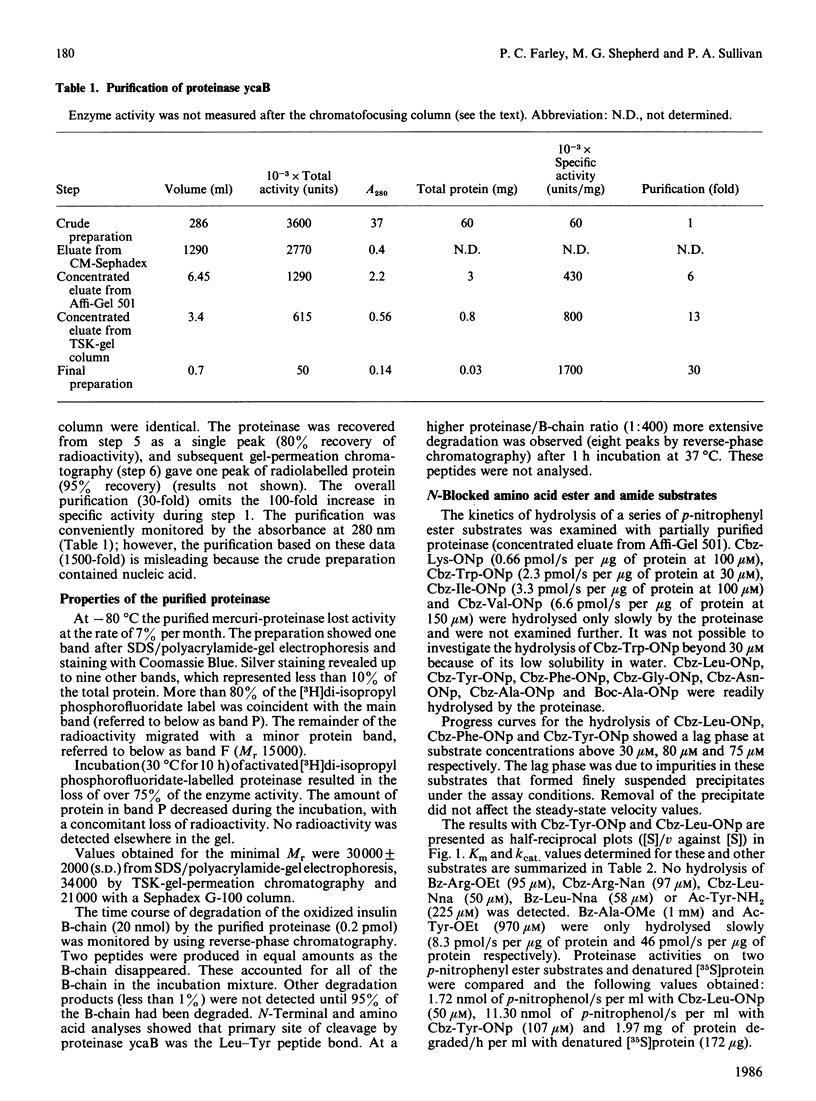
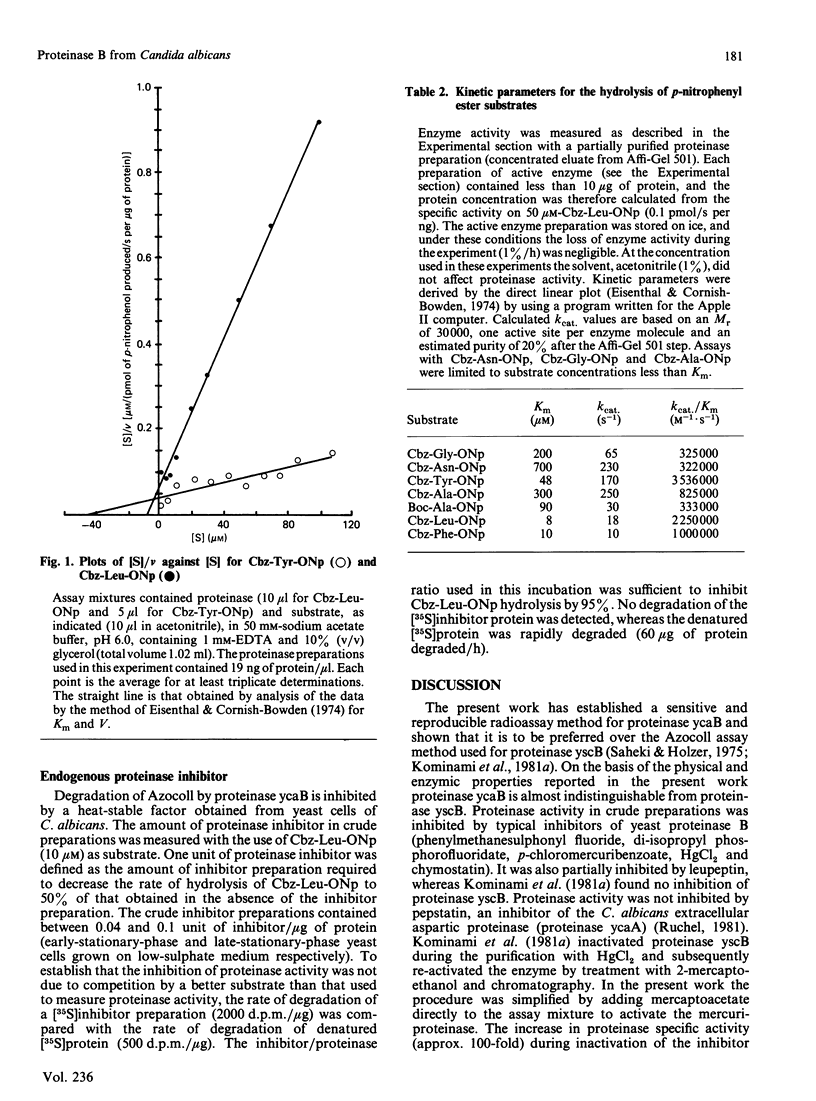
Abstract
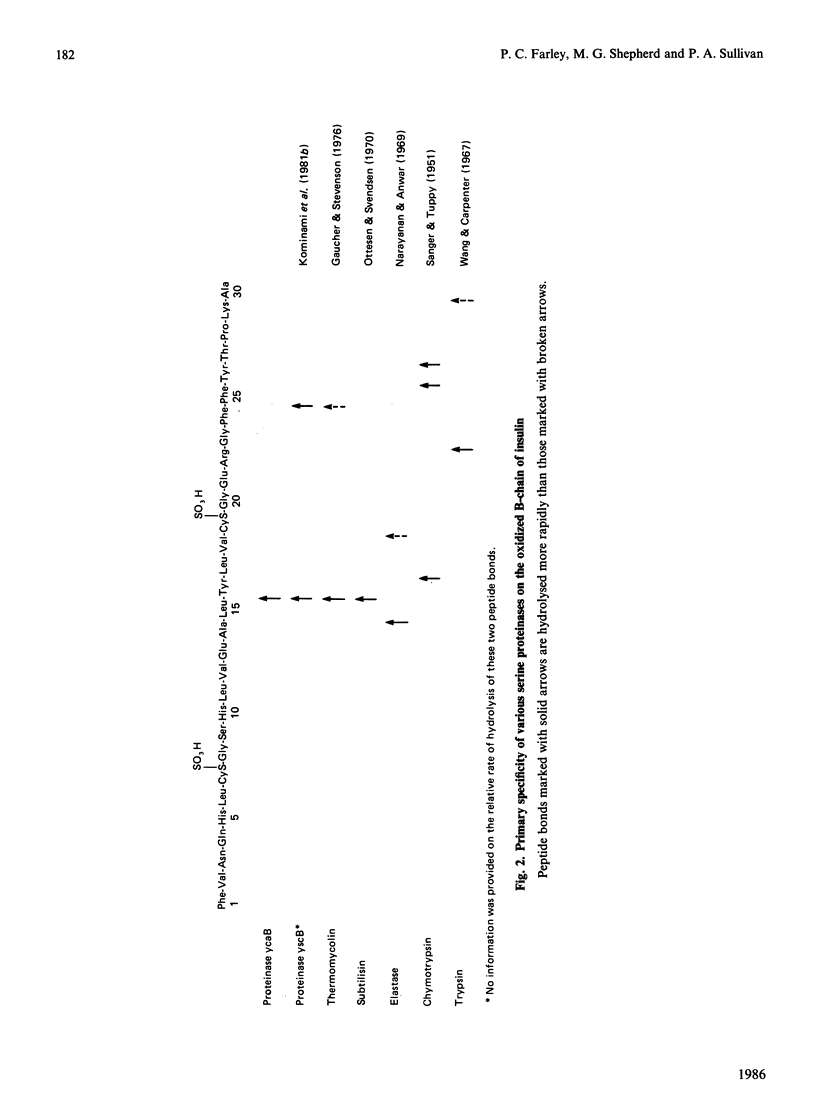
A serine proteinase (ycaB) from the yeast Candida albicans A.T.C.C. 10261 was purified to near homogeneity. The enzyme was almost indistinguishable from yeast proteinase B (EC 3.4.21.48), and an Mr of 30,000 for the proteinase was determined by SDS/polyacrylamide-gel electrophoresis. The initial site of hydrolysis of the oxidized B-chain of insulin, by the purified proteinase, was the Leu-Tyr peptide bond. The preferential degradation at this site, analysed further with N-blocked amino acid ester and amide substrates, demonstrated that the specificity of the proteinase is determined by an extended substrate-binding site, consisting of at least three subsites (S1, S2 and S'1). The best p-nitrophenyl ester substrates were benzyloxycarbonyl-Tyr p-nitrophenyl ester (kcat./Km 3,536,000 M-1 X S-1), benzyloxycarbonyl-Leu p-nitrophenyl ester (kcat./Km 2,250,000 M-1 X S-1) and benzyloxycarbonyl-Phe p-nitrophenyl ester (kcat./Km 1,000,000 M-1 X S-1) consistent with a preference for aliphatic or aromatic amino acids at subsite S1. The specificity for benzyloxycarbonyl-Tyr p-nitrophenyl ester probably reflects the binding of the p-nitrophenyl group in subsite S'1. The presence of S2 was demonstrated by comparison of the proteolytic coefficients (kcat./Km) for benzyloxycarbonyl-Ala p-nitrophenyl ester (825,000 M-1 X S-1) and t-butyloxycarbonyl-Ala p-nitrophenyl ester (333,000 M-1 X S-1). Cell-free extracts contain a heat-stable inhibitor of the proteinase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Emter O., Ehmann C., Wolf D. H. Proteolysis in eukaryotic cells. Identification of multiple proteolytic enzymes in yeast. J Biol Chem. 1984 Nov 10;259(21):13334–13343. [PubMed] [Google Scholar]
- Atlas D., Berger A. On the specificity of elastase. Hydrolysis of peptide p-nitrobenzyl esters. Biochemistry. 1972 Dec 5;11(25):4719–4723. doi: 10.1021/bi00775a013. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Odds F. C., Barlow A. J. An examination of the production of hydrolytic enzymes and toxins by pathogenic strains of Candida albicans. J Gen Microbiol. 1971 Aug;67(3):255–263. doi: 10.1099/00221287-67-3-255. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Every D. Quantitative measurement of protease activities in slab polyacrylamide gel electrophoretograms. Anal Biochem. 1981 Sep 15;116(2):519–523. doi: 10.1016/0003-2697(81)90396-1. [DOI] [PubMed] [Google Scholar]
- Gaucher G. M., Stevenson K. J. Thermomycolin. Methods Enzymol. 1976;45:415–433. [PubMed] [Google Scholar]
- Gopal P., Sullivan P. A., Shepherd M. G. Enzymes of N-acetylglucosamine metabolism during germ-tube formation in Candida albicans. J Gen Microbiol. 1982 Oct;128(10):2319–2326. doi: 10.1099/00221287-128-10-2319. [DOI] [PubMed] [Google Scholar]
- Kominami E., Hoffschulte H., Holzer H. Purification and properties of proteinase B from yeast. Biochim Biophys Acta. 1981 Sep 15;661(1):124–135. doi: 10.1016/0005-2744(81)90091-7. [DOI] [PubMed] [Google Scholar]
- Kominami E., Hoffschulte H., Leuschel L., Maier K., Holzer H. The substrate specificity of proteinase B from baker's yeast. Biochim Biophys Acta. 1981 Sep 15;661(1):136–141. doi: 10.1016/0005-2744(81)90092-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Levine N., Hatcher V. B., Lazarus G. S. Proteinases of human epidermis; a possible mechanism for polymorphonuclear leukocyte chemotaxis. Biochim Biophys Acta. 1976 Dec 8;452(2):458–467. doi: 10.1016/0005-2744(76)90196-0. [DOI] [PubMed] [Google Scholar]
- Magni G., Natalini P., Santarelli I., Vita A. Bakers' yeast protease A purification and enzymatic and molecular properties. Arch Biochem Biophys. 1982 Feb;213(2):426–433. doi: 10.1016/0003-9861(82)90568-9. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murphy C. J., Shepherd M. G., Sullivan P. A. Chemical modification of L-lactate 2-monooxygenase with fluorodinitrobenzene: evidence for two essential histidine residues. Biochemistry. 1983 Mar 29;22(7):1665–1669. doi: 10.1021/bi00276a022. [DOI] [PubMed] [Google Scholar]
- Narayanan A. S., Anwar R. A. The specificity of purified porcine pancreatic elastase. Biochem J. 1969 Aug;114(1):11–17. doi: 10.1042/bj1140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi M., Tsuboi R., Matsui T., Ogawa H. Isolation and characterization of proteinase from Candida albicans: substrate specificity. J Invest Dermatol. 1984 Jul;83(1):32–36. doi: 10.1111/1523-1747.ep12261656. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Remold H., Fasold H., Staib F. Purification and characterization of a proteolytic enzyme from Candida albicans. Biochim Biophys Acta. 1968 Oct 8;167(2):399–406. doi: 10.1016/0005-2744(68)90219-2. [DOI] [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- SANGER F., TUPPY H. The amino-acid sequence in the phenylalanyl chain of insulin. 2. The investigation of peptides from enzymic hydrolysates. Biochem J. 1951 Sep;49(4):481–490. doi: 10.1042/bj0490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Proteolytic activities in yeast. Biochim Biophys Acta. 1975 Mar 28;384(1):203–214. doi: 10.1016/0005-2744(75)90109-6. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schep G. P., Shepherd M. G., Sullivan P. A. Purification and properties of a beta-1,6-glucanase from Penicillium brefeldianum. Biochem J. 1984 Nov 1;223(3):707–714. doi: 10.1042/bj2230707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. Organomercurial agarose. Methods Enzymol. 1974;34:544–547. doi: 10.1016/s0076-6879(74)34070-0. [DOI] [PubMed] [Google Scholar]
- Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton M. D., Sullivan P. A., Shepherd M. G. The inhibition of ornithine transcarbamoylase from Escherichia coli W by phaseolotoxin. Biochem J. 1984 Dec 1;224(2):379–388. doi: 10.1042/bj2240379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser L., Blout E. R. The use of p-nitrophenyl N-tert-butyloxycarbonyl-L-alaninate as substrate for elastase. Biochim Biophys Acta. 1972 Apr 7;268(1):257–260. doi: 10.1016/0005-2744(72)90223-9. [DOI] [PubMed] [Google Scholar]
- Wang S. S., Carpenter F. H. Kinetics of the tryptic hydrolysis of the oxidized B chain of bovine insulin. Biochemistry. 1967 Jan;6(1):215–224. doi: 10.1021/bi00853a034. [DOI] [PubMed] [Google Scholar]
- Wolf D. H. Control of metabolism in yeast and other lower eukaryotes through action of proteinases. Adv Microb Physiol. 1980;21:267–338. doi: 10.1016/s0065-2911(08)60358-6. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Jones E. W. Protein degradation, meiosis and sporulation in proteinase-deficient mutants of Saccharomyces cerevisiae. Genetics. 1981 Jan;97(1):45–64. doi: 10.1093/genetics/97.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


