Abstract
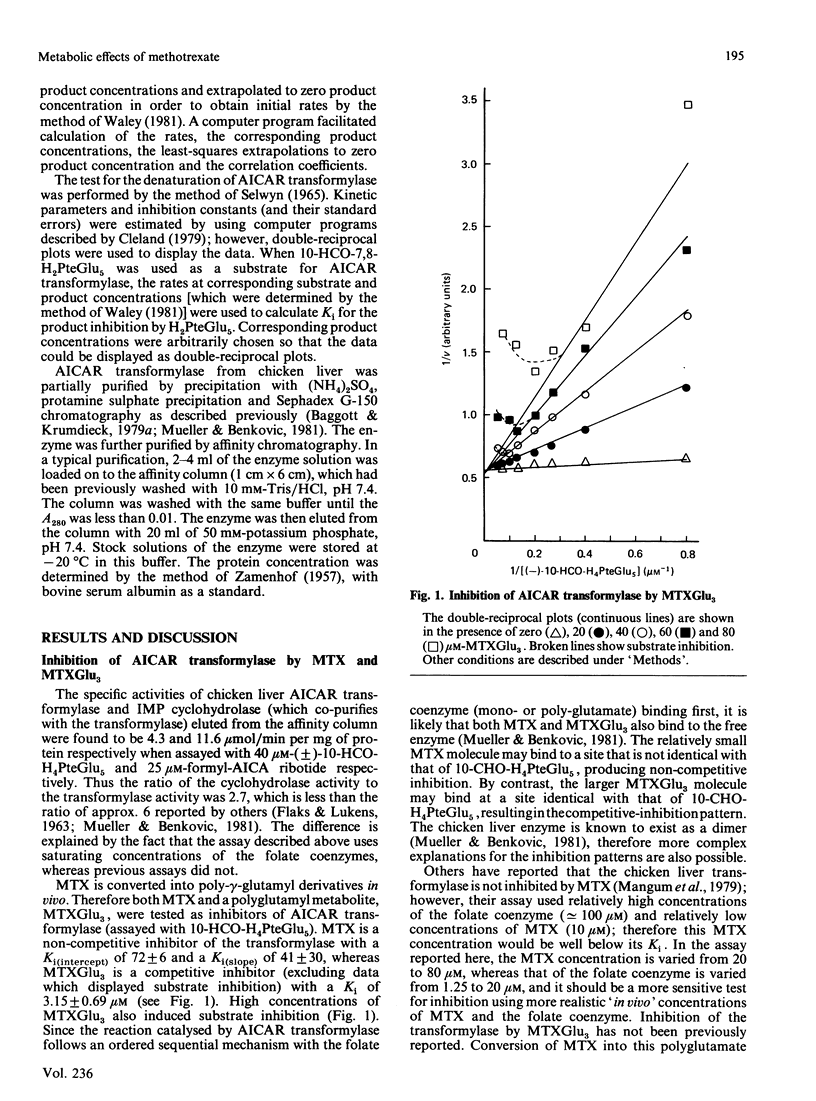
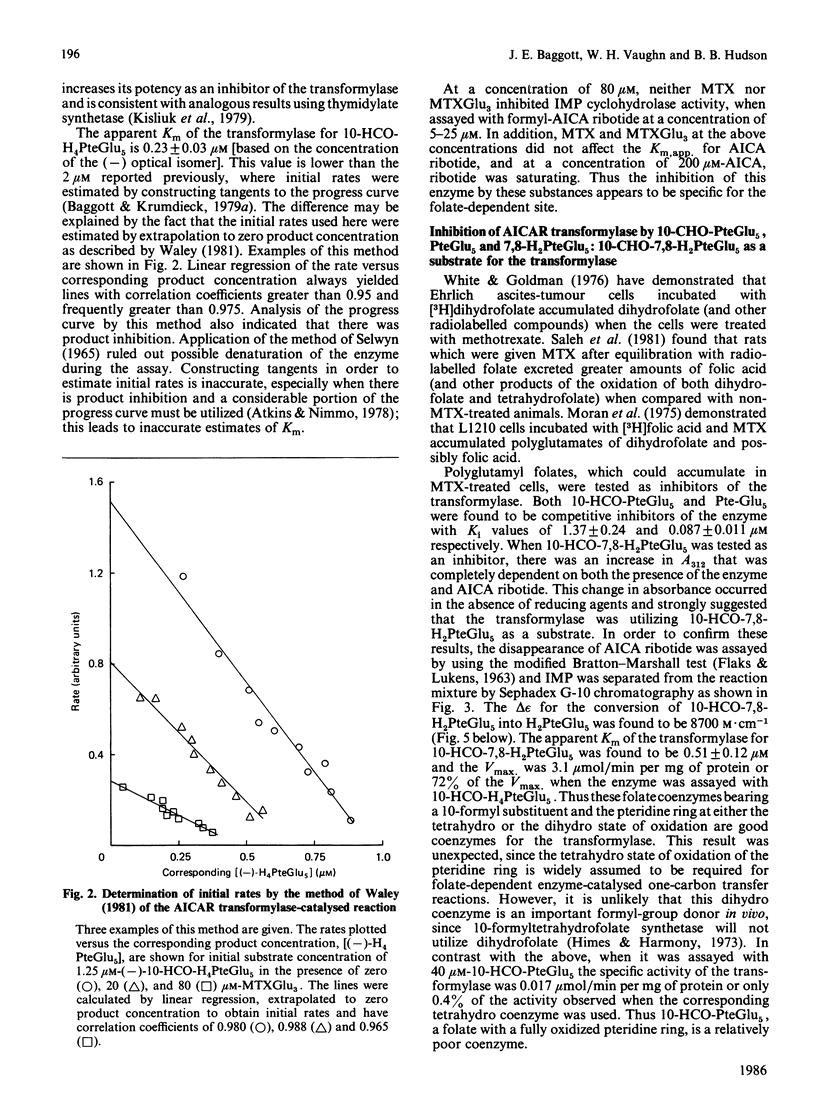
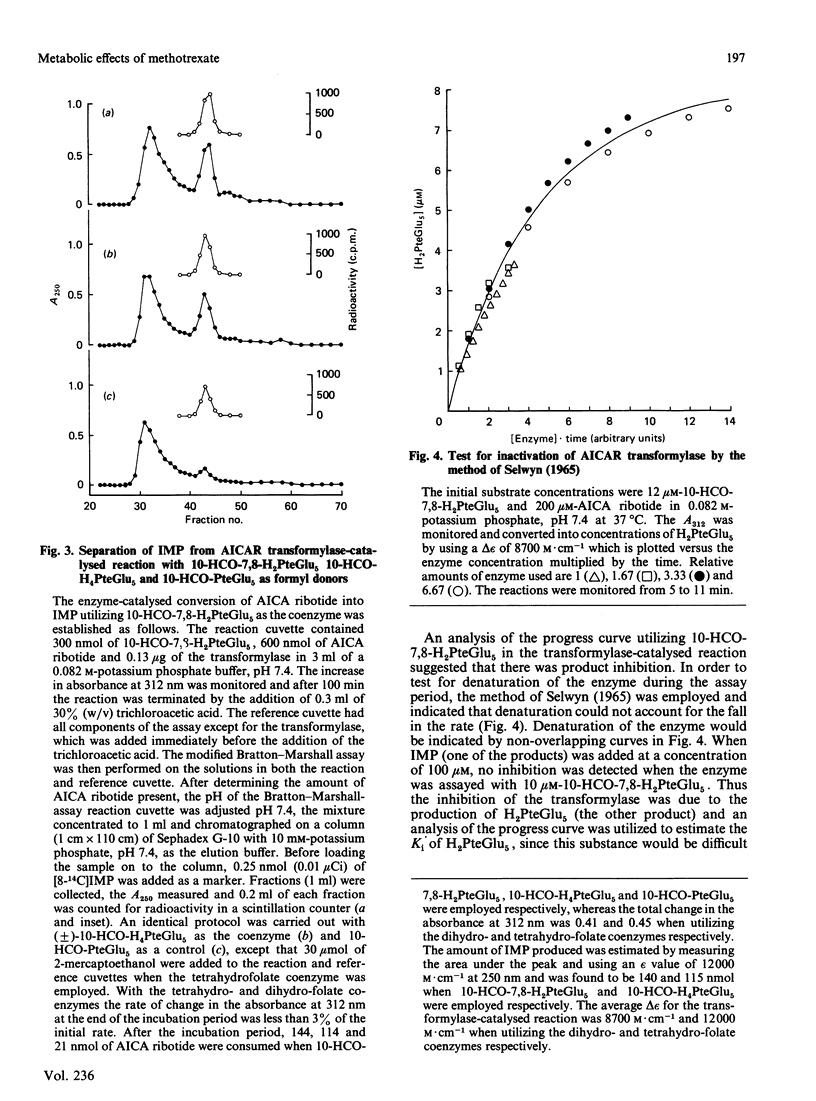
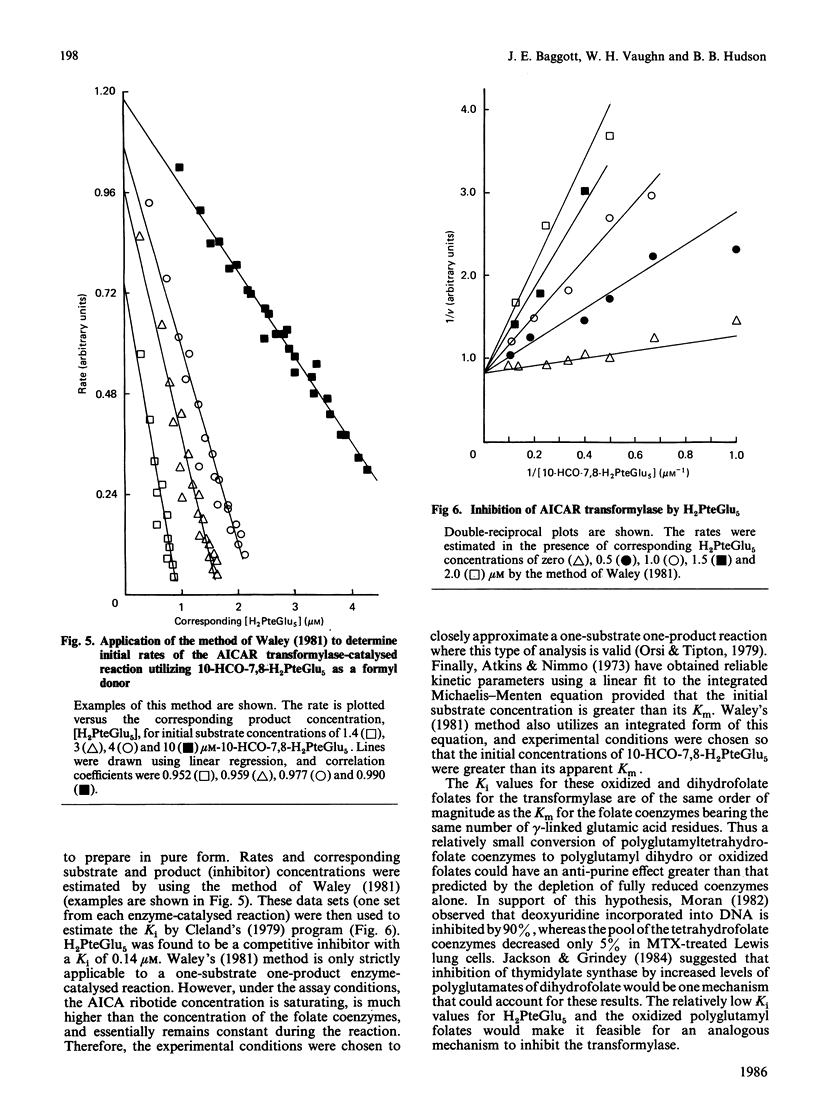
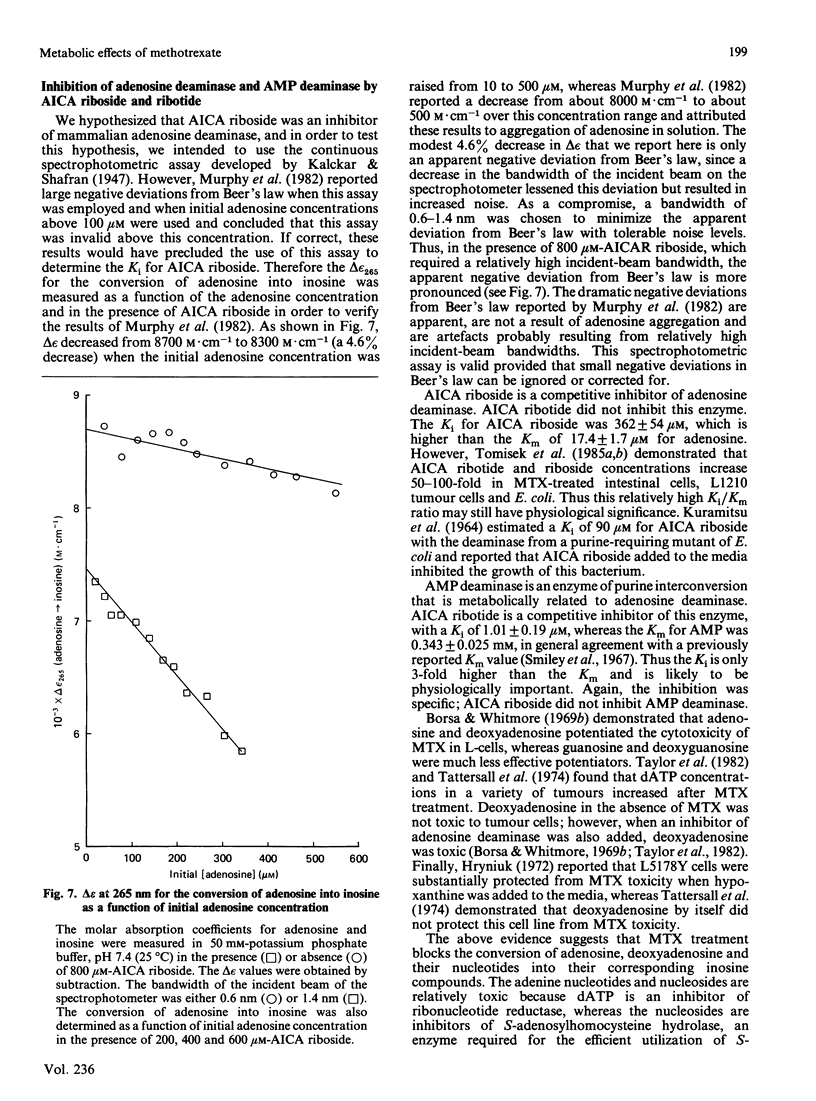
With the use of a continuous spectrophotometric assay and initial rates determined by the method of Waley [Biochem. J. (1981) 193, 1009-1012] methotrexate was found to be a non-competitive inhibitor, with Ki(intercept) = 72 microM and Ki(slope) = 41 microM, of 5-aminoimidazole-4-carboxamide ribotide transformylase, whereas a polyglutamate of methotrexate containing three gamma-linked glutamate residues was a competitive inhibitor, with Ki = 3.15 microM. Pentaglutamates of folic acid and 10-formylfolic acid were also competitive inhibitors of the transformylase, with Ki values of 0.088 and 1.37 microM respectively. Unexpectedly, the pentaglutamate of 10-formyldihydrofolic acid was a good substrate for the transformylase, with a Km of 0.51 microM and a relative Vmax. of 0.72, which compared favourably with a Km of 0.23 microM and relative Vmax. of 1.0 for the tetrahydro analogue. An analysis of the progress curve of the transformylase-catalysed reaction with the above dihydro coenzyme revealed that the pentaglutamate of dihydrofolic acid was a competitive product inhibitor, with Ki = 0.14 microM. The continuous spectrophotometric assay for adenosine deaminase based on change in the absorbance at 265 nm was shown to be valid with adenosine concentrations above 100 microM, which contradicts a previous report [Murphy, Baker, Behling & Turner (1982) Anal. Biochem. 122, 328-337] that this assay was invalid above this concentration. With the spectrophotometric assay, 5-aminoimidazole-4-carboxamide riboside was found to be a competitive inhibitor of adenosine deaminase, with (Ki = 362 microM), whereas the ribotide was a competitive inhibitor of 5'-adenylate deaminase, with Ki = 1.01 mM. Methotrexate treatment of susceptible cells results in (1) its conversion into polyglutamates, (2) the accumulation of oxidized folate polyglutamates, and (3) the accumulation of 5-aminoimidazole-4-carboxamide riboside and ribotide. The above metabolic events may be integral elements producing the cytotoxic effect of this drug by (1) producing tighter binding of methotrexate to folate-dependent enzymes, (2) producing inhibitors of folate-dependent enzymes from their tetrahydrofolate coenzymes, and (3) trapping toxic amounts of adenine nucleosides and nucleotides as a result of inhibition of adenosine deaminase and 5'-adenylate deaminase respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. L., Nimmo I. A. The determination of the initial velocities of enzyme-catalysed reactions from stopped-assay data [proceedings]. Biochem Soc Trans. 1978;6(3):545–548. doi: 10.1042/bst0060545. [DOI] [PubMed] [Google Scholar]
- Atkins G. L., Nimmo I. A. The reliability of Michaelis constants and maximum velocities estimated by using the integrated Michaelis-Menten equation. Biochem J. 1973 Dec;135(4):779–784. doi: 10.1042/bj1350779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott J. E., Krumdieck C. L. Folylpoly-gamma-glutamates as cosubstrates of 10-formyltetrahydrofolate:5'-phosphoribosyl-5-amino-4-imidazolecarboxamide formyltransferase. Biochemistry. 1979 Mar 20;18(6):1036–1041. doi: 10.1021/bi00573a016. [DOI] [PubMed] [Google Scholar]
- Baggott J. E., Krumdieck C. L. trans-alpha, beta-Diformamido-beta-(5'-phosphoribosylamino)acrylamide: a possible new intermediate in de novo purine biosynthesis. Biochemistry. 1979 Aug 7;18(16):3501–3506. doi: 10.1021/bi00583a010. [DOI] [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Cell killing studies on the mode of action of methotrexate on L-cells in vitro. Cancer Res. 1969 Apr;29(4):737–744. [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Studies relating to the mode of action of methotrexate. 3. Inhibition of thymidylate synthetase in tissue culture cells and in cell-free systems. Mol Pharmacol. 1969 Jul;5(4):318–332. [PubMed] [Google Scholar]
- Chan V. T., Baggott J. E. Polyglutamyl folate coenzymes and inhibitors of chicken liver glycinamide ribotide transformylase. Biochim Biophys Acta. 1982 Mar 18;702(1):99–104. doi: 10.1016/0167-4838(82)90031-0. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Connor M. J., Blair J. A. The identification of the folate conjugates found in rat liver 48 h after the administration of radioactively labelled folate tracers. Biochem J. 1980 Jan 15;186(1):235–242. doi: 10.1042/bj1860235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolnick B. J., Cheng Y. C. Human thymidylate synthetase. II. Derivatives of pteroylmono- and -polyglutamates as substrates and inhibitors. J Biol Chem. 1978 May 25;253(10):3563–3567. [PubMed] [Google Scholar]
- Eto I., Krumdieck C. L. Determination of three different pools of reduced one-carbon-substituted folates. 1. A study of the fundamental chemical reactions. Anal Biochem. 1980 Nov 15;109(1):167–184. doi: 10.1016/0003-2697(80)90026-3. [DOI] [PubMed] [Google Scholar]
- Galivan J. Evidence for the cytotoxic activity of polyglutamate derivatives of methotrexate. Mol Pharmacol. 1980 Jan;17(1):105–110. [PubMed] [Google Scholar]
- Himes R. H., Harmony J. A. Formyltetrahydrofolate synthetase. CRC Crit Rev Biochem. 1973 Sep;1(4):501–535. doi: 10.3109/10409237309105441. [DOI] [PubMed] [Google Scholar]
- Hryniuk W. M. Purineless death as a link between growth rate and cytotoxicity by methotrexate. Cancer Res. 1972 Jul;32(7):1506–1511. [PubMed] [Google Scholar]
- KURAMITSU H. K., UDAKA S., MOYED H. S. INDUCTION OF INOSINE 5'-PHOSPHATE DEHYDROGENASE AND XANTHOSINE 5'-PHOSPHATE AMINASE BY RIBOSYL-4-AMINO-5-IMIDAZOLECARBOXAMIDE IN PURINE-REQUIRING MUTANTS OF ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3425–3430. [PubMed] [Google Scholar]
- Kisliuk R. L., Gaumont Y., Baugh C. M. Polyglutamyl derivatives of folate as substrates and inhibitors of thymidylate synthetase. J Biol Chem. 1974 Jul 10;249(13):4100–4103. [PubMed] [Google Scholar]
- LUHBY A. L., COOPERMAN J. M. Aminoimidazolecarboxamide excretion in vitamin-B12 and folic-acid deficiencies. Lancet. 1962 Dec 29;2(7270):1381–1382. doi: 10.1016/s0140-6736(62)91051-6. [DOI] [PubMed] [Google Scholar]
- Mackenzie R. E., Baugh C. M. Tetrahydropterolypolyglutamate derivatives as substrates of two multifunctional proteins with folate-dependent enzyme activities. Biochim Biophys Acta. 1980 Jan 11;611(1):187–195. doi: 10.1016/0005-2744(80)90054-6. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Jr, Gelfand E. W. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981;50:845–877. doi: 10.1146/annurev.bi.50.070181.004213. [DOI] [PubMed] [Google Scholar]
- Mueller W. T., Benkovic S. J. On the purification and mechanism of action of 5-aminoimidazole-4-carboxamide-ribonucleotide transformylase from chicken liver. Biochemistry. 1981 Jan 20;20(2):337–344. doi: 10.1021/bi00505a017. [DOI] [PubMed] [Google Scholar]
- Murphy J., Baker D. C., Behling C., Turner R. A. A critical reexamination of the continuous spectrophotometric assay for adenosine deaminase. Anal Biochem. 1982 May 15;122(2):328–337. doi: 10.1016/0003-2697(82)90291-3. [DOI] [PubMed] [Google Scholar]
- Nair M. G., Baugh C. M. Synthesis and biological evaluation of poly-gamma-glutamyl derivatives of methotrexate. Biochemistry. 1973 Sep 25;12(20):3923–3927. doi: 10.1021/bi00744a021. [DOI] [PubMed] [Google Scholar]
- Nathans G. R., Chang D., Deuel T. F. AMP deaminase from human erythrocytes. Methods Enzymol. 1978;51:497–502. doi: 10.1016/s0076-6879(78)51068-9. [DOI] [PubMed] [Google Scholar]
- Nixon P. F., Slutsky G., Nahas A., Bertino J. R. The turnover of folate coenzymes in murine lymphoma cells. J Biol Chem. 1973 Sep 10;248(17):5932–5936. [PubMed] [Google Scholar]
- Orsi B. A., Tipton K. F. Kinetic analysis of progress curves. Methods Enzymol. 1979;63:159–183. doi: 10.1016/0076-6879(79)63010-0. [DOI] [PubMed] [Google Scholar]
- Saleh A. M., Pheasant A. E., Blair J. A. Folate catabolism in tumour-bearing rats and rats treated with methotrexate. Br J Cancer. 1981 Nov;44(5):700–708. doi: 10.1038/bjc.1981.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn M. J. A simple test for inactivation of an enzyme during assay. Biochim Biophys Acta. 1965 Jul 29;105(1):193–195. doi: 10.1016/s0926-6593(65)80190-4. [DOI] [PubMed] [Google Scholar]
- Smiley K. L., Jr, Berry A. J., Suelter C. H. An improved purification, crystallization, and some properties of rabbit muscle 5'-adenylic acid deaminase. J Biol Chem. 1967 May 25;242(10):2502–2506. [PubMed] [Google Scholar]
- Smith G. K., Benkovic P. A., Benkovic S. J. L(-)-10-Formyltetrahydrofolate is the cofactor for glycinamide ribonucleotide transformylase from chicken liver. Biochemistry. 1981 Jul 7;20(14):4034–4036. doi: 10.1021/bi00517a013. [DOI] [PubMed] [Google Scholar]
- Smith G. K., Mueller W. T., Benkovic P. A., Benkovic S. J. On the cofactor specificity of glycinamide ribonucleotide and 5-aminoimidazole-4-carboxamide ribonucleotide transformylase from chicken liver. Biochemistry. 1981 Mar 3;20(5):1241–1245. doi: 10.1021/bi00508a029. [DOI] [PubMed] [Google Scholar]
- TOMISEK A. J., KELLY H. J., REID M. R., SKIPPER H. E. Chromatographic studies of purine metabolism. II. The mechanism of E. coli inhibition by A-methopterin. Arch Biochem Biophys. 1958 Jul;76(1):45–55. doi: 10.1016/0003-9861(58)90117-6. [DOI] [PubMed] [Google Scholar]
- TOMISEK A. J., KELLY H. J., REID M. R., SKIPPER H. E. Chromatographic studies of purine metabolism. III. Effects of A-methopterin on formate-C14 utilization in mice bearing susceptible and dependent L1210 leukemia. Arch Biochem Biophys. 1958 Nov;78(1):83–94. doi: 10.1016/0003-9861(58)90316-3. [DOI] [PubMed] [Google Scholar]
- Tattersall M. H., Jackson R. C., Jackson S. T., Harrap K. R. Factors determining cell sensitivity to methotrexate: studies of folate and deoxyribonucleoside triphosphate pools in five mammalian cell lines. Eur J Cancer. 1974 Dec;10(12):819–826. doi: 10.1016/0014-2964(74)90140-6. [DOI] [PubMed] [Google Scholar]
- Taylor I. W., Slowiaczek P., Francis P. R., Tattersall M. H. Purine modulation of methotrexate cytotoxicity in mammalian cell lines. Cancer Res. 1982 Dec;42(12):5159–5164. [PubMed] [Google Scholar]
- Waley S. G. An easy method for the determination of initial rates. Biochem J. 1981 Mar 1;193(3):1009–1012. doi: 10.1042/bj1931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. C., Goldman I. D. Mechanism of action of methotrexate. IV. Free intracellular methotrexate required to suppress dihydrofolate reduction to tetrahydrofolate by Ehrlich ascites tumor cells in vitro. Mol Pharmacol. 1976 Sep;12(5):711–719. [PubMed] [Google Scholar]
- Whitfield C. D., Steers E. J., Jr, Weissbach H. Purification and properties of 5-methyltetrahydropteroyltriglutamate-homocysteine transmethylase. J Biol Chem. 1970 Jan 25;245(2):390–401. [PubMed] [Google Scholar]


