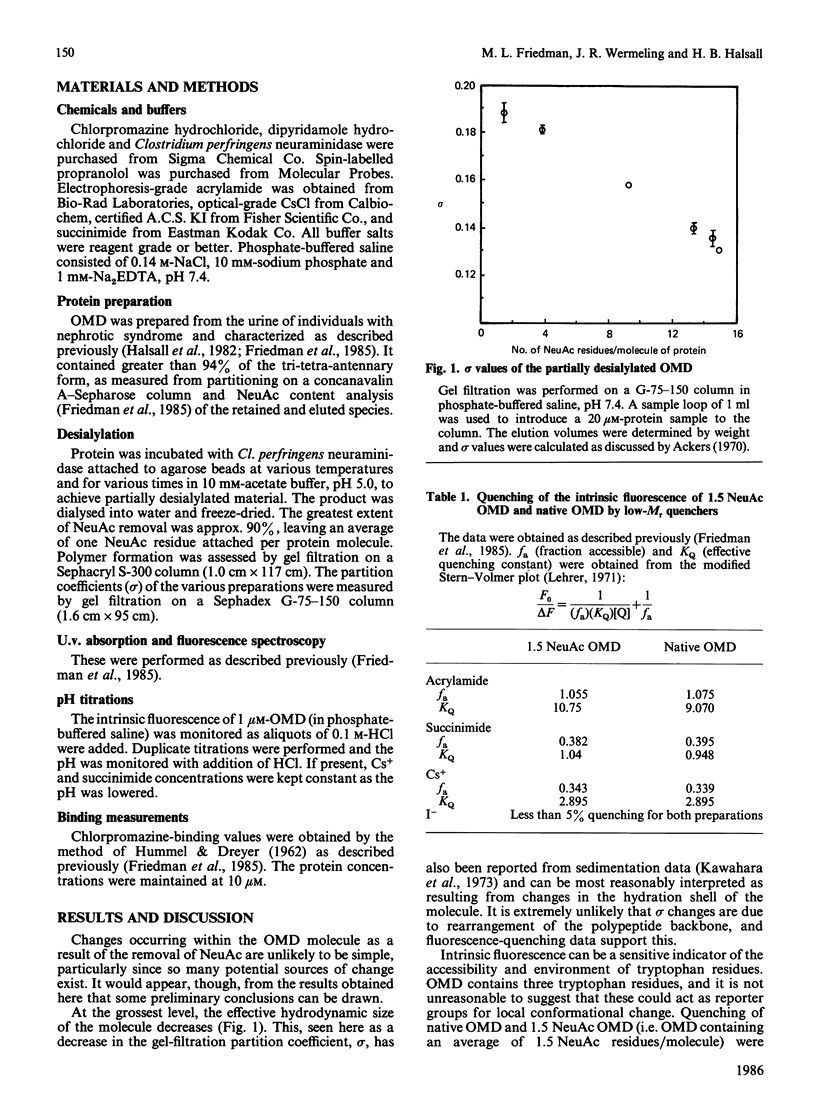
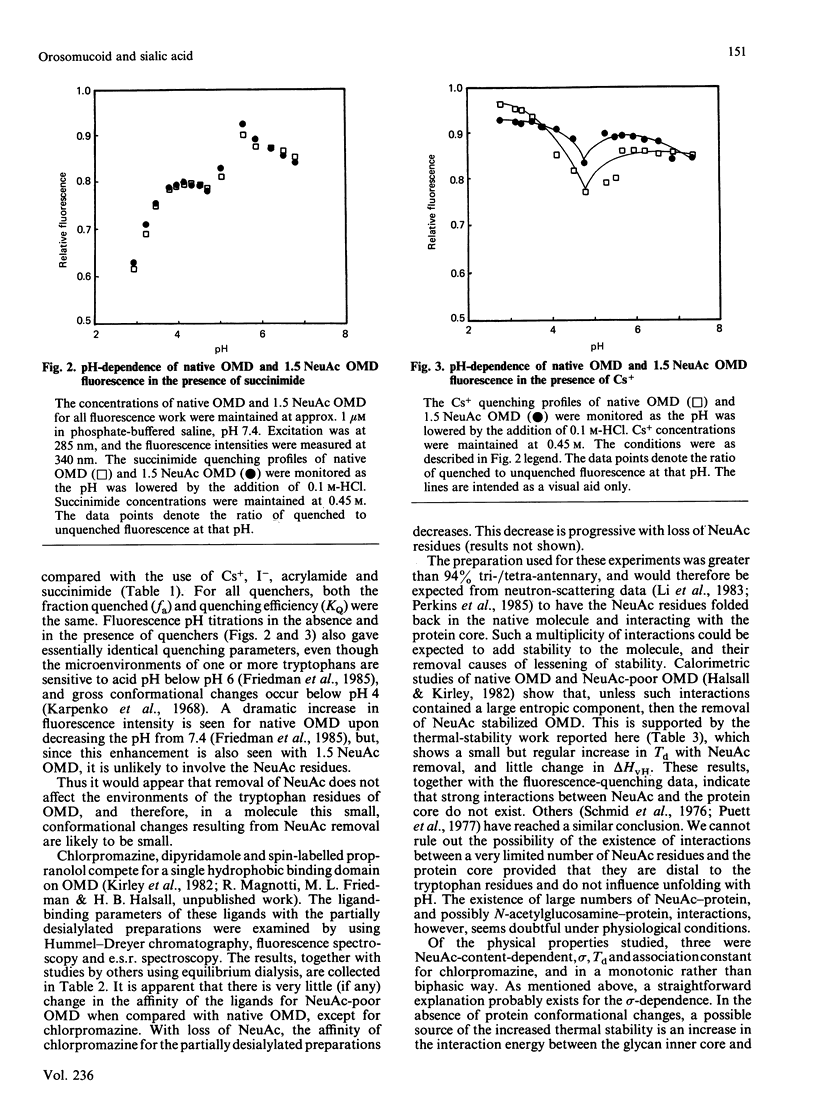
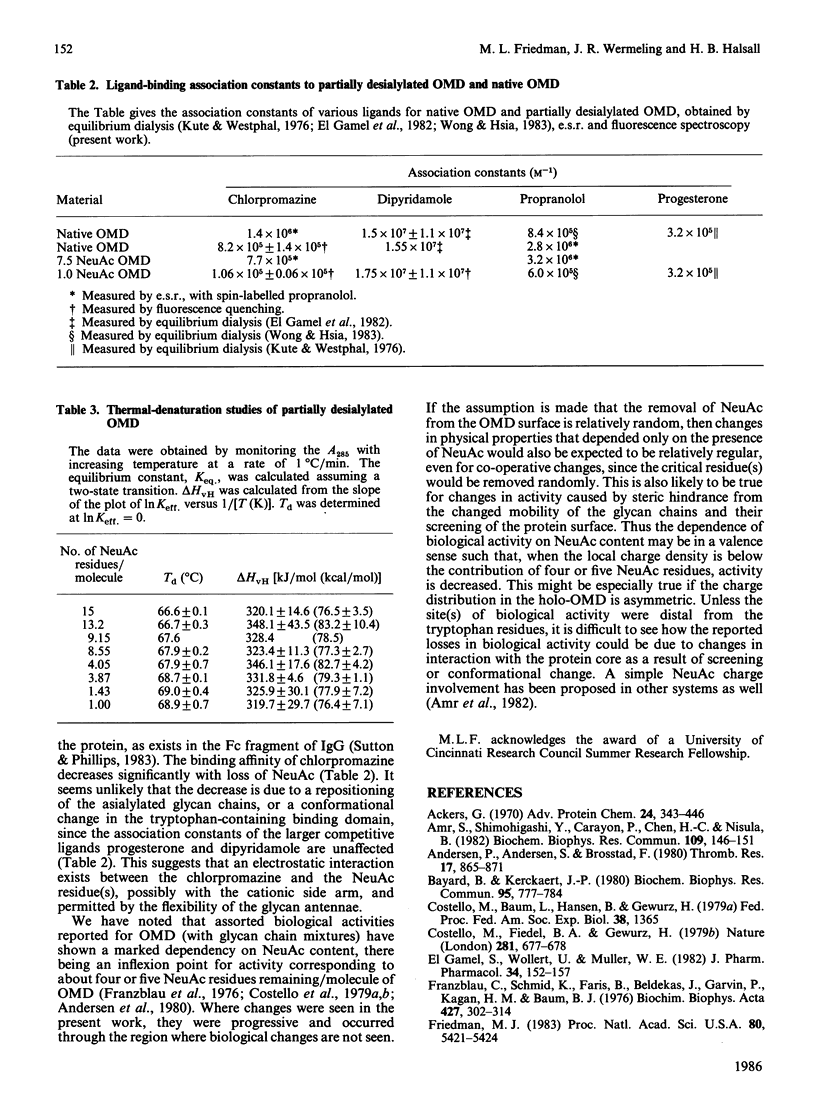
Abstract
Little is known of the relationships that may exist among the three principal functionalities of glycoproteins. Orosomucoids of closely defined N-acetylneuraminic acid content were examined for evidence of influence of N-acetylneuraminic acid content on the physical properties of the glycoprotein. Fluorescence spectroscopy gave no indication of conformational change in the protein core upon desialylation. Small changes in the chromatographic partition coefficient, sigma, and thermal stability, Td, are interpreted to reflect loss of water of hydration and increased glycan stem-protein interaction without a major repositioning of the chains. Ligand-binding measurements indicate no alteration in the hydrophobic binding domain and a possible interaction between chlorpromazine and N-acetylneuraminic acid. All changes seen are progressive and occur through a region where changes in biological activity are not found. It is suggested that the dependence of biological activity on N-acetylneuraminic acid content in orosomucoid reflects, not coupled changes in protein conformation, but a charge-density-related interaction such that, below a contribution of four or five N-acetylneuraminic acid residues, activity is modified.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K. Analytical gel chromatography of proteins. Adv Protein Chem. 1970;24:343–446. doi: 10.1016/s0065-3233(08)60245-4. [DOI] [PubMed] [Google Scholar]
- Amr S., Shimohigashi Y., Carayon P., Chen H. C., Nisula B. Sialic acid residues of the alpha-subunit are required for the thyrotropic activity of HCG. Biochem Biophys Res Commun. 1982 Nov 16;109(1):146–151. doi: 10.1016/0006-291x(82)91577-7. [DOI] [PubMed] [Google Scholar]
- Andersen P., Andersen S., Brosstad F. Reduced antiheparin effect of desialyzed alpha 1-acid glycoprotein. Thromb Res. 1980 Mar 15;17(6):865–871. doi: 10.1016/0049-3848(80)90251-0. [DOI] [PubMed] [Google Scholar]
- Bayard B., Kerckaert J. P. Evidence for uniformity of the carbohydrate chains in individual glycoprotein molecular variants. Biochem Biophys Res Commun. 1980 Jul 31;95(2):777–784. doi: 10.1016/0006-291x(80)90854-2. [DOI] [PubMed] [Google Scholar]
- Costello M., Fiedel B. A., Gewurz H. Inhibition of platelet aggregation by native and desialised alpha-1 acid glycoprotein. Nature. 1979 Oct 25;281(5733):677–678. doi: 10.1038/281677a0. [DOI] [PubMed] [Google Scholar]
- El-Gamel S., Wollert U., Müller W. E. Optical studies on the specific interaction of dipyridamole with alpha 1-acid glycoprotein (orosomucoid). J Pharm Pharmacol. 1982 Mar;34(3):152–157. doi: 10.1111/j.2042-7158.1982.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Franzblau C., Schmid K., Faris B., Beldekas J., Garvin P., Kagan H. M., Baum B. J. The interaction of collagen with alpha1-acid glycoprotein. Biochim Biophys Acta. 1976 Mar 18;427(1):302–314. doi: 10.1016/0005-2795(76)90306-8. [DOI] [PubMed] [Google Scholar]
- Friedman M. J. Control of malaria virulence by alpha 1-acid glycoprotein (orosomucoid), an acute-phase (inflammatory) reactant. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5421–5424. doi: 10.1073/pnas.80.17.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. L., Schlueter K. T., Kirley T. L., Halsall H. B. Fluorescence quenching of human orosomucoid. Accessibility to drugs and small quenching agents. Biochem J. 1985 Dec 15;232(3):863–867. doi: 10.1042/bj2320863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Halsall H. B., Kirley T. L. The denaturation of orosomucoid. Arch Biochem Biophys. 1982 Jul;216(2):392–399. doi: 10.1016/0003-9861(82)90227-2. [DOI] [PubMed] [Google Scholar]
- Hawahara K., Ikenaka T., Nimberg R. B., Schmid K. The effect of the sialyl residues on the thermodynamic and hydrodynamic properties of 1 -acid glycoprotein. Biochim Biophys Acta. 1973 Feb 21;295(2):505–513. doi: 10.1016/0005-2795(73)90046-9. [DOI] [PubMed] [Google Scholar]
- Karpenko V., Pavlícek Z., Kalous V. Behavior of human orosomucoid in acid media. Biochim Biophys Acta. 1968 Jan 22;154(1):245–247. doi: 10.1016/0005-2795(68)90283-3. [DOI] [PubMed] [Google Scholar]
- Kirley T. L., Sprague E. D., Halsall H. B. The binding of spin-labeled propranolol and spin-labeled progesterone by orosomucoid. Biophys Chem. 1982 Jun;15(3):209–216. doi: 10.1016/0301-4622(82)80004-5. [DOI] [PubMed] [Google Scholar]
- Kute T., Westphal U. Steroid-protein interactions. XXXIV. Chemical modification of alpha1-acid glycoprotein for characterization of the progesterone binding site. Biochim Biophys Acta. 1976 Jan 20;420(1):195–213. doi: 10.1016/0005-2795(76)90358-5. [DOI] [PubMed] [Google Scholar]
- Lawson E. Q., Hedlund B. E., Ericson M. E., Mood D. A., Litman G. W., Middaugh R. Effect of carbohydrate on protein solubility. Arch Biochem Biophys. 1983 Feb 1;220(2):572–575. doi: 10.1016/0003-9861(83)90449-6. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Li Z. Q., Perkins S. J., Loucheux-Lefebvre M. H. alpha 1 acid glycoprotein: a small-angle neutron scattering study of a human plasma glycoprotein. Eur J Biochem. 1983 Feb 1;130(2):275–279. doi: 10.1111/j.1432-1033.1983.tb07147.x. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Williams S. Serum inhibitors of desialylated glycoprotein binding to hepatocyte membranes. Biochim Biophys Acta. 1978 Sep 21;543(1):41–52. doi: 10.1016/0304-4165(78)90452-x. [DOI] [PubMed] [Google Scholar]
- Olden K., Parent J. B., White S. L. Carbohydrate moieties of glycoproteins. A re-evaluation of their function. Biochim Biophys Acta. 1982 May 12;650(4):209–232. doi: 10.1016/0304-4157(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Kerckaert J. P., Loucheux-Lefebvre M. H. The shapes of biantennary and tri/tetraantennary alpha 1 acid glycoprotein by small-angle neutron and X-ray scattering. Eur J Biochem. 1985 Mar 15;147(3):525–531. doi: 10.1111/j.0014-2956.1985.00525.x. [DOI] [PubMed] [Google Scholar]
- Puett D., Holladay L. A., Ford J. D., Cunningham L. W. Circular dichroism of glycopeptide fractions from alpha1-acid glycoprotein, thyroglobulin, and ovalbumin. Biochim Biophys Acta. 1977 Mar 28;491(1):129–136. doi: 10.1016/0005-2795(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Rudman D., Treadwell P. E., Vogler W. R., Howard C. H., Hollins B. An abnormal orosomucoid in the plasma of patients with neoplastic disease. Cancer Res. 1972 Sep;32(9):1951–1959. [PubMed] [Google Scholar]
- Schmid K., Chen L. H., Occhino J. C., Foster J. A., Sperandio K. Topography of human plasma alpha1-acid glycoprotein. Biochemistry. 1976 Jun 1;15(11):2245–2254. doi: 10.1021/bi00656a001. [DOI] [PubMed] [Google Scholar]
- Sutton B. J., Phillips D. C. The three-dimensional structure of the carbohydrate within the Fc fragment of immunoglobulin G. Biochem Soc Trans. 1983 Apr;11(2):130–132. [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Wong A. K., Hsia J. C. In vitro binding of propranolol and progesterone to native and desialylated human orosomucoid. Can J Biochem Cell Biol. 1983 Oct;61(10):1114–1116. doi: 10.1139/o83-142. [DOI] [PubMed] [Google Scholar]


