Abstract
As wound healing is an extremely complicated process, consisting of a cascade of interlocking biological events, successful wound healing requires a multifaceted approach to support appropriate and rapid transitions from the inflammatory to proliferative and remodeling phases. In this regard, here the potential use of bovine milk extracellular vesicles (EVs) to enhance wound healing is investigated. The results show that milk EVs promote fibroblast proliferation, migration, and endothelial tube formation. In particular, milk EVs derived from colostrum (Colos EVs) contain various anti‐inflammatory factors facilitating the transition from inflammation to proliferation phase, as well as factors for tissue remodeling and angiogenesis. In an excisional wound mouse model, Colos EVs promote re‐epithelialization, activate angiogenesis, and enhance extracellular matrix maturation. Interestingly, Colos EVs are further found to be quite resistant to freeze‐drying procedures, maintaining their original characteristics and efficacy for wound repair after lyophilization. These findings on the superior stability and excellent activity of milk Colos EVs indicate that they hold great promise to be developed as anti‐inflammatory therapeutics, especially for the treatment of cutaneous wounds.
Keywords: angiogenesis, anti‐inflammation, milk extracellular vesicles, tissue remodeling, wound healing
The potential use of milk extracellular vesicles (EVs) to enhance wound healing is investigated. Milk EVs, especially derived from colostrum contain a cocktail of anti‐inflammatory cytokines and factors for tissue remodeling, facilitating the inflammatory‐tissue regeneration phase transition of wound healing. Based on their superior stability and excellent activity, milk EVs hold infinite promise to be developed as anti‐inflammatory therapeutics.

1. Introduction
Wound healing is the process whereby damaged tissue is replaced by newly formed tissue. The proper transitions from inflammation to tissue regeneration (inflammation‐proliferation‐remodeling phase) for restoration of destroyed extracellular matrix (ECM) and blood vessels is particularly critical for wound healing. During the process of wound repair, macrophages act as versatile players to orchestrate all phase transitions of wound healing. Furthermore, fibroblasts and endothelial cells are the main cells that participate in ECM remodeling and angiogenesis for wound closure.[ 1 ] In addition, transcription factors and epigenetic regulators such as microRNAs (miRNAs) are known to be involved with inflammatory‐proliferation phase transition phase.[ 2 ]
Current conventional methods, such as placenta growth factor‐2 and platelet‐derived growth factor, to reduce scar formation and accelerate healing include the local application of some growth factors; however, locally injected factors can be prone to degradation by enzymes present in the body fluids and their concentration and dosage are often highly variable at the wound site.[ 3 ] Although these issues may be addressed by ECM embedding or nanoparticle/virus‐mediated delivery to prevent degradation or control release of growth factors, direct growth factor‐based therapeutics for wound healing may also carry cancer risk.[ 4 ] In addition, the wound healing process is a delicate and remarkably complex process composed of a cascade of interlocking biological events,[ 5 ] in that it requires the presence of multiple factors that can work on the wound site simultaneously in a temporally and spatially controlled manner.[ 6 ] In this regard, here we tested the wound‐repair‐promoting ability of extracellular vesicles (EVs) from bovine milk, which provide a cocktail of anti‐inflammatory cytokines and factors for ECM remodeling.
EVs are membrane‐encased vesicles that are secreted from most cell types. They are present in biological fluids, where they play key roles in intercellular communication.[ 7 ] The cargo contents of EVs depend on the source cell type, and may comprise proteins, microRNA (miRNA), mRNA, and/or lipids. Recently, a number of studies have shown that EVs can contribute to pathogenesis, tissue regeneration, diagnosis, and drug delivery.[ 8 ]
Various cell‐derived EVs have been considered for therapeutic use in clinical applications. Recently, EVs from bovine milk have garnered attention, in part because they are ingestable and thus are generally considered safe.[ 9 ] In addition, agricultural products such as milk are relatively economical and scalable sources for separating EVs.[ 10 ] With advantages such as excellent safety profile and high yield, milk‐derived EVs do not affect host immune systems.[ 11 ] For the reasons mentioned above, here we used EVs released from mammary gland epithelial cells of dairy cows (milk EVs) and explored their potential to facilitate wound healing (Figure 1A).
Figure 1.

Characterization of milk EVs and their in vitro wound healing effect. A) Schematic diagram of milk EVs‐mediated cutaneous wound healing. B) Western blot analysis of common EV markers (Tsg101, Alix, CD9) and milk‐specific proteins (MFG‐E8). C) Representative TEM image and size distribution diagram of Colos EVs. D,E) Evaluation of cell proliferation by CCK‐8 assay at 24h after EV treatment (100 µg mL−1: 5×107 particles mL−1, n = 6) in (D) NIH‐3T3 cells and (E) SVEC4‐10 cells. F) Wound scratch migration assay in NIH‐3T3 cells (100 µg mL−1: 5×107 particles mL−1). Cells were visualized with fluorescent dye (CellTracker Green CMFDA). G) Quantification bar graph of wound closure area (n = 3). H) Representative pictures of tube formation assays performed using SVEC4‐10 cells. Cells were seeded, treated with EVs, and incubated for 8 h (n = 5). I) Quantitative graphs of the total branch point numbers and tube length at 8 h (n = 5). All data are presented as mean ± SD (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs control; Dunnett's multiple comparisons test).
2. Results
2.1. Isolation and Characterization of EVs Derived from Bovine Milk
EVs derived from milk have gained attention for their intrinsic anti‐inflammatory properties.[ 12 ] In particular, colostrum is known to be full of immune, developmental, and tissue repair factors generated by the mammary gland during late pregnancy immediately before parturition.[ 13 ] In this paper, to examine the potential of bovine milk‐derived EVs to promote wound healing, we isolated and characterized two types of milk EVs, namely those derived from colostrum obtained at early stages of lactation (Colos EVs) and those derived from a commercial dairy product, low‐temperature‐pasteurized milk with low fat (mature milk EVs, Mat Milk EVs). For comparison, EVs isolated from fetal bovine serum (Serum EVs) were selected as a control because serum contains a non‐negligible amount of endogenous bovine EVs.[ 14 ] EVs were isolated using slight modifications of a previously reported ultracentrifugation method.[ 15 ]
To confirm that the EVs were properly extracted, western blot analysis was used to examine the marker proteins, Alix, TSG101, and CD9, which are involved in the biogenesis of intraluminal vesicles and EVs (Figure 1B).[ 16 ] With the exception of Alix, which is known to be rarely expressed in mature and commercial milk,[ 17 ] TSG101 and CD9 were found in all of the tested samples, including Serum EVs. The expression of milk fat globule EGF factor 8 (MFG‐E8), which is a major protein of milk EVs,[ 18 ] was clearly identified in both types of milk EVs. We also confirmed that milk and serum EVs have the expected typical spherical shape and lipid bilayer, and found that the average size of Colos EVs was ≈57.9 nm (Figure 1C; Figure S1A,B, Supporting Information). To assess the presence of contaminants of colostrum, western blot analysis was performed using GM130 (Golgi apparatus marker) antibody. GM130 was not detected in the colostrum‐derived EV samples, thus confirming the absence of contaminating vesicles arising from apoptosis (Figure S1C, Supporting Information). Notably, bovine milk represents a low‐cost and scalable source from which a high yield of EVs can be prepared.[ 17 , 19 ] Using Nanoparticle Tracking Analysis, we found that higher concentrations of EVs were present in biological fluids (serum or milk) compared to cell culture medium (CCM). In particular, the yield of EVs from colostrum was about 60 times higher than that from CCM. The total protein concentration per 1010 vesicles of Colos EVs was also calculated as ≈15 mg (Figure S1D, Supporting Information). The Colos EVs displayed efficient cellular uptake (NIH 3T3 cells) in a concentration and time‐dependent manner (Figure S1E, Supporting Information). It was also confirmed that Colos EVs were successfully internalized by observing the co‐localization between early endosomal markers and EVs in cells, and the percent of internalization of each EV was shown to be similar level (Figure S1F,G, Supporting Information).
2.2. Activation of Proliferation Phase in Wound Healing by Milk EVs
Next, we investigated the potential activity of milk EVs in the proliferation phase of wound healing. The proliferative phase begins as fibroblasts and endothelial cells migrate to damaged areas, proliferate and form blood vessels.[ 20 ] Therefore, we examined how each type of EV affected the proliferation of NIH‐3T3 fibroblasts and SVEC‐4‐10 endothelial cells (Figure 1D,E). Surprisingly, at a concentration of 100 µg mL−1 (5×107 particles mL−1), all of the tested EVs improved the proliferation of these cell types with the milk EVs increasing proliferation by about 40–50% in fibroblasts and 20–30% in endothelial cells. The proliferation of NIH‐3T3 cells was significantly and concentration‐dependently increased by milk EVs (Figure S2A, Supporting Information). We also explored whether the migration ability of fibroblasts was altered by EVs using wound scratch analysis (Figure 1F,G). As with the change in proliferation ability, a significant increase in cell migration was observed in the milk EV‐treatment groups. In particular, Colos EVs treatment caused an approximately fourfold increase in scratch closure.
To assess the effect of EVs on angiogenesis ability, we monitored the branch point number and total tube length in a tube formation assay performed using endothelial cells (Figure 1H,I). Similar to the results of the wound scratch assay, milk EVs caused an approximately 2–30% increase in tube formation compared to that seen in the control group. Overall, our in vitro results revealed that both types of bovine milk EVs stimulated the proliferation and migration of murine fibroblasts (NIH‐3T3 cells) and promoted the tube formation of endothelial cells (SVEC4‐10 cells), whereas serum EVs had no such effect. The enhanced proliferation and migration of fibroblast were also confirmed in non‐immortalized cells, Hs68, and PMEF (Figure S2C,D, Supporting Information). In addition, SVEC‐4‐10 proliferation rate of the Serum EV‐treated group was markedly lower than those of the milk EV groups in a concentration‐dependent manner. (Figure S2B, Supporting Information). We speculated that this tendency may have reflected the presence of multiple pro‐inflammatory factors in serum. We examined this possibility through further experiments, which are discussed below (Figure S2E, Supporting Information).
2.3. Identification of Key Molecules Associated with Milk EV‐Mediated Wound Healing
In order to identify the key molecules responsible for the milk EVs‐mediated promotion of wound healing, we performed proteomic analysis (Figure 2A; Table S1, Supporting Information). The data were expressed as a heatmap to compare the protein abundance associated with each type of EV. Consistent with the previous results,[ 17 ] we found that many wound healing‐related proteins were up‐regulated in Colos EVs and Mat Milk EVs compared to Serum EVs. Moreover, compared to Mat Milk EVs, Colos EVs had significantly higher levels of anti‐inflammatory factors (clusterin and CD5L protein),[ 21 ] cell proliferation and migration factors (lactadherin and IGFBP7 protein),[ 22 ] collagen and elastin deposition factors (peptidoglycan‐recognition protein and B4GALT1),[ 23 ] and re‐epithelization and angiogenesis factors (apolipoprotein E).[ 24 ] In addition, plasminogen, which plays pivotal roles in all stages of cutaneous wound healing (inflammation, proliferation, remodeling), was most highly expressed in Colos EVs compared to the other EV types.[ 25 ] Among them, clusterin, CD5L, lactadherin, and IGFBP7 proteins are deemed important for resolving inflammation and promoting proliferation, while peptidoglycan‐recognition protein, B4GALT1, and apolipiprotein E will facilitate successful transition from proliferation to remodeling phase during skin wound healing.
Figure 2.
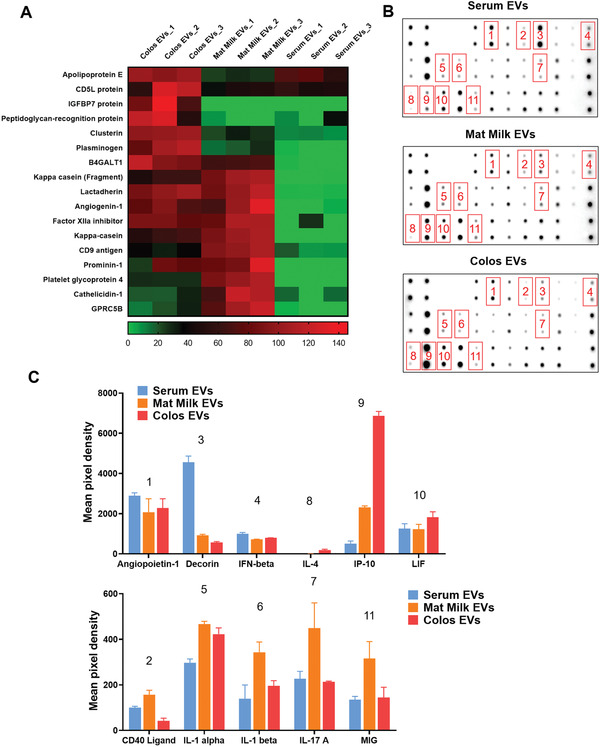
Protein and cytokine expression profiles of milk EVs. A) Heatmap data showing the results from our comparative analysis of protein expression levels among the Serum EVs, Mat Milk EVs, and Colos EVs groups. Specific proteins known to be involved in wound healing were assessed by mass spectrometry, and each value was calculated in log scale. B,C) Cytokine expression profiles of Serum EVs, Mat Milk EVs, and Colos EVs, as determined by antibody analysis. (B) Representative membrane images of bovine cytokine antibody array results. (C) Comparison of mean pixel density measurements between cytokines of each EV group (n = 3). All data are presented as mean ± SD.
We also examined the cytokine expression profile of EVs by antibody array (Figure 2B,C). Compared to the other EV types, Colos EVs exhibited increased expression levels of IL‐4, which promotes anti‐inflammation and ECM accumulation; CXCL10 (IP‐10), which is a chemokine expressed in tissue remodeling; and leukemia inhibitory factor (LIF), which is expressed in response to keratin damage.[ 26 ] Collectively, our proteomics and cytokine array results revealed that a large number of tissue remodeling and anti‐inflammatory factors were highly expressed in Colos EVs. These factors targeting the inflammatory‐tissue regeneration phase transition can improve the outcome of wound healing. To determine whether some of these cytokines are simply soluble contaminants of colostrum, we also evaluated the potential activity of EV‐free fractions from colostrum on proliferation and migration of murine fibroblasts (NIH‐3T3 cells) (Figure S2E, Supporting Information). The results showed that the EV‐free fraction of colostrum could not improve proliferation and migration of NIH‐3T3 cells, suggesting that potential cytokines involved in wound healing are derived from colostrum “EVs”.
Meanwhile, various cytokines (CD40, IL‐1, IL‐17A, and MIG) that contribute to cellular immunity by affecting cells such as macrophages and T cells were highly expressed in Mat Milk EVs (Figure 2B,C).[ 27 ] Several cytokines related to pro‐inflammation, such as angiopoietin‐1, CD40 ligand, and IFN‐β were highly expressed in Serum EVs.[ 28 ] Indeed, the expression levels of the pro‐inflammatory cytokines TNF‐α and IL‐6 were reduced in M1‐polarized macrophages treated with milk EVs, while the anti‐inflammatory cytokine, IL‐10, was increased (Figure S2F, Supporting Information). On the other hand, TNF‐α was significantly increased in M1 macrophages treated with Serum EVs. These results suggest that milk EVs may influence the repolarization of M1 macrophages in the pro‐inflammatory state to the M2 state, which is anti‐inflammatory macrophages. Also, decorin,[ 29 ] which regulates angiogenesis by inhibiting angiogenic growth factors (e.g., vascular endothelial growth factor, hepatocyte growth factor, and platelet‐derived growth factor), was expressed 9 times more in Serum EVs than in Colos EVs. The lower angiogenic activity and fibroblast proliferation rate of the Serum EV‐treated groups (Figure 1H,I, Figure S2B, Supporting Information), may reflect their expression of specific cytokines. Serum is a circulating fluid that contains all endogenous and exogenous molecules derived from whole cells.[ 30 ] It also contains large and ill‐defined amounts of protein that can cause unwanted cellular stimulation, and thus should be applied cautiously for therapeutic purposes.[ 31 ]
Wound healing is regulated by diverse molecules, cytokines, and intercellular signaling pathways. TGF‐β signaling, which is the most canonical signaling pathway in wound epithelialization, is crucially governed by its major intracellular mediators, the Smad proteins.[ 32 ] Smad7 is a major inhibitory regulator of TGF‐β signaling; in the context of cutaneous wound healing, elevated endogenous Smad7 contributes to delayed wound re‐epithelialization.[ 33 ] Also, phosphorylated Smad 2/3 plays important role in cell growth and ECM formation. TGF‐β is probably best known for acting as an upstream activator of Smad‐mediated signaling cascades, and such signals have been shown to induce ECM deposition for both non‐specific scar formation and tissue‐specific regeneration. Here we found that the expression of Smad7 was decreased in the milk EV‐treated groups compared to the control groups (Figure 3A,B). Interestingly, the significant reduction of Smad7 in the Colos EVs group was associated with increased phosphorylation of Smad2, which is a downstream protein of Smad7. Colos EV‐mediated activation of Smad pathways was found to be at a level comparable to TGF‐β stimulation (Figure 3B).
Figure 3.

Identification of key molecules associated with milk EVs‐mediated wound healing. A) Representative western blot of three (n = 3) independent experiments showing the expression levels of proteins in the Smad2/3 signaling pathway (Smad2/3, p‐Smad2, Smad7, and GAPDH). NIH‐3T3 cells were treated with PBS, EVs (100 µg mL−1), or TGF‐β (10 ng mL−1) and incubated for 24 h. B) Western blot quantification of Smad protein levels. C) Quantitative PCR analysis of the relative expression levels of miRNA‐21 in each EV group. D) Wound scratch migration assay in NIH‐3T3 cells (100 µg mL−1: 5×107 particles mL−1) treated with anti‐miRNA‐21. All data are presented as mean ± SD of three independent experiments (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs saline control, Serum EVs group; Dunnett's multiple comparisons test).
Recently, miRNA21 was shown to promote wound healing via the Smad7‐Smad2/3‐elastin pathway.[ 8 , 34 ] In this regard, the expression level of miRNA‐21 was analyzed by quantitative real‐time PCR (qRT‐PCR), and it was found that miRNA‐21 was significantly up‐regulated in Colos EVs compared to other EVs (Figure 3C). In addition, it was confirmed that the wound healing ability of Colos EVs was significantly reduced when anti‐miRNA‐21 was treated (Figure 3D). Given that miRNAs are small endogenous RNA molecules that can bind to multiple mRNAs and enable post‐transcriptional gene regulation through mRNA degradation or suppression of translation, miRNA‐21 might play a pivotal role in the proliferative phase of skin wound healing.[ 35 ]
2.4. In Vivo Therapeutic Effect of Milk EV Derived from Colostrum on Wound Repair
Prior to examining the wound healing ability of milk EVs in a mouse excisional wound healing model (Figure 4A), we measured the in vivo biodistribution of EVs to determine the optimal treatment schedule. Real‐time fluorescence image analysis revealed that Colos EVs labeled with Flamma 675 N‐hydroxysuccinimide (NHS) were retained in the skin for >3 days, with a gradual reduction seen thereafter (Figure 4B). EVs were found to be distributed to the organs of mice sacrificed the day after subcutaneous injection; skin tissues showed a notably higher level of EV accumulation compared to other organs (e.g., more than 4 times higher than in the liver). (Figure S3A,B, Supporting Information).
Figure 4.
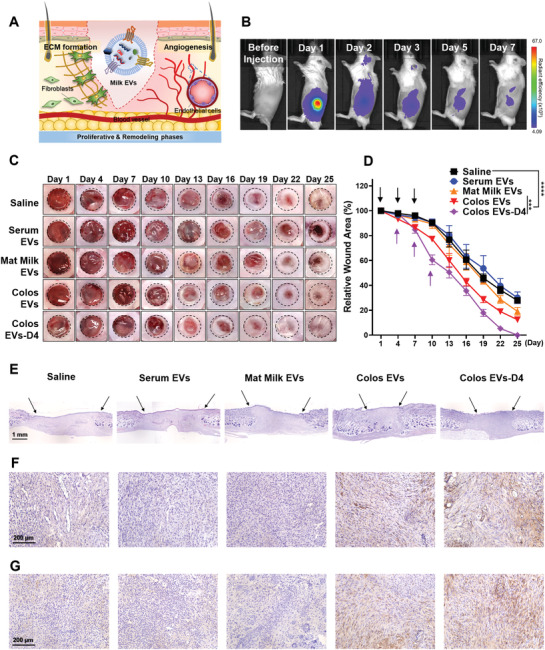
In vivo therapeutic effect of milk EVs on a cutaneous wound. A) Schematic diagram of milk EVs‐mediated ECM formation and angiogenesis during skin wound healing. B) Images of in vivo biodistribution analysis results obtained for 7 days after subcutaneous injection of Flamma 675 labeled Colos EVs (n = 3). C) Representative images of wound closure after local injection of saline, Serum EVs, Mat Milk EVs, Colos EVs, and Colos EVs‐D4. Mice were injected subcutaneously with PBS or EVs (100 µg per 100 µL PBS) three times at 3‐day intervals. D) Wound size was quantified every 3 days post‐wounding (n = 6). The purple arrows represent the day of injection (days 4, 7, and 10) of Colos EVs‐D4 group. The other groups began EVs treatment from day‐1, as indicated by the black arrows. Data are presented as the mean ± SD (***p < 0.001, ****p < 0.0001 vs saline control; Dunnett's multiple comparisons test). E) H&E staining images of vertically sectioned skin tissues from the excisional wound healing mouse model. The area between the black arrows indicates newly formed dermal tissue. F,G) Representative images of immunostaining for (F) CD31 and (G) elastin proteins in wound tissues from mice treated with saline, Serum EVs, Mat Milk EVs, Colos EVs, and Colos EVs‐D4. All skin tissues were dissected at day 13 post‐wounding and analyzed.
We then examined the wound closure rate according to EV treatment using an excisional wound mouse model (Figure 4C,D). EVs were injected subcutaneously into mice every 3 days for a total of three injections, as suggested by our in vivo biodistribution analysis. The wound healing process is generally composed of four sequential and overlapping phases: hemostasis, inflammation, proliferation, and remodeling.[ 36 ] Notably, the transition from the inflammatory to the proliferative phase begins at about day 4 and represents a key step during wound healing.[ 37 ] To examine the treatment schedule‐dependency of the wound healing rate, we thus added another group (Colos EVs‐D4) in which EV treatment began on day 4 after wounding. Compared with the control (saline‐treated) groups, the wound closure was significantly accelerated in the Colos EV‐treated groups. Surprisingly, the Colos EVs‐D4 group showed a markedly improved wound closure rate compared to the original Colos EVs group. From these results, it can be inferred that Colos EVs can support the effective transition from inflammation to proliferation.
Histological analysis performed 13 days post‐wound showed that both Colos EVs‐treated groups showed a thicker dermal layer and had enhanced reepithelization and more granulation tissue compared to the other group (Figure 4E–G, Figure S3C, Supporting Information). Similar to the in vitro data, our results indicated that the Colos EVs treatments induced elastin deposition and CD31 expression, which are indicative of increased angiogenesis (Figure S3D, Supporting Information).
In contrast to our in vitro results, Mat Milk EVs did not significantly accelerate wound closure in our in vivo model, instead yielded results similar to the controls. We speculate that this interesting result may reflect the cytokines present in Milk EVs (CD40, IL‐1, IL‐17A, and MIG), which are expected to affect cellular immunity via immune cells (Figure 2B,C). In mice treated with Serum EVs, we observed a strong inflammatory reaction and a decreased wound recovery rate, compared to the control group. Note that cells positive for the inflammatory enzymes, cyclooxygenase‐2 (COX‐2) and CD16, which would include inflammatory NK cells and macrophages, were clearly stained in skin tissues of Serum EVs‐treated model mice. As expected, it was confirmed that a large number of CD45 positive immune cells were recruited to the wound site in the Serum EVs‐treated group (Figure S3E,F, Supporting Information).
Our cytokine array results indicated that Serum EVs include high levels of pro‐inflammatory molecules (angiopoietin‐1, CD40 ligand, and IFN‐β). Serum also contains numerous growth factors that may carry some cancer risk.[ 4 , 38 ] These features could contribute to the ability of Serum EVs to lengthen the wound healing process by inhibiting the transition from the inflammatory to proliferative phases. Therefore, treatment of a wound site with a high concentration of Serum EVs is likely to delay the wound closure. In contrast, the application of Colos EVs appeared to accelerate wound healing in a concentration‐dependent manner. Indeed, a high concentration of Colos EVs (4 µg µL−1: 2×106 particles µL−1) induced healing of wound more rapidly than recombinant human epidermal growth factor (rhEGF, Nepidermin), which is commonly used as a drug to promote skin wound healing (Figure S4A,B, Supporting information).
2.5. Effect of Lyophilization on the Quality of Milk EVs for Wound Repair
Milk, which contains various bioactive factors, serves as an utter nutritional package for infants and a part of the human diet. Based on that these factors stably pass through the digestive system to properly absorb into the body,[ 39 ] the superior stability of milk EVs compared to EVs derived from CCM can be demonstrated. In this study, we investigated whether the stable molecular structure of milk EVs could be altered by freeze‐drying procedures. Lyophilization is desired to avoid the instability of EVs in suspension and improve their long‐term stability for drug delivery. To do this, Colos EVs were freeze‐dried at −80 °C to form a lyophilized powder (Figure 5A, the EV powder was dissolved in PBS (FD‐Colos EVs), and assays were performed to assess the morphology, protein content, and wound healing promotion ability of the processed EVs. Our results showed that FD‐Colos EVs still expressed the same EV markers (i.e., TSG101, Alix, and MFG‐E8) as Colos EVs (Figure 5B). FD‐Colos EVs were about 49.98 nm in size and had a spherical shape similar to that of Colos EVs, and no aggregation was observed related to the freeze‐drying process (Figure 5C). Furthermore, our in vivo experiments showed that Colos EVs before and after freeze‐drying showed similar wound healing promotion abilities and surprising wound closure rates (Figure 5D,E). On the other hand, Serum EVs changed to a two‐peak distribution after lyophilization, and showed significantly reduced wound healing ability (Figure S5A,B, Supporting Information). These results clearly demonstrate that Colos EVs have higher structural and functional stability than Serum EVs. Heat‐sensitive materials, such as EVs, vaccines, and proteins, can be easily kept in a stable storage by lyophilization and reconstituted by simply adding water. Therefore, the excellent stability of milk EVs under low temperature and vacuum conditions for dehydration and drying could be an advantage for the convenient storage and transportation of EV‐based drugs.
Figure 5.
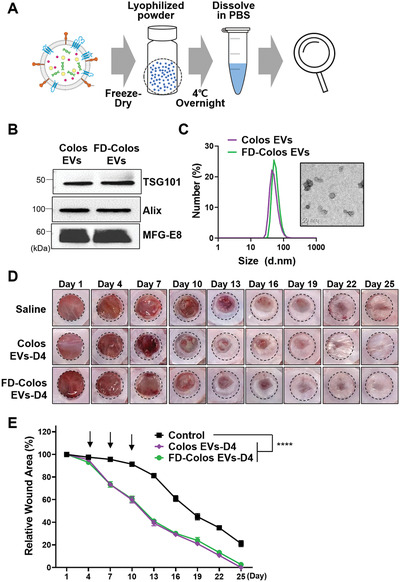
Characterization of lyophilized colostrum‐derived EVs and conservation of in vivo wound healing ability. A) Schematic diagram of the lyophilization procedure. Colostrum EVs were freeze‐dried and stored at −20 °C until use. B) Western blot analysis of EV markers (TSG101, Alix) and MFG‐E8. C) Representative TEM image and size distribution diagram of lyophilized colostrum‐derived EVs. D) Representative images of wound closure after local injection of saline, Colos EVs‐D4, and FD‐Colos EVs‐D4. E) Quantification of wound size at 3‐day intervals post‐wounding (n = 6). The black arrows represent the day of injection (days 4, 7, and 10). Data are presented as mean ± SD (****p < 0.0001 versus saline control; Dunnett's multiple comparisons test).
3. Discussion
Deregulation of any wound healing phase transition results in chronic hard‐to‐heal ulcers or excessive scarring. The current therapies used to address impaired wound healing mainly focus on infection removal, mechanical protection, and nutritional aid. Several targeted therapies have been investigated, including topical application of growth factors, but unfortunately their clinical efficacy has proven limited.[ 40 ] Recently, EVs derived from stem cells have increasingly been used as a tissue regeneration material to support wound healing or decrease scarring. Although these cells are naturally produced, and can control the inflammatory response, biosafety concerns (e.g., immunogenicity) and/or limited yield should be considered when utilizing stem cells or stem cell‐derived EVs in clinical applications. As a safe and alternative biostable material that could be used to develop a more effective treatment, milk EVs have attracted attention. The relevance of milk‐derived EVs in interspecies communication is derived from their biomacromolecule contents (miRNAs, proteins, and lipids), easy internalization by mammalian cells, non‐toxicity, and favorable properties (e.g., anti‐inflammatory, immunomodulatory, and regenerative).[ 8 , 41 ] The high stability and resilience of milk EVs are important factors making them a natural choice for therapeutic applications.
Based on that breast milk helping build a baby's immune system is considered to be a potential source of bioactive components for immune‐boosting and tissue healing, our study suggests that milk Colos EVs with the natural healing power could be one of the most efficient and successful approaches for wound healing. A successful wound healing therapy should consider the complex nature of the process, including dynamic intercellular interactions, cytokines, growth factors, and matrix components interacting in an inflammatory environment.[ 42 ] Therefore, the rationale behind the wound repair ability of milk EVs is that they can simultaneously provide a “cocktail” of anti‐inflammatory cytokines and factors for ECM remodeling. Our proteomic data provide compelling evidence that milk EV‐based therapy could be an attractive approach for promoting wound repair.
While the mechanisms underlying scar‐free wound healing are not completely understood, our data provide evidence that milk EV‐induced wound healing might be regulated by TGF‐β/Smad signaling. Although studies have shown that pasteurized commercial cow milk contains physically stable EVs that express immunomodulatory TGF‐β,[ 43 ] note that a single protein factor might have only a transient effect, and cannot counteract the vicissitudes and complexities of wound healing. On the other hand, miRNA‐21, known to regulate multiple genes related to promoting wound healing via the Smad7‐Smad2/3‐elastin pathway[ 8 , 34 ] was found to be overexpressed in Colos Exo.
Since milk EVs are very stable in the gastrointestinal tract, they have been considered as promising oral delivery systems for both endogenous biomacromolecules and loaded drugs. Indeed, previous studies have demonstrated that milk EVs can be used as efficient delivery systems for therapeutic small molecules and/or nucleic acids.[ 10 , 44 ] Despite these advantages, it should be noted that raw milk is highly heterogeneous. For example, raw bovine milk often includes probiotic lactic acid bacteria and pathogenic bacteria such as Staphylococcus aureus and Escherichia coli.[ 45 ] Although many bacteria are removed during low‐temperature pasteurization, small amounts of bacterial‐derived microvesicles, such as outer membrane vesicles, may remain in milk and could even be precipitated as pellets during ultracentrifugation. Therefore, to support the clinical use of milk EVs in the industry, specific processes will be needed to purify milk EVs. In addition, the introduction of EV engineering, which could enable tissue targeting, sustained release, and/or topical application, is expected to diversify the utility of milk EVs as a promising biomaterial for drug delivery.
4. Experimental Section
Extracellular Vesicles (EVs) Preparation and Characterization
The colostrum used in the experiments was mainly obtained from Cheong‐Sol Farm (Korea), Sa‐rang (Korea), Yeo‐won (Korea), and Ha‐yan farms (Korea). All replicates in each experiment were from independent EVs batches as above. Mature milk was provided by Sangha farm (Korea). Bovine milk EVs, such as colostrum EVs (Colos EVs) and mature milk EVs (Mat Milk EVs), were isolated by serial centrifugation and filtering steps. All centrifugation processes were performed at 4 °C. Initially, milk fat was separated into an upper layer by centrifugation at 5000 g and 12 000 g (Set to max acceleration and deceleration rate in Avanti J‐E with fixed‐angle JA‐20 rotor, Beckman Coulter), and the supernatant was stored at −80 °C. After being thawed for use, the supernatant was ultracentrifuged at 35 000 g for 1 h and at 70 000 g for 3 h to remove the remaining fat, cells, and debris. The supernatant was then size‐filtered using 0.8, 0.45, and 0.2 µm pore‐size filters. EV pellet was obtained by ultracentrifugation at 100 000 g for 1 h (Set to max acceleration and deceleration rate in Optima XE‐100 with fixed‐angle Type 70 Ti rotor, Beckman Coulter), and then washed and resuspended in phosphate‐buffered saline (PBS). Serum‐derived EVs were also collected by ultracentrifugation. Fetal bovine serum (FBS, Gibco, #F2442, USA) was filtered at 0.2 µm and ultracentrifuged at 150 000 g for 3 h. The pellet was resuspended as described above. EV size was measured by dynamic light scattering (DLS) and transmission electron microscopy (TEM) was used to confirm the size and shape of EVs.
Dynamic Light Scattering
The size distribution of EVs was examined by DLS (Zetasizer Nano; Malvern Instruments, UK). Disposable cuvettes (Kartell Labware, Italy) were used, and the measurement angle was 173° backscatter. The data were analyzed using the software provided with the instrument.
Transmission Electron Microscopy
The morphologies and actual sizes of the studied EVs were examined by TEM (Tecnai F20 G2; FEI, USA). EVs were suspended in pure distilled water or (as indicated for colostrum EVs) lyophilized for TEM analysis. EV suspension solution (10 µL of 1 µg µL−1) was placed on the EM grid (Carbon Film 200Mesh copper; Electron Microscopy Sciences, USA) and incubated for 90 s at room temperature. Uranyl acetate (UA, 2%) solution, which was used as a negative stain, was loaded onto the surface of the grid for 1 min, and then the EM grids were placed in a petri dish to dry for 10 min at room temperature.
Immunoblotting
NIH‐3T3 cells were seeded at a density of 8 × 105 cells in 60 mm culture dishes, and 18 h after cells were adhered, the medium was replaced with a serum‐free medium containing EVs (100 µg mL−1: 5×107 particles mL−1) and TGF‐β (10 ng mL−1, Sigma‐Aldrich, USA, T7039). Protein samples were prepared at 24 h time point by lysis with RIPA buffer (Thermo Fisher Scientific, USA) containing a protease/phosphatase inhibitor cocktail (Cell Signaling Technology, USA). The total proteins of EVs and cell lysates were quantified by bicinchoninic acid (BCA) protein assays, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE), and then transferred to nitrocellulose membranes with a Turbo transfer system (Bio‐Rad, USA). The membranes were blocked with 5% skim milk in 1x TBS‐T (0.1% Tween 20) for 1 h at room temperature, and then incubated overnight at 4 °C with primary antibodies. Horseradish peroxidase (HRP)‐conjugated secondary antibodies were applied for 1 h at room temperature, and the results were visualized with chemiluminescence (iBright; Invitrogen, USA). The antibodies used in this study were as follows; TSG101 (Abcam, UK, ab83, 1:1000), Alix (Novus, USA, NB100‐65678, 1:1000), CD9 (Invitrogen, USA, MA1‐19301, 1:2000), MFG‐E8 (R&D Systems, USA, AF2805, 1:1000), GM130 (Invitrogen, USA, PA1‐077, 1:500), GAPDH (R&D Systems, USA, MAB5718, 1:1000), Smad2/3 (CST, USA, #8685, 1:1000), p‐Smad2 (CST, USA, #3108, 1:1000), and Smad7 (Santa Cruz, USA, sc‐365846, 1:500).
Proteome Sample Preparation
The proteome samples were denatured with 8 m urea, the disulfide bonds were reduced using 5 mm dithiothreitol, and the samples were alkylated using 15 mm iodoacetamide. The alkylated proteins were digested with trypsin (Promega, USA) at an enzyme:protein ratio of 1:20 (w/w) for 16 h at 37 °C, then 5% formic acid was added to lower the pH and stop digestion. The samples were desalted using HLB cartridges (Waters, USA) and two‐step elution was sequentially performed using 30% and 80% ACN. The peptides were completely dried by a vacuum concentrator (SAVANT SPD 2010, Thermo Scientific, USA), and the dried peptides were reconstituted in 100 µL of 4% ACN and used for LC‐MS.
Proteomic Analysis by Nano LC‐MS
Proteomic analysis was performed using an Ultimate 3000 nano HPLC system (Thermo Scientific, USA) coupled with a Q‐Exactive Orbitrap mass spectrometer (Thermo Scientific, USA). Mobile phase A consisted of 0.1% formic acid in water and mobile phase B consisted of ACN. Each sample (4 µL) was separated on an EASY‐spray column (75 µm × 2 cm C18, 100 Å, Thermo Scientific, USA) using gradient elution from 4% to 95% solvent B at a flow rate of 350 mL min−1 over 35 min. Mass spectra were collected using the data‐dependent MS2 (ddMS2) mode, and the full‐scan mode was used to quantify proteins. The data acquisition parameters were: resolution, 17 500; isolation window, 4.0 m/z; and maximum injection time, 50 ms. The full‐scan MS settings were: resolution, 70 000; scan range, 400–1500 m/z; and maximum injection time, 100 ms.
Proteomic Data Processing
Proteins and their peptides were identified using the SEQUEST search algorithm of the Proteome Discoverer 2.3 software (Thermo Scientific, USA). The target false detection rate (FDR) was set at 0.01 for the identification of peptides. The mass tolerances used for precursor and fragment ions were 10 ppm and 0.02 Da, respectively. The m/z values of peptide ions and the retention times were confirmed using Proteome Discoverer, and each protein was quantified by comparison to the representative peptide peak area from the full‐scan data files, using the Xcalibur software.
Cell Culture
NIH‐3T3 (murine fibroblast cell line; ATCC, USA), SVEC4‐10 (murine endothelial cell line; ATCC, USA), HS68 (human fibroblast cell line; ATCC, USA), and PMEF (primary mouse embryonic fibroblast cells) cells were grown in Dulbecco's modified Eagle's medium (DMEM; Hyclone, USA) supplemented with 10% FBS (Gibco, USA) and 1% antibiotics‐antimycotics (Gibco, USA). The cells were maintained in a 37 °C incubator with 5% CO2 in air. The cells (particularly the SVEC4‐10 and PMEF cells) were used only up to passage 7.
EV Labeling and Cellular Uptake Analysis of EVs
A fluorescent dye (Flamma 675 NHS ester; BioActs, Korea) was used for lipid‐membrane‐specific labeling. EVs were incubated overnight at 4 °C with the staining solution at a final concentration of 65 µm and washed twice to remove unattached dyes, using an airfuge (Beckman Coulter, USA). The EV pellet was resuspended in PBS buffer and the suspension was used for examination of the cellular uptake of EVs. NIH‐3T3 cells were seeded in a 35‐mm confocal dish, and the labeled colostrum‐derived EVs were applied for 24 h at 37 °C. Staining with 4′,6‐diamidino‐2‐phenylindole (DAPI) was performed to visualize cell nuclei. The samples were observed with a confocal microscope (Leica TCS SP5; Leica, Germany) at 0, 1, 4, 16, and 24 h. EEA1 (Abcam, UK, ab206860, 1:200) was used for staining of early endosomal marker. The percent of internalization of EVs was quantified by counting co‐localization between early endosomal markers and EVs in cells in three randomly taken confocal images.
In Vitro Cytotoxicity Study
To measure the toxicity of the purified EVs, the CCK‐8 assay (Dojindo Laboratory, Japan) was performed. NIH‐3T3, SVEC4‐10, HS68, and PMEF cells were seeded in a 96‐well plate at a density of 6 × 103 cells per well and incubated overnight at 37 °C to allow the cells to stabilize and adhere on the plate. EVs or EV‐free milk were applied at a concentration of 100 µg mL−1 in serum‐free media, and the plate was placed in a CO2 incubator for 24 h. Then, CCK‐8 solution (20 µL, one‐tenth of total media volume) was added to each well, and cell viability was evaluated with a microplate reader (SpectraMax 34; Molecular Devices, USA).
Wound Scratch Migration Assay
NIH‐3T3 cells (3 × 105 cells) were plated in a 35‐mm confocal dish and incubated overnight at 37 °C to enable the formation of a compacted monolayer. The cells were stained with CellTracker Green CMFDA (5‐chloromethylfluorescein diacetate) dye (Invitrogen, USA) in serum‐free medium for 20 min at 37 °C, according to the manufacturer's protocol. The cell layer was scratched with a scratcher (SPLScar; SPL Life Sciences, Korea) and carefully washed with a culture medium. Then, EVs were added to each dish with serum‐free media and maintained for 24 h. Fluorescent images were obtained by a confocal microscope (Leica TCS SP5; Leica, Germany) at 0 and 24 h. NIH‐3T3, HS68, and PMEF cells were seeded in 6‐well plates at a density of 2 × 105 cells per one well and cultured overnight in a 37 °C incubator to stabilize the cells. Each cell monolayer was scraped in a straight line and washed once with PBS buffer. EVs or EV‐free milk were treated at a concentration of 100 µg mL−1 (5×107 particles mL−1) in each well and incubated for 24 h. Anti‐miR21 (5′‐ACGGCAACACCAGUCGAUGGGCUGU‐3′) and Colos EVs (100 µg mL−1: 5×107 particles mL−1) were simultaneously treated to NIH 3T3 cells by using Lipofectamine 3000 (Invitrogen, USA). As following the manufacturer's guide, anti‐miR21 were added at a concentration of 50 pmole per each well. All images were taken with a CK40 culture microscope (Olympus, Japan) and occluded wound areas were measured with Image J software (NIH).
Tube Formation Assay
Matrigel (50 µL, Corning matrigel matrix; Corning, USA) was added to each well of a 96‐well plate and allowed to polymerize for 30 min at 37 °C. SVEC4‐10 cells were seeded to the solid matrigel matrix at a density of 2 × 104 cells per well, and incubated with EVs for 8 h. The degree of tube formation was captured with a CK40 culture microscope (Olympus, Japan) and assessed by measuring the tube length and number of branch points via the ImageJ software (NIH).
Cytokine Antibody Array
A Bovine Cytokine Array C1 (Raybiotech, USA) was used to profile the protein expression of EVs, as described by the manufacturer. The immunoblotting results were detected with chemiluminescence (iBright; Invitrogen, USA), and the intensity of each spot was quantified with the Image J software (NIH)
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT‐PCR)
To measure the expression level of microRNA21 (miR21), qRT‐PCR was performed. Total RNA was obtained from 2 mg of each EVs via an RNA purification kit (RNeasy Plus mini kit, Qiagen, Germany), and 4 ng of total RNA was used for reverse transcription (RT) to synthesize cDNA. The RT step was carried out with TaqMan MicroRNA RT Kit (Thermo Fisher Scientific, USA) according to the manufacturer's protocol. Primers for bovine miR21 were included in a kit (TaqMan MicroRNA Assay, bta‐miR‐21, Thermo Fisher Scientific, USA) and SYBR green (Enzynomics, Korea) was used as a DNA binding dye for qRT‐PCR. The cycling mode was set according to the SYBR green protocol. For inflammatory cytokines of macrophages, Raw 264.7 macrophages were stimulated with LPS (10 ng mL−1) and IFN‐γ (50 ng mL−1) for 24 h, and the expression data were normalized to GAPDH levels and assessed using ΔΔCt method.
In Vivo Biodistribution Study of EVs
All experiments with live animals were performed with the permission of the institutional committee, in compliance with the relevant laws and institutional guidelines of the Korea Institute of Science and Technology (KIST) (approval number KIST‐2020‐043). To determine the optimal treatment schedule, the biodistribution study was performed using two groups: one group for checking the duration in the skin of total Colos EVs and another group for confirming the tissue distribution of EVs 48 h after injection. EVs were labeled as described above and subcutaneously injected into the middle of the back skin of BALB/c mice (100 µg per 100 µL PBS). Blood from the injected mice was withdrawn at 0.25, 0.5, 1, 3, 6, 12, 24, and 48 h of post‐administration with heparin tubes. The blood samples were plated to a 96‐well black plate and the fluorescence of EVs was quantified by an in vivo imaging system (IVIS) (Lumina Series III; PerkinElmer, USA). At 48 h after injection, another group of mice was sacrificed and their skin tissues and major visceral organs (liver, spleen, lung, kidney, and heart) were collected and lysed using radio‐immunoprecipitation assay buffer. The fluorescence intensities of skin and tissue lysates were measured using the IVIS Lumina Series III and quantified as the EV amounts.
In Vivo Skin Excisional Wound Healing Mouse Model and EV treatment
BALB/c mice (male, 7 weeks) were provided by Orient Bio (Korea). Mice were anesthetized by intra‐peritoneal injection of Zoletil50 and Rompun, and the dorsal surface was shaved with an electric clipper and cleaned neatly with 70% ethanol. To create the wound pattern, a sterile biopsy punch tool (8‐mm size) was pushed into the middle of the back skin. A donut‐shaped silicon splint was attached around the wound. PBS or EVs were applied three times at 3‐day intervals (Day1, 4, 7 or Day 4, 7, 10), and the trend of wound closure was observed every 3 days for 25 days. The wound closure area was calculated with the ImageJ software (NIH) and plotted as a percentage.
H&E Staining
The dorsal skin around the wound was dissected at day 25 post‐wounding and fixed in 4% paraformaldehyde solution. The dehydrated tissues were embedded in melted paraffin and sliced at 6‐µm using a microtome (Accu‐cut SRM200 Rotary Microtome; Sakura Finetek, Japan). Tissue sections were stained with an H&E staining kit (Abcam, UK) according to the manufacturer's protocol. The nuclei were stained with hematoxylin for 1 min, and the sections were rinsed in tap water for at least 5 min. After a brief (15‐sec) incubation in bluing reagent, eosin was applied for 10 s to stain cytoplasm. The sections were thoroughly washed with tap water, dehydrated, and mounted with toluene‐free medium.
DAB (3,3'‐Diaminobenzidine) Staining
Immunohistochemistry (IHC) was performed using a commercially available kit according to the instructions provided by the manufacturer (Abcam, UK). The skin tissue around the wound was excised and fixed on day 13 post‐wound. Tissues were dehydrated, embedded in paraffin, and paraffin blocks were horizontally sectioned to a thickness of 6 µm using a microtome. (Accu‐cut SRM200 Rotary Microtome; Sakura Finetek, Japan) Tissue sections were made from the middle part of the skin tissue, and staining was performed on the middle part of the wound. Briefly, the skin‐tissue sections were deparaffinized and rehydrated, and antigen retrieval was carried out with 1x Citrate buffer (pH 6, Abcam, UK) in accordance with the manufacturer's protocol. Next, tissues were blocked and primary antibodies were applied overnight at 4 °C. Subsequently, biotinylated secondary antibodies and streptavidin peroxidase were applied, followed by DAB staining. Counterstaining was performed with hematoxylin staining (Abcam, UK). The antibodies used in this study were as follows: CD31/PECAM‐1 (NOVUS, USA, NB100‐2284) and Elastin (Santa Cruz, USA, sc‐58756).
Immunofluorescence Staining
The incised skin tissues were fixed, embedded in an optimal cutting temperature compound (Thermo Fisher Scientific, USA), and sectioned with a freezing microtome (LEICA CM‐1900; Leica, Germany). The sections were incubated with 3% BSA containing 0.5% Triton X‐100 for 1 h at room temperature, and then with primary antibodies (CD16 and COX‐2) at 4 °C overnight. Other skin tissues embedded in paraffin blocks were sliced with a microtome (Accu‐cut SRM200 Rotary Microtome; Sakura Finetek, Japan), then deparaffinized and rehydrated. Antigen retrieval steps were performed with 1x citrate solution (Abcam, UK). Sections were blocked with 1% BSA in PBS‐T (0.05% tween 20) for 30 min and incubated overnight at 4 °C with primary antibodies (F4/80 and CD45). Alexa Fluor 488‐conjugated secondary antibodies were applied for CD16, COX‐2, and F4/80 antibodies, and the results were observed under a confocal microscope (Leica TCS SP5; Leica, Germany). The antibodies used in this study were as follows: CD16/CD32 (BD Pharmingen, USA, 553 142), COX‐2 (Abcam, UK, ab15191), F4/80 (Biolegend, USA, 123 149), CD45 (Biolegend, USA, 130 132), Alexa Fluor 488 anti‐ rabbit IgG (Invitrogen, USA, A11008, UK).
Lyophilization of Milk EVs
Colostrum and Serum EVs were prepared in PBS buffer at a concentration of 20 µg mL−1 (1×107 particles mL−1). After putting 1 mg of EVs into a 1.5 mL tube with a buffer solution, the tube was completely frozen in liquid nitrogen, and then dehydrated in a freeze dryer (Ilshin Biobase, Korea) with the lid open for 2 days at −85 °C and 58 mTorr or less.
Statistical Analysis
All data were indicated as the mean ± SD. All groups were statistically compared with ordinary one‐way or two‐way ANOVA followed by Tukey's multiple comparison post‐hoc test. A p‐value less than 0.05 was considered statistically significant. Statistical analysis was carried out using Graphpad Prism software.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Supporting Table
Acknowledgements
H.K. and D.E.K. contributed equally to this work. This work was supported by the Mid‐career Researcher Program (NRF‐2019R1A2C2010408), Brain Pool Program (NRF‐2020H1D3A1A02081401), and Intramural Research Program of Korea Institute of Science and Technology (KIST).
Kim H., Kim D. E., Han G., Lim N. R., Kim E. H., Jang Y., Cho H., Jang H., Kim K. H., Kim S. H., Yang Y., Harnessing the Natural Healing Power of Colostrum: Bovine Milk‐Derived Extracellular Vesicles from Colostrum Facilitating the Transition from Inflammation to Tissue Regeneration for Accelerating Cutaneous Wound Healing. Adv. Healthcare Mater. 2022, 11, 2102027. 10.1002/adhm.202102027
Contributor Information
Sun Hwa Kim, Email: sunkim@kist.re.kr.
Yoosoo Yang, Email: ysyang@kist.re.kr.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.a) Krzyszczyk P., Schloss R., Palmer A., Berthiaume F., Front. Physiol. 2018, 9, 419; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Olczyk P., Mencner L., Komosinska‐Vassev K., Biomed Res. Int. 2014, 2014, 747584; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tonnesen M. G., Feng X., Clark R. A., J. Invest. Dermatol. Symp. Proc. 2000, 5, 40. [DOI] [PubMed] [Google Scholar]
- 2. Landén N. X., Li D., Ståhle M., Cell. Mol. Life Sci. 2016, 73, 3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu L., Wang J., Zhou X., Xiong Z., Zhao J., Yu R., Huang F., Zhang H., Chen L., Sci. Rep. 2016, 6, 32993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Briquez P. S., Hubbell J. A., Martino M. M., Adv. Wound Care 2015, 4, 479; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Das S., Baker A. B., Front. Bioeng. Biotechnol. 2016, 4, 82; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Demidova‐Rice T. N., Hamblin M. R., Herman I. M., Adv. Skin Wound Care 2012, 25, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Velnar T., Bailey T., Smrkolj V., J. Int. Med. Res. 2009, 37, 1528. [DOI] [PubMed] [Google Scholar]
- 6.a) Barrientos S., Stojadinovic O., Golinko M. S., Brem H., Tomic‐Canic M., Wound Repair Regen. 2008, 16, 585; [DOI] [PubMed] [Google Scholar]; b) Werner S., Grose R., Physiol. Rev. 2003, 83, 835. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y., Liu Y., Liu H., Tang W. H., Cell Biosci. 2019, 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Yang Z., Shi J., Xie J., Wang Y., Sun J., Liu T., Zhao Y., Zhao X., Wang X., Ma Y., Malkoc V., Chiang C., Deng W., Chen Y., Fu Y., Kwak K. J., Fan Y., Kang C., Yin C., Rhee J., Bertani P., Otero J., Lu W., Yun K., Lee A. S., Jiang W., Teng L., Kim B. Y. S., Lee L. J., Nat. Biomed. Eng. 2020, 4, 69; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Manca S., Upadhyaya B., Mutai E., Desaulniers A. T., Cederberg R. A., White B. R., Zempleni J., Sci. Rep. 2018, 8, 11321; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Haney M. J., Klyachko N. L., Zhao Y., Gupta R., Plotnikova E. G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A. V., Batrakova E. V., J. Controlled Release 2015, 207, 18; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Huang C. C., Narayanan R., Alapati S., Ravindran S., Biomaterials 2016, 111, 103; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Masyuk A. I., Masyuk T. V., Larusso N. F., J. Hepatol. 2013, 59, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benmoussa A., Diallo I., Salem M., Michel S., Gilbert C., Sevigny J., Provost P., Sci. Rep. 2019, 9, 14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Sanwlani R., Fonseka P., Chitti S. V., Mathivanan S., Proteomes 2020, 10.3390/proteomes8020011; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Adriano B., Cotto N. M., Chauhan N., Jaggi M., Chauhan S. C., Yallapu M. M., Bioact. Mater. 2021, 6, 2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luan X., Sansanaphongpricha K., Myers I., Chen H. W., Yuan H. B., Sun D. X., Acta Pharmacol. Sin. 2017, 38, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Buzas E. I., Gyorgy B., Nagy G., Falus A., Gay S., Nat. Rev. Rheumatol. 2014, 10, 356; [DOI] [PubMed] [Google Scholar]; b) Robbins P. D., Dorronsoro A., Booker C. N., J. Clin. Invest. 2016, 126, 1173; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tang T. T., Wang B., Lv L. L., Liu B. C., Theranostics 2020, 10, 8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rathe M., Muller K., Sangild P. T., Husby S., Nutr. Rev. 2014, 72, 237. [DOI] [PubMed] [Google Scholar]
- 14. Lässer C., Alikhani V. S., Ekström K., Eldh M., Paredes P. T., Bossios A., Sjöstrand M., Gabrielsson S., Lötvall J., Valadi H., J. Transl. Med. 2011, 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Benmoussa A., Ly S., Shan S. T., Laugier J., Boilard E., Gilbert C., Provost P., J. Extracell. Vesicles 2017, 6, 1401897; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yamauchi M., Shimizu K., Rahman M., Ishikawa H., Takase H., Ugawa S., Okada A., Inoshima Y., Drug Dev. Ind. Pharm. 2019, 45, 359. [DOI] [PubMed] [Google Scholar]
- 16.a) van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P., Marks M. S., Rubinstein E., Raposo G., Dev. Cell 2011, 21, 708; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zoller M., Nat. Rev. Cancer 2009, 9, 40. [DOI] [PubMed] [Google Scholar]
- 17. Samuel M., Chisanga D., Liem M., Keerthikumar S., Anand S., Ang C. S., Adda C. G., Versteegen E., Jois M., Mathivanan S., Sci. Rep. 2017, 7, 5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Veron P., Segura E., Sugano G., Amigorena S., Thery C., Blood Cells, Mol., Dis. 2005, 35, 81. [DOI] [PubMed] [Google Scholar]
- 19. Vaswani K., Koh Y. Q., Almughlliq F. B., Peiris H. N., Mitchell M. D., Reprod. Biol. 2017, 17, 341. [DOI] [PubMed] [Google Scholar]
- 20. Rodrigues M., Kosaric N., Bonham C. A., Gurtner G. C., Physiol. Rev. 2019, 99, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Sanjurjo L., Aran G., Tellez E., Amezaga N., Armengol C., Lopez D., Prats C., Sarrias M. R., Front. Immunol. 2018, 9, 480; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Weng X., Zhao H., Guan Q., Shi G., Feng S., Gleave M. E., Nguan C. C., Du C., Immunol. Cell Biol. 2020, 99, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a) Bu H. F., Zuo X. L., Wang X., Ensslin M. A., Koti V., Hsueh W., Raymond A. S., Shur B. D., Tan X. D., J. Clin. Invest. 2007, 117, 3673; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nousbeck J., Sarig O., Avidan N., Indelman M., Bergman R., Ramon M., Enk C. D., Sprecher E., J. Invest. Dermatol. 2010, 130, 378. [DOI] [PubMed] [Google Scholar]
- 23.a) Mori R., Kondo T., Nishie T., Ohshima T., Asano M., Am J Pathol 2004, 164, 1303; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stuart K., Paderi J., Snyder P. W., Freeman L., Panitch A., PLoS One 2011, 6, e22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gordts S. C., Muthuramu I., Amin R., Jacobs F., De Geest B., Pharmaceuticals 2014, 7, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.a) Fallah M., Viklund E., Backman A., Broden J., Lundskog B., Johansson M., Blomquist M., Wilczynska M., Ny T., Cell Death Dis. 2020, 11, 201; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shen Y., Guo Y., Mikus P., Sulniute R., Wilczynska M., Ny T., Li J., Blood 2012, 119, 5879; [DOI] [PubMed] [Google Scholar]; c) Sulniute R., Shen Y., Guo Y. Z., Fallah M., Ahlskog N., Ny L., Rakhimova O., Broden J., Boija H., Moghaddam A., Li J., Wilczynska M., Ny T., Thromb. Haemost. 2016, 115, 1001. [DOI] [PubMed] [Google Scholar]
- 26.a) Akita S., Daian T., Ishihara H., Fujii T., Akino K., J. Dermatol. Sci. 2004, 36, 11; [DOI] [PubMed] [Google Scholar]; b) Bunemann E., Hoff N. P., Buhren B. A., Wiesner U., Meller S., Bolke E., Muller‐Homey A., Kubitza R., Ruzicka T., Zlotnik A., Homey B., Gerber P. A., Eur. J. Med. Res. 2018, 23, 4; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Salmon‐Ehr V., Ramont L., Godeau G., Birembaut P., Guenounou M., Bernard P., Maquart F. X., Lab. Invest. 2000, 80, 1337. [DOI] [PubMed] [Google Scholar]
- 27.a) Jin W., Dong C., Emerging Microbes Infect. 2013, 2, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Metzemaekers M., Vanheule V., Janssens R., Struyf S., Proost P., Front. Immunol. 2017, 8, 1970; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Rider P., Carmi Y., Guttman O., Braiman A., Cohen I., Voronov E., White M. R., Dinarello C. A., Apte R. N., J. Immunol. 2011, 187, 4835; [DOI] [PubMed] [Google Scholar]; d) Zirlik A., Maier C., Gerdes N., MacFarlane L., Soosairajah J., Bavendiek U., Ahrens I., Ernst S., Bassler N., Missiou A., Patko Z., Aikawa M., Schonbeck U., Bode C., Libby P., Peter K., Circulation 2007, 115, 1571. [DOI] [PubMed] [Google Scholar]
- 28.a) Kopitar‐Jerala N., Front. Immunol. 2017, 8, 873; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Seok S. H., Heo J. I., Hwang J. H., Na Y. R., Yun J. H., Lee E. H., Park J. W., Cho C. H., Mol. Cells 2013, 35, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jarvelainen H., Sainio A., Wight T. N., Matrix Biol. 2015, 43, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.a) Caby M. P., Lankar D., Vincendeau‐Scherrer C., Raposo G., Bonnerot C., Int. Immunol. 2005, 17, 879; [DOI] [PubMed] [Google Scholar]; b) Ochieng J., Pratap S., Khatua A. K., Sakwe A. M., Exp. Cell Res. 2009, 315, 1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.a) Dessels C., Potgieter M., Pepper M. S., Front. Cell Dev. Biol. 2016, 4, 115; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kirikae T., Tamura H., Hashizume M., Kirikae F., Uemura Y., Tanaka S., Yokochi T., Nakano M., Int. J. Immunopharmacol. 1997, 19, 255. [DOI] [PubMed] [Google Scholar]
- 32.a) Gilbert R. W. D., Vickaryous M. K., Viloria‐Petit A. M., J. Dev. Biol. 2016, 10.3390/jdb4020021; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang X. J., Han G., Owens P., Siddiqui Y., Li A. G., J. Invest. Dermatol. Symp. Proc. 2006, 11, 112. [DOI] [PubMed] [Google Scholar]
- 33. Reynolds L. E., Conti F. J., Silva R., Robinson S. D., Iyer V., Rudling R., Cross B., Nye E., Hart I. R., Dipersio C. M., Hodivala‐Dilke K. M., J. Clin. Invest. 2008, 118, 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li X., Guo L., Liu Y., Su Y., Xie Y., Du J., Wang S., Wang H., Liu Y., Exp. Cell Res. 2018, 362, 245. [DOI] [PubMed] [Google Scholar]
- 35.a) Soliman A. M., Das S., Abd Ghafar N., Teoh S. L., Front. Genet. 2018, 9, 38; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang T., Feng Y., Sun H., Zhang L., Hao L., Shi C., Wang J., Li R., Ran X., Su Y., Zou Z., Am J Pathol 2012, 181, 1911; [DOI] [PubMed] [Google Scholar]; c) Yang X., Wang J., Guo S. L., Fan K. J., Li J., Wang Y. L., Teng Y., Yang X., Int. J. Biol. Sci. 2011, 7, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Landen N. X., Li D., Stahle M., Cell. Mol. Life Sci. 2016, 73, 3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zomer H. D., Trentin A. G., J. Dermatol. Sci. 2018, 90, 3. [DOI] [PubMed] [Google Scholar]
- 38.a) Brahmkhatri V. P., Prasanna C., Atreya H. S., Biomed Res. Int. 2015, 2015, 538019; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Witsch E., Sela M., Yarden Y., Physiology 2010, 25, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rani P., Vashisht M., Golla N., Shandilya S., Onteru S. K., Singh D., J. Funct. Foods 2017, 34, 431. [Google Scholar]
- 40. Sun B. K., Siprashvili Z., Khavari P. A., Science 2014, 346, 941. [DOI] [PubMed] [Google Scholar]
- 41.a) Ahn G., Kim Y.‐H., Ahn J.‐Y., Nanoscale Adv. 2021, 3, 528; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hata T., Murakami K., Nakatani H., Yamamoto Y., Matsuda T., Aoki N., Biochem. Biophys. Res. Commun. 2010, 396, 528. [DOI] [PubMed] [Google Scholar]
- 42. Eming S. A., Martin P., Tomic‐Canic M., Sci. Transl. Med. 2014, 6, 265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pieters B. C., Arntz O. J., Bennink M. B., Broeren M. G., van Caam A. P., Koenders M. I., van Lent P. L., van den Berg W. B., de Vries M., van der Kraan P. M., van de Loo F. A., PLoS One 2015, 10, e0121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Munagala R., Aqil F., Jeyabalan J., Gupta R. C., Cancer Lett. 2016, 371, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karami S., Roayaei M., Hamzavi H., Bahmani M., Hassanzad‐Azar H., Leila M., Rafieian‐Kopaei M., Int. J. Pharm. Invest. 2017, 7, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Table
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


