Abstract
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of a transmissible lung cancer of sheep known as ovine pulmonary carcinoma. Recently, we have found that the expression of the JSRV envelope (Env) is sufficient to transform mouse NIH 3T3 cells in classical transformation assays. To further investigate the mechanisms of JSRV oncogenesis, we generated a series of envelope chimeras between JSRV and the JSRV-related endogenous retroviruses of sheep (enJSRVs) and assessed them in transformation assays. Chimeras containing the exogenous JSRV SU region and the enJSRV TM region were unable to transform NIH 3T3 cells. Additional chimeras containing only the carboxy-terminal portion of TM (a region that we previously identified as VR3) of the endogenous envelope with SU and the remaining portion of TM from the exogenous JSRV were also unable to transform NIH 3T3 cells. The VR3 region includes the putative membrane-spanning region and cytoplasmic tail of the JSRV TM glycoprotein; this suggested that the cytoplasmic tail of the JSRV Env mediates transformation, possibly via a cell signaling mechanism. Mutations Y590 and M593 in the cytoplasmic tail of the JSRV envelope were sufficient to inhibit the transforming abilities of these constructs. Y590 and M593 are part of a Y-X-X-M motif that is recognized by the phosphatidylinositol 3-kinase (PI-3K). PI-3K initiates a cell signaling pathway that inhibits apoptosis and is required for a number of mitogens during the G1-to-S-phase transition of the cell cycle. PI-3K activates Akt by phosphorylation of threonine 308 and serine 473. We detected by Western blot analysis phosphorylated Akt in serum-starved MP1 cells (NIH 3T3 cells transformed by JSRV) but not in the parental NIH 3T3 cells. These data indicate that the cytoplasmic tail of the JSRV TM is necessary for cell transformation and suggest a new mechanism of retroviral transformation. In addition, the ability to dissociate the function of the JSRV envelope to mediate viral entry from its transforming capacity has direct relevance for the design of JSRV-based vectors that target the differentiated epithelial cells of the lungs.
Jaagsiekte sheep retrovirus (JSRV) is the etiological agent of a contagious lung cancer of sheep known as ovine pulmonary carcinoma (OPC) or sheep pulmonary adenomatosis (4, 16, 30, 36). OPC is a naturally occurring disease in most countries of the world and is characterized by the transformation of the differentiated epithelial cells of the lungs, type II pneumocytes and Clara cells (17, 33). OPC is being extensively studied for its similarities with a particular type of human lung cancer known as bronchioloalveolar carcinoma (BAC) (17, 21, 23, 33). The causes of BAC are unknown. BAC is not strongly associated with cigarette smoking, and its incidence appears to be increasing in the United States (1, 5, 6, 51). Thus, the JSRV/OPC model can offer a foundation for understanding the molecular mechanisms of type II pneumocytes and Clara cell transformation, especially considering the role played by retroviruses in the discovery of cellular oncogenes (46).
Cell transformation by JSRV is offering a new paradigm for retroviral oncogenesis (45). The JSRV genome does not contain a cell-derived oncogene, which would suggest that JSRV induces cell transformation by insertional activation of proto-oncogenes (46). However, the incubation period in experimentally inoculated lambs can be as short as 2 to 3 weeks (47, 52), which appears too short for insertional activation to occur. Our recent study showed that the expression of the envelope (Env) protein of JSRV is able to transform rodent fibroblasts in vitro (27). Thus, besides mediating viral entry through the interaction with the cellular receptor, the JSRV Env might also function directly as a viral oncogene. Recently, the cellular receptor for JSRV has been identified as the hyaluronidase-2 (Hyal-2) (42), a glycosylphosphatidylinositol-anchored protein whose function is not completely known (25). Interestingly, Hyal-2 is contained within a region on human chromosome 3p21.3 that is deleted in a substantial frequency of lung tumors, which suggests that it might function as a tumor suppressor gene (26).
In this study we initially mapped the determinants of cell transformation of the JSRV envelope. We approached this task by generating a series of chimeric constructs between the envelopes of JSRV21 (an infectious JSRV molecular clone) (36) and of two JSRV-related endogenous retroviruses of sheep (enJS56A1 and enJS5F16) (34). The results indicated that the cytoplasmic tail of the transmembrane (TM) protein of the JSRV Env is essential for transformation. Further experiments indicated that a YXXM motif in the cytoplasmic tail is necessary for transformation and that in JSRV-transformed cells the PI-3K pathway is activated. These results suggest a new mechanism of retroviral transformation.
MATERIALS AND METHODS
Plasmids.
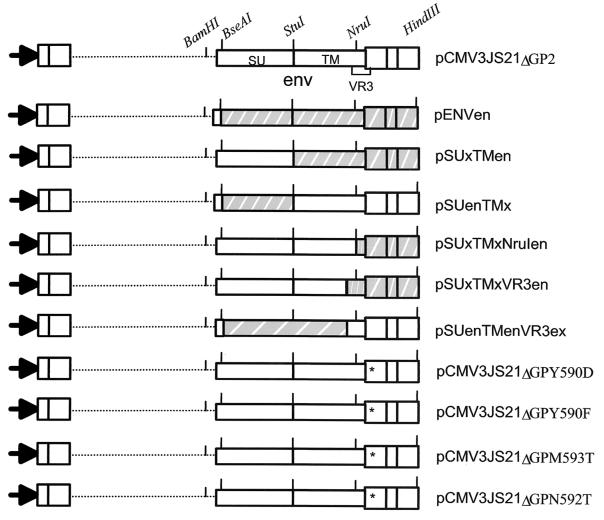
A diagram of the plasmids employed in this study is shown in Fig. 1. Molecular cloning and mutagenesis were performed by established procedures (2). Plasmid pCMV3JS21ΔGP expressing the JSRV21 envelope under the control of the cytomegalovirus (CMV) immediate-early promoter has been described previously (27).
FIG. 1.
Schematic of the chimeric and some of the mutant JSRV Env constructs used in this study. The restriction sites that facilitated the cloning strategy are indicated. All the constructs have the same promoter, splice donor, and splice acceptor.
To facilitate cloning procedures described below, we generated pCMV3JS21ΔGP2 by removing most of the cellular 3′-flanking sequences present in the pCMV3JS21ΔGP plasmid. pEnvEn and pEnvEn2 have the CMV immediate-early promoter, R, U5, and the splice donor and splice acceptor sites of pCMV3JS21ΔGP but express the entire env of the enJSRV clones en56A1 and en5F16, respectively (34). The other chimeric plasmids were derived from pCMV3JS21ΔGP2 and pEnvEn. The chimeras were named by identifying the envelope region followed by either “x” for the exogenous pCMV2JS21ΔGP or “en” for the endogenous plasmid. For example, pSUxTMen has the surface (SU) region from the exogenous envelope and the transmembrane (TM) from the endogenous clone, while pSUenTMx is the opposite chimera. pSUxTMxVR3en has the SU and most of TM from the exogenous envelope and the VR3 from the endogenous one, while pSUenTMenVR3x is the reciprocal chimera. pSUxTMxNruIen has SU, TM, and the first portion of the VR3 from the exogenous envelope and the last portion of the VR3 from the endogenous envelope. Single point mutations of the JSRV Env in the pCMV3JS21ΔGP construct were performed by site-directed mutagenesis using standard PCR-based procedures (2). By conventional nomenclature, pCMV3JS21ΔGPY590D and pCMV3JS21ΔGPY590F have a single point mutation changing the tyrosine at position 590 to aspartic acid and phenylalanine, respectively.
Transformation assays and development of JSRV-transformed cell lines.
Transformation assays were performed as previously described (27). Briefly, NIH 3T3 cells (3 × 105 per 10-cm tissue culture plate) were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) and 10% calf serum. Cells were transfected with 28 μg of plasmid DNA using the CalPhos mammalian transfection kit (Clontech) as recommended by the manufacturer. Each plasmid was tested in at least three independent transformation assays, with two independent plasmid preparations for each construct. Approximately 12 h after transfection, cells were washed three times with phosphate-buffered saline (PBS), and fresh medium was added. Cells were maintained in culture for 4 to 5 weeks, with the medium replaced every 3 days, and monitored microscopically. Foci of transformed cells were counted 1 month after transfection. Two transformed foci (MP1 and MP2, from two separate transformation assays) were picked and expanded to give JSRV-transformed cell lines.
mRNA analysis.
To analyze the pattern and level of expression of some of the plasmids used in this study, 293T cells (approximately 106 cells per 10-cm plate) were transfected with 28 μg of plasmid DNA and the CalPhos mammalian transfection kit (Clontech) as recommended by the manufacturer. Forty-eight hours after transfection, total RNA was extracted using the RNAqueous-Midi kit (Ambion). RNA preparations were treated with RNase-free DNase (Qiagen). From 6 to 10 μg of total RNA was denatured with glyoxal-dimethyl sulfoxide, run in a 1% agarose gel in 10 mM sodium phosphate, and blotted to nylon membranes (Hybond-N Plus; Amersham) using established methods (2). Membranes were hybridized with 32P-labeled JSRV env probes and subjected to autoradiography by exposure to X-ray film (28).
Entry assays.
Entry mediated by the mutant and chimeric envelopes was assessed by the ability of the JSRV envelope to pseudotype murine leukemia virus (MLV)-based vectors (41). A 293-based cell line expressing MLV Gag and Pol and an MLV luciferase vector (GP-293-luc; Clontech) was transfected as described above with various JSRV env expression plasmids. Supernatants were collected and stored at −70°C. Subsequently, serial dilutions of the vector supernatants were used to infect NIH 3T3 cells stably expressing the human Hyal-2. These cells were prepared by infection of NIH 3T3 cells with an MLV-based vector expressing the human Hyal-2 protein (provided by Dusty Miller), followed by selection for the drug resistance marker in the vector (M. Palmarini and H. Fan, unpublished results).
Experiments were performed twice, with duplicate experiments for each dilution. Cells were lysed after 72 h, and luciferase assays were performed using the luciferase assay kit as recommended by the manufacturer (Promega).
Western blot analysis.
For the detection of Akt, cells were grown at 37°C and 5% CO2 in DMEM and 10% calf serum until they reached approximately 80% confluence. Cells were then washed twice with PBS and grown for another 16 h in DMEM lacking calf serum. Cells were then lysed in lysis buffer containing 0.5% NP-40, 50 mM HEPES buffer (pH 7.8), 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1 protease inhibitor cocktail tablet (Roche) per 50 ml of lysis buffer. From 5 to 10 μg of cell lysate was subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE)-Western blotting following standard procedures (2). Filters were incubated with polyclonal rabbit antiserum to either Akt (cell signaling), Akt phosphorylated in serine 473 (cell signaling), or Akt phosphorylated in threonine 308 (cell signaling) and detected with a donkey anti-rabbit immunoglobulin labeled with horseradish peroxidase (Amersham), followed by an enhanced chemiluminescence detection system (Supersignal; Pierce) as recommended by the manufacturers.
RESULTS
TM region of JSRV envelope is a determinant of cell transformation in vitro.
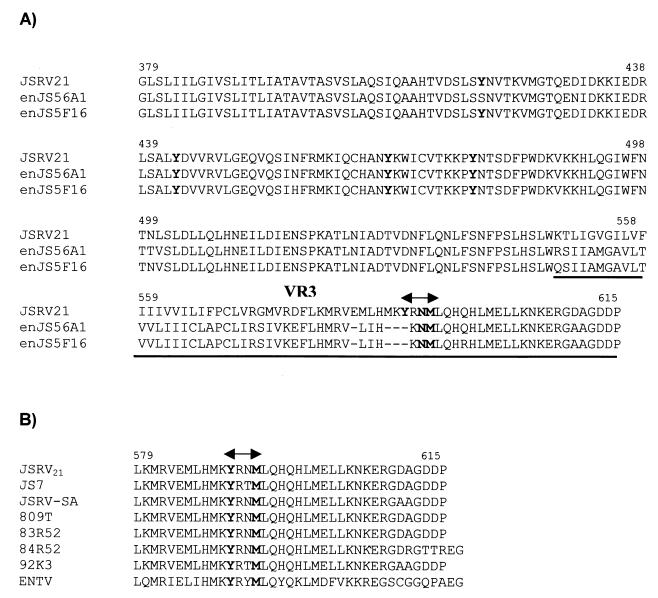
The JSRV envelope precursor (as for all retroviruses) is cleaved by cellular proteases into two proteins, surface (SU) and transmembrane (TM). SU is primarily responsible for the interaction with the cell surface receptor, while TM anchors the complex to the virion envelope and is involved in later steps of viral entry (e.g., fusion). The exogenous JSRV envelope is highly related to its known endogenous counterparts (3, 34). Between the exogenous and the endogenous envelopes used in this study, there is 96% identity in the SU region and 86% identity in the TM, but only approximately 50% identity in VR3 (Fig. 2). We therefore reasoned that the VR3 might be important for viral transformation. To this end we generated a series of chimeric envelopes between the exogenous JSRV21 and the related endogenous enJS56A1 in the context of the pCMV2JS21ΔGP envelope expression plasmid (Fig. 1). These plasmids were tested in the standard NIH 3T3 cell focus formation assay.
FIG. 2.
Amino acid sequences of the TM region of JSRV and enJSRV. (A) Alignment of the amino acid sequence of the TM region of the exogenous JSRV21 and the endogenous enJS56A1 and enJS5F16 clones. A dash (–) indicates lack of an amino acid. In bold are the amino acids mutated in this study. The VR3 region is underlined, and the putative PI-3K docking site is indicated by a double arrow. (B) The putative PI-3K docking site is conserved among various JSRV isolates (double arrow). GenBank accession numbers for the exogenous sequences are AF105220 (JSRV21), AF357971 (JS7), NC001494 (JSRV-SA), Y18302 (809T), Y18303 (83R52), Y18304 (84R52), Y18305 (92K3), and Y16627 (ENTV).
As expected, pCMV3JS21ΔGP transformed NIH 3T3 cells (23.7 ± 12.7 foci [mean ± standard deviation]), while pEnvEn and pEnvEn2 did not. The chimeric constructs pSUxTMen, pSUxTMxVR3en, and pSUxTMxNruIen, containing, respectively, the TM, the VR3, or only the carboxy-terminal 36 amino acids from the endogenous loci, were not able to transform NIH 3T3 cells. This indicated that sequences downstream from the NruI site in the VR3 of JSRV are necessary for transformation, in agreement with our initial hypothesis.
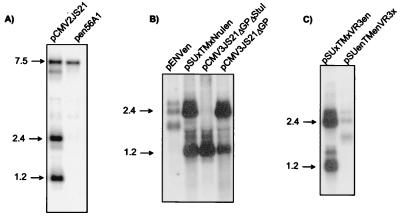
However, the reciprocal chimeras pSUenTMx and pSUenTMenVR3x also did not transform NIH 3T3 cells. These results could reflect an additional requirement for exogenous SU to transform NIH 3T3 cells, or they could simply reflect differences in expression and/or mRNA stability. To address this, we carried out Northern blot analysis for env RNA from 293T cells transiently transfected with pCMV2JS21, the exogenous JSRV21 molecular clone driven by the CMV immediate-early promoter (36), and with pen56A1 (34), a full-length endogenous molecular clone also driven by the CMV immediate-early promoter (Fig. 3A). The results showed a great difference in expression of the spliced env mRNA, but there was no difference in the levels of full-length unspliced viral RNAs. Thus, either the endogenous spliced env mRNA is relatively unstable or its expression is repressed. This was supported by Northern blot analysis of 293T cells transfected with either pEnvEn, pCMV2JS21ΔGP, or pSUxTMxNruIen. These constructs are all driven by the same CMV promoter and have the same splice donor and splice acceptor. However, spliced env mRNA from pEnvEn was not expressed as efficiently as pCMV2JS21ΔGP or pSUxTMxNruIen, which contain various amounts of exogenous JSRV env sequences (Fig. 3B). Similarly, spliced env mRNA from the chimera containing the endogenous env with the exogenous VR3 was expressed at much lower levels than the full-length exogenous counterpart; the latter is instead expressed as efficiently as the wild-type pCMV2JS21ΔGP (Fig. 3C). A full description of the JSRV mRNA splicing patterns and of the relative instability of the endogenous mRNA transcripts will be discussed elsewhere (M. Palmarini, C. Murgia, and H. Fan, submitted for publication). Briefly, all chimeras that contained endogenous SU sequences showed substantially lower levels of spliced env mRNA. Therefore, the failure of these chimeras to transform could be simply attributed to low levels of env mRNA, and they were not informative with regard to env domains for transformation.
FIG. 3.
Northern blotting analysis of exogenous JSRV21 full-length clone, endogenous en56A1 full-length clone, and some chimeric Env constructs. 293T cells were transiently transfected with the plasmids indicated below, and total RNA was obtained 48 h after transfection and analyzed by Northern blotting as described in Materials and Methods. (A) Comparison of full-length exogenous pCMV2JS21 and pen56A1 clones. Three main transcripts are visible in the pCMV2JS21 lane: a 7.5-kb band corresponding to the canonical full-length mRNA, a 2.4-kb band corresponding to the spliced mRNA encoding Env, and a 1.2-kb band corresponding to the prematurely polyadenylated tr-env transcript (28). In the pen56A1 lane, the full-length mRNA band has approximately the same intensity as the equivalent band of the exogenous pCMV2JS21 clone, but no spliced env mRNA is visible. (B and C) Western blotting as above using various Env constructs. In panel B, note the difference in expression between the endogenous pEnvEn (first lane) and the exogenous pCMV3JS21ΔGP (last lane). The chimeric construct pSUxTMxNruIen is expressed at a level comparable to that of the exogenous pCMV3JS21ΔGP. pCMV2JS21ΔGPΔStuI expresses the prematurely polyadenylated env transcript and is not relevant for this study. (C) Difference in expression between the chimera pSUxTMxVR3en and pSUenTMenVR3x. pSUenTMenVR3x is not an informative construct because its expression is downregulated or the resulting mRNA is unstable. pSUxTMxVR3en has a level of expression comparable to that of the exogenous pCMV2JS21ΔGP.
Tyrosine residue in cytoplasmic tail of TM is necessary for transformation.
The preceding experiments pointed to the VR3 of JSRV as a main determinant of viral oncogenesis. The VR3 region includes the cytoplasmic tail of the JSRV TM glycoprotein. Thus, the cytoplasmic tail of the JSRV Env might mediate transformation by a cell signaling mechanism. It was noteworthy that the VR3 region from all exogenous strains of JSRV sequences contains a tyrosine residue (Y590 in JSRV21) (Fig. 2B), while the two endogenous envelopes do not (Fig. 2A). Moreover, Y590 is in an amino acid sequence that could potentially bind SH2 domain-containing proteins (see below). To test if Y590 might be important for JSRV transformation, we mutated Y590 in pCMV2JS21ΔGP to either phenylalanine (pCMV2JS21ΔGPY590F) or aspartic acid (pCMV2JS21ΔGPY590D). Both the Y590 mutants were completely unable to transform cells (Table 1). This indicated that Y590 is essential for transformation.
TABLE 1.
Transformation assays with JSRV Env constructs carrying various tyrosine mutations in TM
| Plasmid | No. of foci observed
|
||
|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | |
| pCMV3JS21ΔGP | 32 | 22 | 31 |
| pCMV3JS21ΔGPY590D | 0 | 0 | 0 |
| pCMV3JS21ΔGPY590F | 0 | 0 | 0 |
| pCMV3S21ΔGPY419F | 25 | 34 | 16 |
| pCMV3JS21ΔGPY443F | 17 | 20 | 15 |
| pCMV3JS21ΔGPY468F | 10 | 17 | 13 |
| pCMV3JS21ΔGPY478F | 8 | 5 | 6 |
There are five tyrosine residues in the TM protein of JSRV. To test if the requirement for Y590 for transformation was specific, the other tyrosines were also individually mutated to phenylalanines in the context of pCMV2JS21ΔGP (Y419F, Y443F, Y468F, and Y478F). All of these mutants resulted in transformation (Table 1). Thus, mutation of only Y590 abolished transformation, indicating that the requirement for Y590 for transformation was quite specific.
Docking site for PI-3K but not Grb-2 is essential for transformation.
The amino acid sequence surrounding the Y590 in the JSRV TM cytoplasmic tail is YRNM. This sequence contains potential binding sites for two classes of SH2 domain-containing proteins: YXXM for the regulatory alpha subunit of PI-3K (p85) and YXN for the growth factor receptor binding protein 2 (Grb-2) (9, 43). Both of these proteins are at the beginning of important signal transduction cascades. Mutation of the methionine at position 593 (M593T) completely abolished transformation, while mutation of the asparagine at residue 592 (N592T) did not. These results indicated that binding of Grb-2 and signaling through the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway was not essential for JSRV transformation, although we cannot rule out that the Ras/MAPK pathway could still be activated in a different manner at some other level. In contrast, the results also supported the possibility that docking of PI-3K to the cytoplasmic tail of JSRV TM protein is essential for transformation.
Mutations of Y590 do not abolish ability of JSRV Env to bind receptor and mediate viral entry.
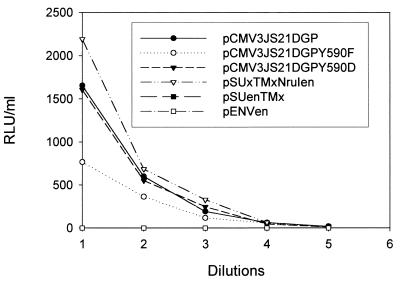
We next considered whether the mutations abolishing the transforming phenotype of the JSRV envelope were merely causing conformational changes in the JSRV envelope protein that might prevent the correct expression and/or interaction with the cellular receptor. To address this, we tested the capacity of the critical Y590 envelope mutants to mediate viral entry. Entry assays were performed using MLV-based vectors expressing the luciferase genes pseudotyped with various envelope constructs. The viral pseudotypes were then used to infect 3T3 cells stably expressing the JSRV cellular receptor (M. Palmarini and H. Fan, unpublished results), and luciferase activity was measured 3 days postinfection (Fig. 4). In the same experiment we used the wild-type pCMV2JS21ΔGP and plasmids pSUxTMxNruIen, pEnvEn, and pSUxTMen.
FIG. 4.
Entry assays employing MLV-luciferase vectors pseudotyping some of the chimeric and mutant constructs described in this study. Values are expressed as relative light units (RLU); threefold dilutions were used in this experiment. PCMV3JS21ΔGP, pCMV3JS21ΔGPY590D, and pSUxTMxNruIen all gave comparable values. pCMV3JS21Y590F gives values approximately 50% of those of the above plasmids but well above the background given by pSUenTMx and pEnvEn, which, as expected, represent the baseline close to zero (considering that these constructs are not efficiently expressed, as established by Northern blotting). The values are the averages of duplicate experiments for each dilution. The experiments were repeated once with a different DNA plasmid preparation and gave essentially the same results (not shown).
No significant differences were observed in the luciferase values between the wild-type pCMV2JS21ΔGP, pCMV2JS21ΔGPY590D, and pSUxTMxNruIen. pCMV2JS21ΔGPY590F showed decreased luciferase values with respect to the wild-type pCMV2JS21ΔGP. pSUenTMex and pEnvEn showed no luciferase activity; this was presumably due to the inability of these constructs to be expressed efficiently. However, it is also possible that the endogenous envelope does not bind the same cellular receptor as the exogenous counterpart. In any event, these experiments show that the putative docking site for PI-3K in the JSRV VR3 region is absolutely necessary for transformation but not for the viral envelope interaction with the cellular receptor and subsequent entry into the cell. This also indicates that the Y590F or Y590D mutations did not have global effects on envelope conformation.
We also tested the functionality of the Y590 envelope mutants in viral replication. The Y590F mutation was cloned into the pCMV2JS21 plasmid, which contains a complete provirus under control of the CMV immediate-early promoter. We generated Y590F mutant virus by transfection of 293T cells, as described previously (36). The resulting virus was then used to infect sheep choroid plexus (CP) cells in vitro, as described previously (37). PCR tests of the infected CP cells indicated that the Y590F mutant virus was capable of replication (not shown). However, due to the nature of the PCR-based assay (the only one available for assaying JSRV infectivity in vitro), quantitative assessment of the relative infectivity of the mutant virus was not possible.
Phosphorylated Akt is present in JSRV-transformed but not parental NIH 3T3 cells.
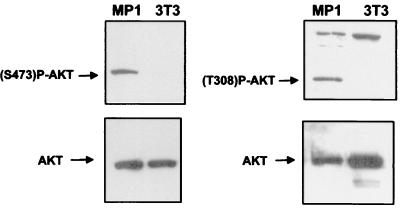
The results in Table 2 strongly suggested that JSRV Env protein transforms cells by docking PI-3K to the cytoplasmic tail of TM. The Akt protein kinase is an important intermediate in the PI-3K signaling pathway. PI-3K activates Akt by phosphorylation of serine 473 and threonine 308 via the intermediate PDK-1 (14). If JSRV transforms cells via the PI-3K pathway, transformed cells would be expected to show activation of intermediates in this pathway. Therefore we tested two JSRV-transformed cell lines (MP1 and MP2) for phosphorylation of Akt by Western blotting with Akt antibodies specific for S473 and T308 phosphorylation (Fig. 5). Phosphorylated Akt was detected in serum-starved MP1 cells but not in the parental NIH 3T3 cells. Akt in MP1 cells is in its fully active form, because we could detect both phosphorylated serine 473 and phosphorylated threonine 308 (Fig. 5). Similar results were shown in MP2 cells (not shown). These results strongly support the hypothesis that the cytoplasmic tail of the JSRV envelope mediates transformation via the PI-3K/Akt cell signaling pathway.
TABLE 2.
Transformation assays with JSRV Env constructs carrying mutations in the SH-2 site of the cytoplasmic tail
| Plasmid | No. of foci observed
|
||
|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | |
| pCMV3JS21ΔGP | 12 | N.T.a | 56 |
| pCMV3JS21ΔGPN592T | 40 | 38 | 105 |
| pCMV3JS21ΔGPM593T | 0 | 0 | 0 |
N.T., not tested.
FIG. 5.
Activation of Akt in MP1 cells. Cell lysates of serum-starved MP1 and NIH 3T3 cells were analyzed by SDS-PAGE–Western blotting as described in Materials and Methods. Akt phosphorylated both in serine and in threonine is visible in MP1 cells but not in the parental NIH 3T3 cells. The same lysates were analyzed by using an antiserum to Akt as a loading control.
DISCUSSION
Our previous work has established that the JSRV envelope has the capacity to transform NIH 3T3 cells in classical transformation assays. In this study we have demonstrated that (i) the cytoplasmic tail of the transmembrane region of the JSRV envelope is critical for mediating virus-induced transformation of NIH 3T3 cells and (ii) the PI-3K/Akt pathway is activated in JSRV-transformed NIH 3T3 cells. These conclusions have been drawn by abolishing the transforming phenotype of JSRV through single point mutations (Y590F or Y590D and M593T) in a putative PI-3K docking site (Y-X-X-M) of the TM cytoplasmic tail. In some cells, PI-3K initiates a cell signaling pathway that appears to inhibit apoptosis and be required for a number of mitogens during the G1-to-S-phase transition of the cell cycle (43). Akt (also known as protein kinase B) is a central effector of the PI-3K pathway. PI-3K activates Akt by phosphorylation of serine 473 and threonine 308 via the intermediate PDK-1 (14). We detected phosphorylation of Akt both in serine and in threonine in JSRV-transformed cells but not in the parental NIH 3T3 cells (Fig. 5).
These results suggest that a central event in JSRV-induced transformation of NIH 3T3 cells is the activation of the PI-3K/Akt pathway resulting from the expression of the JSRV envelope and the phosphorylation of Y590 in the cytoplasmic tail of the transmembrane region (Fig. 6). This would furnish a novel mechanism of transformation for retroviruses. However, the PI-3K/Akt pathway has been implicated in retrovirus-induced cell transformation in other systems. Both PI-3K and Akt have been described as retrovirus-transduced oncogenes. The catalytic subunit of PI-3K has been transduced by avian sarcoma virus 16 (11), while Akt has been transduced by an ecotropic MLV (7, 49). In addition, the PI-3K/Akt pathway has recently been found to be involved in the induction of erythropoietin independence of erythroid cells following infection with Friend spleen focus-forming virus (29). Thus, it is plausible that the PI-3K/Akt pathway could be the cause of the transformation events that occur in JSRV-transformed cells. Moreover, PI-3K/Akt cell signaling has been shown to be activated in a number of solid human tumors, including lung cancers (24, 28, 38, 48).
FIG. 6.
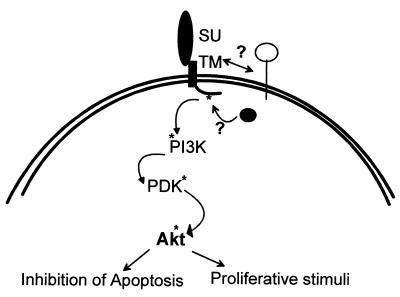
Model of JSRV-induced transformation of NIH 3T3 cells. The cytoplasmic tail of JSRV mediates transformation of NIH 3T3 cells possibly via activation of the PI-3K/Akt pathway. Y590 in the cytoplasmic tail of the JSRV Env needs to be phosphorylated in order to activate the PI-3K pathway. It is not yet clear which interactions at the membrane and/or at the cytoplasmic level are necessary in order for transformation to occur.
Akt promotes cell survival by inhibiting apoptosis by phosphorylating several targets, primarily the proapoptotic BCL-2 family member BAD (10), forkhead transcription factors (8), and the cell death pathway enzyme caspase-9 (44). In addition, Akt activates the ribosomal S6 kinase (p70S6K) (13) via FRAP (also known as mTOR) phosphorylation. Activation of p70S6K regulates a wide variety of cellular processes involved in the mitogenic response (12, 15, 18).
One impediment to this research is the fact that no effective antibodies specific for JSRV envelope protein have been developed yet. The development of such reagents will be necessary in order to further dissect the cell signaling activated in JSRV-transformed NIH 3T3 cells and to establish if indeed the JSRV Env is phosphorylated. A first priority will be to test if the tyrosine at 590 is in fact phosphorylated in transformed cells, a prerequisite for docking of PI-3K. Other important issues to address in the future are to identify the particular proteins that interact with the JSRV Env (e.g., PI-3K) and to determine if interaction with the JSRV cellular receptor Hyal-2 is also necessary for JSRV-induced transformation. The murine Hyal-2 does not mediate efficient JSRV entry (41); however, this does not necessarily imply that there is not an interaction between JSRV Env and murine Hyal-2 that could be necessary for transformation. Abundant JSRV Env expression appears to be necessary for transformation of NIH 3T3 cells. Indeed, if the JSRV Env is driven by the JSRV long terminal repeat (LTR), which is a relatively weak promoter in NIH 3T3 cells, transformation is inefficient (27). An analogous situation might pertain to in vivo infection. In OPC-affected animals, JSRV infects a wide variety of lymphoid tissues and cells (20, 22, 35), but viral antigen is consistently easily detected only in differentiated lung epithelial cells (32). This is because the JSRV LTR is specifically active in the target cells for viral transformation (31). Thus, activation of the PI-3K/Akt pathway will preferentially occur in lung epithelial cells, where the JSRV LTR is efficiently expressed.
Recent data suggest that in type II pneumocytes, surfactant protein A (SP-A) regulates the production of pulmonary surfactant secretion via activation of the PI-3K/Akt pathway (53). In turn, SP-A increases transcription of another surfactant protein, SP-B, by enhancing the activity of lung-specific transcription factors like hepatocyte nuclear factor HNF-3 (50). Thus, it might be envisaged that JSRV expression in transformed type II pneumocytes involves an autocrine loop in which lung-specific transcription factors activate the JSRV LTR; the resulting Env expression leads to constitutive activation of the PI-3K/Akt pathway, which in turn enhances expression of lung-specific transcription factors. Activation of the PI-3K/Akt pathway might be only one of several hits required for transformation in vivo, particularly in naturally occurring OPC, where the incubation period can be several months or even years. There are other examples of retroviral oncogenesis that would support this view. For instance, Abelson MLV induces cell transformation in vitro by the expression of a Gag-Abl fusion protein (19). In vivo, however, it has been shown that insertional activation by a Moloney MLV helper virus is also required to induce leukemia (39, 40). Experimental inoculation of newborn lambs with replication-competent JSRV Y590/M593 mutants will directly address whether activation of the PI-3K/Akt pathway is also essential for transformation in vivo. Furthermore, studies on OPC tumor-derived cell lines might help to address this issue.
Given the unique tropism for the differentiated epithelial cells of the lungs, JSRV is also being considered as a potential vector for gene transfer in lung cells. However, the ability of JSRV envelope protein to induce transformation is clearly a point of concern for development of JSRV-based vectors. This study provides a way to uncouple the unwanted transforming properties of the JSRV envelope from the desired ability to mediate viral entry.
ACKNOWLEDGMENTS
We are grateful to Paula Cannon for suggestions regarding the Y590 mutation.
M.P. was a recipient of an American Cancer Society Ray and Estelle Spehar Fellowship. This work was supported by NIH grant RO1CA82564 to H.F. and by the University of Georgia (to M.P.). Support from the UCI Cancer Research Institute and the DNA sequencing core of the Chao Family Comprehensive Cancer Center is acknowledged.
REFERENCES
- 1.Auerbach O, Garfinkel L. The changing pattern of lung carcinoma. Cancer. 1991;68:1973–1977. doi: 10.1002/1097-0142(19911101)68:9<1973::aid-cncr2820680921>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Son; 2000. [Google Scholar]
- 3.Bai J, Bishop J V, Carlson J O, DeMartini J C. Sequence comparison of JSRV with endogenous proviruses: envelope genotypes and a novel ORF with similarity to a G-protein-coupled receptor. Virology. 1999;258:333–343. doi: 10.1006/viro.1999.9728. [DOI] [PubMed] [Google Scholar]
- 4.Bai J, Zhu R Y, Stedman K, Cousens C, Carlson J, Sharp J M, DeMartini J C. Unique long terminal repeat U3 sequences distinguish exogenous Jaagsiekte sheep retroviruses associated with ovine pulmonary carcinoma from endogenous loci in the sheep genome. J Virol. 1996;70:3159–3168. doi: 10.1128/jvi.70.5.3159-3168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkley J E, Green M R. Bronchioloalveolar carcinoma. J Clin Oncol. 1996;14:2377–2386. doi: 10.1200/JCO.1996.14.8.2377. [DOI] [PubMed] [Google Scholar]
- 6.Barsky S H, Cameron R, Osann K E, Tomita D, Holmes E C. Rising incidence of bronchioloalveolar lung carcinoma and its unique clinicopathologic features. Cancer. 1994;73:1163–1170. doi: 10.1002/1097-0142(19940215)73:4<1163::aid-cncr2820730407>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 8.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 9.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 10.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 11.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 12.Chou M M, Blenis J. The 70 kDa S6 kinase: regulation of a kinase with multiple roles in mitogenic signalling. Curr Opin Cell Biol. 1995;7:806–814. doi: 10.1016/0955-0674(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 13.Chung J, Grammer T C, Lemon K P, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 14.Datta S R, Brunet A, Greenberg M E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 15.de Groot R P, Ballou L M, Sassone-Corsi P. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: an alternative route to mitogen-induced gene expression. Cell. 1994;79:81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 16.DeMartini J C, Bishop J V, Allen T E, Jassim F A, Sharp J M, de Las Heras M, Voelker D R, Carlson J O. Jaagsiekte sheep retrovirus proviral clone JSRV(JS7), derived from the JS7 lung tumor cell line, induces ovine pulmonary carcinoma and is integrated into the surfactant protein A gene. J Virol. 2001;75:4239–4246. doi: 10.1128/JVI.75.9.4239-4246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMartini J C, York D F. Retrovirus-associated neoplasms of the respiratory system of sheep and goats. Ovine pulmonary carcinoma and enzootic nasal tumor. Vet Clin N Am Food Anim Pract. 1997;13:55–70. doi: 10.1016/s0749-0720(15)30364-9. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari S, Thomas G. S6 phosphorylation and the p70s6k/p85s6k. Crit Rev Biochem Mol Biol. 1994;29:385–413. doi: 10.3109/10409239409083485. [DOI] [PubMed] [Google Scholar]
- 19.Goff S P, Witte O N, Gilboa E, Rosenberg N, Baltimore D. Genome structure of Abelson murine leukemia virus variants: proviruses in fibroblasts and lymphoid cells. J Virol. 1981;38:460–468. doi: 10.1128/jvi.38.2.460-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez L, Garcia-Goti M, Cousens C, Dewar P, Cortabarria N, Extramiana A B, Ortin A, De Las Heras M, Sharp J M. Jaagsiekte sheep retrovirus can be detected in the peripheral blood during the preclinical period of sheep pulmonary adenomatosis. J Gen Virol. 2001;82:1355–1358. doi: 10.1099/0022-1317-82-6-1355. [DOI] [PubMed] [Google Scholar]
- 21.Greco R J, Steiner R M, Goldman S, Cotler H, Patchefsky A, Cohn H E. Bronchoalveolar cell carcinoma of the lung. Ann Thorac Surg. 1986;41:652–656. doi: 10.1016/s0003-4975(10)63082-2. [DOI] [PubMed] [Google Scholar]
- 22.Holland M J, Palmarini M, Garcia-Goti M, Gonzalez L, Mckendrick I, De las Heras M, Sharp J M. Jaagsiekte retrovirus is widely distributed in both T and B lymphocytes and mononuclear phagocytes of sheep with naturally and experimentally acquired pulmonary adenomatosis. J Virol. 1999;73:4004–4008. doi: 10.1128/jvi.73.5.4004-4008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ives J C, Buffler P A, Greenberg S D. Environmental associations and histopathologic patterns of carcinoma of the lung: the challenge and dilemma in epidemiologic studies. Am Rev Respir Dis. 1983;128:195–209. doi: 10.1164/arrd.1983.128.1.195. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi M, Nagata S, Iwasaki T, Yanagihara K, Saitoh I, Karouji Y, Ihara S, Fukui Y. Dedifferentiation of adenocarcinomas by activation of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1999;96:4874–4879. doi: 10.1073/pnas.96.9.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepperdinger G, Strobl B, Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem. 1998;273:22466–22470. doi: 10.1074/jbc.273.35.22466. [DOI] [PubMed] [Google Scholar]
- 26.Lerman M I, Minna J D. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000;60:6116–6133. [PubMed] [Google Scholar]
- 27.Maeda N, Palmarini M, Murgia C, Fan H. Direct transformation of rodent fibroblasts by Jaagsiekte sheep retrovirus DNA. Proc Natl Acad Sci USA. 2001;98:4449–4454. doi: 10.1073/pnas.071547598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore S M, Rintoul R C, Walker T R, Chilvers E R, Haslett C, Sethi T. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer Res. 1998;58:5239–5247. [PubMed] [Google Scholar]
- 29.Nishigaki K, Hanson C, Ohashi T, Thompson D, Muszynski K, Ruscetti S. Erythroid cells rendered erythropoietin independent by infection with Friend spleen focus-forming virus show constitutive activation of phosphatidylinositol 3-kinase and Akt kinase: involvement of insulin receptor substrate-related adapter proteins. J Virol. 2000;74:3037–3045. doi: 10.1128/jvi.74.7.3037-3045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmarini M, Cousens C, Dalziel R G, Bai J, Stedman K, DeMartini J C, Sharp J M. The exogenous form of Jaagsiekte retrovirus is specifically associated with a contagious lung cancer of sheep. J Virol. 1996;70:1618–1623. doi: 10.1128/jvi.70.3.1618-1623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmarini M, Datta S, Omid R, Murgia C, Fan H. The long terminal repeats of Jaagsiekte sheep retrovirus are preferentially active in type II pneumocytes. J Virol. 2000;74:5776–5787. doi: 10.1128/jvi.74.13.5776-5787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmarini M, Dewar P, De las Heras M, Inglis N F, Dalziel R G, Sharp J M. Epithelial tumour cells in the lungs of sheep with pulmonary adenomatosis are major sites of replication for Jaagsiekte retrovirus. J Gen Virol. 1995;76:2731–2737. doi: 10.1099/0022-1317-76-11-2731. [DOI] [PubMed] [Google Scholar]
- 33.Palmarini M, Fan H, Sharp J M. Sheep pulmonary adenomatosis: a unique model of retrovirus-associated lung cancer. Trends Microbiol. 1997;5:478–483. doi: 10.1016/S0966-842X(97)01162-1. [DOI] [PubMed] [Google Scholar]
- 34.Palmarini M, Hallwirth C, York D, Murgia C, de Oliveira T, Spencer T, Fan H. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveals a different cell tropism from that of the highly related exogenous Jaagsiekte sheep retrovirus. J Virol. 2000;74:8065–8076. doi: 10.1128/jvi.74.17.8065-8076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmarini M, Holland M J, Cousens C, Dalziel R G, Sharp J M. Jaagsiekte retrovirus establishes a disseminated infection of the lymphoid tissues of sheep affected by pulmonary adenomatosis. J Gen Virol. 1996;77:2991–2998. doi: 10.1099/0022-1317-77-12-2991. [DOI] [PubMed] [Google Scholar]
- 36.Palmarini M, Sharp J M, De las Heras M, Fan H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J Virol. 1999;73:6964–6972. doi: 10.1128/jvi.73.8.6964-6972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmarini M, Sharp J M, Lee C, Fan H. In vitro infection of ovine cell lines by Jaagsiekte sheep retrovirus. J Virol. 1999;73:10070–10078. doi: 10.1128/jvi.73.12.10070-10078.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips W A, St. Clair F, Munday A D, Thomas R J, Mitchell C A. Increased levels of phosphatidylinositol 3-kinase activity in colorectal tumors. Cancer. 1998;83:41–47. doi: 10.1002/(sici)1097-0142(19980701)83:1<41::aid-cncr6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Poirier Y, Jolicoeur P. Distinct helper virus requirements for Abelson murine leukemia virus-induced pre-B- and T-cell lymphomas. J Virol. 1989;63:2088–2098. doi: 10.1128/jvi.63.5.2088-2098.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poirier Y, Kozak C, Jolicoeur P. Identification of a common helper provirus integration site in Abelson murine leukemia virus-induced lymphoma DNA. J Virol. 1988;62:3985–3992. doi: 10.1128/jvi.62.11.3985-3992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rai S K, DeMartini J C, Miller A D. Retrovirus vectors bearing Jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J Virol. 2000;74:4698–4704. doi: 10.1128/jvi.74.10.4698-4704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rai S K, Duh F M, Vigdorovich V, Danilkovitch-Miagkova A, Lerman M I, Miller A D. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for Jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci USA. 2001;98:4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roche S, Koegl M, Courtneidge S A. The phosphatidylinositol 3-kinase alpha is required for DNA synthesis induced by some, but not all, growth factors. Proc Natl Acad Sci USA. 1994;91:9185–9189. doi: 10.1073/pnas.91.19.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rommel C, Clarke B A, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos G D, Glass D J. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg N. New transformation tricks from a barnyard retrovirus: implications for human lung cancer. Proc Natl Acad Sci USA. 2001;98:4285–4287. doi: 10.1073/pnas.091097698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg N, Jolicoeur P. Retroviral pathogenesis. In: Coffin J M, Hughes S, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 475–585. [PubMed] [Google Scholar]
- 47.Sharp J M, Angus K W, Gray E W, Scott F M. Rapid transmission of sheep pulmonary adenomatosis (Jaagsiekte) in young lambs. Arch Virol. 1983;78:89–95. doi: 10.1007/BF01310861. [DOI] [PubMed] [Google Scholar]
- 48.Shayesteh L, Lu Y, Kuo W L, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills G B, Gray J W. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 49.Staal S P, Hartley J W, Rowe W P. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci USA. 1977;74:3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strayer D S, Korutla L. Activation of surfactant protein-B transcription: signaling through the SP-A receptor utilizing the PI3 kinase pathway. J Cell Physiol. 2000;184:229–238. doi: 10.1002/1097-4652(200008)184:2<229::AID-JCP11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 51.Valaitis J, Warren S, Gamble D. Increasing incidence of adenocarcinoma of the lung. Cancer. 1981;47:1042–1046. doi: 10.1002/1097-0142(19810301)47:5<1042::aid-cncr2820470535>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Verwoerd D W, De Villiers E M, Tustin R C. Aetiology of Jaagsiekte: experimental transmission to lambs by means of cultured cells and cell homogenates. Onderstepoort J Vet Res. 1980;47:13–18. [PubMed] [Google Scholar]
- 53.White M K, Strayer D S. Surfactant protein A regulates pulmonary surfactant secretion via activation of phosphatidylinositol 3-kinase in type II alveolar cells. Exp Cell Res. 2000;255:67–76. doi: 10.1006/excr.1999.4764. [DOI] [PubMed] [Google Scholar]