 with the same DNA dose. A control group was immunized with the pcDNA3 plasmid. Total immunoglobulin titers to purified Gag protein were determined by ELISA. Vaccination with both the p-syngag and UTRgagRRE/Rev vector system induced substantial Gag-specific antibody responses (Fig. 3). However, the antibody titers obtained after injection of p-syngag appeared more rapid and were higher than those induced after coimmunization with the UTRgagRRE and Rev plasmid. Mice immunized with p-syngag developed measurable levels of p24(CA)-specific antibodies already 3 weeks after the primary immunization (Fig. 3). Reactive antibodies appeared to increase almost 100-fold 2 weeks after the first booster immunization and reached Gag-specific endpoint titers of 1:81,000 1 week after the second booster injection.
FIG. 3.
Kinetics and strength of humoral responses induced in BALB/c mice by i.m. injection of 100 μg of various Gag expression vectors, respectively. Each point represents the mean value (n = 5) ± the SD of individual groups for anti-Gag immunoglobulin antibodies as determined by endpoint dilution ELISA assay. Endpoint titers of the immune sera were defined as the reciprocal of the highest plasma dilution that resulted in an absorbance value (OD = 495) three times greater than that of a preimmune serum with a cutoff value of 0.05. The arrows indicate the time points of booster immunizations. Symbols: ♦, gagRRE + Rev; ▪, UTRgagRRE + Rev; ▴, syngag; ×, pcDNA.
In contrast, Gag-specific serum antibody responses raised in mice after repeated coadministration of UTRgagRRE and pRev were significantly delayed and reached poor maximum titers of 1:5,500 1 week after the second booster immunization. No Gag-specific antibody response was detectable at any time point in the sera of pcDNA3 plasmid-immunized mice and animals coinjected with gagRRE and the Rev expression vector.
Anti-Gag isotype responses in mice immunized by different routes with modified Gag expression vectors.
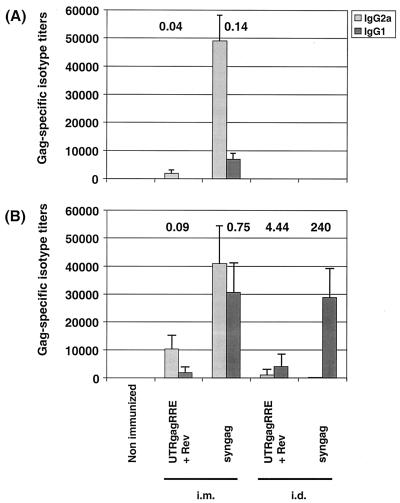
The method of DNA delivery is an important parameter for optimizing the DNA immunization. Thus, we compared i.m. needle injection and bombardment of abdominal skin with plasmid DNA-coated gold particles (gene gun) with respect to the induction of humoral and cellular immune responses. Antibody isotype responses to Gag were assessed 2 weeks after the first (Fig. 4A) and second (Fig. 4B) booster immunizations.
FIG. 4.
Influence of immunization route on the Gag-specific isotype responses induced by i.m and i.d. immunization with various Gag expression vectors. Mice were immunized either by i.m. injection or by gene gun immunization and boostered twice in 3-week intervals. IgG1 and IgG2a antibody titers in serum were determined 2 weeks after the first (A) and second (B) booster immunizations of mice that were immunized with Gag expression plasmids, as indicated. Gag-specific IgG1 and IgG2a antibodies were measured in serum by ELISA and are expressed as the reciprocal of the highest plasma dilution that resulted in an absorbance value (OD = 495) three times greater than that of the same dilution of the corresponding preimmune serum with a cutoff value of 0.05. Each bar represents the group mean (n = 5) for anti-Gag titers, and vertical lines represent the SD. The numbers above each bar pair represent the calculated ratio of IgG1 to IgG2a anti-Gag antibodies.
Mice, which were i.m. immunized with p-syngag produced a significant Th1 mediated anti-Gag response, characterized by high titers of IgG2a isotypes with substantial mean titers of 1:49,000, but only minute quantities of Gag-reactive IgG1 antibodies (1:7,000) corresponding to an IgG1/IgG2a ratio of 0.14 (Fig. 4A). A strong Th1 response (IgG1/IgG2a ratio of 0.04), but with substantially lower IgG2a (1:1,930) and IgG1 titers (1:80), was obtained after i.m. coinjection of UTRgagRRE and pRev. In contrast, at that time point no anti-Gag response was detectable from mice, in which particle gun was used to administer either p-syngag or UTRgagRRE and pRev (Fig. 4A).
At 2 weeks after the second booster immunization by i.m. needle injection, the p-syngag group of mice developed increased levels of IgG1 isotypes (1:30,720) but slightly decreased IgG2a isotypes (1:40,950), with an IgG1/IgG2a ratio of 0.75. At that time point increased IgG2a (1:10,230) and IgG1 (1:1,920) titers, with an IgG1/IgG2a ratio of 0.09 were detectable from the group of mice coimmunized with UTRgagRRE and pRev by the i.m. route (Fig. 4B). In contrast, i.d. immunization of these Gag expression plasmids with the particle gun resulted in a Th2-type antibody response. Thus, substantial titers of anti-Gag IgG1 isotypes (1:28,800), but only low levels of specific IgG2a isotypes (1:120), with an IgG1/IgG2a ratio of 240, were observed after i.d. p-syngag injection (Fig. 4B). Furthermore, significant titers of IgG1 antibodies (1:4,800) and IgG2a antibodies (1:1,080), with an IgG1/IgG2a ratio of 4.44, were detectable from the serum of mice coimmunized with UTRgagRRE and pRev by particle gun. At all time points, no Gag-specific antibodies were measurable from the serum of mice from the control group.
In vitro cytokine release of splenocytes from mice immunized with plasmid DNA.
The antigen-specific cytokine secretion as a measure of T helper cell subpopulations was determined by specific stimulation of splenic cells, obtained from mice 5 days after the second booster immunization. Splenocytes from mice i.m. immunized with both the p-syngag and UTRgagRRE/Rev vector systems showed a substantial gamma interferon (IFN-γ) production upon specific in vitro stimulation with purified Gag proteins (Table 1). However, only a weak, but specific IFN-γ secretion was observed after i.d. administration of p-syngag with the particle gun. In contrast, no detectable amounts of IFN-γ were secreted from restimulated splenocytes of mice i.d. coimmunized with UTRgagRRE and a Rev expression vector (Table 1) and nonstimulated splenocytes of all experimental groups of mice (data not shown).
TABLE 1.
Cytokine profile of in vitro Gag-stimulated splenocytes from mice immunized i.m. by needle injection or i.d. by particle gun with various Gag expression vectors
| DNA vaccine (immunization route) | Mean cytokine concn (pg/ml)a ± SD
|
|
|---|---|---|
| IL-5 | IFN-γ | |
| UTRgagRRE + Rev (i.m.) | <16 | 3,220 ± 840 |
| p-syngag (i.m.) | <16 | 3,520 ± 1020 |
| UTRgagRRE + Rev (i.d.) | <16 | <32 |
| p-syngag (i.d.) | <16 | 80 ± 32 |
Means ± the SD of splenocytes of five mice per experiment.
To assess Th2 differentiation, ELISA was performed from aliquots of the same cell culture supernatants to quantify the concentrations of secreted interleukin-4 (IL-4) and IL-5. In all groups of immunized and nonimmunized mice, independent of the immunization route, no IL-4 and IL-5 secretion was detectable from the supernatants of specifically restimulated, as well as nonstimulated, splenocytes.
Thus, an i.m. immunization of vectors containing the modified Gag expression cassettes induced a strong Th1 cytokine profile, whereas particle gun injection of these plasmid constructs resulted in a cytokine response near background levels.
Induction of CTL responses in mice immunized with modified Gag expression plasmids.
In order to analyze the capacity of p-syngag plasmids to induce a Gag-specific CTL response, splenic cells, derived from immunized mice 3 weeks after the primary immunization, were specifically restimulated in a 5-day mixed lymphocyte tumor cell culture and tested for cytotoxic activity. The AMQMLKETI 9-mer p24(CA)-derived peptide used in this assay is known to constitute a Dd-restricted CTL epitope in BALB/c mice. Gag-specific CTL were detectable after a single i.m. injection of p-syngag, whereas the administration of UTRgagRRE-pRev and gagRRE-pRev combinations was not sufficient to induce detectable CTL responses. Furthermore, no CTL priming was observed after an i.d. injection of p-syngag, as well as of the UTRgagRRE-pRev and gagRRE-pRev combinations by particle gun immunization (Fig. 5). These results showed that i.m. injection of p-syngag was the most efficient vaccination strategy to induce both substantial humoral and cellular Gag-specific immune responses.
FIG. 5.
CTL activity in splenocytes from mice immunized i.m. by needle injection or i.d. by particle gun with the indicated Gag expression vectors. Lymphoid cells obtained from mice 5 days after the booster injection were cocultured with Gag peptide-pulsed syngeneic P815 mastocytoma cells (irradiated with 20,000 rads). Control assays included splenocytes of nonimmunized mice stimulated in vitro with peptide-pulsed P815 cells. Cytotoxic effector populations were harvested after 5 days of in vitro culture. The cytotoxic response was read against 9-mer Gag-peptide-pulsed A20 cells and untreated A20 negative target cells in a standard 51Cr release assay. The data shown are mean values of triplicate cultures. The standard errors of the means of triplicate data were always less than 15% of the mean.
Contribution of CpG motifs within syngag on the immunogenicity of the p-syngag expression vector.
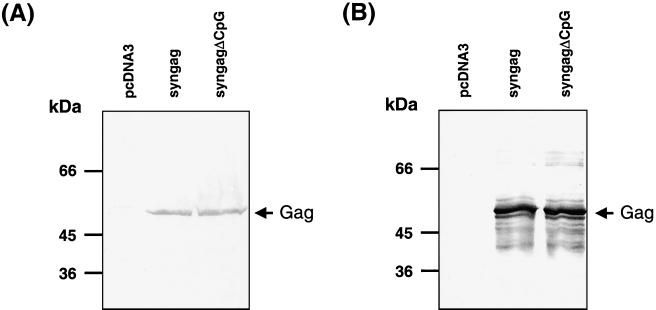
The HIV-1 genome differs from that of most human genes in terms of a noticeably high A+U content and by the predominant use of adenine and uracile in the third codon position. Adaption of the wt HIV-1 gag codon usage to that of highly expressed human genes leads to a significant increase in the G+C content and the generation of four typical CpG motifs (RRCGYY), which are not present in the wt gag sequence. In order to investigate the contribution of that CpG motifs on the high immunogenicity of p-syngag, a modified syngag gene was constructed, in which all cytosines within consensus CpG motifs were mutated, without altering the amino acid sequence (p-syngagΔCpG; Fig. 1). The mutations within syngag had no influence on the Gag expression and secretion levels in murine C2C12 muscle cells (Fig. 6), as well as in human (H1299) and monkey (COS-7) cells (data not shown). Furthermore, comparative examination of the in vitro stimulatory properties of p-syngag and p-syngagΔCpG in splenic single cell cultures of nonimmunized mice revealed nonsignificant differences of both vector constructs (Table 2). In this assay system, in contrast to CpG ODNs, neither p-syngag nor p-syngagΔCpG could induce significant concentrations of Th1-type (IFN-γ) or Th2-type (IL-5 and IL-6) cytokines. Occasionally, increased IL-12 production was detectable in cultures of splenic cells, stimulated with p-syngag, compared to those stimulated with p-syngagΔCpG. However, with respect to the high SDs of individual IL-12 values, there is no statistical proof that the IL-12 production from both vectors is substantially different. The lack of immune stimulation by the used pcDNA3 vector constructs may be explained by the presence of a number of inhibitory sequence motifs within the plasmid backbone.
FIG. 6.
Comparative Gag expression in transfected C2C12 cells with p-syngag and p-syngagΔCpG constructs. C2C12 cells were transiently transfected by calcium phosphate precipitation with the indicated plasmids, and cells (A) and cell culture supernatants (B) were harvested 48 h posttransfection. Control C2C12 cells were transfected with the pcDNA3 plasmid. Cell lysates (50 μg of total protein) and sucrose-sedimented proteins from the culture supernatant were separated by SDS–10% PAGE and analyzed by immunoblotting with a p24-specific monoclonal antibody. Arrows at the right indicate the position of the Gag polyprotein. Sizes are indicated in kilodaltons.
TABLE 2.
Cytokine secretion from splenic cells of nonimmunized mice upon plasmid stimulationa
| DNA vaccine (50 μg/2 × 106 cells) | Mean cytokine concn (pg/ml) ± SE
|
|||
|---|---|---|---|---|
| IL-5 | IL-6 | IL-12 | IFN-γ | |
| syngag | 18 ± 11 | <16 | 210 ± 195 | <16 |
| syngagΔCpG | 21 ± 18 | <16 | 28 ± 27 | <16 |
| pcDNA3.1 | <16 | <16 | 92 ± 64 | <16 |
| 1668 (CpG ODN) | 292 ± 216 | 1,305 ± 105 | 413 ± 377 | 97 ± 51 |
| PBS | 31 ± 40 | <16 | 107 ± 115 | <16 |
Splenic cells were isolated from nonimmunized female BALB/c mice and stimulated with 50 μg of different plasmids or CpG ODNs, respectively, and supernatants were collected for cytokine assay after 36 h. Each value represents the mean cytokine level ±the SD of four independent experiments.
In order to assess the influence of CpG motifs on the high immunogenicity of the p-syngag expression vector, groups of BALB/c mice were inoculated with p-syngag or p-syngagΔCpG and tested for the specific induction of antibody, cytokine, and CTL responses. For that purpose groups of each 20 BALB/c were immunized in two separate experiments and serum samples, obtained 2 weeks after the second booster immunization, were analyzed for Gag-specific total immunoglobulin and isotype responses. In these experiments, p-syngagΔCpG induced high titers of specific total immunoglobulin (1:153,600), IgG1 (1:114,300), and IgG2a (1:128,000) isotypes, which were similar to those induced by p-syngag (Table 3).
TABLE 3.
Humoral and cellular immune responses induced by syngag and syngagΔCpG expression vectors
| Vector | Humoral response (titer)a
|
Cytokine secretionb (mean pg/ml ± SE)
|
CTL response (% lysis)c at E/T ratio
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total immunoglobulin | IgG1 | IgG2a | IL-4 | IL-5 | IL-6 | IL-12 | TNF-α | IFN-γ | 50 | 25 | 12.5 | 6.25 | |
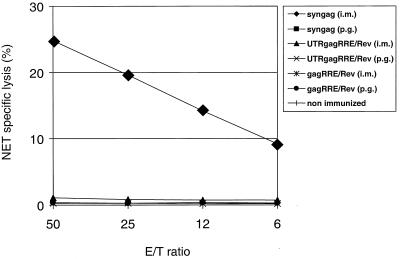
| syngag | 109,227 | 120,614 | 100,124 | <8 | 23 ± 15 | 1,668 ± 95 | 415 ± 240 | <16 | 13,449 ± 1,123 | 29 | 20 | 8 | 4 |
| syngagΔCpG | 153,600 | 114,300 | 128,000 | <8 | 21 ± 15 | 701 ± 187 | 400 ± 298 | <16 | 12,441 ± 1,314 | 39 | 26 | 12 | 8 |
| pcDNA3 | 3 | 2 | 2 | 2 | |||||||||
Serum samples obtained 2 weeks after the second booster immunization were serially diluted and tested for HIV-1 Gag-specific antibodies by ELISA. Titers are expressed as the reciprocal of the serum dilution that gave an absorbance value three times higher than the value obtained with the corresponding preimmune sera. The results are the means of five mice per group.
Mice were killed 7 days after the second booster injection and cultured splenic cells were specifically restimulated in vitro for 36 h. Only background levels of the indicated cytokines were detected in the supernatants of nonstimulated splenocytes. Each value represents the mean cytokine level ± the standard error of four independent experiments.
Spleen cells of BALB/c mice were primed by an i.m. injection of the indicated Gag expression vectors. Spleen cells obtained from mice 14 days postimmunization were restimulated in vitro with a 9-mer p24(CA) peptide-pulsed H-2d P815 cells. The cytotoxic response was read out against H-2d A20 cells pulsed for 1 h with 10−8 M of a p24(CA)-derived, synthetic 9-mer peptid. The cytotoxic response is indicated as the net specific lysis (%). The mean values of three individual mice in each group are shown. The standard error of the mean of triplicate data was always <15% of the mean.
We used an ELISA assay to compare antigen-specific IFN-γ secretion as a measure of Th1 memory cells induced after immunization with p-syngag and p-syngagΔCpG plasmids. Ten days after i.m. immunization of BALB/c mice, splenocytes were isolated, incubatedin vitro for 36 h with or without purified Gag protein, and assayed for cytokine production. Upon restimulation with Gag protein, splenocytes from mice immunized with both p-syngag and p-syngagΔCpG showed comparably high IFN-γ secretion, with mean concentrations of 13,499 and 12,441 pg/ml. Furthermore, significant concentrations of IL-6 and IL-12, but no detectable levels of IL-4, IL-5, and tumor necrosis factor alpha (TNF-α), were secreted from splenic cells of both groups of immunized mice (Table 3). These findings clearly demonstrate that both tested syngag expression vectors induced a specific Th1-dominated immune response, which is not significantly modulated by the presence or absence of the five CpG motifs within or directly 3′ to the codon optimized gag.
Moreover, a specific CTL response of BALB/c mice to the Gag protein was induced to comparable efficiency with the p-syngag and the p-syngagΔCpG expression vectors (Table 3). The p24(CA)-specific CTL reactivity was detectable as soon as 10 days after the primary i.m. injection of the syngag vector constructs. These data strongly indicate that the additional CpG motifs within syngag do not significantly contribute to the strong capacity of p-syngag to induce specific CD8+ CTL responses.
Modulation of antibody induction from DNA vaccines by coadministered CpG ODNs.
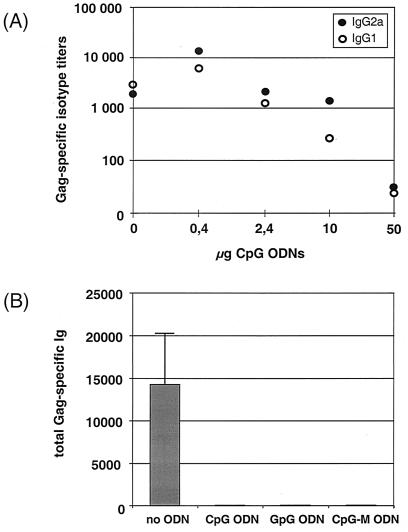
To study the potential of CpG motifs to serve as an adjuvant for DNA vaccines, we used a CpG ODN that was previously described to possess potent effects on the murine immune system in vitro and to augment immune responses in vivo (34). BALB/c mice were coimmunized with 80 μg of p-syngag and increasing concentrations of CpG ODNs ranging from 0 to 50 μg per injection and the antibody responses were measured 2 weeks after the booster immunization with the same plasmid-ODN combination. The anti-Gag response was drastically lower in mice injected with p-syngag plus high doses of CpG ODNs than in those immunized with DNA vaccine alone, and the observed negative effect was dose dependent. For example, the mean titers of anti-Gag antibodies were reduced ca. 2- to 10-fold and greater than 100-fold by the addition of 10 or 50 μg of CpG ODN, respectively. Thus, direct adjuvation of the DNA vaccine with high doses of ODNs appears to hinder the induction of antibody responses against the expressed antigen (Fig. 7A). However, the decrease in antibody production was also observed after coadministration of high concentrations of nonstimulatory GpG and CpG-M ODNs (Fig. 7B), indicating that the observed inhibition of immune responses is not caused by CpG motifs. However, at low CpG ODN concentrations of 0.4 μg per dose, a 5- to 10-fold increase in Gag-specific antibody response was induced by the coadministered p-syngag expression vector. The enhancement of humoral responses was not observed after adjuvation of syngag plasmids with low doses of the control ODNs GpG or CpG-M, indicating that the CpG motif rather than other ODN-specific properties, such as the backbone modification by using phosphorothioate ODNs, was responsible for the observed weak enhancing effect of CpG ODN. In addition, these results indicate a dose-dependent interference of CpG and non CpG ODNs with the DNA vaccine.
FIG. 7.
Effect of coadministered phosphorothioate ODNs to the immunogenicity of the p-syngag expression vector. (A) Various concentrations of immunostimulatory CpG ODNs were coadministered i.m. with 80 μg of p-syngag. Serum samples were obtained 2 weeks after the booster immunization. Titers of Gag-specific IgG1 and IgG2a antibodies were analyzed by isotype ELISA and are expressed as the reciprocal of the highest plasma dilution that resulted in an absorbance value (OD = 495) three times greater than that of a corresponding preimmune serum with a cutoff value of 0.05. Each bar represents the mean values derived from 18 animals. (B) Each 50 μg of the indicated immunostimulatory and nonstimulatory ODNs was i.m. coadministered with 80 μg of p-syngag, and the specific antibody isotypes were measured 2 weeks after the booster immunization. Each bar represents the mean values ± the SD derived from six mice.
DISCUSSION
Recent results by several groups have clearly demonstrated that vaccine vectors exploiting codon-optimized genes elicit increased humoral and cellular responses than did vector constructs encoding the corresponding wt gene (1, 29, 43). However, the mechanisms underlying the enhanced efficacy and immunogenicity of synthetic gene-based DNA vaccines are not yet clear so far. Thus, in the present study we analyzed the potential impact of enhanced protein expression, sequence modifications within the foreign gene, and the mode of gene delivery on the immunogenicity of HIV-1 Gag expression vectors.
We have shown that syngag and wt gag expression units were equally efficient to mediate Gag protein expression and secretion from human H1299 lung carcinoma cells and African green monkey COS-7 kidney cells. In contrast, we achieved >10-fold-increased Gag expression levels with the codon-optimized syngag plasmids in murine C2C12 muscle cells compared to the corresponding wt gag vector system. The mechanisms supporting substantial Gag expression from syngag, but not from wt gag constructs, in the murine C2C12 muscle cells are still not clear. However, this observation may be explained by previous results by others, suggesting either a lack or nonfunctionality of specific cellular (3, 24) and viral (28, 40) factors to be responsible for defects in Gag protein production and lack of virion assembly in murine cells. We have previously reported that the marked differences between wt gag and syngag expression are clearly due to alterations in the nuclear translocation of viral mRNAs and not based on modifications in transcriptional regulation. More specifically, the findings by our group and others that, unlike wt gag-derived mRNAs, syngag-derived mRNAs are not exported via the exportin-1 nuclear export pathway (12, 17) supports the suggestion that the absence or nonfunctionality of cellular or viral components, which are involved in the Rev–CRM-1-dependent mRNA export or associated cellular mechanisms may also account for the observed differences in Gag expression in murine C2C12 muscle cells. However, this hypothesis remains to be validated in future experiments.
In the light of the differences in Gag production from C2C12 cells, further studies were conducted in the BALB/c mouse model to compare the immunogenicity of both wt gag and syngag expression plasmids. The results presented here show that sequence-optimized syngag-based plasmids induce substantially increased humoral and cellular responses compared to wt gag-based DNA vaccine, independent of the route of vector administration. The improved potency of syngag over the wt gag system in terms of inducing anti-Gag responses correlated obviously with the more efficient in vitro Gag protein expression observed in cultures of murine C2C12 cells. These results indicate that the increase in protein expression may be an important parameter for the enhanced immunogenicity of the syngag constructs.
In addition, a number of studies have shown that the method of immunization can influence both the strength and the nature of immune responses. In our studies, i.m. needle injection was superior to i.d. particle gun immunization with respect to the priming of humoral and cellular immune responses, independent of the type of Gag expression vectors used in the vaccination study. The i.m. needle injection of both Gag plasmid systems in saline produced a Th1-biased immune response with mostly IgG2a isotypes. In contrast, the same DNA vectors delivered i.d. by gene gun produced a more Th2-like immune response with predominantly IgG1 antibodies. Moreover, i.m. immunization was more efficient than particle gun injection in priming of humoral responses, due to elevated antibody titers and the occurrence of antibodies at earlier times postimmunization. The i.m. needle injection of plasmid DNA differs considerably from i.d. DNA inoculation by gene gun immunization in several aspects, such as the method and route of DNA delivery, the concentration of applicated plasmids, and the efficacy of cell transfection by different routes of DNA entry. All of these factors may contribute to the observed differences in T helper cell differentiation and the strength and polarization of the immune response. Previous work has shown that the differentiation of naive Th cells into Th1 or Th2 cells can be affected by many factors, the most important of which are cytokines. Herein, IFN-γ is a key regulator of Th1 differentiation, whereas IL-4 is a key regulator of Th2 differentiation. In the experiments presented here, IFN-γ production was much higher in i.m. immunized mice than in animals injected by particle gun, independent on the vector constructs used, thus supporting the enhanced capacity of the i.m. route of DNA administration to prime Th1 immunity. However, the tendency of gene gun-mediated DNA immunization to elicit predominantly IgG1 subclass responses, while i.m. needle inoculation yields mixed or predominantly IgG2a responses could also reflect significant differences in the amounts of soluble antigen synthesized after DNA delivery by the i.m and i.d. routes.
An other consideration for optimizing DNA vaccines is through the adjuvant effects provided by immune stimulatory CpG motifs within the inoculated gene (19). In a recent report it was suggested that a higher CpG content obtained by mammalian codon usage may contribute to a higher antibody and CTL response observed after synthetic versus wt gene vaccination (1, 29). However, since neither antibody isotypes nor cytokine profiles were analyzed, any influence on the Th type of immune response was difficult to evaluate in that investigation. When applying the definition of two 5′ purines, an unmethylated CpG motif and two 3′ pyrimidines (RRCGYY), as consensus putative immune stimulatory CpG motifs, as many as seven and three CpG motifs are present in syngag and wt gag genes, respectively. In order to define the role of CpG motifs within the codon-optimized syngag on the observed strong immunogenicity of p-syngag, we generated a variant of syngag, in which all CpG motifs, which have been newly created within syngag in consequence of codon optimization, were inactivated by silent point mutations. Our transfection studies with various mammalian cell lines did not indicate significantly altered yields of Gag protein expression by p-syngagΔCpG relative to p-syngag. Furthermore, our data strongly indicate that the few CpG motifs generated within syngag by codon optimization did not significantly influence the immunological properties of the plasmid and the capacity of p-syngag to generate strong humoral and cellular immune responses in the mouse model. Thus, the few additional CpG motifs generated within syngag by codon optimization seem to have no significant influence on the biological properties of the syngag plasmid. The exact effects of CpG motifs within the plasmid are difficult to predict since the precise arrangement, spacing, and sequences on the flank of immune-stimulatory CpG motifs may influence CpG adjuvant effects. Furthermore, the addition of large numbers of CpG motifs may even have negative effects on the priming of immune responses, due to the overstimulation of IFN-α/β and other factors that downregulate plasmid gene expression (34). This field is even more complicated by the recent description of putative immune-neutralizing motifs, which oppose the ability of an immune-stimulatory CpG motive to induce cytokine expression and may interfere with the induction of the desired immune responses (20). These inhibitory sequence motifs, consisting of direct repeats of CpG dinucleotides and/or CpGs preceded by a C and followed by a G, are present in the used pcDNA3 vaccine vectors in multiple copies. In addition, since more than 400 nucleotide substitutions have been conducted throughout wt gag gene to generate a completely codon-optimized Gag gene (12), we cannot exclude that other, not-yet-characterized activities have been generated, which may contribute to the increased capacity of p-syngag to induce humoral and cellular responses, compared to wt gag plasmids.
To study the potential of synthetic ODNs that contain unmethylated CpG motifs (CpG ODNs) to serve as an adjuvant for DNA vaccines, we used a CpG ODN (ISS) that is known to possess potent effects on the murine immune system in vitro and to augment in vivo immune responses (34). Surprisingly, at high CpG ODN concentrations, the anti-Gag response detected at any given time point after immunization was lower in mice injected with p-syngag plus CpG than in those injected with the p-syngag plasmid alone, and the negative effect was dose dependent. Thus, mixing ODNs directly with a DNA vaccine appears to hinder the induction of antibody response against the expressed antigen. These data support a previous work, which demonstrated that the coadministration of CpG ODNs and a DNA vaccine encoding hepatitis B surface antigen had a negative effect on the priming of specific immune responses (39). The observed immunosuppressive effects of CpG ODNs at higher concentrations could be a result of interference with either antigen expression or the immune response against the antigen. However, the dose dependency of CpG ODN interference and the fact that nonstimulatory ODNs reduced gene expression as much as an equivalent dose of CpG ODN suggests that reduced gene expression rather than cytokine mediated downregulation of the cytomegalovirus promoter plays a crucial role in the observed decrease in antibody production. The reduced immunogenicity of p-syngag plasmid DNA mixed with ODNs may be due to the interference of uptake into cells. However, our data also indicate that, at low concentrations, the specific immunostimulatory effects of CpG ODNs dominate over the less-specific suppressive effects of ODNs on the immunogenicity of the p-syngag vector. Further studies are needed to evaluate the specific mechanism by which ODNs mediate dose-dependent promotion or suppression of foreign gene expression from plasmid DNA. The results of the present study clearly indicate that increased Gag expression levels rather than altered vector immunity by codon adaption is responsible for the strong immunogenicity of the p-syngag plasmid.
ACKNOWLEDGMENTS
The excellent technical assistance of Ingrid Kirst and Elke Perthen is appreciated. We also thank Marcus Neumann (GSF, Munich, Germany) for providing the Rev expression plasmid pCsRevsg25-GFP.
This work was supported by DLR grant 01 KI 97 65/3 to R.W.
REFERENCES
- 1.Andre S, Seed B, Eberle J, Schraut W, Bultmann A, Haas J. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J Virol. 1998;72:1497–1503. doi: 10.1128/jvi.72.2.1497-1503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betts M R, Krowka J, Santamaria C, Balsamo K, Gao F, Mulundu G, Luo C, N′Gandu N, Sheppard H, Hahn B H, Allen S, Frelinger J A. Cross-clade human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte responses in HIV-infected Zambians. J Virol. 1997;71:8908–8911. doi: 10.1128/jvi.71.11.8908-8911.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieniasz P D, Cullen B R. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J Virol. 2000;74:9868–9877. doi: 10.1128/jvi.74.21.9868-9877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer J D, Cohen A D, Vogt S, Schumann K, Nath B, Ahn L, Lacy K, Bagarazzi M L, Higgins T J, Baine Y, Ciccarelli R B, Ginsberg R S, MacGregor R R, Weiner D B. Vaccination of seronegative volunteers with a human immunodeficiency virus type 1 env/rev DNA vaccine induces antigen-specific proliferation and lymphocyte production of beta-chemokines. J Infect Dis. 2000;181:476–483. doi: 10.1086/315229. [DOI] [PubMed] [Google Scholar]
- 6.Boyer J D, Wang B, Ugen K E, Agadjanyan M, Javadian A, Frost P, Dang K, Carrano R A, Ciccarelli R, Coney L, Williams W V, Weiner D B. In vivo protective anti-HIV immune responses in non-human primates through DNA immunization. J Med Primatol. 1996;25:242–250. doi: 10.1111/j.1600-0684.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 7.Deml L, Schirmbeck R, Reimann J, Wolf H, Wagner R. Immunostimulatory CpG motifs trigger a T helper-1 immune response to human immunodeficiency virus type-1 (HIV-1) gp 160 envelope proteins. Clin Chem Lab Med. 1999;37:199–204. doi: 10.1515/CCLM.1999.037. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 9.Durali D, Morvan J, Letourneur F, Schmitt D, Guegan N, Dalod M, Saragosti S, Sicard D, Levy J P, Gomard E. Cross-reactions between the cytotoxic T-lymphocyte responses of human immunodeficiency virus-infected African and European patients. J Virol. 1998;72:3547–3553. doi: 10.1128/jvi.72.5.3547-3553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 11.Ferbas J, Kaplan A H, Hausner M A, Hultin L E, Matud J L, Liu Z, Panicali D L, Nerng Ho H, Detels R, Giorgi J V. Virus burden in long-term survivors of human immunodeficiency virus (HIV) infection is a determinant of anti-HIV CD8+ lymphocyte activity. J Infect Dis. 1995;172:329–339. doi: 10.1093/infdis/172.2.329. [DOI] [PubMed] [Google Scholar]
- 12.Graf M, Bojak A, Dem L, Bieler K, Wolf H, Wagner R. Concerted action of multiple cis-acting sequences is required for Rev dependence of late human immunodeficiency virus type 1 gene expression. J Virol. 2000;74:10822–10826. doi: 10.1128/jvi.74.22.10822-10826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurunathan S, Klinman D M, Seder R A. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 14.Harrer E, Harrer T, Barbosa P, Feinberg M, Johnson R P, Buchbinder S, Walker B D. Recognition of the highly conserved YMDD region in the human immunodeficiency virus type 1 reverse transcriptase by HLA-A2-restricted cytotoxic T lymphocytes from an asymptomatic long-term nonprogressor. J Infect Dis. 1996;173:476–479. doi: 10.1093/infdis/173.2.476. [DOI] [PubMed] [Google Scholar]
- 15.Harrer E, Harrer T, Buchbinder S, Mann D L, Feinberg M, Yilma T, Johnson R P, Walker B D. HIV-1-specific cytotoxic T lymphocyte response in healthy, long-term nonprogressing seropositive persons. AIDS Res Hum Retrovir. 1994;10(Suppl. 2):S77–S78. [PubMed] [Google Scholar]
- 16.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotsopoulou E, Kim V N, Kingsman A J, Kingsman S M, Mitrophanous K A. A Rev-independent human immunodeficiency virus type 1 (HIV-1)-based vector that exploits a codon-optimized HIV-1 gag-pol gene. J Virol. 2000;74:4839–4852. doi: 10.1128/jvi.74.10.4839-4852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koup R A, Ho D D. Shutting down HIV. Nature. 1994;370:416. doi: 10.1038/370416a0. [DOI] [PubMed] [Google Scholar]
- 19.Krieg A M. Lymphocyte activation by CpG dinucleotide motifs in prokaryotic DNA. Trends Microbiol. 1996;4:73–76. doi: 10.1016/0966-842X(96)81515-0. [DOI] [PubMed] [Google Scholar]
- 20.Krieg A M, Wu T, Weeratna R, Efler S M, Love H L, Yang L, Yi A K, Short D, Davis H L. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc Natl Acad Sci USA. 1998;95:12631–12636. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyard M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch J A, deSouza M, Robb M D, Markowitz L, Nitayaphan S, Sapan C V, Mann D L, Birx D L, Cox J H. Cross-clade cytotoxic T cell response to human immunodeficiency virus type 1 proteins among HLA disparate North Americans and Thais. J Infect Dis. 1998;178:1040–1046. doi: 10.1086/515652. [DOI] [PubMed] [Google Scholar]
- 23.MacGregor R R, Boyer J D, Ugen K E, Lacy K E, Gluckman S J, Bagarazzi M L, Chattergoon M A, Baine Y, Higgins T J, Ciccarelli R B, Coney L R, Ginsberg R S, Weiner D B. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 24.Mariani R, Rutter G, Harris M E, Hope T J, Krausslich H G, Landau N R. A block to human immunodeficiency virus type 1 assembly in murine cells. J Virol. 2000;74:3859–3870. doi: 10.1128/jvi.74.8.3859-3870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mossman S P, Pierce C C, Robertson M N, Watson A J, Montefiori D C, Rabin M, Kuller L, Thompson J, Lynch J B, Morton W R, Benveniste R E, Munn R, Hu S L, Greenberg P, Haigwood N L. Immunization against SIVmne in macaques using multigenic DNA vaccines. J Med Primatol. 1999;28:206–213. doi: 10.1111/j.1600-0684.1999.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 26.Niedrig M, Rabanus J P, L'Age Stehr J, Gelderblom H R, Pauli G. Monoclonal antibodies directed against human immunodeficiency virus (HIV) Gag proteins with specificity for conserved epitopes in HIV-1: HIV-2 and simian immunodeficiency virus. J Gen Virol. 1988;69:2109–2114. doi: 10.1099/0022-1317-69-8-2109. [DOI] [PubMed] [Google Scholar]
- 27.Pertmer T M, Eisenbraun M D, McCabe D, Prayaga S K, Fuller D H, Haynes J R. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13:1427–1430. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 28.Pomerantz R J, Trono D, Feinberg M B, Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 29.Qiu J T, Song R, Dettenhofer M, Tian C, August T, Felber B K, Pavlakis G N, Yu X F. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J Virol. 1999;73:9145–9152. doi: 10.1128/jvi.73.11.9145-9152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimann J, Bellan A, Conradt P. Development of autoreactive L3T4+ T cells from double-negative (L3T4−/Ly-2−) Thy-1+ spleen cells of normal mice. Eur J Immunol. 1988;18:989–999. doi: 10.1002/eji.1830180704. [DOI] [PubMed] [Google Scholar]
- 31.Rinaldo C, Huang X L, Fan Z F, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann-Cottrill J M, et al. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinaldo C R, Jr, Beltz L A, Huang X L, Gupta P, Fan Z, Torpey D J. Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res Hum Retrovir. 1995;11:481–489. doi: 10.1089/aid.1995.11.481. [DOI] [PubMed] [Google Scholar]
- 33.Robinson H L, Torres C A. DNA vaccines. Semin Immunol. 1997;9:271–283. doi: 10.1006/smim.1997.0083. [DOI] [PubMed] [Google Scholar]
- 34.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 35.Tang D C, DeVit M, Johnston S A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 36.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 37.Wagner R, Deml L, Fliessbach H, Wanner G, Wolf H. Assembly and extracellular release of chimeric HIV-1 Pr55gag retrovirus-like particles. Virology. 1994;200:162–175. doi: 10.1006/viro.1994.1175. [DOI] [PubMed] [Google Scholar]
- 38.Wagner R, Leschonsky B, Harrer E, Paulus C, Weber C, Walker B D, Buchbinder S, Wolf H, Kalden J R, Harrer T. Molecular and functional analysis of a conserved CTL epitope in HIV-1 p24 recognized from a long-term nonprogressor: constraints on immune escape associated with targeting a sequence essential for viral replication. J Immunol. 1999;162:3727–3734. [PubMed] [Google Scholar]
- 39.Weeratna R, Brazolot M C, Krieg A M, Davis H L. Reduction of antigen expression from DNA vaccines by coadministered oligodeoxynucleotides. Antisense Nucleic Acid Drug Dev. 1998;8:351–356. doi: 10.1089/oli.1.1998.8.351. [DOI] [PubMed] [Google Scholar]
- 40.Winslow B J, Trono D. The blocks to human immunodeficiency virus type 1 Tat and Rev functions in mouse cell lines are independent. J Virol. 1993;67:2349–2354. doi: 10.1128/jvi.67.4.2349-2354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf H, Modrow S, Soutschek E, Motz M, Grunow R, Döbl H. Production, mapping, and biological characterisation of monoclonal antibodies to the core protein (p24) of the human immunodeficiency virus type 1. AIFO. 1990;1:24–29. [Google Scholar]
- 42.Yankauckas M A, Morrow J E, Parker S E, Abai A, Rhodes G H, Dwarki V J, Gromkowski S H. Long-term anti-nucleoprotein cellular and humoral immunity is induced by intramuscular injection of plasmid DNA containing NP gene. DNA Cell Biol. 1993;12:771–776. doi: 10.1089/dna.1993.12.771. [DOI] [PubMed] [Google Scholar]
- 43.Zur M J, Chen M C, Doe B, Schaefer M, Greer C E, Selby M, Otten G R, Barnett S W. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J Virol. 2000;74:2628–2635. doi: 10.1128/jvi.74.6.2628-2635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]