ABSTRACT
Follicular lymphoma (FL), the second most common subtype of non-Hodgkin lymphoma, relies on interactions with immune elements in the tumor microenvironment, including T-follicular helper cells and follicular dendritic cells, for its survival and progression. Despite its initial responsiveness to chemoimmunotherapy, FL is generally considered incurable. Strategies to improve immune-mediated control of FL could significantly benefit this population, particularly as it includes many elderly and comorbid patients. Immune cell engagers, especially bispecific antibodies (BsAbs), are crucial in targeting FL by bridging tumor and effector cells, thereby triggering T-cell activation and cytotoxic killing. CD3 × CD20 BsAbs have shown the most promise in clinical development for B-NHL patients, with structural variations affecting their target affinity and potency. This review summarizes the current clinical trials of BsAbs for relapsed/refractory FL, highlighting the approval of some agents, their role in first-line treatment or combination therapies, their toxicity profiles, and the future of this therapeutic approach compared to other immune cell therapies.
KEYWORDS: Bispecific antibodies, follicular lymphoma, immunotherapy, epcoritamab, glofitamab, mosunetuzumab, odronextamab
Introduction
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma (NHL) subtype and is characterized by the abnormal proliferation of mature B cells in the germinal center of lymphoid follicles1. While indolent in behavior and exquisitely responsive to initial chemoimmunotherapy, FL is considered incurable in the majority of patients.2,3
Recent research into the pathobiology of FL has revealed that these tumor cells require interactions with immune elements in the microenvironment (ME) which foster their survival and promote tumor progression.4 Previous studies have suggested that T-follicular helper cells and follicular dendritic cells may contribute to FL lymphomagenesis and that the degree or patterns of infiltration of some nonmalignant cell subsets may correlate with treatment outcomes.5,6 Additionally, in a retrospective study, Tobin et al.7 classified newly diagnosed FL samples as having high or low pre-treatment immune infiltration based on the expression of 12 immune effector, immune checkpoint, and macrophage genes. Low programmed death ligand-2 expression, indicating low immune infiltration, was more commonly found in patients with progression within 24 months of chemoimmunotherapy (POD24) suggesting a more aggressive behavior. Thus, understanding the interplay between FL cells and ME T-cell populations is critically important for designing rational and effective therapeutic strategies for this disease.8
Immune cell engagers are antibodies with multiple binding sites that bridge tumor cells and effector cells by co-targeting one or more tumor antigens and effector cell-surface molecules, thereby promoting the formation of an immunological synapse, T-cell activation, and cytotoxic killing in a major histocompatibility complex-independent fashion.9 Most bispecific antibodies (BsAb) have a full-length, IgG-like structure, and share pharmacologic properties with monoclonal antibodies. These agents are produced through various technologies, such as “knobs-into-holes”, which ensures consistent pairing of heavy chains through complementary mutations in the CH3 domain.10 Another approach uses similar technology to facilitate heterodimerization and address light-chain mispairing through domain crossover.11 Additionally, many BsAbs feature Fc region silencing mutations to prevent unwanted T-cell activation and “fratricidal” killing via antibody-dependent cellular cytotoxicity and complement fixation.12
So far, BsAbs against CD3 and CD20 (CD3 × CD20 BsAbs) have undergone the most extensive clinical development in patients with B-NHL. Structurally, these agents may possess one or more CD20-binding Fabs in different spatial configurations, conferring different target affinity and in vitro potency.13
The safety profile of BsAbs has been generally manageable and consistent across clinical trials, with most adverse events (AEs) attributable to T-cell overactivation. The frequency of AEs varies depending on the agent, route of administration, and dosing schedule. Cytokine release syndrome (CRS) is among the most frequently reported AEs, typically manifesting as chills, fever, skin rash, hypotension, hypoxia, and confusion. Neurological toxicities have been rarely observed, and included delirium, dysphasia, tremor, lethargy, impaired concentration, agitation, confusion, aphasia, depressed consciousness, encephalopathy, seizures, and cerebral edema.12
In this review, we provide an overview of the current clinical trial landscape of BsAb for patients with relapsed/refractory (R/R) FL, both as monotherapy and in combinations, and offer our perspective on the future of the field.
Relapsed/refractory FL
Single-agent trials
Mosunetuzumab, an IgG1-like CD20 × CD3 BsAb, marked a milestone as the first BsAb to receive United States Food and Drug Administration (US FDA) accelerated approval for treating adult patients with R/R FL after at least two prior lines of systemic therapy.14 Intravenous (IV) mosunetuzumab was administered in 21-day cycles with cycle 1 consisting of step-up dosing: 1 mg on cycle 1 day 1, 2 mg on cycle 1 day 8, 60 mg on cycle 1 day 15 and cycle 2 day 1, and 30 mg on day 1 of cycle 3 and onwards. Treatment was stopped after cycle 8 for patients in complete response (CR), whereas patients with a partial response (PR) or stable disease (SD) continued treatment for up to 17 cycles (Figure 1).15 In the extended follow-up from the phase 1/2 study (NCT02500407), mosunetuzumab showed a favorable safety profile with an overall response rate (ORR) of 65.7%, including a 49.3% CR rate and a median duration of the response (DOR) of 23.2 months (95% CI: 13.8–not reached) in the indolent NHL cohort.16 In the pivotal phase 2 study involving 90 patients with R/R FL, the observed ORR and CR rates were 77.8% (95% CI: 67.8–85.9) and 60% (95% CI: 49.1–70.2), respectively. The median DOR was 35.9 months (95% CI: 20.7–not reached) overall, and it was not reached among patients who achieved a CR, with a median progression-free survival (PFS) of 24 months (95% CI: 12.0–not reached). CRS was the predominant AE in 44% of the patients, but only 2% of the patients experienced grade 3–4 episodes. It occurred primarily during cycle 1, particularly on step-up day 1 (23%) or the target-dose administration on day 15 (36%). Among other frequently reported AEs, fatigue was noted in 37% of the cases, headache in 30%, and pyrexia in 28%. Neurological toxicity was rarely observed (5%) and always of grade 1–2 (Table 1). The most prevalent grade 3–4 AE was neutropenia (25% of the patients).17
Figure 1.
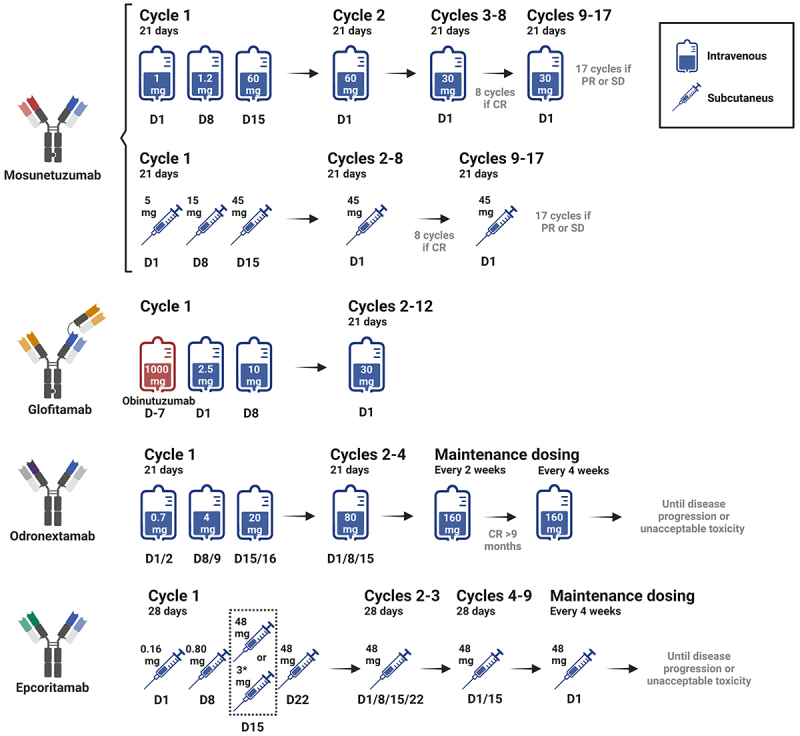
BsAbs doses and schedule administration.
Table 1.
BsAb studies in R/R FL patients.
| BsAb | N | Phase | ORR% (CR) | DOR, mo | PFS, mo | OS, mo | Follow-up, mo | CRS/NEUROTOXICITY |
|---|---|---|---|---|---|---|---|---|
| Mosunetuzumab≠ 15 | 90 | II | 77.8 (60) | 35.9 (20.7 - NR) | 24 (12–NR) | NR | 37.4 | CRS: 44% (G ≥ 3: 25) Neurotoxicity: 5% (G ≥ 3: 0) |
| Glofitamab21 | 44 | I | 70.5 (47.7) | 10.8 (3.8-NR) | 11.8 (6.3–24.2) | NA | 13.5* | CRS: 50.3% (G ≥ 3: 4.6)* Neurotoxicity : 5.3% (G ≥ 3: 0)* |
| Epcoritamab≠ 19 | 128§ | I/II | 82 (63) | NR (13.7-NR) | 15.4 (10.9-NR) | NR | 17.4 | CRS: 65% (G ≥ 3: 2) Neurotoxicity : 6% (G ≥ 3: 0) |
| 86‡ | I/II | 86 (64) | NA | NA | NA | 5.7 | CRS: 49% (G ≥ 3: 0) Neurotoxicity : 0% |
|
| Odronextamab23 | 128 | II | 80 (72) | 22.6 | 20.7 (16.7–26.5) | NR | 26.6 | CRS: 56% (G ≥ 3: 4) Neurotoxicity : 1% (G ≥ 3: 0) |
| Mosunetuzumab + Lenalidomide24 | 29 | Ib | 89.7 (65.5) | NA | NA | NA | 5.4 | CRS: 27.6% (G ≥ 3:0) Neurotoxicity : 3.4% (G ≥ 3: 0) |
| Epcoritamab + Rituximab + Lenalidomide26 | 109 | I/II | 97(86) | NA | NA | NA | 8.8 | CRS: 46% (G ≥ 3:2) Neurotoxicity : 1.8% (G ≥ 3: 0) |
≠FDA approved; *Reported for all glofitamab cohorts including 171 with B-NHL; §Pivotal cohort and ‡optimization cohorts from EPCORE NHL-1 study; ORR = overall response rate; CR = complete response; DOR = duration of response; PFS = progression-free survival; OS = overall survival; CRS = cytokine release syndrome; G = grade; NA = not available; NR = not reached.
Epcoritamab is a full-length IgG1-like CD20 × CD3 BsAb derived from a humanized mouse anti-human CD3 monoclonal antibody and obtained through controlled Fab exchange technology.18 It is administered exclusively via subcutaneous (SC) injection and recently received accelerated approval by the US FDA for the treatment of adult patients with R/R FL after two or more lines of systemic therapy. In the EPCORE NHL-1 phase 1/2 trial (NCT03625037), epcoritamab was administered in step-up doses during cycle 1 (in the pivotal cohort, 0.16 mg priming dose on day 1 and a 0.80 mg intermediate dose on day 8; in the optimization cohort, a second intermediate dose of 3 mg on day 15 and prophylactic dexamethasone in lieu of prednisone were added), followed by full doses of 48 mg in 28-day cycles until disease progression or unacceptable toxicity (Figure 1). Among 128 patients treated in the pivotal expansion cohort, the ORR and CR rates were, 82%, and 63%, respectively. The median PFS was 15.4 months, while the median DOR and overall survival (OS) were not reached. Among the observed AEs, CRS was the most common, occurring in 67% of the patients (40% G1, 25% G2, 2% G3), followed by injection-site reactions (57%), fatigue (30%), neutropenia (29%), diarrhea (27%), and pyrexia (25%). Neurotoxicity was reported in 6% of the patients, all cases being of grade 1–2. Clinically meaningful reductions in the rate and severity of CRS were observed in the optimization cohort, with CRS reported in 42 (49%) of 86 patients, exclusively of grade 1 or 2 (Table 1).19
Glofitamab is a third full-length Ig-like CD20 × CD3 BsAb, with a unique 2:1 configuration that enables bivalent binding to CD20 on B cells and monovalent binding to CD3 on T cells, improving target-effector cell binding.20 In a phase 1 trial (NCT03075696), 171 patients with R/R B-NHL were enrolled, including a cohort of 44 patients with FL. Patients received IV obinutuzumab (1000 mg) 7 days before the first dose of glofitamab, with a step-up dosing IV infusion of glofitamab in 14- or 21-day cycles (Figure 1). In the FL cohort, the ORR was 70.5%, including 47.7% achieving a CR. The median PFS of the FL cohort was 11.8 months (95% CI, 6.3 to 24.2). Among the 31 responders, the median DOR was 10.8 months (95% CI, 3.8 - NR).21 Morschhauser et al.22 explored the safety and efficacy of glofitamab either as monotherapy (N = 53 patients) or in combination with obinutuzumab (N = 19 patients), for up to 12 cycles every 21 days in patients with R/R FL, both following a pre-treatment fixed 1000 mg dose of obinutuzumab. Response rates were similar in the monotherapy and combination arms (ORR 81% and 100%, respectively; CR rate 70% and 73.7%, respectively). CRS, predominately grade 1–2, emerged again as the most common AE, affecting 66% and 79% of the patients in the monotherapy and combination cohorts, respectively. Notably, no neurotoxicity events related to glofitamab were reported. Other common AEs included infusion-related reactions and pyrexia (28%) as well as neutropenia (26% in monotherapy and 58% in the combination group).
Finally, odronextamab, a hinge-stabilized, fully human IgG4-based CD20 × CD3 BsAb, exhibited promising results in the phase 2 ELM-2 study (NCT03888105).23 Odronextamab was administered IV weekly in 21-day cycles during cycles 1–4 with step-up doses of 0.7/4/20 mg on days 1/2, 8/9, and 15/16 with a split infusion of 4 h per day during C1, followed by 80 mg in C2–4, and then continued at 160 mg every 2 weeks until disease progression or unacceptable toxicity. Patients who achieved a durable CR for ≥9 months transitioned to every-4-week dosing (Figure 1). The ORR and CR rates were 80% and 72%, respectively. The median DOR was 22.6 months, and the median duration of CR duration was 25.1 months. The median PFS was 20.7 months (95% CI: 17.2–27.5), and the median OS was not reached. The most common AEs were CRS (56%), neutropenia (39%), and pyrexia (38%). Notably, with the step-up regimen 98% of CRS events were grade 1/2, and only one low-grade neurotoxicity event was reported. Grade ≥3 infections occurred in 54 (42%) of patients, including fatal events in 14 (11%), eight of which were due to COVID-19 infections (Table 1). The findings supported the approval of odronextamab by the European Medicines Agency.
Combination trials
Lenalidomide is an immunomodulatory agent with robust activity in FL and it has been investigated as a combination partner for BsAbs, leveraging the non-overlapping toxicity profiles and potential immunologic synergism between the two agents (Table 1). In a phase 1b study (NCT04246086) involving 29 patients with R/R FL, mosunetuzumab was administered concurrently with lenalidomide at the starting dose of 20 mg orally daily on days 1 through 21 of cycles 2 through 12. This combination produced an ORR of 89.7% and CR rate of 65.5%. CRS events occurred in eight patients (27.6%), all grade 1–2. Five patients (19%) experienced grade 3–4 neutropenia, but no febrile neutropenia events were reported.24 Building upon these findings, the CELESTIMO trial (NCT04712097), an ongoing phase 3 randomized, multicenter, open-label study, is evaluating the efficacy and safety of mosunetuzumab and lenalidomide versus rituximab and lenalidomide in patients with R/R FL who have received at least one line of systemic therapy.25
Epcoritamab was tested in combination with lenalidomide and rituximab within the phase 1/2 EPCORE NHL-2 trial (NCT04663347). The BsAb was administered weekly during the first 2 or 3 cycles, then every 4 weeks for a total of up to 2 years, while rituximab and lenalidomide were given for up to 6 and 12 4-week cycles, respectively. The triplet demonstrated remarkable efficacy with a manageable and predictable safety profile. Specifically, among 109 patients treated, the ORR was 98%, with an observed CR rate of 87%. These results proved consistent across high-risk subgroups, including patients with POD24, those with primary refractory lymphoma (lacking response or experiencing relapse within 6 months of initial treatment), and those with disease refractory to both anti-CD20 antibodies and an alkylating agent.26 These encouraging data constituted the rationale for the ongoing phase 3 EPCORE FL-1 trial (NCT05409066), which compares epcoritamab plus rituximab-lenalidomide versus rituximab-lenalidomide in patients with R/R FL who have received at least one prior line of anti-lymphoma therapy with a treatment duration limited at twelve 28-day cycles.27
Moving BsAbs to the front-line setting
While the standard of care for advanced-stage high-burden FL is still represented by traditional chemoimmunotherapy, BsAbs-based therapies have the potential to challenge this paradigm.28–33 The impressive results of these chemotherapy-free regimens created the rationale for their use in patients with newly diagnosed FL with the added potential benefit of reducing long-term toxicities, including secondary malignancies (Table 2).
Table 2.
Clinical trials of BsAbs in monotherapy and/or combinations including untreated FL.
| Clinical Trial | Phase | Drugs | Comments | Mechanism of action | Primary Endpoints | Key Secondary Endpoints | Estimated Enrollment |
|---|---|---|---|---|---|---|---|
| NCT05389293 | II | Mosunetuzumab | Untreated FL grade 1-3A | CD3 x CD20 BsAb | CR rates | ORR, TEAE, PFS | 76 |
| NCT05169658 | II | Mosunetuzumab ± Polatuzumab Vedotin + Obinutuzumab | Untreated FL grade 1-3A, or MZL | CD3 x CD20 BsAb ± anti-CD79b ADC + anti-CD20 | CR rates | ORR | 42 |
| NCT05410418 | II | Mosunetuzumab + Polatuzumab Vedotin | Untreated FL grade 1-3A | CD3 x CD20 BsAb + CD79b ADC | CR rates | ORR, PFS, OS, TNTT, TEAEs, DOR, TTR | 34 |
| NCT05994235 | II | Mosunetuzumab + Tazemetostat | Untreated FL grade 1-3A | CD3 x CD20 BsAb + EZH inhibitor | CR rates | ORR, PFS, OS, DOR | 50 |
| NCT04246086 | Ib/II | Mosunetuzumab + Lenalidomide | Untreated FL grade 1-3A | CD3 x CD20 BsAb + IMID | DLT, TEAE | Pharmacokinetics, ORR, CR, DOR | 187* |
| NCT04663347 | II | Mosunetuzumab + Lenalidomide | Untreated FL grade 1-3A, or MZL | CD3 x CD20 BsAb + IMID | CR rates | PFS | 52 |
| NCT06191744 | III | Epcoritamab + Lenalidomide + Rituximab vs. Rituximab or Obinutizumab + Chemotherapy | Untreated FL grade 1-3A | CD3 X CD20 BsAb + IMID + anti-CD20 | CR30 rates | PFS, OS, MRD negativity, quality of life, best OR, EFS, DOR, DOCR, TNTT, PRO, PGIC/S | 900 |
| NCT05783609 | II | Epcoritamab + Rituximab | Untreated FL grade 1-3A | CD3 x CD20 BsAB + anti-CD20 | EOT CR | Best ORR, best PR, EOT PR, EOT ORR, DOR, PFS, TNTT, toxicities | 35 |
| NCT06112847 | II | Epcoritmab + Lenalidomide | Untreated FL grade 1-3A | CD3 x CD20 BsAB + IMID | CR rates | ORR, PFS, DOR, AEs | 27 |
| NCT06091254 | III | Odronextamab vs. Rituximab + Chemotherapy | DLT, TEAE, CR30 | Pharmacokinetics, ADA, PFS, EFS, CR30, OS, Quality of life, DOR, TEAE, TNTT, PRO, PGIC/S | 473 | ||
| NCT06097364 | III | Odronextamab + chemotherapy vs. Rituximab + chemotherapy | Untreated FL grade 1-3A | CD3 x CD20 BsAb | DLTs, TEAE, CR30 | Pharmacokinetics, ADA, best ORR, PFS, CR30, Quality of life, EFS, OS, DOR, TNTT, TEAE, PGIC/S | 733 |
*Include patients in the cohort for R/R FL; CR = complete metabolic response; ORR = overall response rate; TEAE = treatment emergent adverse effects; PFS = progression-free survival; DOR = duration of response; DLT = drug limiting toxicities; TTR = time to response; OS = overall survival; TNTT = time to next treatment; CR30 = complete response rate at 30 months; MRD = minimal residual disease; PRO= patient-related outcomes; DOCR = duration of complete response; EFS = event-free survival; PGIC = patient global impression of change/severity; EOT = end of treatment; ADA = anti-drug antibodies.
Mosunetuzumab is currently being evaluated as a single agent in a phase 2 multicenter trial (NCT05389293) in patients with newly diagnosed grade 1–3A FL requiring systemic therapy according to the Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria. The drug is administered SC at the initial dose of 5 mg on day 1, followed by the full dose of 45 mg on days 8 and 15, then 45 mg on day 1 of each 21-day cycle for up to eight cycles in patients achieving CR, and up to 17 cycles in those with PR (Figure 1). Data from the first 43 patients showed promising early results, with a best ORR of 96% and CR rate of 81% among 26 evaluable patients. Notably, these responses remained consistent across high-risk subgroups characterized by high-risk FLIPI, grade 3A FL, bulky disease, or higher SUVmax (≥13). Fifty-four percent of patients experienced CRS, almost exclusively grade 1, with only two patients developing a grade 2 episode.34 A similar approach was used in trial NCT05169658 involving patients with untreated FL or MZL. In that study, patients in SD or PR after eight cycles of mosunetuzumab could receive six cycles of polatuzumab vedotin and obinutuzumab. In a preliminary report, 27 patients completed mosunetuzumab therapy, achieving an ORR of 100% and CR rate of 71%. Among 35 patients evaluable for safety, 66% experienced grade 1 CRS, with no grade 2 or higher CRS or neurotoxicity.35
A third trial (NCT05207670) is investigating SC mosunetuzumab in patients with previously untreated, low-tumor burden FL.36 Among the first 24 efficacy evaluable patients, 23 achieved an objective response (95.8%; 95% CI: 78.9–99.9), and 20 a CR (83.3%; 95% CI: 62.6–95.3). CRS occurred in 43.3% of the patients, and neurotoxicity was reported in two cases.
The combination of SC mosunetuzumab (45 mg) and lenalidomide (20 mg) was also investigated in previously untreated FL (NCT04246086). Among 27 evaluable patients, 24 (89%) achieved an objective response and 22 (82%) a CR.37 This combination is also being evaluated in a separate phase 2 trial (NCT04792502) including FL and MZL in need of first-line systemic therapy.38 Other combinations under investigation include mosunetuzumab combined with tazemetostat (NCT05994235), mosunetuzumab plus polatuzumab vedotin (NCT05410418), and the same combination with the addition of obinutuzumab in patients with untreated indolent B-NHL (NCT05169658).
The EPCORE NHL-2 trial (NCT04663347) included two arms looking at epcoritamab in combination with either rituximab and bendamustine (arm 3) or rituximab and lenalidomide (arm 6) in previously untreated FL. Recent results from 41 patients treated in arm 6 showed a 95% ORR and 85% CR rate, with an estimated 18-month PFS at 89% and OS at 90%. The most common AEs reported were COVID-19 infection (63%), CRS (56%, all grade 1–2), and neutropenia (56%), with no neurotoxicity events reported.39 A randomized trial (NCT06191744) is underway comparing the efficacy of epcoritamab plus rituximab and lenalidomide to chemoimmunotherapy as a first-line FL treatment.
Finally, two additional randomized phase 3 studies (NCT06091254 and NCT06097364) are testing single-agent odronextamab or odronextamab plus chemotherapy against standard chemoimmunotherapy in patients with previously untreated FL.
Beyond CD20 × CD3 BsAb: novel agents
Targeting single-surface molecules with T-cell-based therapies imposes significant selective pressure on the lymphoma clonal architecture, potentially facilitating the emergence of new or preexisting antigen-negative variants.40 For example, Schuster et al.41 analyzed tumor samples from the initial phase 1/2 mosunetuzumab trial, identifying loss of CD20 expression in 34% of the biopsies collected upon disease progression attributable to acquired mutations in MS4A1, the gene encoding CD20. Other mechanisms of CD20 loss may include alternative splicing, leading to the production of translation-competent or -deficient isoforms and ultimately reducing CD20 translation,42 or other post-transcriptional modifications.
To overcome this mechanism of resistance, new BsAbs against alternative targets have been developed and are currently being tested in clinical trials (Table 3). AZD0486 binds to CD19 on the target B-cell and CD3 on the T-cell. Early results of a phase 1 trial (NCT04594642) involving 17 patients with heavily pretreated (3 L+) FL, showed an ORR and CR rate of 91% at a median follow-up of 7 months (range 1–22). Among patients with POD24 or those whose lymphoma lost the expression of CD20 after CD20 BsAb, the ORR and CR rate were 100%. All CRS reported were grade 1 (59%) or grade 2 (12%). Neurotoxicity was reported in 24% of the patients, and it was of grades 1–2 except for 1 patient with a grade 3 event. The recommended phase 2 dose of this drug is yet to be determined.43 The introduction of a step-up dosing approach led to a decrease in the incidence of grade 1–2 CRS from 62.5% to 22.2%, and grade 1–2 neurological AEs from 20% to 5.6%. No grade 3 events occurred in either category.44
Table 3.
Clinical trials involving novel agents in B-NHL.
| Clinical Trial | Phase | Drugs | Comments | Mechanism of action | Primary Endpoints | Key Secondary Endpoints | Estimated Enrollment |
|---|---|---|---|---|---|---|---|
| NCT05424822 | I | JNJ-80948543 | B-NHL and CLL | CD3 x CD20 x CD79b | DLT, AEs | Pharmacokinetics, ORR, CR, TTR, DOR | 180 |
| NCT04594642 | I | TNB-486 | R/R B-NHL | CD19 x CD3 | DLT | ORR, PFS, DOR | 116 |
| NCR04077723 | I/II | RO7227166 | R/R B-NHL | 41BBL + CD20 X CD3 | DLT, AEs, ORR, DCR, DOR, PFS, OS, CR | Pharmacokinetics, AEs, ADA, TFR, Quality of life | 498 |
| NCT05397496 | I | PIT565 | R/R- B-NHL | CD19 x CD3 x CD2 | DLT | Pharmacokinetics, ORR, CR, OS,PFS, EFS, | 140 |
| NCT05219513 | I | RO7443904 | R/R- B-NHL | CD19 x CD28 + CD20 X CD3 | DLT, AEs | Pharmacokinetics | 53 |
DLT = Drug limiting toxicities; AEs = adverse effects; ORR = overall response rate; CR = complete metabolic response; TTR = time to response; DOR = duration of response; PFS = progression-free survival; OS = overall survival; DOCR = duration of complete response; ADA = anti-drug antibodies; EFS = event-free survival; DCR = Disease control rate; TFR = time to response.
Another approach to overcome antigen loss is to target two separate tumor markers simultaneously. JNJ-80948543, a trispecific Ab targeting CD20 and CD79b on B-cells and CD3 on T-cells, is being investigated in a phase 1 study in patients with B-NHL or chronic lymphocytic leukemia (NCT05424822).45
In patients experiencing relapse while maintaining antigen expression, a notable expansion of T cells within the tumor ME was observed, albeit with signs of exhaustion, reflecting deficiencies in the effector cell compartment. Fixed-duration, intermittent BsAb therapy may mitigate T-cell exhaustion and enhance tumor control while reducing patient toxicity compared to intense or prolonged treatment schedules.46,47 IPH6501 is a tetraspecific antibody-based NK cell engager that targets both CD16a and NKp46 receptors on NK cells, as well as the IL-2 receptor via an engineered IL-2 variant (IL2v), and CD20 on B-NHL cells. This multifaceted approach enhances NK cell activation, proliferation, cytotoxicity against tumor cells, and cytokine production. Preclinical in vivo studies in non-human models have demonstrated potent activity across various B-NHL cell lines, including those with low CD20 expression, while reducing cytokine release, suggesting a potentially improved safety profile. IPH6501 is currently under investigation in a global, first-in-human phase 1/2 clinical trial (NCT06088654)48
Combination therapies involving immune checkpoint blockade or other immunomodulator agents have demonstrated the ability to reinvigorate immune responses and enhance anti-tumor activity. Additionally, combining some chemotherapy agents with immunotherapy holds promise in augmenting the efficacy of cell engagers, promoting immunogenic cell death, depleting regulatory T cells, facilitating T cell proliferation, and reducing myeloid-derived suppressor cells.9
Another approach to reinvigorating anti-tumor T cell responses involves targeting co-stimulatory receptors. Englumafusp alfa (RO7227166) is an antibody-like fusion protein simultaneously targeting CD19 on B-cells and 4-1BB on T-cells and other immune cells. Preclinical and correlative studies have shown that the combination of glofitamab and englumafusp alfa enhances anti-tumor efficacy by promoting increased infiltration of activated CD8 T cells into the tumor microenvironment.49 This combination is being evaluated in a first-in-human multicenter open-label phase 1 trial (NCT04077723). The preliminary report encompassed 24 patients with R/R indolent B-cell NHL (23 FL and 1 MZL), and the observed best ORR for FL patients was 91%, with a CR rate of 73%.50 RO7443904 (also known as RG6333), a bispecific CD19-targeted CD28 agonist (CD19-CD28), significantly enhanced T-cell effector functions in ex vivo assays and enhanced glofitamab-mediated regression of aggressive lymphomas in humanized mouse models and is currently being evaluated in a phase 1 clinical trial in combination with glofitamab in R/R B-NHL (NCT05219513).51 PIT565, a CD3 × CD2 × CD19 trispecific antibody, designed to elicit a more robust and enduring antitumor response and mitigate T-cell exhaustion is being explored in a multicenter phase 1 study with R/R CD19-positive B-cell hematologic malignancies after the failure of more than two lines of therapy.52
Topics of special interest
Managing adverse events
CD20 × CD3 BsAbs have demonstrated promising results in both frontline and relapse settings with a manageable toxicity profile. As previously mentioned, the most common AE following the administration of CD3 × CD20 BsAbs is CRS. Recently, consensus-based guidelines have been developed to assist clinicians in safely managing BsAb-related immune activation toxicities.53 Some of the strategies recommended to reduce the incidence and severity of CRS include: 1) Adequate pre-medication, including the use of prophylactic corticosteroids, which should be administered per the prescribing label for each BsAb; 2) use of a step-up dosing schedule in cycle 1, in order to more gradually activate the immune system and mitigate the risk of an early, uncontrolled inflammatory response leading to CRS or other immune-related toxicities; 3) assessment of pre-treatment patient- and disease-related risk factors, early recognition of CRS symptoms, and prompt referral to a nearby facility with expertise in the management of high-grade CRS. Other measures, such as subcutaneous administration, extended corticosteroid prophylaxis, or pre-treatment anti-CD20 antibody therapy, have been employed for further CRS risk mitigation and should implemented following each product’s package insert.
Patients receiving BsAbs should undergo close monitoring for infections due to the immunosuppressive potential of these agents. BsAbs can cause cytopenias as ‘off-tumor on-target’ effects, such as B-cell hypoplasia and T-cell exhaustion, resulting in significant immunosuppression.54,55 BsAb interruption and aggressive anti-infectious therapy are recommended in patients with active infections. Regular monitoring of immunoglobulin levels is also advised, and intravenous immunoglobulin replacement therapy should be considered for individuals with hypogammaglobulinemia and recurrent infections. Prophylaxis against Pneumocystis jirovecii pneumonia and varicella-zoster virus reactivation is universally recommended during and after BsAb therapy. Specific antiviral therapy is recommended for patients with a history of latent hepatitis B.53
Sequencing BsAbs and CD19 CAR T-cell therapy
Chimeric antigen receptor (CAR) T-cells are generated by viral transduction of autologous T-cells to express the CAR construct, which binds to a specific antigen in tumor cells and generates an antitumor immune response. Three CD19-targeted CAR T-cell products are currently approved by the US FDA for the treatment of patients with R/R FL who have received at least two prior lines of systemic therapy: axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel. CAR T-cell therapy has demonstrated promising results in patients with R/R FL, with ORR of 86–97% and CR rates ranging from 68% to 94%.56–58 CD3 × CD20 BsAbs and anti-CD19 CAR T-cell therapy were not directly compared in clinical trials and inter-trial comparisons have significant inherent limitations. BsAbs are off-the-shelf products that may be associated with a lower incidence and severity of CRS and neurotoxicity compared to CAR T-cell therapy, and thus have the potential to be administered to a broader patient population, largely in an outpatient setting.53 Optimal sequencing between CD20 × CD3 BsAb and CAR T-cell therapy is unknown. However, a small number of patients who relapsed after CAR T-cell therapy have been included in pivotal BsAb trials. Notably, in a phase 2 trial of epcoritamab, among six patients with prior CAR T-cell treatment, four responded, with three achieving a CR.19 In addition, Crochet et al.59 recently reported that prior exposure to BsAb treatment targeting different antigens does not negatively impact safety, responses, or survival outcomes after CAR T-cell therapy in patients with R/R aggressive B-cell lymphoma. While similar clinical studies have not yet been done in patients with R/R FL, it is conceivable that the efficacy of BsAb and CAR T-cell therapy are not significantly affected by the order in which these are given.60 Finally, studies evaluating the cost-effectiveness of BsAbs versus CAR T-cell therapy in treating R/R FL, have yielded mixed, if not contradictory, results.61–63
Conclusions
BsAbs have revolutionized the treatment landscape of B-NHL, including FL. The single-agent activity of these drugs is among the most promising in the field and has led to multiple regulatory approvals. BsAb-based combinations produced incremental improvements in rates and duration of responses without new or excessive toxicity and are challenging the existing standards of care. Future research will be aimed at defining the optimal role of these agents in the FL treatment algorithm, analyze and address relevant toxicities, including cytopenias, infections, and high cost, identify mechanisms of resistance and, ideally, suggest strategies to overcome them.
Schematic representation of the different administration schedules, including step-up dosing during cycle 1, for the different bispecific antibodies (BsAbs) used in monotherapy. Mosunetuzumab is available for both intravenous and subcutaneous administration. *Depicts the dose-optimization protocol for epcoritamab. Imagen created with BioRender.com
Funding Statement
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. A.R-D was supported by a grant from Fundación Española de Hematología y Hemoterapia.
Disclosure statement
L.F. has served on advisory boards for ADC Therapeutics, Seagen, Ipsen, AbbVie, and Genentech, has consulted for and received Honoraria from Genmab, Roche, AbbVie, Sanofi, Genetech, and Evolveimmune, has received research fundings from Genmab, Roche, AbbVie, Genetech, and Innate Pharma. I. L. has served on advisory boards for Janssen and received travel support from Kite/Gilead. A. R-D. has no conflict of interest to disclose.
Data availability statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.
References
- 1.Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, Brousset P, Cerroni L, de Leval L, Dirnhofer S. et al. The international consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood. 2022;140(11):1229–9. doi: 10.1182/BLOOD.2022015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivas-Delgado A, Magnano L, Moreno-Velázquez M, García O, Nadeu F, Mozas P, Dlouhy I, Baumann T, Rovira J, González-Farre B. et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2019;184(5):753–759. doi: 10.1111/bjh.15708. [DOI] [PubMed] [Google Scholar]
- 3.Batlevi CL, Sha F, Alperovich A, Ni A, Smith K, Ying Z, Soumerai JD, Caron PC, Falchi L, Hamilton A. et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020;10(7). doi: 10.1038/S41408-020-00340-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo-Ayala F, Pérez-Galán P, Campo E. Vulnerabilities in the tumor and microenvironment in follicular lymphoma. Hematol Oncol. 2021;39(Suppl S1):83–87. doi: 10.1002/HON.2855. [DOI] [PubMed] [Google Scholar]
- 5.Abe Y. Follicular lymphoma microenvironment: insights provided by single-cell analysis. J Clin Exp Hematop. 2023;63(3):143–151. doi: 10.3960/JSLRT.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurent C, Dietrich S, Tarte K. Cell cross talk within the lymphoma tumor microenvironment: follicular lymphoma as a paradigm. Blood. [2024 Mar 21]. 143(12):1080–1090. doi: 10.1182/blood.2023021000. [DOI] [PubMed] [Google Scholar]
- 7.Tobin JWD, Keane C, Gunawardana J, Mollee P, Birch S, Hoang T, Lee J, Li L, Huang L, Murigneux V. et al. Progression of disease within 24 months in follicular lymphoma is associated with reduced intratumoral immune infiltration. J Clin Oncol. 2019;37(34):3300–3309. doi: 10.1200/JCO.18.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han G, Deng Q, Marques-Piubelli ML, Dai E, Dang M, Ma MCJ, Li X, Yang H, Henderson J, Kudryashova O. et al. Follicular lymphoma microenvironment characteristics associated with tumor cell mutations and MHC class II expression. Blood Cancer Discov. 2022;3(5):428–443. doi: 10.1158/2643-3230.BCD-21-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenis A, Demaria O, Gauthier L, Vivier E, Narni-Mancinelli E. New immune cell engagers for cancer immunotherapy. Nat Rev Immunol. 2024;24(7):471–486. doi: 10.1038/S41577-023-00982-7. [DOI] [PubMed] [Google Scholar]
- 10.Atwell S, Ridgway JBB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol. 1997;270(1):26–35. doi: 10.1006/JMBI.1997.1116. [DOI] [PubMed] [Google Scholar]
- 11.Klein C, Schaefer W, Regula JT. The use of CrossMAb technology for the generation of bi- and multispecific antibodies. MAbs. 2016;8(6):1010–1020. doi: 10.1080/19420862.2016.1197457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falchi L, Vardhana SA, Salles GA. Bispecific antibodies for the treatment of B-cell lymphoma: promises, unknowns and opportunities. Blood. [2022 Nov 2]. 141(5):467–480. doi: 10.1182/BLOOD.2021011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassanello G, Luna de Abia A, Falchi L. Trial watch: bispecific antibodies for the treatment of relapsed or refractory large B-cell lymphoma. Oncoimmunology. 2024;13(1). doi: 10.1080/2162402X.2024.2321648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E. et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 2015;7(287). doi: 10.1126/SCITRANSLMED.AAA4802. [DOI] [PubMed] [Google Scholar]
- 15.Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, Kuruvilla J, Canales M, Dietrich S, Fay K. et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055–1065. doi: 10.1016/S1470-2045(22)00335-7. [DOI] [PubMed] [Google Scholar]
- 16.Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon S-S, Yoon DH, Matasar MJ, Bosch F, Kim WS, Nastoupil LJ. et al. Durable responses with mosunetuzumab in relapsed/refractory indolent and aggressive B-Cell Non-Hodgkin Lymphomas: extended follow-up of a phase I/II study. J Clin Oncol. [2024 Jul 1]. 42(19):2250–2256. doi: 10.1200/JCO.23.02329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster SJ, Sehn LH, Bartlett NL, Matasar M, Assouline S, Giri P, Kuruvilla J, Shadman M, Cheah CY, Dietrich S. et al. Mosunetuzumab monotherapy continues to demonstrate durable responses in patients with relapsed and/or refractory follicular lymphoma after ≥2 prior therapies: 3-year follow-up from a pivotal phase II study. Blood. 2023;142(Supplement 1):603–603. doi: 10.1182/BLOOD-2023-173692. [DOI] [Google Scholar]
- 18.Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, Lewis DJ, Sureda Balari A, Cunningham D, Oliveri RS. et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. The Lancet. 2021;398(10306):1157–1169. doi: 10.1016/S0140-6736(21)00889-8. [DOI] [PubMed] [Google Scholar]
- 19.Linton KM, Vitolo U, Jurczak W, Lugtenburg PJ, Gyan E, Sureda A, Christensen JH, Hess B, Tilly H, Cordoba R. et al. Epcoritamab monotherapy in patients with relapsed or refractory follicular lymphoma (EPCORE NHL-1): a phase 2 cohort of a single-arm, multicentre study. Lancet Haematol. 2024. June. 11(8):e593–e605. doi: 10.1016/S2352-3026(24)00166-2. [DOI] [PubMed] [Google Scholar]
- 20.Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, Bianchi R, Richard M, Schoenle A, Nicolini V. et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24(19):4785–4797. doi: 10.1158/1078-0432.CCR-18-0455. [DOI] [PubMed] [Google Scholar]
- 21.Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, Salles G, Martinez-Lopez J, Crump M, Thomas DN. et al. Glofitamab, a novel, bivalent CD20-targeting T-Cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-Cell lymphoma: a phase I trial. J Clin Oncol. 2021;39(18):1959–1970. doi: 10.1200/JCO.20.03175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morschhauser F, Carlo-Stella C, Dickinson M, Phillips T, Houot R, Offner F, Haioun C, Corradini P, Hutchings M, Sureda A. et al. Glofitamab as monotherapy and in combination with obinutuzumab induces high complete response rates in patients (pts) with multiple relapsed or refractory (R/R) follicular lymphoma (FL). Blood. 2021;138(Supplement 1):128–128. doi: 10.1182/BLOOD-2021-148778. [DOI] [Google Scholar]
- 23.Kim TM, Taszner M, Novelli S, Cho SG, Villasboas JC, Merli M, Jiménez-Ubieto A, Tessoulin B, Poon LM, Tucker D. et al. Safety and efficacy of odronextamab in patients with relapsed or refractory follicular lymphoma. Ann Oncol. 2024; doi: 10.1016/J.ANNONC.2024.08.2239. [DOI] [PubMed] [Google Scholar]
- 24.Morschhauser F, Bishton M, Eyre TA, Bachy E, Cartron G, Ysebaert L, Bobillo S, Gutierrez NC, Budde LE, Fox CP. et al. Mosunetuzumab in combination with lenalidomide has a manageable safety profile and encouraging activity in patients with relapsed/refractory follicular lymphoma: initial results from a phase ib study. Blood. 2021;138(Supplement 1):129. doi: 10.1182/BLOOD-2021-145694. [DOI] [Google Scholar]
- 25.Nastoupil LJ, Morschhauser F, Scholz CW, Bishton M, Yoon S-S, Giri P, Wei MC, Knapp A, Li C-C, Bottos A. et al. CELESTIMO: a phase III trial evaluating the efficacy and safety of mosunetuzumab plus lenalidomide versus rituximab plus lenalidomide in patients with relapsed or refractory follicular lymphoma who have received ≥ 1 line of systemic therapy. J Clin Oncol. 2022;40(16_suppl):TPS7588–TPS7588. doi: 10.1200/JCO.2022.40.16_SUPPL.TPS7588. [DOI] [Google Scholar]
- 26.Sureda A, Falchi L, Leppa S, Vermaat J, Holte H, Hutchings M, Lugtenburg P, de VS, Abrisqueta P, Nijland M. et al. S222: Epcoritamab with rituximab + lenalidomide (r2) provides durable responses in patients with high-risk follicular lymphoma, regardless of pod24 status. Hemasphere. 2023;7(Suppl):e5547136. doi: 10.1097/01.HS9.0000967800.55471.36. [DOI] [Google Scholar]
- 27.Falchi L, Morschhauser F, Linton K, Huang H, Galderisi F, Quadri S, Zeng L, Hoehn D, Seymour JF. EPCORE FL-1: phase 3 trial of subcutaneous epcoritamab with rituximab and lenalidomide (R 2) Vs R 2 alone in patients with relapsed or refractory follicular lymphoma. Blood. 2023;142(Supplement 1):3053. doi: 10.1182/BLOOD-2023-180092. [DOI] [Google Scholar]
- 28.Flinn IW, Van Der Jagt R, Kahl B, Wood P, Hawkins T, MacDonald D, Simpson D, Kolibaba K, Issa S, Chang J. et al. First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J Clin Oncol. 2019;37(12):984–991. doi: 10.1200/JCO.18.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus R, Imrie K, Solal-Celigny P, Catalano JV, Dmoszynska A, Raposo JC, Offner FC, Gomez-Codina J, Belch A, Cunningham D. et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 30.Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF. et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331–1344. doi: 10.1056/NEJMOA1614598. [DOI] [PubMed] [Google Scholar]
- 31.Morschhauser F, Nastoupil L, Feugier P, De Colella JMS, Tilly H, Palomba ML, Bachy E, Fruchart C, Libby EN, Casasnovas RO. et al. Six-year results from RELEVANCE: lenalidomide plus rituximab (R2) versus rituximab-chemotherapy followed by rituximab maintenance in untreated advanced follicular lymphoma. J Clin Oncol. 2022;40(28):3239–3245. doi: 10.1200/JCO.22.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rummel MJ, Niederle N, Maschmeyer G, Banat GA, Von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C. et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. The Lancet. 2013;381(9873):1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 33.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch-Becker S. et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German low-grade lymphoma study group. Blood. 2005;106(12):3725–3732. doi: 10.1182/BLOOD-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 34.Falchi L, Okwali M, Ghione P, Owens C, Hamlin PA, Lue JK, Epstein-Peterson ZD, Palomba ML, Kumar A, Torka P. et al. Subcutaneous (SC) mosunetuzumab (mosun) as first-line therapy for patients (pts) with high tumor-burden follicular lymphoma (FL): first results of a multicenter phase 2 study. Blood. 2023;142(Supplement 1):604–604. doi: 10.1182/BLOOD-2023-179906. [DOI] [Google Scholar]
- 35.Lynch R, Poh C, Shadman M, Till B, Ujjani C, Di M, Raghunathan V, Smith S, Maloney D, Gausman D. et al. A. Response-adapted treatment with mosunetuzumab with or without obinutuzumab and polatuzumab vedotin in treatment Naïve follicular and marginal zone lymphoma: interim results and phased-seq mrd analysis. Hemasphere. 2024;8(Suppl):e104. doi: 10.1002/hem3.104. [DOI] [Google Scholar]
- 36.Flinn IW, Budde LE, Tun AM, Burke JM, Anz B, Peles S, Sharman JP, Tumula PK, Biondo JML, Jani P. et al. Subcutaneous mosunetuzumab is active and has a manageable safety profile in patients with Previously untreated, low-tumor burden follicular lymphoma: initial results from the phase II morning sun study. Blood. 2023;142(Supplement 1):3029–3029. doi: 10.1182/BLOOD-2023-177917. [DOI] [Google Scholar]
- 37.Morschhauser F, Patel K, Bobillo S, Cordoba R, Eyre TA, Bishton M, Houot R, Zhang H-L, Zou L, Osborne W. et al. Preliminary findings of a phase Ib/ii trial indicate manageable safety and promising efficacy for mosunetuzumab in combination with lenalidomide (M+len) in Previously untreated (1L) follicular lymphoma (FL). Blood. 2023;142(Supplement 1):605–605. doi: 10.1182/BLOOD-2023-174432. [DOI] [Google Scholar]
- 38.Olszewski AJ, Huntington SF, Ollila T, Pelcovits A, McMahon JB, Yakirevich I, Sturtevant A, Chorzalska A, Morgan J, Dubielecka P. A phase 2 trial of mosunetuzumab with lenalidomide augmentation as first-line therapy for follicular (FL) and marginal zone lymphoma (MZL). J Clin Oncol. 2023;41(16_suppl):7524–7524. doi: 10.1200/JCO.2023.41.16_SUPPL.TPS7588. [DOI] [Google Scholar]
- 39.Falchi L, Leppä S, Wahlin BE, Nijland M, Christensen JH, De Vos S, Holte H, Linton KM, Abbas A, Wang L. et al. Subcutaneous epcoritamab with rituximab + lenalidomide (R 2) in patients (pts) with relapsed or refractory (R/R) follicular lymphoma (FL): update from phase 1/2 trial. J Clin Oncol. 2022;40(16_suppl):7524–7524. doi: 10.1200/JCO.2022.40.16_SUPPL.7524. [DOI] [Google Scholar]
- 40.Duell J, Leipold AM, Appenzeller S, Fuhr V, Rauert-Wunderlich H, Da via M, Dietrich O, Toussaint C, Imdahl F, Eisele F. et al. Sequential antigen loss and branching evolution in lymphoma after CD19- and CD20-targeted T-cell–redirecting therapy. Blood. 2024;143(8):685–696. doi: 10.1182/BLOOD.2023021672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuster SJ, Huw LY, Bolen CR, Maximov V, Polson AG, Hatzi K, Lasater EA, Assouline SE, Bartlett NL, Budde LE. et al. Loss of CD20 expression as a mechanism of resistance to mosunetuzumab in relapsed/refractory B-cell lymphomas. Blood. 2024;143(9):822–832. doi: 10.1182/BLOOD.2023022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ang Z, Paruzzo L, Hayer KE, Schmidt C, Torres Diz M, Xu F, Zankharia U, Zhang Y, Soldan S, Zheng S. et al. Alternative splicing of its 5′-UTR limits CD20 mRNA translation and enables resistance to CD20-directed immunotherapies. Blood. 2023;142(20):1724–1739. doi: 10.1182/BLOOD.2023020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair R, Jacobs R, Cho S-G, Devata S, Gaballa S, Yoon DH, Stevens DA, Kim JS, Shah NN, Brennan DM. et al. High complete response rate with TNB-486 in relapsed/refractory follicular lymphoma: interim results from an ongoing phase 1 study. J Clin Oncol. 2023;41(16_suppl):7524–7524. doi: 10.1200/JCO.2023.41.16_SUPPL.7524. [DOI] [Google Scholar]
- 44.Gaballa S, Nair R, Jacobs RW, Devata S, Cho S-G, Stevens DA, Yoon DH, Shah NN, Brennan D, Law J. et al. Double step-up dosing (2SUD) regimen mitigates severe icans and CRS while maintaining high efficacy in subjects with relapsed/refractory (R/R) B-Cell non-Hodgkin lymphoma (NHL) treated with AZD0486, a novel CD19xCD3 T-Cell engager (TCE): updated safety and efficacy data from the ongoing first-in-human (FIH) phase 1 trial. Blood. 2023;142(Supplement 1):1662. doi: 10.1182/BLOOD-2023-174668. [DOI] [Google Scholar]
- 45.Kuchnio A, Yang D, Vloemans N, Lowenstein C, Cornelissen I, Amorim R, Han C, Sukumaran S, Janssen L, Suls T. et al. Characterization of JNJ-80948543, a novel CD79bxCD20xCD3 trispecific T-Cell redirecting antibody for the treatment of B-Cell Non-Hodgkin Lymphoma. Blood. 2022;140(Supplement 1):3105–3106. doi: 10.1182/BLOOD-2022-168739. [DOI] [Google Scholar]
- 46.Toffalori C, Vago L. Bispecifics need a mindful pause. Blood. 2022;140(10):1056–1058. doi: 10.1182/BLOOD.2022017726. [DOI] [PubMed] [Google Scholar]
- 47.Philipp N, Kazerani M, Nicholls A, Vick B, Wulf J, Straub T, Scheurer M, Muth A, Hänel G, Nixdorf D. et al. T-cell exhaustion induced by continuous bispecific molecule exposure is ameliorated by treatment-free intervals. Blood. 2022;140(10):1104–1118. doi: 10.1182/BLOOD.2022015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demaria O, Vetizou M, Remark R, Chiossone L, Vagne C, Courtois R, Denis C, Maguer A, Le Floch F, Represa A. et al. Preclinical assessment of IPH6501, a first-in-class IL2v-armed tetraspecific NK cell engager directed against CD20 for R/R B-NHL, in comparison with a CD20-targeting T cell engager. J Clin Oncol. 2024;42(16_suppl):7030–7030. doi: 10.1200/JCO.2024.42.16_SUPPL.7030. [DOI] [Google Scholar]
- 49.Claus C, Ferrara C, Xu W, Sam J, Lang S, Uhlenbrock F, Albrecht R, Herter S, Schlenker R, Hösser T. et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med. 2019;11(496). doi: 10.1126/SCITRANSLMED.AAV5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutchings M, Carlo-Stella C, Gritti G, Bosch F, Morschhauser F, Townsend W, Offner F, Walter HS, Ghesquieres H, Houot R. et al. CD19 4-1BBL (RO7227166) a novel costimulatory bispecific antibody can be safely combined with the T-Cell-engaging bispecific antibody glofitamab in relapsed or refractory B-Cell Non-Hodgkin Lymphoma. Blood. 2022;140(Supplement 1):9461–9463. doi: 10.1182/BLOOD-2022-157011. [DOI] [Google Scholar]
- 51.Sam J, Hofer T, Kuettel C, Claus C, Thom J, Herter S, Georges G, Korfi K, Lechmann M, Eigenmann MJ. et al. CD19-CD28: an affinity-optimized CD28 agonist for combination with glofitamab (CD20-TCB) as off-the-shelf immunotherapy. Blood. 2024;143(21):2152–2165. doi: 10.1182/BLOOD.2023023381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu H, Oka A, Coulson M, Polli JR, Aardalen K, Ramones M, Walker DB, Carrion A, Alexander D, Klopfenstein M. et al. PIT565, a first-in-class anti-CD19, anti-CD3, anti-CD2 trispecific antibody for the treatment of B cell malignancies. Blood. 2022;140(Supplement 1):3148–3148. doi: 10.1182/BLOOD-2022-168904. [DOI] [Google Scholar]
- 53.Crombie JL, Graff T, Falchi L, Karimi YH, Bannerji R, Nastoupil LJ, Thieblemont C, Ursu R, Bartlett NL, Nachar VR. et al. Consensus recommendations on the management of toxicity associated with CD3xCD20 bispecific antibody therapy. Blood. [2024 Apr 18]. 143(16):1565–1575. doi: 10.1182/blood.2023022432. [DOI] [PubMed] [Google Scholar]
- 54.Longhitano AP, Slavin MA, Harrison SJ, Teh BW. Bispecific antibody therapy, its use and risks for infection: bridging the knowledge gap. Blood Rev. 2021;49:49. doi: 10.1016/J.BLRE.2021.100810. [DOI] [PubMed] [Google Scholar]
- 55.Radhakrishnan VS, Davies AJ. Bispecific antibodies in indolent B-cell lymphomas. Front Immunol. 2023;14:1295599. doi: 10.3389/fimmu.2023.1295599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dreyling M, Fowler NH, Dickinson M, Martínez-López J, Kolstad A, Butler J, Ghosh M, Popplewell L, Chavez JC, Bachy E. et al. Durable response after tisagenlecleucel in adults with relapsed/refractory follicular lymphoma: ELARA trial update. Blood J. [2024 Jan 9]. 143(17):1713–1725. doi: 10.1182/BLOOD.2023021567/507096/DURABLE-RESPONSE-AFTER-TISAGENLECLEUCEL-IN-ADULTS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neelapu SS, Chavez JC, Sehgal AR, Epperla N, Ulrickson M, Bachy E, Munshi PN, Casulo C, Maloney DG, de Vos S. et al. Three-year follow-up analysis of axicabtagene ciloleucel in relapsed/refractory indolent non-hodgkin lymphoma (ZUMA-5). Blood. 2024;143(6):496–506. doi: 10.1182/BLOOD.2023021243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morschhauser F, Dahiya S, Palomba ML, Martin Garcia-Sancho A, Reguera JL, Kuruvilla J, Jaeger U, Cartron G, Izutsu K, Dreyling M. et al. TRANSCEND FL: phase 2 study primary analysis of lisocabtagene maraleucel as second-line therapy in patients with high-risk relapsed or refractory follicular lymphoma. Blood. 2023;142(Supplement 1):602–602. doi: 10.1182/BLOOD-2023-179474. [DOI] [Google Scholar]
- 59.Crochet G, Iacoboni G, Couturier A, Bachy E, Iraola-Truchuelo J, Gastinne T, Cartron G, Fradon T, Lesne B, Kwon M. et al. Efficacy of CAR T-Cell therapy is not impaired by previous bispecific antibody treatment in large B-Cell lymphoma. Blood. [2024 Apr 24]. 144(3):334–338. doi: 10.1182/BLOOD.2024024526. [DOI] [PubMed] [Google Scholar]
- 60.Olszewski AJ. When B cells rebuff bispecifics. Blood. 2024;143(9):744–746. doi: 10.1182/BLOOD.2023023312. [DOI] [PubMed] [Google Scholar]
- 61.Lin M, Weiss J, Phillips TJ, Sano D, Carty SA, Ghosh M, Ahmed I, Nachar V, Hutton D, Karimi YH. Cost effectiveness of mosunetuzumab and CAR-T cell therapy in relapsed/refractory follicular lymphoma. Blood. 2023;142(Supplement 1):256–256. doi: 10.1182/BLOOD-2023-182244. [DOI] [Google Scholar]
- 62.Oluwole OO, Ray MD, Zur R, Little A, Ferrufino C, Doble B, Patel AR, Bilir P. Cost-effectiveness of axicabtagene ciloleucel versus mosunetuzumab in relapsed/refractory follicular lymphoma in the US. Blood. 2023;142(Supplement 1):5082. doi: 10.1182/BLOOD-2023-186548. [DOI] [Google Scholar]
- 63.Nastoupil LJ, Bonner A, Wang P, Almuallem L, Desai J, Fasan O, Farazi T, Kumar J, Dahiya S. Matching-adjusted indirect comparison (MAIC) of efficacy and safety of Lisocabtagene Maraleucel (liso-cel) and Mosunetuzumab for the treatment (tx) of third line or later (3L+) relapsed or refractory (R/R) follicular lymphoma (FL). Blood. 2023;142(Supplement 1):2338. doi: 10.1182/BLOOD-2023-178786. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.


