Abstract
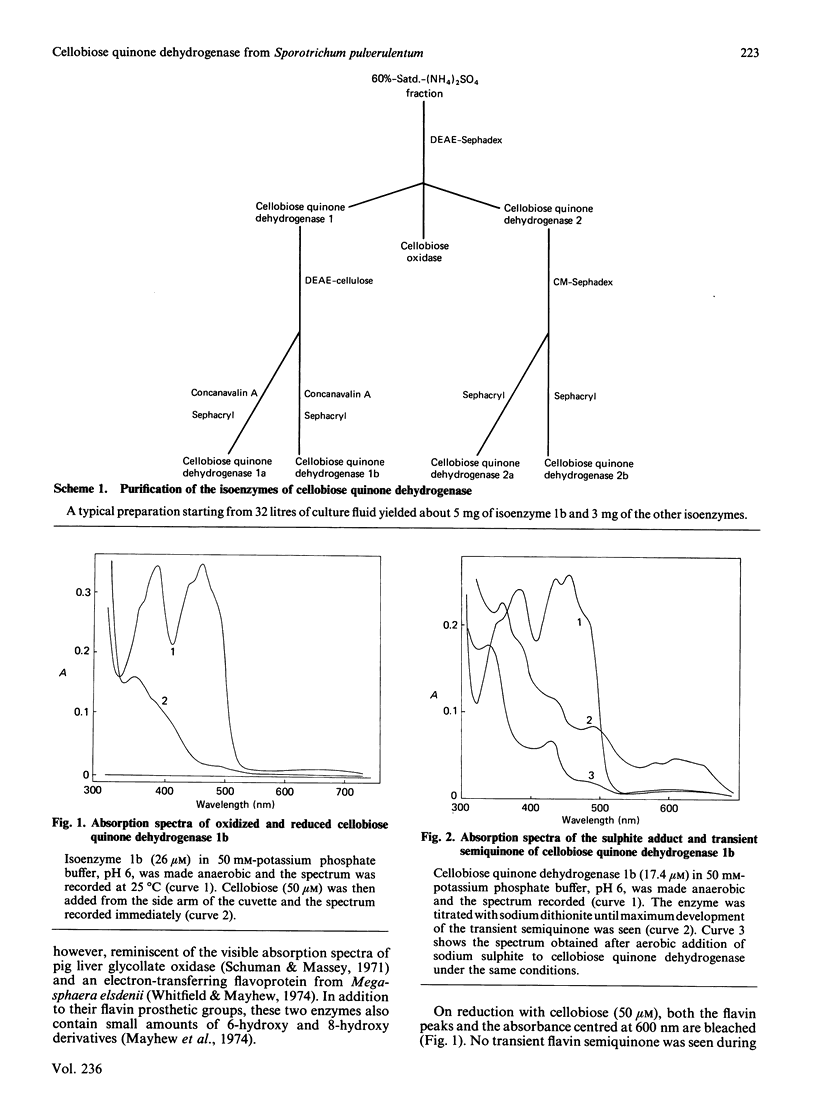
Four forms of cellobiose quinone dehydrogenase have been purified from the white-rot fungus Sporotrichum pulverulentum. The Mr of the enzyme has been estimated by sedimentation equilibrium to be 57,800 and by SDS/polyacrylamide-gel to be 60,000. These enzymes are clearly monomers. Cellobiose quinone dehydrogenases contain FAD and variable amounts of a green chromophore which we suggest is 6-hydroxy-FAD. The superoxide anion and H2O2 are the products of its reaction with oxygen. All of the isoenzymes from any one preparation display similar kinetic parameters. However, these vary between preparations. The only apparent difference between the four separable isoenzymes is their neutral-sugar content.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ayers S. B., Eriksson K. E. Cellobiose oxidase, purification and partial characterization of a hemoprotein from Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):171–181. doi: 10.1111/j.1432-1033.1978.tb12588.x. [DOI] [PubMed] [Google Scholar]
- Coudray M. R., Canevascini G., Meier H. Characterization of a cellobiose dehydrogenase in the cellulolytic fungus Sporotrichum (Chrysosporium) thermophile. Biochem J. 1982 Apr 1;203(1):277–284. doi: 10.1042/bj2030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Berrieman S. The separation of sheep liver cytoplasmic and mitochondrial aldehyde dehydrogenases by isoelectric focusing, and observations on the purity of preparations of the cytoplasmic enzyme, and their sensitivity towards inhibition by disulfiram. Biochem J. 1979 Jun 1;179(3):709–712. doi: 10.1042/bj1790709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 1. Separation, purification and physico-chemical characterization of five endo-1,4-beta-glucanases. Eur J Biochem. 1975 Feb 3;51(1):193–206. doi: 10.1111/j.1432-1033.1975.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Janatova J., Fuller J. K., Hunter M. J. The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content. J Biol Chem. 1968 Jul 10;243(13):3612–3622. [PubMed] [Google Scholar]
- Kersten P. J., Tien M., Kalyanaraman B., Kirk T. K. The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. J Biol Chem. 1985 Mar 10;260(5):2609–2612. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundquist K., Kristersson P. Exhaustive laccase-catalysed oxidation of a lignin model compound (vanillyl glycol) produces methanol and polymeric quinoid products. Biochem J. 1985 Jul 1;229(1):277–279. doi: 10.1042/bj2290277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Hemmerich P. Active-site probes of flavoproteins. Biochem Soc Trans. 1980 Jun;8(3):246–257. doi: 10.1042/bst0080246. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Whitfield C. D., Ghisla S., Schuman-Jörns M. Identification and properties of new flavins in electron-transferring flavoprotein from Peptostreptococcus elsdenii and pig-liver glycolate oxidase. Eur J Biochem. 1974 May 15;44(2):579–591. doi: 10.1111/j.1432-1033.1974.tb03515.x. [DOI] [PubMed] [Google Scholar]
- Morpeth F. F. Some properties of cellobiose oxidase from the white-rot fungus Sporotrichum pulverulentum. Biochem J. 1985 Jun 15;228(3):557–564. doi: 10.1042/bj2280557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Massey V. Flavin-sulfite complexes and their structures. J Biol Chem. 1969 Aug 10;244(15):4007–4016. [PubMed] [Google Scholar]
- Pajot P. Fluroescence of proteins in 6-M guanidine hydrochloride. A method for the quantitative determination of tryptophan. Eur J Biochem. 1976 Mar 16;63(1):263–269. doi: 10.1111/j.1432-1033.1976.tb10228.x. [DOI] [PubMed] [Google Scholar]
- Schuman M., Massey V. Purification and characterization of glycolic acid oxidase from pig liver. Biochim Biophys Acta. 1971 Mar 10;227(3):500–520. doi: 10.1016/0005-2744(71)90003-9. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark U., Eriksson K. E. Purification and properties of cellobiose: quinone oxidoreductase from Sporotrichum pulverulentum. Acta Chem Scand B. 1975;29(4):419–424. [PubMed] [Google Scholar]
- Whitfield C. D., Mayhew S. G. Purification and properties of electron-transferring flavoprotein from Peptostreptococcus elsdenii. J Biol Chem. 1974 May 10;249(9):2801–2810. [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The cellulase of Trichoderma koningii. Purification and properties of some endoglucanase components with special reference to their action on cellulose when acting alone and in synergism with the cellobiohydrolase. Biochem J. 1978 Apr 1;171(1):61–72. doi: 10.1042/bj1710061. [DOI] [PMC free article] [PubMed] [Google Scholar]


