Abstract
Background
Worldwide about 2.3 million newborns still die in the neonatal period and the majority occurs in low- and middle-income countries (LMICs). Intrapartum-related events account for 24% of neonatal mortality. Of these events, intrapartum birth asphyxia with subsequent neonatal encephalopathy is the main cause of child disabilities in LMICs. Data on neurodevelopmental outcome and early risk factors are still missing in LMICs. This study aimed at investigating the factors associated with mortality, risk of neurodevelopmental impairment and adherence to follow-up among asphyxiated newborns in rural Tanzania.
Methods
This retrospective observational cohort study investigated mortality, neurodevelopmental risk and adherence to follow-up among asphyxiated newborns who were admitted to Tosamaganga Hospital (Tanzania) from January 2019 to June 2022. Neurodevelopmental impairment was assessed using standardized Hammersmith neurologic examination. Admission criteria were Apgar score < 7 at 5 min of life and birth weight > 1500 g. Babies with clinically visible congenital malformations were excluded. Comparisons between groups were performed using the Mann-Whitney test, the Chi-square test, and the Fisher test.
Results
Mortality was 19.1% (57/298 newborns) and was associated with outborn (p < 0.0001), age at admission (p = 0.02), lower Apgar score at 5 min (p = 0.003), convulsions (p < 0.0001) and intravenous fluids (IV) (p = 0.003). Most patients (85.6%) were lost to follow-up after a median of 1 visit (IQR 0–2). Low adherence to follow-up was associated with female sex (p = 0.005). The risk of neurodevelopmental impairment at the last visit was associated with longer travel time between household and hospital (p = 0.03), female sex (p = 0.04), convulsions (p = 0.007), respiratory distress (p = 0.01), administration of IV fluids (p = 0.04), prolonged oxygen therapy (p = 0.004), prolonged hospital stay (p = 0.0007) and inappropriate growth during follow-up (p = 0.0002).
Conclusions
Our findings demonstrated that mortality among asphyxiated newborns in a rural hospital in Tanzania remains high. Additionally, distance from home to hospital and sex of the newborn correlated to higher risks of neurodevelopmental impairment. Educational interventions among the population about the importance of regular health assessment are needed to improve adherence to follow-up and for preventive purposes. Future studies should investigate the role of factors affecting the adherence to follow-up.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06837-w.
Keywords: Neonatal asphyxia, Low- and middle-income countries, Mortality, Risk factors, Post-discharge follow up, Neurodevelopmental impairment
Background
Worldwide, the mortality rate of children under five years has been dramatically reduced in the last 20 years, while neonatal mortality now accounts for the majority of such deaths [1]. Around 7,000 neonates die every day and 99% of these deaths occur in low- and middle-income countries (LMICs) [1–3].
Intrapartum-related events account for 24% of all neonatal deaths and represent the second cause of neonatal mortality after complications due to prematurity [4, 5]. Of note, the neonatal mortality rate is nine times higher in LMICs with respect to high-income countries (HICs) [4].
Within the intrapartum related events, birth asphyxia represents an acute insult to the fetus or newborn which is characterized mainly by tissue hypoxia that may be associated with ischemia [6].
In HICs, hypoxic-ischemic injury (HII) is defined based on clinical appearance and biochemical markers. HII may lead to various degrees of acute illness such as metabolic derangements, poor muscle tone, breathing problems, alteration in consciousness and seizures which may ultimately result in early neonatal death. Long term morbidities and chronic conditions are mostly represented by neurodevelopmental disorders such as disability (learning and behavioral), poor feeding and cerebral palsy [6].
The standard care for neonates with birth asphyxia in LMICs should remain normothermia, avoiding both hyper- and hypothermia [7, 8]. Every deviation from euthermia (36.5°-37.5°) at admission in the neonatal unit has been associated with an increased risk of mortality [9]. A recent study in Tanzania suggested that deviations from euthermia at admission may also be associated with an increased risk of developing cerebral palsy in survivors at one month of life [10].
In LMICs, the diagnosis of perinatal asphyxia is usually performed according to clinical assessments such as the Apgar score and the neurological evaluation by Sarnat&Sarnat [11]. This neurological evaluation assesses level of consciousness, motility, posture, tone, reflexes and the pupil, leading to the classification in different stages of HII (mild, moderate and severe) that have been associated with early neurological status and short-term prognosis [11].
Early recognition of risk for neurodevelopmental impairment is highly desirable in the first period of life because of the prognostic role of early interventions in the period of greatest brain development and neuroplasticity. However, a detailed neurologic examination may be insufficient when used as the only diagnostic tool and a more comprehensive assessment should ideally encompass brain imaging such as functional Magnetic Resonance Imaging (fMRI) or cranial ultrasound, tools that are mostly not available in LMICs [12].
Before five months of corrected age, the most predictive tools to detect risk of neurodevelopmental impairment are magnetic resonance imaging (86−89% sensitivity), the Prechtl Qualitative Assessment of General Movements (98% sensitivity), and the Hammersmith Infant Neurological Examination (90% sensitivity). After five months of corrected age, the most predictive tools for detecting risk are magnetic resonance imaging (86−89% sensitivity) (where safe and feasible), the Hammersmith Infant Neurological Examination (90% sensitivity), and the Developmental Assessment of Young Children (83% C index) [13].
In LMICs, the most used clinical evaluation in the first year of life is the Hammersmith Infant Neurological Examination performed by skilled personnel according to different stages of life [14].
Child disability is still a serious problem worldwide with 1.3 million newborns surviving each year with major disabilities and 1 million with long-term moderate or mild disability, such as learning and behavior difficulties. In LMICs almost 80% of child disabilities are related to a perinatal brain injury, of which neonatal encephalopathy is the most prevalent cause leading to a substantial burden of preventable childhood neurodisabilities such as cerebral palsy, epilepsy, deafness, and blindness. Limited high quality evidence is available on prevention and management of neonatal encephalopathy in these settings [15].
Simiyu et al. analyzed a similar cohort of asphyxiated newborns in a tertiary hospital in northern Tanzania. Severity of HII was classified according to the Sarnat and Sarnat scoring at birth and at seventh day of life. Mortality among asphyxiated newborns was 12.3%. Newborns with mild and moderate HII showed improvement at Sarnat and Sarnat scoring at 1 week of life [16].
In low-resource countries, data about long term disabilities of babies surviving intrapartum complications are mostly not even available and rehabilitation services are very limited [17–21]. In HICs, two in three individuals with cerebral palsy will walk, three in four will talk, and one in two will have normal intelligence [13].
The objective of this study was to investigate the risk factors for mortality among asphyxiated newborns in a Neonatal Intensive Care Unit (NICU) in rural Tanzania. Furthermore, this study aimed at analysing the risk factors for neurodevelopmental impairment at follow-up and the factors associated to adherence to follow-up.
Methods
Study design
This was a retrospective observational cohort study on the risk factors formortality, neurodevelopmental impairment, and adherence to follow-up among asphyxiated newborns in rural Tanzania.
Institutional context and institutional background
The study was performed at the Tosamaganga Regional Referral Hospital (Tosamaganga Hospital), which is a non-profit, faith-based secondary-level hospital located in the Iringa District Council (IDC), Iringa region in Tanzania. IDC is a rural district in South-Western Tanzania that covers approximately 320,000 people [22]. In 2023, Tosamaganga Hospital registered 3,503 deliveries and 715 admissions to the NICU unit.
Doctors with Africa CUAMM (University College of Aspiring Medical Missionaries) is an Italian Non-Governmental Organization (NGO) supporting health service delivery in Africa for more than 70 years. In the region of Iringa, Doctors with Africa CUAMM has implemented several projects to strengthen the local health system, particularly in the field of maternal and child health.
Patients
All neonates admitted to the NICU of Tosamaganga Hospital between 1st January 2019 and 30st June 2022 with a diagnosis of asphyxia and birth weight > 1500 g were retrospectively evaluated for inclusion in the study. According to Tanzanian guidelines [23], neonatal asphyxia was diagnosed if the Apgar score at 5 min of life was minor than 7. Babies with clinically visible congenital malformations were excluded.
Data collection
All data were extrapolated from the Health Information Management System (HMIS) currently in use at Tosamaganga Hospital and collected in a dedicated electronic spreadsheet after being anonymised. This process involved clinical staff employed by both Tosamaganga Hospital and CUAMM. All analyses were performed in the second semester of 2023.
The analysis of risk factors for mortality included discharge status, mode of delivery, being outborn or inborn, age at admission in NICU, sex, birth weight, twin pregnancy, Apgar score at 5 min of life, presence of meconium at delivery, body temperature at admission, presence of respiratory distress, convulsions, administration of IV fluids, antibiotic therapy, oxygen therapy and length of hospital stay.
Follow-up information included number of follow-up visits, age and weight at last visit, inappropriate growth, and risk of neurodevelopmental impairment at first and last visits. Inappropriate growth at follow-up was assessed by comparing weight at discharge and at last follow-up visit using growth percentiles according to the World Health Organization (WHO) growth charts. The neurological status was assessed during a 12-month follow-up using the Hammersmith neonatal or infant neurologic examination charts according to age [14]. Infants were classified as at risk of neurodevelopmental impairment if two or more abnormal items were found.
The analysis of factors associated with neurodevelopmental impairment risk at follow-up included travel distance and time from household to hospital, delivery information (mode of delivery, outborn/inborn), neonatal data (age at admission, sex, birth weight, twin pregnancy, Apgar score at 5 min of life, presence of meconium at delivery, body temperature at admission, presence of respiratory distress and convulsions), information during hospital stay (administration of IV fluids, antibiotic therapy, oxygen therapy and length of stay) and inappropriate growth at follow up.
The analysis of factors associated with adherence to follow-up included travel distance and time from household to hospital, sex, and birth weight.
Statistical analysis
Numerical data were summarized as median and interquartile range (IQR), while categorical data as absolute and relative frequency (percentage). Comparisons between groups were performed using the Mann-Whitney test, the Chi-square test, and the Fisher test, as appropriate. Multivariable analyses were not performed due to the small number of events compared to the candidate risk factors of interest. All tests were two-sided and a p-value less than 0.05 was considered statistically significant. The statistical analysis was carried out with R 4.3 (R Foundation for Statistical Computing, Vienna, Austria) [24].
Results
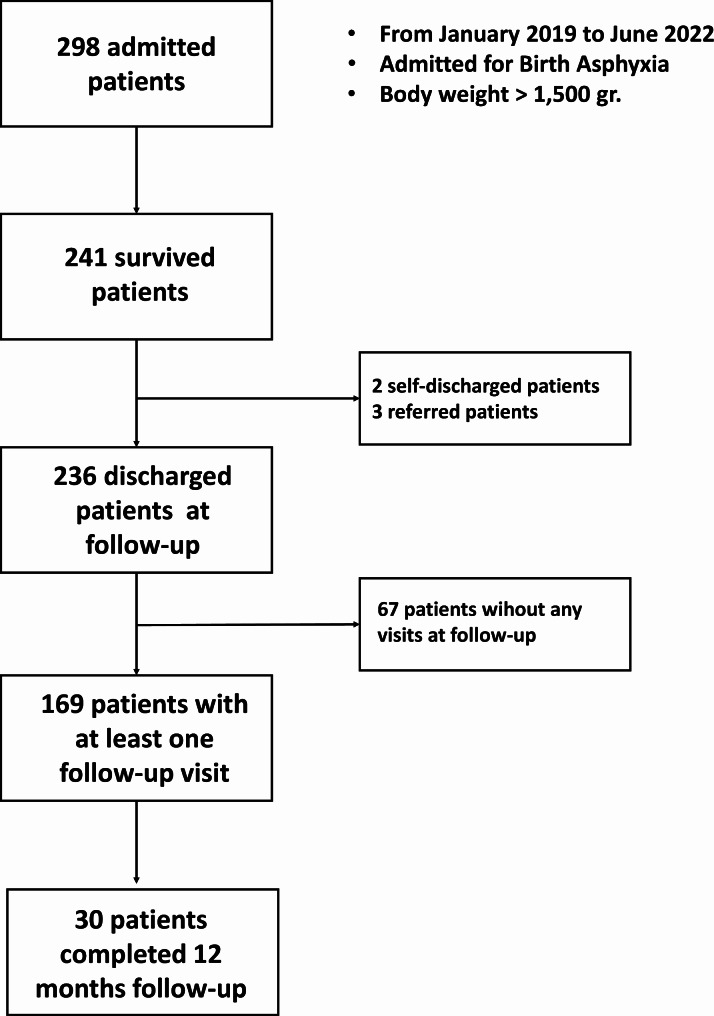
The analysis included 298 children who were hospitalized during the study period and met the inclusion criteria. Patient characteristics are summarized in Table 1. Additionally, we reported the study’s patient flow chart in Fig. 1.
Table 1.
Patient characteristics in the whole sample and stratified by mortality outcome
| All patients (n = 298) | Discharged/transferred (n = 241) | Deceased (n = 57) | p-value | |
|---|---|---|---|---|
|
Delivery: Spontaneous vaginal Assisted vaginal C-section |
124/275 (45.1%) 44/275 (16.0%) 107/275 (38.9%) |
105/223 (47.1%) 33/223 (14.8%) 85/223 (38.1%) |
19/52 (36.5%) 11/52 (21.2%) 22/52 (42.3%) |
0.32 |
| Outborn | 17/297 (5.7%) | 7/240 (2.9%) | 10 (17.5%) | < 0.0001 |
| Age at admission, days | 1 (1–1) | 1 (1–1) | 1 (1–2) | 0.02 |
| Males | 179/297 (60.3%) | 143/240 (59.6%) | 36 (63.2%) | 0.73 |
| Twins | 8 (2.7%) | 8 (3.3%) | 0 (0.0%) | 0.36 |
| Apgar score at 5 min | 5 (4–6) | 5 (4–6) | 5 (3–5) | 0.003 |
| Birth weight, gr | 2995 (1670–3290) | 3000 (2660–3300) | 2950 (2670–3270) | 0.75 |
| Temperature at admission, °C | 35.4 (34.7–36.0) | 35.4 (34.7–36.0) | 35.3 (34.7–36.2) | 0.87 |
| Convulsions | 96 (32.2%) | 64 (26.6%) | 32 (56.1%) | < 0.0001 |
| Meconium-stained amniotic fluid (not available in 2019) | 89/187 (47.6%) | 74/151 (49.0%) | 25/36 (41.7%) | 0.54 |
| IV fluids | 192 (64.4%) | 145 (60.2%) | 47 (82.5%) | 0.003 |
| Antibiotic therapy | 283 (95.0%) | 228 (94.6%) | 55 (96.5%) | 0.74 |
| Respiratory distress | 202 (67.8%) | 160 (66.4%) | 42 (73.7%) | 0.35 |
| Oxygen therapy, days | 2 (1–5) | 2 (1–7) | 2 (1–3) | 0.87 |
Data summarized as n/N (%) or median (IQR)
Fig. 1.
Patient Flow Chart of the study
Overall, 61.1% of babies were born through vaginal delivery (45.1% from spontaneous delivery and 16% assisted via vacuum extraction) and 38.9% through a C-section (almost all emergency C-sections). Only 5.7% of babies were outborn and referred to the study hospital for birth complications. Median birth weight was 2,995 g (IQR 1,670-3,290). Of note, 47.6% of the births in 2020–2022 presented meconium-stained fluid at delivery, while such information was not available in the records of infants born in 2019. Median temperature at admission was 35.4 °C (IQR 34.7–36.0). Clinical diagnosis of convulsion was present in 32.2% of babies. As per local protocol, almost all babies (95%) received antibiotic treatment at admission, while IV fluids were administered to 64.4% of babies.
Median length of hospital stay was 7 days (IQR 5–10). Overall, 57 patients died (19.1%) while 241 were alive at discharge (80.9%). Alive patients included 236 discharged at home by a doctor, two self-discharged, and three transferred patients. Mortality was associated with outborn (p < 0.0001), older age at admission (p = 0.02), lower Apgar score at 5 min (p = 0.003), convulsions (p < 0.0001), and administration of IV fluids (p = 0.003) (Table 1).
Figure 1 reports follow-up adherence in 236 improved patients. After discharge, most patients (202/236, 85.6%) were lost to follow-up after a median of 1 visit (IQR 0–2), and 33.2% of them (67/202) did not even attend the first follow-up visit. Thirty patients attended a regular follow-up with a median of 5 visits (IQR 5–6), while four patients were readmitted after a median of 3 visits (IQR 2–3). No follow-up information was available for self-discharged (n = 2) and transferred (n = 3) patients.
Follow-up information in 169 improved patients who attended at least one follow-up visit is reported in the Supplementary Material. In 30 closed patients (those who completed the 12 months follow-up), inappropriate growth was found in 50% and the risk of neurodevelopmental impairment decreased from 50% at first visit to 13.3% at last visit. In 135 defaulted patients (those who were lost to follow-up), inappropriate growth was found in 34.6% and the risk of neurodevelopmental impairment was around 30%. Four patients were readmitted at median 2 months of age with half of them showing inappropriate growth and were at risk of neurodevelopmental delay.
The factors associated with the risk of neurodevelopmental impairment at the last visit are reported in Table 2. The risk of neurodevelopmental impairment at the last visit was associated with longer travel time between household and hospital (p = 0.03), female sex (p = 0.04), convulsions (p = 0.007), respiratory distress (p = 0.01), administration of IV fluids (p = 0.04), prolonged oxygen therapy (p = 0.004), prolonged hospital stay (p = 0.0007) and inappropriate growth during follow-up (p = 0.0002).
Table 2.
Factors associated with the risk of neurodevelopmental impairment at the last visit
| Patients without neurodevelopmental risk at the last visit (n = 122) | Patients with neurodevelopmental risk at the last visit (n = 45) | p-value | |
|---|---|---|---|
| Distance between household and hospital, km | 29 (12–40) | 35 (20–52) | 0.07 |
| Travel time between household and hospital, min | 41 (20–55) | 46 (31–72) | 0.03 |
|
Delivery: Spontaneous vaginal Assisted vaginal C-section |
48/112 (42.9%) 20/112 (17.8%) 44/112 (39.3%) |
26 (57.8%) 3 (6.7%) 16 (35.5%) |
0.11 |
| Outborn | 3/121 (2.5%) | 3 (6.7%) | 0.35 |
| Age at admission, days | 1 (1–1) | 1 (1–1) | 0.82 |
| Males | 69/121 (43.0%) | 11 (24.4%) | 0.04 |
| Twins | 5 (4.1%) | 0 (0.0%) | 0.33 |
| Apgar score at 5 min | 5 (4–6) | 5 (4–6) | 0.24 |
| Birth weight, gr | 2995 (2662–3308) | 3100 (2730–3300) | 0.47 |
| Body temperature at admission, °C | 35.4 (34.7–36.0) | 35.3 (34.8–35.9) | 0.85 |
| Convulsions | 29 (23.8%) | 21 (46.7%) | 0.007 |
| Meconium (not available in 2019) | 37/82 (45.1%) | 16/28 (57.1%) | 0.38 |
| IV fluids | 70 (57.4%) | 34 (75.6%) | 0.04 |
| Antibiotic therapy | 117 (95.9%) | 42 (93.3%) | 0.78 |
| Respiratory distress | 80 (65.6%) | 39 (86.7%) | 0.01 |
| Oxygen therapy, days | 2 (1–6) | 5 (2–13) | 0.004 |
| Length of hospital stay, days | 8 (7–11) | 12 (8–17) | 0.0007 |
| Inappropriate growth | 35 (28.7%) | 28 (62.2%) | 0.0002 |
Data summarised as n (%) or median (IQR)
Mode of delivery (either spontaneous, assisted via vacuum, or cesarean section) was not found to be statistically associated with increased mortality (Table 1) nor with increased risk for developmental impairment during follow-up (Table 2).
Low adherence to follow-up was associated with female sex (p = 0.005) but not with the other factors considered (Table 3).
Table 3.
Factors associated with adherence to follow-up
| Discharged patients: | |||
|---|---|---|---|
| Closed patients* (n = 30) | Defaulted patients** (n = 202) | p-value | |
| Distance between household and hospital, km | 22 (14–36) | 35 (20–54) | 0.07 |
| Distance between household and hospital, min | 34 (20–53) | 46 (30–69) | 0.07 |
| Males | 20 (66.7%) | 76/201 (37.8%) | 0.005 |
| Birth weight < 2500 gr | 6 (16.7%) | 32 (15.8%) | 0.99 |
Data summarised as n (%) or median (IQR)
*Closed = patients who completed 12 months’ follow-up
** Defaulted = patients lost to follow-up
Discussion
Our findings confirmed that neonatal mortality in LMICs remains a significant burden and is associated with preventable and treatable conditions, underlying the importance of improving prenatal and perinatal care among asphyxiated newborns in such settings [9, 25].
In our cohort, around one out of five newborns died after a few days of life, in agreement with previous studies showing that most deaths due to intrapartum-related complications occur in the first week of life [26, 27]. In addition, perinatal complications, such as meconium-stained amniotic fluid at birth and respiratory distress, convulsions and hypothermia were common among asphyxiated newborns. Our findings suggested the association of higher mortality risk with being outborn, older age at admission, lower Apgar score at 5 min, convulsions and administration of IV fluids. Differently from HICs, neonatal mortality in LMICs usually occurs from preventable and treatable conditions [6, 28]. Previous investigations indicated being outborn, low 5-minutes Apgar score, depressed clinical status at NICU admission, occurrence of infection or seizures within 24 h from admission and receiving aminophylline during hospital stay as risk factors for mortality [25]. The prognosis of aspyxiated newborn born outside the hospital was strictly related to the availability of a referral system [29]. Ambulances connecting health centers and Tosamaganga Hospital are often not running due to a lack of maintenance or fuel shortage. Strengthening the referral system is a central issue in the secondary prevention of birth asphyxia [30]. Moreover, adequate neonatal resuscitation skills and equipment are fundamental in the prevention of perinatal asphyxia [30]. In particular, frequent re-training of the health personnel attending women during delivery could be a strategy to maintain a good quality of service [31]. Finally, interventions of primary prevention for birth asphyxia (i.e. improving antenatal care services, developing coverage, equity and quality of the obstetric supply, women’s education, and empowerment) generally have the greatest impact in improving health outcomes [30].
Unfortunately, we were unable to differentiate the primary cause of death (such as meconium aspiration syndrome and persistent pulmonary hypertension as causes of respiratory insufficiency, level of metabolic derangement, confirmation of infection, birth trauma or intracranial hemorrhage) due to the limited availability of diagnostic instruments in the study setting. Thus, the primary diagnosis was based on the clinical evaluation by the attending clinician, while more information about the neonatal neurological status using validated scoring systems, such us Sarnat and Sarnat score, may provide important clues to short- and long-term prognosis [32].
International literature offers scarce information on the follow-up of neonates with perinatal complications in LMICs [13, 33]. In the study setting, neonatal follow-up of discharged patients started in 2019 and, to our knowledge, this is the first study evaluating long-term follow-up characteristics of asphyxiated newborns in Tanzania. Our data highlighted that one of the most important barriers to the study of long-term neurodevelopmental outcomes in LMICs was the low adherence to follow-up after discharge from NICU. Most patients in the present study lost to follow-up after a median of one visit and a considerable proportion never attended any follow-up visits at all.
We analysed the factors associated with low adherence to follow-up and found that male patients were more likely to attend follow-up visits. Thes results agreed with data reported in a systematic review showing that care-seeking rates were lower for female vs. male neonates in south Asia, especially in households with older female children [34]. The authors argued that parents were prone to pay more and seek high-quality care for males than females, and such difference in care-seeking behavior might partially explain the higher mortality among female neonates in South Asia. The promotion of policies supporting gender equality and context-specific strategies with local partners may challenge the socio-economic and cultural norms that led to such inequality [35]. Moreover, further investigations should consider the role of travel distance from the household to the hospital, which may affect the adherence to follow-up [35].
Unfortunately, the limited sample size prevented us from drawing strong conclusions on this matter. Of course, we also acknowledge that other socio-economic and cultural factors may be involved in the low adherence to follow-up.
Results in the small group of those attending the follow-up clinic suggested the association of neurodevelopmental impairment with longer travel time between household and hospital, female sex, convulsions, respiratory distress, administration of IV fluids, prolonged oxygen therapy and hospital stay, and inappropriate growth during follow-up. Of note, these factors may be prevented or reduced by improving antenatal and peripartum care, thus reducing the neonatal burden of morbidity and mortality [30].
Low-income settings suffer from constraints in terms of preventive, diagnostic, and therapeutic strategies, as well as access to adequate health care structures. [34] Most of the population of the sub-Saharan area still live in the rural countryside, where proper and safe means of transportation are often unavailable or unaffordable [29]. From a cultural point of view, educating the population about the importance of health assessment by healthcare personnel for preventive purposes and not only for acute health issues still represents a challenge. This consideration is consistent with the relevant proportion of neonates who were considered at risk of neurodevelopmental impairment at the first follow-up visit. In addition, we found a high proportion of inappropriate growth during follow-up, suggesting that many survivors from perinatal asphyxia may suffer from eating difficulties probably due to neurological compromise [36].
Further evaluations may assess how many of these patients will be affected by malnutrition in their childhood. Overall, we agreed that interventions aiming at improving the comprehensive care of the newborn should focus on strengthening the continuum of care including fetal, intrapartum, postnatal and early childhood period [37].
This study suffers from some limitations that pave the way for further research opportunities. Firstly, the retrospective data collection limited data availability and precluded any causal associations. Seconddly, the single-center design restricted the generalizability of the findings to similar settings. Thirdly, the low adherence to follow-up visits reduced the sample size for the investigation of risk factors for of neurodevelopmental impairment, suggesting caution in the interpretation of such findings.
Conclusions
Our findings confirmed that neonatal mortality in LMICs remains a significant burden and is associated with preventable and treatable conditions, underlying the importance of improving prenatal and perinatal care in such settings. The low adherence to follow-up after discharge, and the magnitude of inappropriate growth and neurodevelopmental impairment found, highlighted the urgent need for educational interventions among the population about the importance of regular health assessment for preventive purposes. Future studies should investigate the role of travel distance from household to hospital on the adherence to follow-up, and evaluate appropriate interventions to improve post-discharge care of neonates who suffered from perinatal complications in LMICs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the parents whose newborns participated in this study, all the local and expatriate health professionals in the Tosamaganga Hospital in Tanzania and Doctors with Africa CUAMM.
Abbreviations
- [LMIC]
Low and middle-income country
- [IV]
Intravenous fluids
- [HIC]
High-income countries
- [HII]
Hypoxic-ischemic injury
- [IDC]
Iringa District Council
- [FMRI]
Functional Magnetic Resonance Image
- [NICU]
Neonatal Intensive Care Unit
- [IDC]
Iringa District Council
- [CUAMM]
University College of Aspiring Medical Missionaries
- [NGO]
Non-Governmental Organization
- [WHO]
World Health Organization
- [IQR]
Interquartile range
Author contributions
EM1, MB and DT designed the study. EK, EM2, CM collected the data. FC, EM1, MB analyzed the data. EM1 wrote the first original draft and MB, BP, FC, DT, and AS contributed to the final version. All authors read and approved the final manuscript.
Funding
There is no funding source for this study.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All data were retrieved from hospital records by hospital staff and were collected in an anonymized dataset. The study was approved by the Review Board of Tosamaganga Hospital (protocol number DOIRA/TVAH/VOL/56/02), which waived the need for written informed consent given the retrospective nature of the study and the use of anonymized data from hospital records.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet [Internet]. 2005;365(9462):891–900. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(05)71048-5/abstract [DOI] [PubMed]
- 2.Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Heal [Internet]. 2022;6(2):106–15. 10.1016/S2352-4642(21)00311-4 [DOI] [PMC free article] [PubMed]
- 3.Abbafati C, Machado DB, Cislaghi B, Salman OM, Karanikolos M, McKee M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devine S, Taylor G. & U. Every child alive: The urgent need to end newborn deaths. 2018.
- 5.Lawn JE, Lee ACC, Kinney M, Sibley L, Carlo WA, Paul VK et al. Two million intrapartum-related stillbirths and neonatal deaths: Where, why, and what can be done? Int J Gynecol Obstet [Internet]. 2009;107(SUPPL.):S5–19. 10.1016/j.ijgo.2009.07.016 [DOI] [PubMed]
- 6.Lee ACC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(SUPPL 1):50–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thayyil S, Pant S, Montaldo P, Shukla D, Oliveira V, Ivain P, et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Heal. 2021;9(9):e1273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan V, Kumar V, Shankaran S, Thayyil S. Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial. Indian J Pediatr [Internet]. 2021;(0123456789). 10.1007/s12098-021-03861-y [DOI] [PubMed]
- 9.Cavallin F, Calgaro S, Brugnolaro V, Wingi OM, Muhelo AR, Da Dalt L et al. Non-linear association between admission temperature and neonatal mortality in a low-resource setting. Sci Rep [Internet]. 2020;10(1):1–8. 10.1038/s41598-020-77778-5 [DOI] [PMC free article] [PubMed]
- 10.Guadagno C, Cavallin F, Brasili L, Maziku DM, Leluko DE, Azzimonti G et al. Relationship between Admission temperature and risk of cerebral palsy in infants admitted to Special Care Unit in a low resource setting: a retrospective single-center study. Children. 2022;9(3). [DOI] [PMC free article] [PubMed]
- 11.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. Obstet Gynecol Surv. 1977;32(5):295–7. [Google Scholar]
- 12.King AR, Hassan M, Imam A, Mcintyre S, Morgan C, Khandaker G et al. Early Diagnosis of Cerebral Palsy in Low- and Middle-Income Countries. Brain Sci [Internet]. 2022;12(5):539. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9138742/ [DOI] [PMC free article] [PubMed]
- 13.Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, Accurate diagnosis and early intervention in cerebral palsy. JAMA Pediatr. 2017;171(9):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzardi A, Romeo DMM, Cioni M, Romeo MG, Guzzetta A. Infant neurological examination from 3 to 12 months: predictive value of the single items. Neuropediatrics. 2008;39(6):344–6. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan V, Kumar V, Variane GFT, Carlo WA, Bhutta ZA, Sizonenko S et al. Need for more evidence in the prevention and management of perinatal asphyxia and neonatal encephalopathy in low and middle-income countries: a call for action. Semin Fetal Neonatal Med. 2021;26(5). [DOI] [PMC free article] [PubMed]
- 16.Simiyu IN, Mchaile DN, Katsongeri K, Philemon RN, Msuya SE. Prevalence, severity and early outcomes of hypoxic ischemic encephalopathy among newborns at a tertiary hospital, in northern Tanzania. BMC Pediatr. 2017;17(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Survive & thrive. [Internet]. Vol. 29, Delicious Living. 2019. 20–37 p. https://apps.who.int/iris/bitstream/handle/10665/326495/9789241515887-eng.pdf
- 18.World Health Organization. United Nations Population Fund. Ending preventable newborn deaths and stillbirths by 2030: moving faster towards high-quality universal health coverage in 2020–2025 [Internet]. World Health Organization. 2020. https://www.unicef.org/media/77166/file/Ending-preventable-newborn-deaths-and-stillbirths-by-2030-universal-health-coverage-in-2020–2025.pdf
- 19.Smythe T, Zuurmond M, Tann CJ, Gladstone M, Kuper H. Early intervention for children with developmental disabilities in low and middle-income countries - the case for action. Int Health. 2021;13(3):222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smythe T, Adelson JD, Polack S. Systematic review of interventions for reducing stigma experienced by children with disabilities and their families in low- and middle-income countries: state of the evidence. Trop Med Int Heal. 2020;25(5):508–24. [DOI] [PubMed] [Google Scholar]
- 21.Adugna MB, Nabbouh F, Shehata S, Ghahari S. Barriers and facilitators to healthcare access for children with disabilities in low and middle income sub-saharan African countries: a scoping review. BMC Health Serv Res. 2020;20(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanzania N. B. S. opulation and housing census: population distribution by administrative areas. 2013.
- 23.Ministry of Health. Community Development, Gender, Elderly and Children (MoHCDGEC) [Tanzania Mainland]. National Guideline for Neonatal Care and establishment of Neonatal Care Unit. 2019.
- 24.C. TR. R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2013.
- 25.Cavallin F, Menga A, Brasili L, Maziku D, Azzimonti G, Putoto G et al. Factors associated with mortality among asphyxiated newborns in a low-resource setting. J Matern Neonatal Med [Internet]. 2022;35(6):1178–83. 10.1080/14767058.2020.1743670 [DOI] [PubMed]
- 26.Farah AE, Pai PG, Menezes V, Srikanth AMS, Shenoy JP. Medical students’ perception of their educational environment. J Clin diagnostic Res JCDR. JCDR Research & Publications Private Limited; 2014;8(1):103. PLoS One. 2018;492:1–10. [DOI] [PMC free article] [PubMed]
- 27.Debelew GT, Afework MF, Yalew AW. Determinants and causes of neonatal mortality in Jimma Zone, Southwest Ethiopia: a multilevel analysis of prospective follow up study. PLoS ONE. 2014;9(9). [DOI] [PMC free article] [PubMed]
- 28.Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nat Rev Dis Prim. 2017;3:17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AC, Lawn JE, Cousens S, Kumar V, Osrin D, Bhutta ZA, et al. Linking families and facilities for care at birth: what works to avert intrapartum-related deaths? Int J Gynaecol Obstet. 2009;107(Suppl 1):S65–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usman F, Imam A, Farouk ZL, Dayyabu AL. Newborn mortality in sub-saharan africa: why is perinatal asphyxia still a major cause? Ann Glob Heal. 2019;85(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niermeyer S. For the global implementation task force: helping babies breathe. Elk Grove Village Acad Pediatr. 2009.
- 32.Stanifer JW, Cleland CR, Makuka GJ, Egger JR, Maro V, Maro H, et al. Prevalence, risk factors, and complications of diabetes in the Kilimanjaro region: a population-based study from Tanzania. PLoS ONE. 2016;11(10):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milner KM, Neal EFG, Roberts G, Steer AC, Duke T. Long-term neurodevelopmental outcome in high-risk newborns in resource-limited settings: a systematic review of the literature. Paediatr Int Child Health. 2015;35(3):227–42. [DOI] [PubMed] [Google Scholar]
- 34.Ismail SA, McCullough A, Guo S, Sharkey A, Harma S, Rutter P. Gender-related differences in care-seeking behaviour for newborns: a systematic review of the evidence in South Asia. BMJ Glob Heal. 2019;4(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Girma D, Dejene H, Adugna L. Predictors of neonatal mortality in Ethiopia: a Comprehensive Review of Follow-Up studies. Int J Pediatr (United Kingdom). 2022;2022:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerac M, Postels DG, Mallewa M, Alusine Jalloh A, Voskuijl WP, Groce N et al. The interaction of malnutrition and neurologic disability in Africa. Semin Pediatr Neurol [Internet]. 2014;21(1):42–9. 10.1016/j.spen.2014.01.003 [DOI] [PubMed]
- 37.Bhutta, Z. A., Das, J. K., Bahl, R., Lawn, J. E., Salam, R. A., Paul, V. K., ... & Walker N. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384(9940):347–70. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.