Abstract
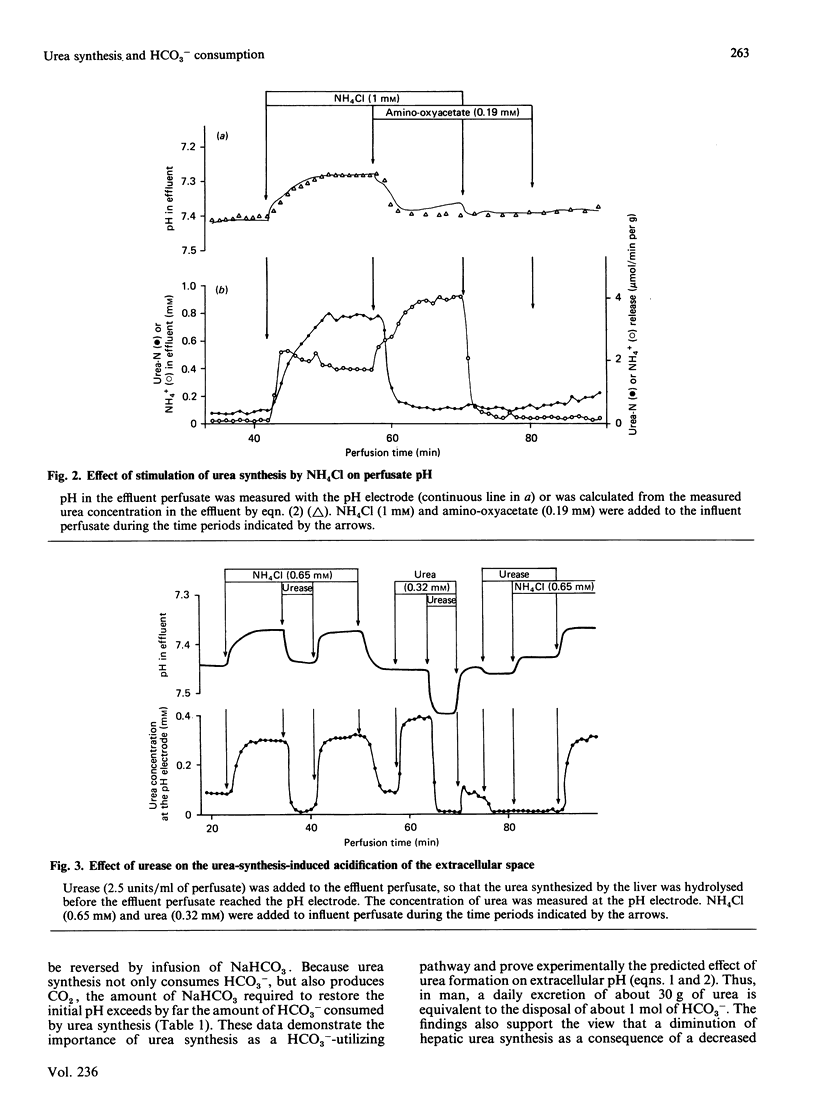
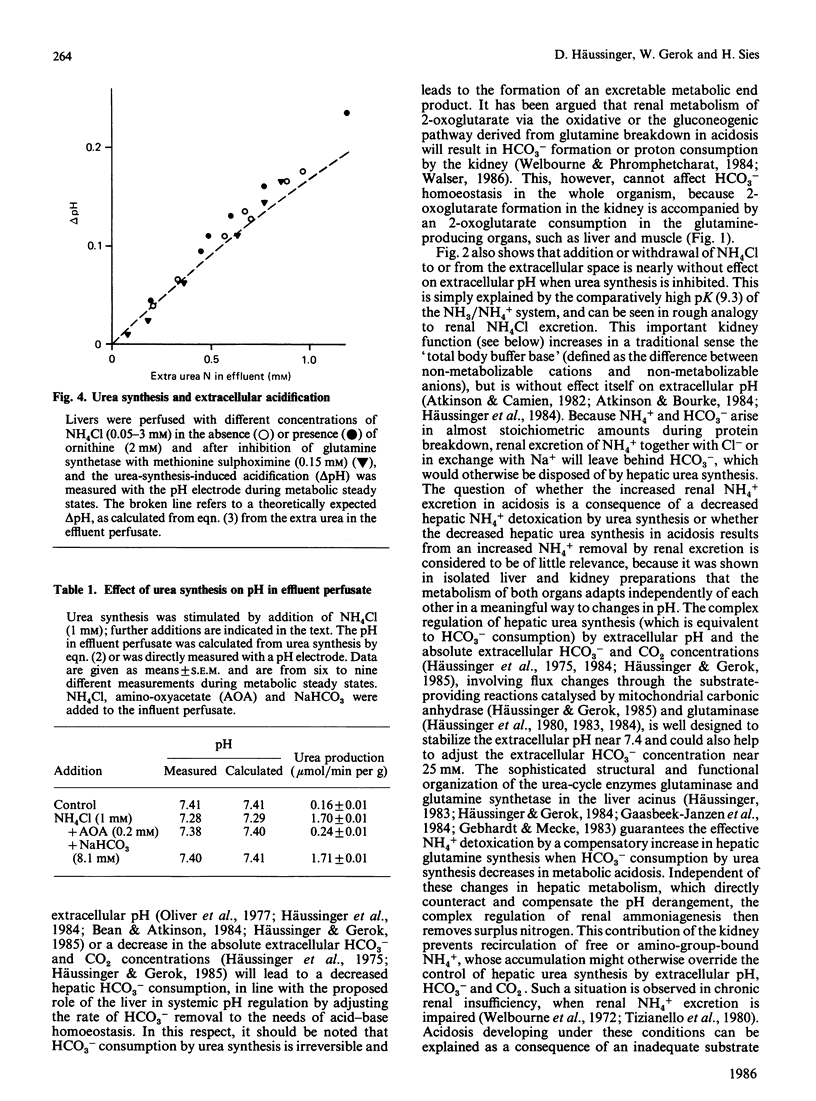
In a non-recirculating system of isolated liver perfusion, stimulation of urea synthesis by NH4Cl is followed by a decrease of effluent pH by up to 0.2 pH unit. This effect is not observed when urea synthesis is inhibited by amino-oxyacetate or norvaline. When the urea formed by the liver is immediately hydrolysed with urease before the effluent perfusate reaches the pH electrode, the urea-synthesis-induced acidification is no longer observed. This indicates that accompanying alterations in hepatic metabolism after stimulation of urea synthesis, such as increased energy provision and consumption, are not responsible for the extracellular acidification, but that the effect is due to the formation of urea itself. The acidification of the extracellular space after stimulation of urea synthesis by NH4Cl is quantitatively explained by the consumption of 2 mol of HCO3-/mol of urea formed: 1 mol being incorporated into urea, the other being protonated to yield CO2 and H2O. The data match the theoretically predicted HCO3- consumption during ureogenesis and underline the role of hepatic urea synthesis for disposal of HCO3- by converting it into the excretable products CO2 and urea.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E., Camien M. N. The role or urea synthesis in the removal of metabolic bicarbonate and the regulation of blood pH. Curr Top Cell Regul. 1982;21:261–302. doi: 10.1016/b978-0-12-152821-8.50014-1. [DOI] [PubMed] [Google Scholar]
- Bean E. S., Atkinson D. E. Regulation of the rate of urea synthesis in liver by extracellular pH. A major factor in pH homeostasis in mammals. J Biol Chem. 1984 Feb 10;259(3):1552–1559. [PubMed] [Google Scholar]
- Gaasbeek Janzen J. W., Lamers W. H., Moorman A. F., de Graaf A., Los J. A., Charles R. Immunohistochemical localization of carbamoyl-phosphate synthetase (ammonia) in adult rat liver; evidence for a heterogeneous distribution. J Histochem Cytochem. 1984 Jun;32(6):557–564. doi: 10.1177/32.6.6373912. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983;2(4):567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin M. L., Jungas R. L. Metabolic production and renal disposal of hydrogen ions. Kidney Int. 1983 Dec;24(6):709–713. doi: 10.1038/ki.1983.217. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Akerboom T. P., Sies H. The role of pH and the lack of a requirement for hydorgencarbonate in the regulation of hepatic glutamine metabolism. Hoppe Seylers Z Physiol Chem. 1980 Jul;361(7):995–101. doi: 10.1515/bchm2.1980.361.2.995. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Gerok W. Hepatic urea synthesis and pH regulation. Role of CO2, HCO3-, pH and the activity of carbonic anhydrase. Eur J Biochem. 1985 Oct 15;152(2):381–386. doi: 10.1111/j.1432-1033.1985.tb09208.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Gerok W. Hepatocyte heterogeneity in ammonia metabolism: impairment of glutamine synthesis in CCl4 induced liver cell necrosis with no effect on urea synthesis. Chem Biol Interact. 1984 Feb;48(2):191–194. doi: 10.1016/0009-2797(84)90120-0. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Gerok W., Sies H. Regulation of flux through glutaminase and glutamine synthetase in isolated perfused rat liver. Biochim Biophys Acta. 1983 Jan 25;755(2):272–278. doi: 10.1016/0304-4165(83)90214-3. [DOI] [PubMed] [Google Scholar]
- Häussinger D. Hepatocyte heterogeneity in glutamine and ammonia metabolism and the role of an intercellular glutamine cycle during ureogenesis in perfused rat liver. Eur J Biochem. 1983 Jun 15;133(2):269–275. doi: 10.1111/j.1432-1033.1983.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Weiss L., Sies H. Activation of pyruvate dehydrogenase during metabolism of ammonium ions in hemoglobin-free perfused rat liver. Eur J Biochem. 1975 Apr 1;52(3):421–431. doi: 10.1111/j.1432-1033.1975.tb04010.x. [DOI] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. II. Multiple binding sites for carbamyl-P and L-norvaline, correlation with steady state kinetics. J Biol Chem. 1972 Mar 25;247(6):1654–1668. [PubMed] [Google Scholar]
- Oliver J., Bourke E. Adaptations in urea ammonium excretion in metabolic acidosis in the rat: a reinterpretation. Clin Sci Mol Med Suppl. 1975 Jun;48(6):515–510. doi: 10.1042/cs0480515. [DOI] [PubMed] [Google Scholar]
- Oliver J., Koelz A. M., Costello J., Bourke E. Acid-base induced alterations in glutamine metabolism and ureogenesis in perfused muscle and liver of the rat. Eur J Clin Invest. 1977 Oct;7(5):445–449. doi: 10.1111/j.1365-2362.1977.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Tannen R. L. Ammonia metabolism. Am J Physiol. 1978 Oct;235(4):F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Sastrasinh S. Response of ammonia metabolism to acute acidosis. Kidney Int. 1984 Jan;25(1):1–10. doi: 10.1038/ki.1984.1. [DOI] [PubMed] [Google Scholar]
- Tizianello A., De Ferrari G., Garibotto G., Gurreri G., Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest. 1980 May;65(5):1162–1173. doi: 10.1172/JCI109771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbourne T., Weber M., Bank N. The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest. 1972 Jul;51(7):1852–1860. doi: 10.1172/JCI106987. [DOI] [PMC free article] [PubMed] [Google Scholar]



