Abstract
Conductive hydrogels (CHs) are emerging as a promising and well‐utilized platform for 3D cell culture and tissue engineering to incorporate electron signals as biorelevant physical cues. In conventional covalently crosslinked conductive hydrogels, the network dynamics (e.g., stress relaxation, shear shining, and self‐healing) required for complex cellular functions and many biomedical utilities (e.g., injection) cannot be easily realized. In contrast, dynamic conductive hydrogels (DCHs) are fabricated by dynamic and reversible crosslinks. By allowing for the breaking and reforming of the reversible linkages, DCHs can provide dynamic environments for cellular functions while maintaining matrix integrity. These dynamic materials can mimic some properties of native tissues, making them well‐suited for several biotechnological and medical applications. An overview of the design, synthesis, and engineering of DCHs is presented in this review, focusing on the different dynamic crosslinking mechanisms of DCHs and their biomedical applications.
Keywords: biomedical applications, conductive hydrogels, extracellular matrix, reversible networks
Dynamic conductive hydrogels (DCHs) are emerging as a promising and well‐utilized platform for 3D cell culture and tissue engineering to incorporate electron signals as biorelevant physical cues. DCHs can provide dynamic environments for cellular functions while maintaining matrix integrity. An overview of the design, synthesis, and engineering of DCHs is presented in this review.
1. Introduction
Cells grow in vivo in a complex and dynamic microenvironment composed of extracellular matrix (ECM), soluble factors, and neighboring cells.[ 1 , 2 , 3 ] For decades, cells have mostly been cultured and studied on 2D culture substrates, which are very different from their native dynamic environments in 3D. There is increasing evidence that cells are modulated by a well‐structured specific microenvironment of adjoining cells, associated ECM, and numerous biochemical and mechanophysical cues.[ 4 , 5 , 6 ] The dynamic microenvironments support structural rearrangement and the transmission of various cell growth and metabolism signals. The cell adhesion ligands, topological features, mechanical support, and degradability of ECM guide the morphology, proliferation, migration, differentiation, and apoptosis of cells.[ 7 , 8 , 9 ] Therefore, the scientific focus is directed at investigating biomaterials that mimic the dynamic structure and functions of natural ECM.[ 10 , 11 , 12 ] Due to their high water content, compatible mechanical properties, and resemblance to biological tissues, hydrogels are attractive candidates for developing 3D ECM mimics.[ 13 , 14 , 15 , 16 ] Soft materials can be tailored to minimize biomechanical mismatch with biological tissues, which can be designed and fabricated to mimic wet and ion‐rich physiological environments.[ 17 ]
Different hydrogels have been developed to meet the requirements for specific biomedical applications. For instance, hydrogels can be tailored to be responsive to environmental stimuli such as temperature, pH, mechanical treatment, and electric as well as magnetic fields and to transfer the signals to cells.[ 18 , 19 , 20 , 21 ] Conductive hydrogels (CHs) have recently attracted much attention in bioelectronics due to the constant endeavor toward the seamless interfacing between biology and electronics. Conductivity is a highly anticipated functionality of hydrogels since conductivity is useful in various fields, such as biomedicine, wearable bioelectronics, and electrical processing (biosensors).[ 22 , 23 , 24 ] Therefore, CHs with sufficient electrical conductivity and flexible mechanical properties similar to biological tissue have generated significant research interest.[ 11 , 25 , 26 , 27 ] However, most conventional CHs lack dynamic properties. Dynamic properties mean that the material can adapt to the dynamic biological environment, coordinate with the tissue engineering process, and rearrange after damage (self‐healing).[ 28 , 29 , 30 ] Dynamic behavior can be realized by designing reversible networks, such as dynamic covalent bonds and noncovalent interactions. Over the past five years, intensive research has been performed on dynamic conductive hydrogels (DCHs) to combine the benefits of electrical conductivity and various biofunctionalities. This review highlights the recent achievements in DCHs (from chemical interactions to biomedical applications). The categories of DCHs are based on the design criteria of DCHs, dynamic crosslinking mechanisms, and their applications in biomedical fields.
2. Design Criteria of Dynamic Conductive Hydrogels
This section discusses the design criteria for fabricating DCHs. As 3D scaffolds for cell culture, it is important to satisfy the requirements for ECM‐mimetic materials, including cytocompatibility, mild crosslinking conditions, a proper gelation rate for cell encapsulation in 3D cell culture, and homogeneous dispersion. Other considerations required for DCHs regarding dynamic reversible linkages include the crosslinking kinetics, tunable mechanical properties, self‐healing properties, and good conductivity. As shown in Figure 1 , various crosslinking mechanisms have been exploited to form DCHs, including chemical and physical crosslinking. Based on the crosslinking mechanisms, DCHs can be classified into two types: physical crosslinked DCHs and reversible covalent crosslinked DCHs. Physical crosslinked DCHs form their 3D networks through dynamic noncovalent interactions, such as ionic and metal‐coordination interactions, hydrogen bonds, π−π stacking, hydrophobic, and host−guest interactions. Reversible covalent crosslinked DCHs form their 3D networks through dynamic covalent bonds, such as Schiff, disulfide, and boronate ester bonds.
Figure 1.
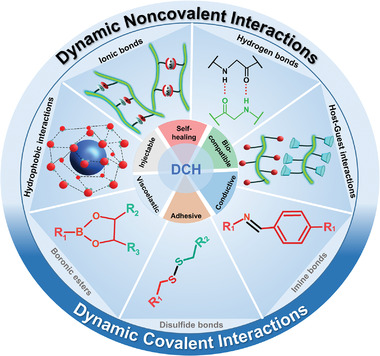
Different reversible covalent bonds and noncovalent interactions used in assembling dynamic conductive hydrogels (DCHs).
2.1. Crosslinking Kinetics and Stability
The kinetic rate constants of the physical interactions or the reversible covalent chemical bonds are important parameters to consider when designing a DCH. The network design should balance its dynamics and stability, where the cells can spread, migrate, and proliferate due to reversible linkages. Due to the weak noncovalent bonds, DCHs are often mechanically less stable than covalently crosslinked polymers, while their stability in cell culture and in vivo can also be largely regulated by their degradability by enzymes. On the one hand, if the covalent bonds are too stable, the linkages do not undergo significant breakage, and the hydrogel will function similarly to a permanently crosslinked CH. On the other hand, the strength of reversible linkages must be sufficient to permit the formation of a stable DCH that can maintain its bulk integrity during cell culture or tissue engineering. This is a particularly important concern for physically crosslinked hydrogels, which can have relatively fast erosion rates when exposed to fluid shear force.[ 31 , 32 , 33 ] A way of enhancing the physical stability of the network is by tuning its degree of crosslinking. This can be achieved by either increasing the relative functional groups per polymer molecule or increasing the relevant crosslinker concentrations. Alternatively, stable DCHs can be designed by combining two or more crosslinking mechanisms to achieve hydrogels with slow erosion rates and by using polymers with low enzymatic degradability.
2.2. Mechanical Properties
The mechanical properties of the ECM affect cell behavior. The influence of biomaterial rigidity on stem cell survival and differentiation has been extensively studied in hydrogels.[ 34 , 35 ] Recent studies have shown that the intensity of the interaction between the substrate and the cell surface receptor, rather than the substrate hardness, may play an important role in cell adhesion and differentiation.[ 36 , 37 , 38 ] These studies are of great significance for understanding the general role of mechanical cues in cell behavior and mechanical transduction. Therefore, mechanical properties are among the most critical considerations when designing DCHs for recapitulating 3D cell microenvironments. Additionally, mechanical and rheological properties are essential for DCH processing, engineering, and application in tissue engineering, including printability for additive manufacturing and injectability for their minimally invasive clinical utility.
-
1)
Elasticity, characterized by stress–strain curves and often described as the stiffness or Young's modulus, is one of the most studied mechanical properties of hydrogels for mimicking cell microenvironments and native tissues.[ 39 ] This reflects a hydrogel's ability to withstand deformation and return to its original state when the external force is withdrawn. Viscoelasticity is the property of materials that exhibit both viscous (characterized by viscosity; the viscosity of a fluid is a measure of its resistance to deformation at a given rate) and elastic characteristics when undergoing deformation.[ 40 , 41 ] Whereas most conductive materials are rigid and nonelastic, such as metal electrodes, the elasticity and viscoelasticity of CHs and DCHs can be controlled by varying the polymer network. CHs with stable covalent crosslinks will typically be stiffer than DCHs with reversible covalent crosslinks, while physically crosslinked DCHs are often the weakest in terms of mechanical strength. Moreover, the desired DCH system should possess good tunability of the crosslinking density to form soft materials to minimize the mismatch between soft tissues and rigid electronics.
-
2)
In addition to elasticity and viscoelasticity, most soft tissues also exhibit stress relaxation properties. DCHs, especially physically crosslinked hydrogels, are viscoelastic and exhibit stress relaxation, which means that in response to applied strain, the stress decreases, also known as creep behavior (i.e., the tendency to undergo permanent deformation in response to applied stress).[ 34 , 37 , 42 ] The DCH stress relaxation can result from numerous dissipative events, such as polymer disentanglement, weak physical interactions, and rearrangement of the reversible network. Additionally, the degradation of the polymer can also affect the network viscoelasticity and cause stress relaxation. The stress relaxation behavior of DCHs can be optimized by varying the hydrogel composition or concentration, polymer chain length, and crosslinking density.
-
3)
Shear‐thinning behavior is an essential rheological property for developing injectable materials and bioinks for 2D/3D printing.[ 43 , 44 ] Noncovalent self‐assembly is the main route to design shear‐thinning hydrogels. Individual interactions are usually relatively weak, but they can jointly lead to a stable network structure. Shear thinning describes the non‐Newtonian behavior of a polymer network whose viscosity decreases under shear strain. The shear‐thinning process is highly nonlinear and can be expressed by a nonsinusoidal response under an applied sinusoidal shear force. In the cases of DCHs, due to their dynamic nature, the formed network can be dissociated under an applied shear force. After the shearing force is removed, these networks will be reassembled into DCHs with almost the same gel properties (self‐healing).
2.3. Self‐Healing
The rheological feature of self‐healing means that a polymer network can autonomously recover its original state after being damaged.[ 29 ] The self‐healing behavior of DCHs is caused by reversible interactions (reversible chemical bonds and noncovalent interactions). Reversible chemical bonds, such as acyl hydrazone, Schiff base, borate ester, and disulfide bonds (S—S), and noncovalent interactions, such as hydrogen bonds, ionic interactions, metal coordination, host–guest interactions, and hydrophobic interactions, have been thoroughly investigated for fabricating self‐healing materials.
Materials with both electroconductivity and self‐healing capability are highly desirable for biomedical applications in vivo. Their structural reliability can be monitored through electronic assessment to identify and quantify defects such as microcracks. Single‐walled carbon nanotubes (SWCNTs) were used by Guo et al. to design DCH by connecting adamantine‐modified poly(2‐hydroxyethyl methacrylate) (PHEMA) and β‐cyclodextrin‐decorated SWCNTs through host‐guest interactions.[ 45 ] This PHEMA–SWCNT DCH exhibits proximity sensitivity, humidity sensitivity, and electrical conductivity and can self‐heal quickly. The electrical healing efficiency for all specimens with different amounts of SWCNTs was nearly 95 %, which indicated that most conduction paths reformed through the healing process. However, unlike the recovery of conductivity, the recovery of mechanical properties of PHEMA–SWCNT DCH was slower and less efficient. Interestingly, the SWCNT contents in the composites affect the mechanical healing efficiency. They have demonstrated that the mechanical healing efficiency increases with an increase in the mass fraction of SWCNTs.
A DCH based on a self‐assembled supramolecular gel and nanostructure polypyrrole (PPy) was developed by Shi et al, which synergizes the dynamic assembly and disassembly nature of the metal–ligand supramolecule and the conductive nanostructure of PPy hydrogel. The material shows high conductivity (12 S m−1) and exhibits mechanical and electrical self‐healing properties.[ 46 ] They demonstrated that a self‐crosslinked supramolecule gel helps to support the PPy network by wrapping and connecting PPy particles as well as building a self‐supported network. The PPy matrix also plays an important role in self‐healing because its hierarchically porous structure could facilitate molecular motion and transportation at the cracks, and its tough nature also favors mechanical healing behavior.
The storage modulus, stress relaxation, shear‐thinning, and self‐healing properties are essential parameters determining the applicability of biomaterials as an injectable material to deliver encapsulated drugs or cells. For drug delivery in vivo, slow self‐healing may lead to DCH degradation under fluid shear flow. For delivering cells with DCHs, in addition to the recovery rate, stress dissipation of the applied force is crucial for cell survival, as shear stress can cause mechanical damage to cells during cell delivery via injection.
2.4. Conductivity
Since many mobile ion species as electrolytes are dissolved in body fluids, biological tissues are considered to be volume conductors with medium conductivity (e.g., conductivity = 10–6 to 10–4 S m–1).[ 47 ] As electrons do not act as charge carriers, the electrical communication between cells mainly depends on ion flux. Metal electrodes are the most widely used terminals to create electrical connections between tissues and external electronics, through which signals and information can be transmitted from cells to electronics and vice versa.[ 48 , 49 , 50 ] Different from tissues, most electronic devices depend on electronically conductive materials, such as metals, in which free electrons serve as carriers of mobile charges. This characteristic disparity results in an interface that distinguishes the tissue from the electrode. Therefore, to minimize the mismatch associated with such interfaces in tissue engineering, it is important to introduce an electroconductive matrix into ion‐conductive matrices.
Electrical communication between cells in tissues is mainly mediated by ion channels. Electroconductive materials and electric stimulations can affect ionic currents, thus influencing various pathophysiological processes, including wound healing and tissue regeneration.[ 51 , 52 , 53 , 54 ] Biomimetic conductive materials are usually made by doping biopolymers with conductive components (e.g., conductive polymers, gold nanoparticles (AuNPs), carbon nanotubes (CNTs), and graphene). In DCHs, these conductive components can be tuned to tailor the material to realize a dynamic, biocompatible, and conductive microenvironment for specific biomedical applications.
Most studies measure biomaterial conductivity without distinguishing the electroconductivity and ionic conductivity. However, conductive biomaterials, especially conductive hydrogels under physiological conditions, are a combination of electronic conductivity and ionic conductivity, which should be characterized separately in the future. This is important for both basic and applied research in the field of electrophysiology.
2.5. Biocompatibility
Biocompatibility refers to the ability of biomaterials to cause an appropriate host response in biological systems, for example, as implants in the human body.[ 55 , 56 ] Modern approaches for testing the compatibility and suitability of biomaterials in vivo increasingly use complementary multimodal analysis by means of dedicated tomography systems for experimental investigations in small animals or with the corresponding clinical setting in humans. As an example in this context, host tissue responses can be investigated by magnetic resonance imaging of inguinal lymph nodes, as swollen lymph nodes indicate systemic inflammatory responses. In addition, the application of fluorescent agents or even radiotracers, such as [18F]fluorodeoxyglucose, enables the analysis of vascularization and inflammatory processes in vivo by optical imaging or positron emission tomography, respectively.[ 57 ] Furthermore, immunohistochemical staining of inflammation, angiogenesis, and proliferation markers or subcutaneous connective tissue layers on resected tissue samples with remaining biomaterials provide ex vivo experimental information on evoked tissue responses, capsule formation, and biocompatibility.[ 57 , 58 , 59 ]
To realize seamless contacts with living tissues, DCH‐based bioelectronics devices require good biocompatibility for long‐term and safe biomedical applications. Most conductive polymers used in the fabrication of DCHs are known to be noncytotoxic. The currently available conductive polymers have allowed the development of various cytocompatible organic electronics. However, the biocompatibility of conductive polymers in vivo is determined by many criteria, including the conductive polymer chemical composition, degradability, polymer charge, and acidity.[ 11 , 52 , 60 , 61 ] Depending on the chosen conductive polymer and the synthetic route, DCHs may contain toxic monomers, solvents, and surfactants, which will be released gradually and become toxic to the surrounding tissue. Therefore, synthetic methods must be optimized to achieve excellent biocompatibility or cytocompatibility of the resulting biomaterials. For instance, poly(3,4‐ethylene dioxythiophene) (PEDOT) has been electrochemically polymerized around neurons and can maintain the high viability of the cells. CHs based on poly(3,4‐ethylene dioxythiophene) polystyrene sulfonate (PEDOT:PSS) have been successfully used as biosensors at both the cell and tissue levels.[ 62 , 63 , 64 ]
To enhance the biocompatibility of conducting polymers, many approaches have been proposed: 1) to modify conductive polymers with biomolecules as synthetic precursors, including peptides, proteins, and polysaccharides[ 65 , 66 , 67 ] and 2) to combine conductive polymers with highly biocompatible biomaterial systems, including ECM basement gels, ECM‐derived collagen, and polysaccharide‐based gels.[ 11 , 68 , 69 ] It is important to note that material biocompatibility studies are at the frontiers of many disciplines, including polymer sciences, bioanalysis and bioimaging, immunology, and stem cell biology. The knowledge‐based rational design approach needs to be further combined with the screening method to develop tailored materials for specific applications.[ 70 ]
3. Mechanism and Preparation of DCHs
3.1. Physically Crosslinked DCHs
Physical crosslinking, such as ionic interactions, metal coordination, molecular recognition (host–guest interactions), and hydrogen bonding, have been widely utilized to synthesize DCHs. Table 1 lists some recently reported DCHs crosslinked primarily through noncovalent interactions.
Table 1.
Physical crosslinks, their compositions, and properties that can be utilized in building DCHs. Selected references are provided
| Physical crosslinks | Composition | Properties | Applications | Conductivity | Ref. |
|---|---|---|---|---|---|
| Ionic and metal‐coordination interactions |
PEG‐peptide PEDOT:PSS |
Self‐healing Injectable Cytocompatible |
MSC 2D and 3D cell culture, MSC 3D differentiation under electrical stimulation | 1.4×10–2 S cm–1 | [ 71 ] |
|
PEDOT:PSS PPY Mg+2 |
Cytocompatible Mg biobattery |
Mechanically robust Cytocompatible biointerface for hADSCs proliferation |
‐ | [ 72 ] | |
|
Alg SiO2 nanofibers Al3+ |
Injectable Compressible Shape memory |
Wearable electronics | 1.85 S cm–1 | [ 73 ] | |
|
PEDOT:PSS Collagen Alginate |
Cytocompatible |
2D and 3D IPS Cell culture IPS cell differentiation in 2D |
35 ± 11 × 10−4 S cm–1 | [ 74 ] | |
| Host–guest interactions |
β‐CD NIPAM CNT PPY |
Self‐healing Compressible Injectable Cytocompatible |
Wearable electronics 2D cell culture |
Up to 34.93 S cm–1 | [ 75 ] |
|
PEDOT:S‐Alg‐Ad Pβ‐CD |
Injectable Self‐healing Cytocompatible |
3D cell culture Cell differentiation in 3D |
0.16 S cm–1 | [ 76 ] | |
|
α‐CD NIPAM PANI |
Temperature‐responsive Stretchable Self‐healing |
Temperature‐triggered electric switch |
0.64 S cm–1 | [ 77 ] | |
| Hydrophobic interactions | PEDOT:PSS |
Injectable Self‐healing Cytocompatible |
Organic electrochemical transistors (OECTs) 2D cell culture |
≈10−1 S cm–1 | [ 78 ] |
| PEDOT:PSS | Stretchable | 3D printing | 20–40 S cm–1 | [ 79 ] | |
| PEDOT:PSS |
3D printability Long‐term stability |
Bioelectronic sensor | 28 S cm–1 | [ 80 ] | |
| Hydrogen bond |
PPGS PEDOT:PSS |
Injectable Cytocompatible Self‐healable Hemocompatible |
2D cell culture Wound‐healing in vivo |
2.22 mS cm–1 | [ 81 ] |
|
Upy PSS PANI |
Stretchability Injectability Self‐healing Thermosensitive |
Wearable electronics | 1.1–13 S m–1 | ||
|
TA CNT Glycerol PVA |
Anti‐freezing Stretchable |
Wearable electronics Electricity generation from moisture |
5.13 S m–1 | [ 83 ] | |
|
TiO2 Chitosan PVA |
UV‐switchable wettability Adhesive |
Wearable electronics |
3.641 × 10−4 to 1.347 × 10−3 S cm–1 |
[ 84 ] |
3.1.1. Ionic and Metal‐Coordination Interactions
Ionic interactions have often been used in hydrogel synthesis to crosslink polysaccharides such as alginate (Alg) and chitosan. PEDOT:PSS is one of the most widely used conductive polymers,[ 85 ] in which hydrophobic PEDOT is coiled and surrounded by hydrophilic PSS shells, resulting in the suspension of nanoparticles in an aqueous buffer. The anionic PSS chains can interact with positively charged metal ions. Wallace et al. reported DCHs based on ionic and metal‐coordination interactions. First, Mg2+ and PEDOT:PSS solutions were mixed to form the pregel solution after ion crosslinking.[ 74 ] Interestingly, even when the concentration of Mg2+ was too low to initiate the gelation of PEDOT:PSS, it was essential to maintain a uniform hydrogel without local gelation upon increasing the Mg2+ concentration. To improve the gel's mechanical strength and electrochemical properties, the degree of crosslinking can be increased along with the concentration of Mg2+. After immersing the gel in pyrrole, additional electropolymerization is performed to form PPy/PEDOT hydrogel. This work demonstrates that ionic interactions can be used to control the properties of DCHs. It is feasible to achieve the desired size and shape of hydrogels by implementing a two‐step crosslinking process. The hydrogel scaffold is reinforced by the addition of PPy, demonstrating the enhancement of mechanical properties for application as a stable biointerface. Due to the 3D porous structure of the DCH, the battery assembled by this DCH demonstrated high energy density and good capacity.
In addition to metal ion interactions, organic ion interactions have also been reported to prepare DCHs. Our group reported a DCH synthesized by using peptide–polyethylene glycol (peptide‐PEG) and PEDOT:PSS.[ 71 ] The noncovalent DCH was gelated fast by utilizing ionic interactions and molecular self‐assembly between the cationic PEG‐peptide and anionic PEDOT:PSS solutions (Figure 2a). The peptide and PEDOT:PSS‐based DCH demonstrate self‐healing and injectable properties as well as excellent biocompatibility in vitro.
Figure 2.
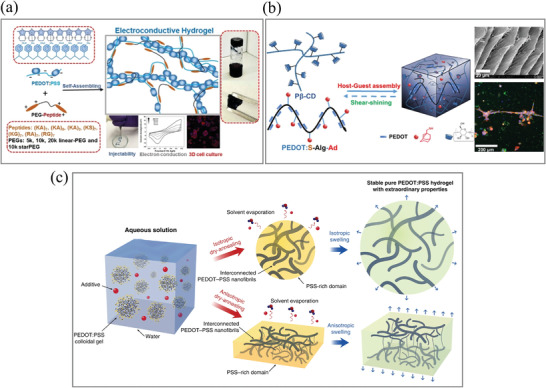
a) PEG–peptide and PEDOT:PSS interaction form a DCH via a self‐assembly process. The DCHs are self‐healable and injectable due to the reversible non‐covalent interactions and are utilized as a 3D cell culture matrix with high biocompatibility in vitro. Adapted with permission.[ 71 ] Copyright 2018, American Chemical Society. b) Pβ‐CD and Ad‐modified conductive polymer (PEDOT:S‐Alg‐Ad) formed a DCH. Adapted with permission.[ 76 ] Copyright 2019, American Chemical Society. c) The process to prepare the pure a PEDOT:PSS hydrogel with DMSO as an additive. Adapted with permission.[ 79 ] Copyright 2019, Springer Nature.
3.1.2. Host–Guest Interactions
Host–guest interactions are a type of noncovalent interaction with broad applications in the fabrication of reversible hydrogels. A host molecule interacts with a guest molecule to bind internally to form an inclusion complex. Cyclodextrins (CDs) are a family of cyclic oligosaccharides formed by six or more glucopyranose molecules. They are produced from starch by cyclodextrin glucosyltransferase.[ 86 ] There are three major types of CDs: α‐cyclodextrin (6 D‐glucose repeating units), β‐cyclodextrin (7 D‐glucose repeating units), and γ‐cyclodextrin (8 D‐glucose repeating units), which are named based on the number of anhydroglucose units.[ 86 , 87 , 88 ] The structure of the CD is similar to a hollow circular table. The hydrogen atoms in its C3 and C5 hydroxyl groups and the oxygen atoms in glycosidic bonds form an internal hydrophobic cavity. The external surface is hydrophilic due to the presence of hydroxyl groups. This unique amphiphilic structure makes CD a suitable host molecule to form inclusion complexes with many hydrophobic small guest molecules.[ 88 , 89 ] Polymer chains modified with hydrophobic groups, such as adamantane (Ad) and azobenzene, and some modified conductive monomers, such as PEDOT[ 90 ] or graphene,[ 91 ] have been reported to form host–guest interaction‐based DCHs when mixed with polymers modified with CD monomers. Such hydrogels have shear‐thinning properties and are thus injectable. However, because of the relatively weak host–guest interaction, self‐assembled host–guest hydrogels are mechanically soft and exhibit high erosion rates, inhibiting their use in long‐term cell culture experiments. The stability of the hydrogel can be increased with secondary covalent crosslinking to further modulate its mechanical properties[ 75 ] at the cost of reducing network reversibility.
Guo et al. reported a DCH that can recover after stretching and compression. The hydrogel is based on the host−guest interactions between β‐cyclodextrin (β‐CD) and the hydrophobic isopropyl group of N‐isopropyl acrylamide (NIPAM). With the addition of nanostructured PPy and multiwalled carbon nanotubes, the resulting hydrogel is responsive to multiple stimuli (heat and near‐infrared light) and has self‐healing properties, tested by rheological step–strain measurements.[ 75 ] Our group developed a DCH through host–guest interactions.[ 78 ] As shown in Figure 2b, in the presence of adamantyl‐modified sulfated alginate (S–Alg–Ad), a bioelectroconductive polymer (PEDOT:S–Alg–Ad) was synthesized by 3,4‐ethylene dioxythiophene (EDOT) oxidative polymerization. In rheological tests, the viscosity of the hydrogels decreases with increasing shear rate, demonstrating their shear‐thinning behavior. The mechanical properties can be further fine‐tuned by adjusting the hydrogel composition (concentrations and ratios of host and guest polymers). These properties allow the DCH to readily encapsulate cells and to be injectable via a 27G needle. This hydrogel demonstrated a quick self‐healing capability that can also be recovered through multiple cycles of alternate step–strain switches via rheological testing. The physical crosslinks at the interface could be restored through host–guest interactions. Due to the involvement of π–π stacking and sulfate anion–π interactions, the mechanical stability of the hydrogel is better than those of many host–guest structures. The viability, proliferation, and differentiation of encapsulated C2C12 cells in vitro are also assisted by the hydrogel matrices. Interestingly, the presence of PEDOT will significantly lead to the formation of myotube‐shaped structures.
3.1.3. Hydrophobic Interactions
Self‐assembly by hydrophobic interactions is a very general mechanism for fabricating DCHs. Although they are less specific than host–guest interactions, when amphiphilic biopolymers are engineered appropriately, they are good candidates for the assembly of noncovalent hydrogel networks. By treating commercially available PEDOT:PSS with H2SO4 (0.1 m) at 90 °C, the self‐assembled pure PEDOT:PSS hydrogels demonstrated a high water content of 99.22 wt% and conductivity of 46 S m−1, as heating and acidic media induce π–π stacking and hydrophobic attractions between the PEDOT particles.[ 92 ] Khademhosseini et al. recently reported a PEDOT:PSS hydrogel formed at room temperature.[ 78 ] The gels were developed by adding a surfactant (4‐dodecyl benzene sulfonic acid, DBSA) to PEDOT:PSS. For gelation, the concentration of DBSA needs to reach a threshold value at which it provides sufficient H+ to protonate the negatively charged PSS. Paired with the remaining surfactant micelles (headed with negatively charged sulfuric acid groups), the increased ionic strength is expected to weaken the electrostatic attraction between PEDOT and PSS chains. In turn, this would facilitate the formation of a 3D network between the exposed PEDOT molecules, promoting the physical crosslinking caused by π‐π–stacking and hydrophobic attractions. The authors have demonstrated that the PEDOT:PSS formed at room temperature can be utilized for developing soft and self‐healable bioelectronic devices based on hydrogels. Zhao et al. reported a pure PEDOT:PSS hydrogel that can be used for 3D printing by drying and swelling a mix of aqueous PEDOT:PSS with volatile dimethyl sulfoxide (DMSO).[ 79 ] By choosing different dry‐annealing methods, the electrical, mechanical, and swelling properties of the resulting hydrogel can be tuned. Another way to methodically tune the properties of hydrogels is by modifying the proportion of DMSO, as it enables the recrystallization of the aqueous PEDOT:PSS solution during the dry‐annealing process. This results in the rearrangement of PEDOT and PSS chains that can assemble into a pure PEDOT:PSS hydrogel due to π–π stacking and hydrophobic interactions.
3.1.4. Hydrogen Bonds
Among the dynamic bonding mechanisms, hydrogen bonds represent the most common interactions.[ 97 ] Individual hydrogen bonds in hydrogel systems are usually weak but can achieve mechanically robust hydrogels by being synergistically combined with other types of chemical interactions. Different materials can be incorporated into a hydrogen bond‐based network to improve the mechanical and conductive properties of hydrogels, such as graphene oxide (GO). These nanosheets contain abundant oxygen‐containing moieties on their surfaces[ 98 ] and can be used as crosslinkers to prepare conductive composite hydrogels.[ 95 , 96 , 97 ] Furthermore, Jiang et al. recently reported PEDOT:PSS/guar slime (PPGS) as an injectable, biocompatible, self‐healable, and conductive material that can be used for wound healing. As a proof‐of‐principle assay, the healing effect of the PPGS DCH was explored in a skin wound model in rats, where it showed a faster healing rate.[ 83 ]
Zeng et al. reported a DCH based on hydrogen bonds by incorporating multiple 2‐ureido‐4[1H]‐pyrimidinone (UPy) groups as crosslinking points into a brittle polyaniline/poly(4‐styrene sulfonate) (PANI/PSS) network. Due to its linear mechanical properties and electronic conductivity, the interpenetrated PANI/PSS network can accurately and reliably detect various human motions. By combining supramolecular chemistry and conductive polymers, it is possible to develop hydrogel networks with multiple functionalities. These new tools provide fresh insights into the design of advanced functional materials that can be applied to multiple fields as 3D printable materials and for the design of wearable and flexible electronic devices.[ 82 ]
3.2. Dynamic Chemical Bonding‐Based DCHs
Reversible covalent bonds, such as Schiff base, disulfide, and borate ester bonds, can be used to prepare DCHs (Table 2 ). The breaking and reformation of these bonds are in an equilibrium state under specific conditions (i.e., pH), which cannot be shifted under mechanical force, thus offering high stability of the resulting dynamic network.
Table 2.
Chemical crosslinks, composition, properties, and dynamic covalent reactions that can be utilized in building DCHs. Selected references are provided
| Chemical crosslinks | Composition | Properties | Applications | Conductivity | Ref. |
|---|---|---|---|---|---|
|
Schiff base (secondary imine) |
Chitosan‐graft‐aniline Benzaldehyde‐capped‐PEG |
Self‐healing Antibacterial Adhesive Injectable In vivo degradable Cytocompatible |
Cell delivery for cell therapy in vivo | 2.29 × 10−3 to 2.42 × 10−3 S cm–1 | [ 98 ] |
|
N‐carboxyethyl chitosan PF127 CNTs |
Self‐healing Adhesive Hemocompatible Cytocompatible |
Wound healing in vivo Drug delivery |
1.37 × 10−3 to 8.45×10−3 S m–1 |
[ 99 ] | |
|
OSA PAM |
Self‐healing | ‐ | 0.00152 S cm–1 | [ 100 ] | |
|
Quaternized chitosan‐g‐polyaniline PEG‐copoly(glycerol sebacate) 4‐Formylbenzoic acid |
Self‐healing Adhesive Hemocompatible Cytocompatible |
In vivo wound healing | Up to 3.5 mS cm–1 | [ 101 ] | |
|
Chitosan‐graft‐PANI Oxidized dextran |
Antibacterial Cytocompatible In vivo degradable In vivo biocompatible. |
Drug release in vitro | 6.9×10–2 S m–1 to 7.9×10–2 S m–1 | [ 102 ] | |
|
Partially oxidized Alg Gelatin TA 2‐Aminopyridine‐5‐thiocarboxamide |
Injectable In vitro degradable Cytocompatible In vivo compatible |
Drug and cell delivery for cell therapy in vivo | Up to 3.7 ± 0.3 × 10−4 S cm–1 | [ 103 ] | |
| Disulfide bonds |
α‐lipoic acid AA Choline chloride Fe3+ |
Stretchable Self‐healing |
Wearable electronics |
2.4 × 10−3 to 2.8 × 10−3 S m–1 |
[ 104 ] |
|
PEGDA Cystamine TA Thiol‐modified HA |
Cytocompatible in vitro Biocompatible in vivo Biodegradable in vitro |
Cell delivery in vivo. | Up to 9.6 × 10−3 S cm–1 | [ 105 ] | |
|
Liquid‐metal‐embedded sulfur polymer |
Self‐healing | ‐ | 0.43–1.8 × 103 S m−1 | [ 106 ] | |
|
2,3‐Dimercapto‐ 1‐propanol meso‐2,3‐Dimercaptosuccinic acid Hydrobromic acid |
Self‐healing Stretchable Injectable Cytocompatible Adhesive Biodegradable in vitro |
0.0028 S cm–1 | [ 107 ] | ||
| Dynamic borate ester bond |
CNT Graphene Silver nanowire |
Self‐healing Stretchable |
Wearable bioelectronics | ‐ | [ 108 ] |
|
Guanosine Potassium tetrahydroxyborate PANI |
3D printable |
Glucose biosensor (GOx) Improved functionality of hemin compared to free enzyme |
‐ | [ 109 ] | |
|
PPy Cellulose nanofibers PVA Borax |
Self‐healing pH‐sensitive Cytocompatibility |
‐ | 1.5 to 4.8 S m–1 | [ 110 ] | |
|
PVA PAA Borax KCl |
Self‐healable | Capacitor prototype | 41.6 mS cm–1 | [ 111 ] |
3.2.1. Schiff Base Bonds
The reversible chemical bond of the Schiff base forms between an amine and an aldehyde, while the acyl hydrazone bond forms between an aldehyde and a hydrazide. The injectability and self‐healing properties of hydrogels formed by Schiff base bonds have been well studied. Recently, Guo et al. reported a conductive self‐healing hydrogel based on Schiff's base reaction (Figure 3a).[ 102 ] First, the aniline oligomer was grafted to chitosan, while PEG was functionalized with benzaldehyde. The two components are then mixed to synthesize hydrogels via Schiff base formation. Self‐healing behavior could be realized in this covalently crosslinked DCH system. Another chitosan‐based DCH was developed using CNTs as the conductive component.[ 116 ] DCHs were prepared by mixing an N‐carboxyethyl chitosan solution and a block polymer PF127‐CHO/CNT suspension. The hydrogel networks were formed by a covalent Schiff base between the aldehyde from the PF127‐CHO micelle and the amino groups from N‐carboxyethyl chitosan. PF127 was selected as a crosslinker because of its good biocompatibility and ability to enhance the dispersity of CNTs. Fu et al. also reported a DCH based on Schiff base crosslinking. Acrylamide (AM) was polymerized in sodium alginate with an aldehyde group to form a dynamic hydrogel.[ 104 ] The synergy of the dynamic Schiff base bond and hydrogen bond in the hydrogel system contributed to the excellent self‐healing, toughness, and stretchability. However, the ability of the amide of AM to form a stable Schiff base remains to be demonstrated. Moreover, a general weakness of crosslinking by the Schiff base is its pH dependency and instability in acidic conditions, which might hinder some applications.[ 117 ]
Figure 3.
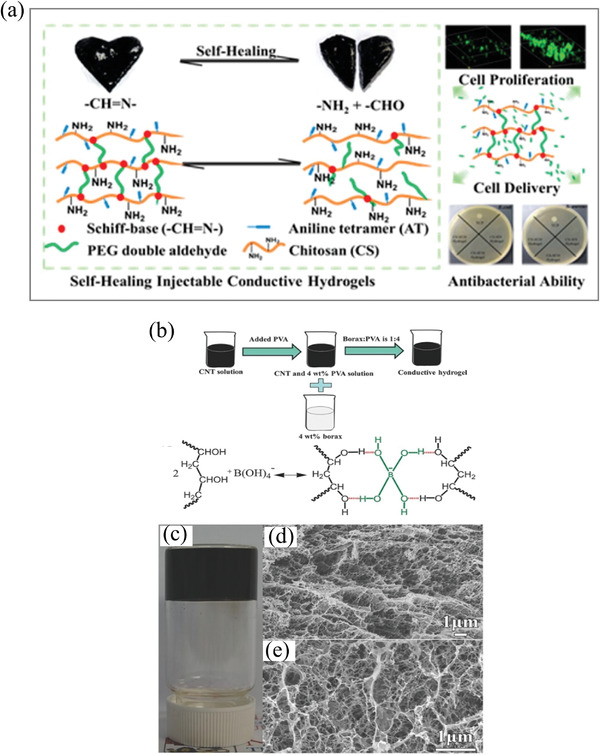
a) DCHs based on Schiff bases. DCHs were demonstrated with cell delivery and antibacterial applications. Adapted with permission.[ 98 ] Copyright 2016, American Chemical Society. b) The dynamic borate ester bond crosslinking reaction formed DCHs between PVA, SWCNT, and borate ester. c) Picture of SWCNT‐containing DCHs. d,e) Scanning electron microscopy (SEM) images of the SWCNT DCHs. Reproduced with permission.[ 108 ] Copyright 2017, Wiley‐VCH GmbH.
3.2.2. Disulfide Bonds
Disulfide bonds play a crucial role in protein folding and assembly. This S—S covalent bond is formed between the thiol side chains of cysteine residues of some proteins in the presence of a mild oxidative environment. However, it can also be adapted to design strategies for smart biomaterials and DCHs. To form disulfide crosslinks in a hydrogel, an oxidation agent must be present; oxygen itself is sufficient to achieve mild, cytocompatible crosslinking.[ 118–116 ] Disulfide cross‐linking has been used in different design strategies for smart biomaterials and can provide an opportunity to develop DCHs.[ 117 ]
Qi et al. reported a novel lipoic acid‐based ionic DCH with acrylic acid (AA), choline chloride, and ferric chloride (Fe3+).[ 108 ] The solvent‐free process is extremely facile, fast, low‐cost, and scalable. Transparent, highly stretchable, and self‐healing properties are simultaneously achieved in this ionic conductor because of the collective contribution from disulfide bonds, hydrogen bonds, and coordination bonds. The resulting material shows high sensitivity as strain sensors, which can be directly used as wearable electronics for detecting motions. Xin et al. also reported recyclable, conductive, and self‐healable materials based on liquid metal and dynamic disulfide bond formation.[ 110 ] Shang et al. reported injectable DCHs with disulfide bonds as a dynamic crosslinking for delivering adipose‐derived stem cells.[ 105 ]
The thiol–disulfide exchange reaction resulted in DCHs with fast gelation kinetics and high cytocompatibility with biomedical applications. Potential drawbacks of this crosslinking mechanism include off‐target reactions that can interfere with their bioactivity with encapsulated proteins and cells. The exchange of disulfide bonds can be accelerated by protein disulfide‐isomerases, a family of enzymes in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria. It will be very interesting to explore enzyme‐mediated network dynamics to develop responsive and adaptive smart materials in the future.
3.2.3. Dynamic Borate Ester Bonds
Boric acid is a neutral triangular plane sp2‐hybrid boron that is bonded with alkyl and two hydroxyl groups, serving as a Lewis acid because of the vacant p‐orbital at the boron center.[ 118 ] Due to the dynamic exchange between the free diol and boronic acid and condensated species, boronic esters are considered dynamic covalent structures.[ 118 , 119 , 120 ] Boronate esters constantly undergo dynamic rearrangement and can be used to form DCHs because of the low‐energy barrier between reactants and products. Lee et al. reported a DCH prepared by dynamic borate ester bonds. The DCH was formed through tetrafunctional borate ion interactions with the hydroxyl groups of polyvinyl alcohol (PVA), and CNTs were utilized as the conductive component (Figure 3b). The hydrogel showed high stretchability (strain up to 1000%) and exhibited good self‐healing capability that restored most of its original conductivity (98 %) in a very short time (3.2 s).[ 108 ] Similar DCHs have been reported by replacing CNTs with other conductive materials, such as PEDOT:PSS, PPy, and graphene.[ 121 , 122 , 123 , 124 , 125 ] All of these dynamic borate ester‐based DCHs exhibit fast self‐healing, good stretchability, and good conductivity.
3.3. Double‐Network DCHs
One of the major challenges of DCH design and development is the conflict among various features, including the mechanical strength, conductivity, stiffness, and high water content (>90 wt%). Highly conductive hydrogels tend to be brittle because of the high content of rigid conductive materials, while highly hydrated materials are often soft and less conductive. Another challenge is to combine fast network dynamics with high mechanical strength. Robust hydrogels are typically achieved by increasing the polymer concentration and degree of crosslinking, which will greatly reduce the dynamic interactions, impairing the shear‐thinning, self‐healing, and stress‐relaxation properties.
The double‐network strategy can be used to overcome some of the problems by combining a mechanically strong nonreversible network with reversible crosslinks. This is similar to the mechanism used by biological materials such as mussels and tendons to generate structural materials that are strong and tough at the same time. Materials fabricated with this strategy demonstrated enhanced mechanical properties, including a high elastic modulus (0.1–1.0 MPa), strength (1–10 MPa), stretchability (100–2000 %), and toughness (100–10 000 J m−2).[ 124 , 125 , 126 , 127 , 128 ] The reversible bonds can reform again after deformation, dissipating the stress accumulated in the matrix. Hence, the resulting network retains good shear‐shining and self‐healing properties. Developing conductive polymers with a double network will lead to conductive materials with robust mechanical properties.
In a recent study, a double‐network DCH was reported by Xing et al. PPy was conjugated to acylated chitosan to form PPy‐grafted chitosan. Acrylic acid was polymerized together with PPy‐grafted chitosan and Fe3+ (Figure 4 ). The synergy of reversible and irreversible bonds results in good mechanical properties that can be stretched up to 1500% and quickly recover its electrical and mechanical properties. In particular, the mechanical self‐healing efficiency was remarkable, and the electrical recovery efficiency was 96% (in 1 min).[ 129 ]
Figure 4.
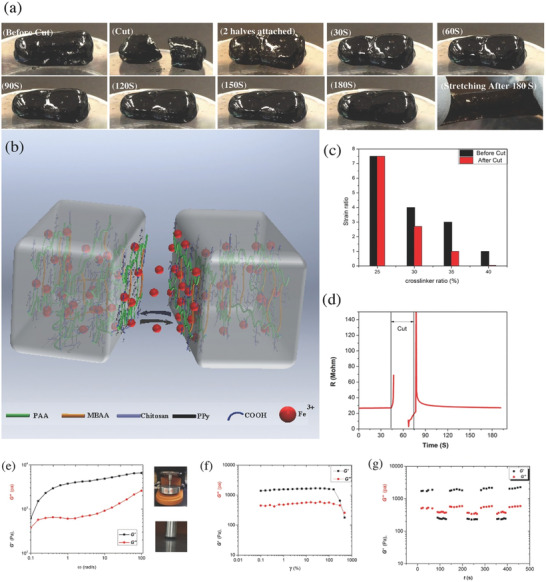
A double‐network DCH based on ionic interactions and radical polymerization. a) Picture of the self‐healing property of the double‐network DCH. b) Schematic demonstration of the self‐healing capability of the double‐network DCH. c) Capability of mechanical recovery after cutting and self‐healing. d) Capability of electrical recovery after cutting, showing that the electrical recovery efficiency is 96% within 1 min. e–g) Rheological testing of the double‐network DCH.Frequency sweep (e) and amplitude (f) sweep test. G′ and G″ of the double‐network DCH with cycle oscillation force (g). Adapted with permission.[ 129 ] Copyright 2017, Wiley‐VCH GmbH.
4. DCHs for Biomedical Applications
In DCHs, various ECM‐mimetic biochemical and mechanophysical cues, cytocompatibility in vitro and biocompatibility in vivo as well as desired electrochemical properties can be realized, enabling their use for different applications in the biomedical field, including 3D cell culture, tissue engineering, drug/cell delivery, wearable bioelectronics, and implantable bioelectronics. We will focus on the new developments of DCHs for biomedical applications since 2015.
4.1. DCHs for 3D Cell Culture
As described in the introduction, the ECMs are hydrated networks consisting of biomacromolecules, which provide biochemical and structural support for the cells. Recapitulating native ECMs with chemically defined synthetic polymers represents a long‐lasting challenge for cell cultures and tissue engineering. In general, desired ECM‐mimetic properties can be achieved by (1) programmable functionalization with modular building blocks to provide the ECM‐like supporting scaffold and (2) modulation of soluble factors. Thus, DCHs can be tailored to resemble a specific native environment and promote cell activities, including cell adhesion, migration, proliferation, differentiation, and protein secretion. Meanwhile, electrical stimulations can be applied, and electrical signals can be recorded.[ 5 , 17 , 130 , 131 ]
Since many types of cells, such as epithelial cells, fibroblasts, and cardiomyocytes, are sensitive to the material stiffness, tuning the mechanical properties of DCH would significantly impact cell behavior. Engel et al. reported a DCH composed of collagen, alginate, and PEDOT:PSS.[ 74 ] Materials with the desired stiffness and stress relaxation properties have been identified, exhibiting ECM–mimetic fibrous structures and enhanced electrical coupling in engineered cardiac tissue, resulting in markedly improved beating characteristics (Figure 5a). Improved cardiomyocyte maturation and beating properties have also been observed in human induced pluripotent stem cell‐derived cardiomyocytes.
Figure 5.
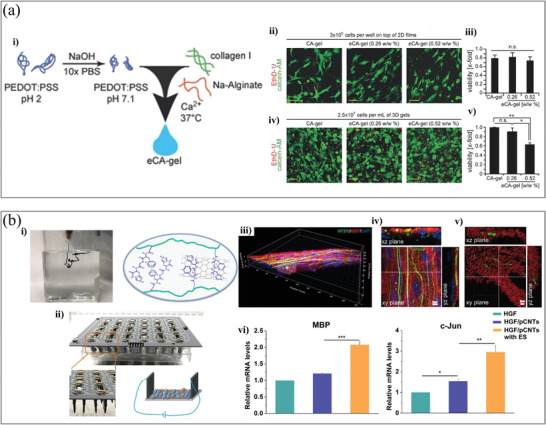
a‐i) Schematic illustration of DCH (eCA‐gel) preparation. ii) Representative live‐dead staining images of neonatal rat cardiac cells cultured on 2D at 48 h. iii) Quantitative analysis of cell viability. iv) Representative live‐dead staining images of neonatal rat cardiac cells in 3D cultured at 48 h. v) Cell viability of live‐dead staining (n = 3). Scale bars: 50 µm. Adapted with permission.[ 74 ] Copyright 2017, Wiley‐VCH. b‐i) The formation of DCH with the injection of pregel into PBS solution and a schematic illustration of the interaction mechanism. ii) Image of the electrical stimulation device for cell culture and a schematic illustration of cell culture under electrical stimulation. iii) The bundled axons in the DCH with 3D cell culture. The asterisk‐marked area in (iii) was investigated by analyzing iv) confocal images with high magnification of single optic section. v) The reconstructed axons and myelinated segments were colored green and red,respectively. vi) mRNA expression of MBP and c‐Jun genes. Adapted with permission.[ 132 ] Copyright 2020, American Chemical Society.
The microstructure can be beneficial for cell growth in 3D. Annabi et al. reported a DCH by gelatin methacryloyl (GelMA) and PEDOT:PSS, which was used to build conductive 3D C2C12 myoblast cell‐laden structures with bioprinting.[ 133 ] The bio‐ink was formed by two steps. First, PEDOT:PSS was crosslinked by bivalent calcium ions and a secondary photopolymerization step with visible light to crosslink GelMA after printing. He et al. developed a nanocomposite supramolecular DCH by homogeneously incorporating pristine carbon nanotubes into a functional self‐assembling peptide‐based matrix as a hybrid hydrogel with good injectability and conductivity.[ 132 ] Changing the type of peptide and concentration of pristine carbon nanotubes in the DCH can control the structural and mechanical properties. They showed that electrical stimulation (ES) can promote axon outgrowth and Schwann cell (SC) migration away from dorsal root ganglia spheres in 3D (Figure 5b), thus demonstrating that ES can enhance the interactions between SCs and axons, resulting in enhanced myelination.
4.2. DCHs Used as Adhesives for Bioelectronic Applications
Recently, adhesive hydrogel‐based bioelectronics has been promoted to enable a more robust and seamless tissue–device connection, particularly for tissues under deformation and stress, such as skin, myocardium, or skeletal muscles.[ 134 ] Adhesive hydrogel bioelectronics can ensure stable and conformal contact with tissue, reduced interface resistance from complex tissue, stable and continuous signal detection from human body motion, and a comfortable wearing experience. There are several considerations in the design of DCH‐based adhesive bioelectronics: 1) tunable adhesive strength, reversible adhesive property, and easy peel‐off without causing trauma to the tissue surfaces and 2) strong adhesion in wet environments, especially for implantable bioelectronics encountering body fluids and bleeding wounds.
Catechols such as dopamine (DA), gallol, and polyphenols (e.g., tannic acid (TA) and polydopamine (PDA)) are widely used in developing adhesives.[ 134 , 135 , 136 , 137 ] DA can be oxidized and adhere to various substrates, resulting in an outstanding adhesive ability via dynamic non‐covalent and covalent interactions with tissue surfaces.[ 136 ] Recently, mussel‐inspired dopamine‐based conductive hydrogels have generated significant interest due to their excellent adhesive performance associated with multiple noncovalent interactions (ionic interactions and hydrogen bonds, and π–π stacking).[ 140 , 141 , 142 , 143 ] Lu et al. reported a polyacrylamide hydrogel incorporating PDA and GO.[ 138 ] To achieve the resulting adhesive DCH, dopamine was polymerized to produce a PDA solution, to which GO was introduced and partially reduced (Figure 6a). This reduced GO (rGO) is the main factor responsible for the high conductivity of the hydrogel. For the acrylamide to polymerize and form the hydrogel, the monomer needs to be added along with an initiator and crosslinker. The final hydrogel showed high electrical conductivity and self‐healing properties when studied by tensile–heal–tensile mechanical tests. The maximum adhesive strength on porcine skin is 30 kPa. Alternatively, with the incorporation of gallol and metal iron, Burdick et al. reported an adhesive DCH by introducing microparticles of a hyaluronic acid (HA) hydrogel (i.e., microgels) into a granular solid through metal coordination.[ 144 ] The resulting DCH is injectable and suitable for 3D printing, which would allow for the development of electroactive components for wearable and flexible devices and ultimately the support of electrophysiological tissues (e.g., myocardium and skeletal muscles).
Figure 6.
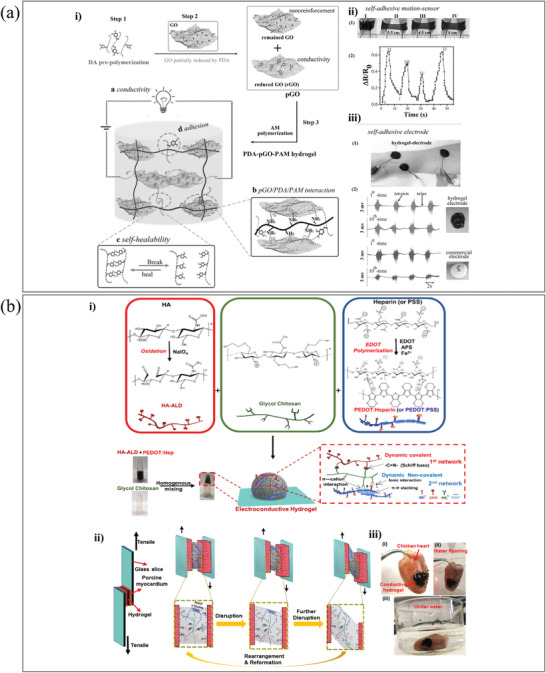
a‐i) The fabrication process of the adhesive PDA‐GO‐PAM based DCH. ii) The DCH acted as an adhesive electrode to detect electromyography signals. Adapted with permission.[ 138 ] Copyright 2017, Wiley‐VCH GmbH. b) The dual cross‐linked DCH adhesive. i) The preparation process of the DCHs. ii) Image of the lap‐shear experimental of DCH and adhesion mechanism. iii) Adhesion of the DCH on muscle tissue. The hydrogel remains adhered to the muscle tissue in water. Adapted with permission.[ 139 ] Copyright 2019, Wiley‐VCH GmbH.
To mimic the ECM and incorporate glycosaminoglycan (GAG) into the synthetic matrix, our group developed and synthesized DCHs based on polysaccharides, which were generated by a hydrogel network of aldehyde‐modified hyaluronic acid (HA‐ALD), glycol chitosan (GC), and PEDOT:Hep (or PEDOT:PSS).[ 139 ] Reversible covalent crosslinking resulted from Schiff base formation between HA‐ALD and GC. Due to the ionic interaction between negatively charged PEDOT:Hep or PEDOT:PSS and positively charged GCC, noncovalent crosslinking was primarily mediated (Figure 6b). The dynamic double‐network resulted in hydrogels with desired rheological properties, such as self‐healing and shear‐thinning behavior, as well as adhesiveness on both glass and wet tissue surfaces.
To achieve a stable integration of bioelectronics with tissues, Zhao et al. reported an e‐bioadhesive interface.[ 145 ] The combination of rGO with PVA results in a graphene nanocomposite hydrogel, which can be further interpenetrated with a network of polyacrylic acids (PAAs) grafted with N‐hydroxysuccinimide ester (PAA‐NHS ester). This rGO–PVA–PAA–NHS ester can be dried to prepare a carboxylic acid‐containing e‐bioadhesive interface. This allows the formation of hydrogen bonds and electrostatic interactions with the wet and complex surface of wet tissue and the interfacial water, which is rapidly absorbed and removed. It is also possible to achieve the long‐term stable integration of bioelectronic devices in physiological environments with the additional contribution of covalent crosslinks with primary amine groups (e.g., lysine residues) on the tissue surface.
Adhesiveness is highly favorable for improving bioelectronic functionality by forming a seamless interface. In addition to excellent toughness and stretchability, high conductivity, self‐healing and stress relaxation, and antibacterial capability are also desired properties for the development of adhesive bioelectronics.
4.3. DCHs for Controlled Drug Release and Cell Delivery
Responsiveness to electrical stimulation represents a unique property of CHs when compared to other biomaterials. Stimulus‐responsive materials allow for controlled and long‐term drug release, which are improved drug delivery systems to treat chronic diseases, as a promising alternative to regular and systematic injections.[ 18 , 146 , 147 , 148 , 149 ] Most premises for drug release from DCH‐based systems rely on a similar mechanism: alterations in charge distribution due to changes in the electrical field cause structural changes in the network.[ 102 , 150 ] These changes reflect the different encapsulation mechanisms of the drug, the structure of the polymer, and its responsiveness to variations in the electrical field.
Zare et al. reported a programmed drug delivery hydrogel fabricated by an electric field responsive material.[ 151 ] A temperature‐sensitive hydrogel is used to encapsulate nanoparticles of a conductive polymer (PPy) loaded with an anticancer drug. The amount and speed of drug release can be controlled by applying a direct external current (DC). The resulting electrochemical reduction or oxidation of PPy changes the overall net charge of the conductive nanoparticles, providing a release profile that can be externally modulated with DCH.
In addition, DCHs can also be utilized as cell delivery systems. Ma et al. proved that injectable DCHs based on chitosan‐aniline conjugated conductive polymer and PEG with benzaldehyde modification had been identified as an injectable conductive hydrogel for cell delivery, which was used for myocardial infarction (MI).[ 98 ] Cells encapsulated in the hydrogel showed good viability and proliferation. The system was tested with C2C12 myoblasts and H9c2 cardiac cells, and the release rate was further tuned by varying the cell density. Also, this DCH demonstrates good antibacterial properties because of the positively charged chitosan and polyaniline, which will be a very promising DCH system for both cell culture and clinical uses.[ 41 , 98 , 152 ]
4.4. DCH‐Based Implantable Hydrogel Bioelectronics
While DCHs have many utilities, such as ECM‐mimetic matrices for 3D cell culture, functional adhesives for tissue engineering, and controlled drug release, these functions can also be combined and used for in vivo applications as implantable materials. Implantable hydrogel bioelectronics have been developed for biomedical applications such as myocardial tissue engineering and neural tissue engineering.
One of the leading causes of morbidity and mortality globally is heart‐related issues, especially the damage caused by MI.[ 153 ] The myocardium is a tissue formed by different cell types, among which electrically excitable cells can be found, making the heart to be an electrically active organ.[ 154 , 155 ] In vivo, myocardial cells contract synchronously when an electric signal is received from the sinoatrial node. If the tissue is damaged and scarred, the transmission of electrical and mechanical signals becomes impaired and may ultimately lead to heart failure.[ 156 , 157 ] One way of coping with this type of injury is by combining biological and chemical approaches. Stevens et al. reported the first functional heart patch for cardiac tissue engineering by coating a phytic acid‐doped chitosan surface with PANI.[ 158 ] This conductive patch is adhesive conductive and has low surface resistivity due to the strong chelation between phytic acid and chitosan. Furthermore, this tissue engineering platform has shown promising results in both ex vivo and in vivo experiments, showing immediate effects on the electrophysiology of the heart without causing any arrhythmias.
Liu et al. also developed a therapeutic patch for MI based on conductive and adhesive hydrogels (Figure 7a). A hyperbranched polymer was synthesized by the reaction between dopamine hydrochloride, pentaerythritol triacrylate, and poly(ethylene glycol) diacrylate (PEGDA). Next, pyrrole was conjugated to the hyperbranched polymer acrylate group through Michael addition as end‐capping.
Figure 7.
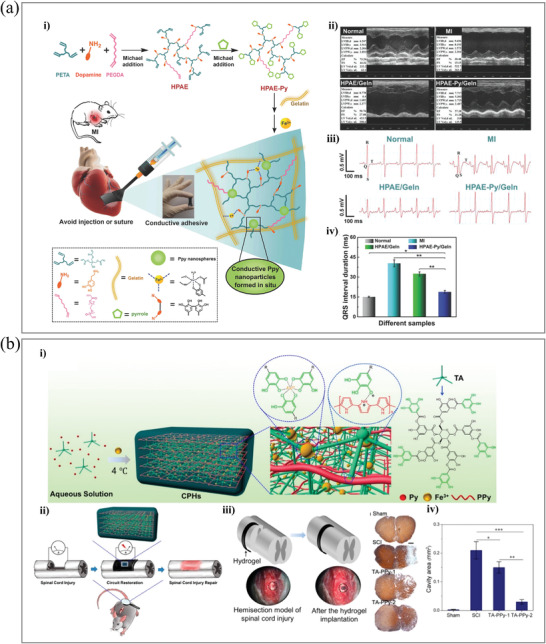
DCH‐based implantable hydrogel bioelectronics. a‐i) Schematic illustration of the formation of DCH and its application as heart patches. ii) Representative images for nontreatment control, MI, nonconductive (HPAE/Geln), and DCH (HPAE–Py/Geln) groups after 4 weeks. iii) Representative ECGs of different groups. iv) Quantification of the QRS interval of different groups. Adapted with permission.[ 159 ] Copyright 2018, Wiley‐VCH GmbH. b‐i) Illustration of the fabrication of a DCH through crosslinking by mixing tannic acid, pyrrole, and Fe3+. ii) DCH induced endogenous neurogenesis in vivo. iii) Schematic demonstration of a DCH that was transimplanted into the spinal cord hemisection gap. iv) Quantification of the average cystic cavity area of animals with SCI and different hydrogel treatments. Adapted with permission.[ 160 ] Copyright 2018, American Chemical Society.
Fe3+ was added to polymerize pyrrole as an oxidant and form a complex with dopamine, leading to gelation.[ 159 ] The conductive hydrogel exhibits good adhesive properties. It can be directly painted onto the heart surface as a patch without external reactions or curing, providing a novel therapeutic biomaterial for cardiac tissue engineering.
Neurological injuries bring huge burdens to patients and society. The nervous system includes the central nervous system and the peripheral nervous system, both of which can suffer from neurological injuries. Although the peripheral nervous system has a moderate capability for autonomic regeneration compared to the central nervous system, which has a very limited regenerative capability, neither the brain nerves nor the spinal cord nerves can regenerate spontaneously in most neurological injuries.[ 161 ] Therefore, autologous transplantation remains the gold standard treatment for neurological injuries.[ 162 , 163 , 164 ] Ning et al. reported an implantable DCH based on PPy, in which TA acts as a crosslinker and dopant and Fe3+ acts as an oxidant and ionic crosslinker.[ 160 ] The therapeutic efficacy of the material on spinal cord injury (SCI) was also investigated (Figure 7b). High electronic conductivity (0.18 S cm–1) and tunable stiffness (0.3–2.2 kPa) provide this DCH with a huge application possibility in the neural regeneration area. Neural stem cells (NSCs) demonstrated accelerated differentiation into neurons on this DCH surface while suppressing astrocyte development in vitro. In vivo, the DCH can stimulate endogenous NSC neurogenesis, resulting in significant locomotor function recovery in an SCI animal model. This work suggests that DCHs can stimulate tissue repair in SCI without the addition of any other therapeutic agents.
For large defects in bone tissues, polymer scaffolds can be implanted to prevent scar formation.[ 165 ] Since bones are electrically active tissues, CH‐based materials can promote bone regeneration.[ 166 , 167 , 168 ] Lu et al. synthesized a multilayered graphene‐containing DCH that exhibited improved mechanical strength, flexibility, and adhesion to osteoblast and bone tissues. The material can maintain the osseous space and promote early osteogenesis in a rat calvarial defect model.[ 169 ] To mimic the physiological microenvironment of bone, Liu et al. reported injectable CNTs and black phosphorus (BP)‐containing DCH with enhanced mechanical strength, electrical conductivity, and continuous phosphate ion release for tissue engineering.[ 170 ] The DCH utilized a biodegradable oligo‐(poly‐(ethylene glycol) fumarate) (OPF) polymer as the crosslinking matrix, with the addition of cross‐linkable CNT‐poly(ethylene glycol)‐acrylate (CNTpega) to grant network formation and electroconductivity. BP nanosheets were incorporated to aid bone regeneration through the steady release of phosphate (through the environmental oxidation of phosphorus in situ). This BP‐CNTpega gel was found to enhance the adhesion, proliferation, and osteogenic differentiation of MC3T3 preosteoblast cells. As monitored with X‐ray imaging in bone defect regeneration tests with rabbits, the BP‐CNTpega gel exhibited excellent capability to fill femur defects, vertebral body cavities, and posterolateral spinal fusion sites via in situ gelation.
5. Conclusion and Outlook
In DCHs, the strength of conventional biomaterials (viscoelastic 3D structure, more than 90% water content, and reliable biocompatibility) is well integrated with conductive polymers to realize highly tunable mechanical properties, high conductivity, and responsiveness to multiple stimuli. Many physical and chemical features can be tailored to resemble various properties and functions of natural tissues. Therefore, DCH‐based biomaterials have become increasingly interesting for biomaterial research and clinical therapy. Nevertheless, there are still several critical challenges in this field.
5.1. Mechanical and Electrochemical Properties and Biocompatibility
In recent years, the electrical and biomechanical properties of hydrogels have been remarkably improved. However, there are still several critical features that require further optimization and improvement. For instance, most reported DCHs possess limited electrical conductivity (mostly less than 1 S cm–1). Many materials require tedious synthesis, thus preventing large‐scale synthesis and high reproducibility. While noncovalent assembly is important for many cell‐based applications, most physical crosslinked hydrogels do not show high stretchability and toughness, which remains a challenge and significantly limits their utilization in bioelectronic devices.
5.2. Advanced Fabrication Technology with DCHs
Fabrication technology for hydrogel‐based bioelectronics has still not been adequately explored. For example, 3D printing, especially bioprinting, can be used to fabricate target tissue‐specific micro‐ and macrostructures. However, there are still many daunting challenges, including high spatial resolution, large‐scale production, the construction of a complex 3D structure, and a highly cytocompatible manufacturing process.
5.3. Further Investigation of the Long‐Term Stability or Tunable Degradability and Biocompatibility In Vivo
Most of the conductive components in DCHs are conductive polymers, carbon materials, and metals. These materials are often very stable when isolated or in vitro, but their electrochemical properties dynamically change when exposed to complex biological environments, especially in vivo. This means that one of the considerations to use DCH‐based biosensors and bioelectronics is whether the DCH can retain its electrochemical properties when constantly exposed to biological environments. As a corequisite, different biocompatibility tests should be performed, at least at first glance, in vitro at the cell culture level. While remarkable advances in the synthesis and functionalization of DCHs have been made, long‐term studies of their in vivo biocompatibility, stability, and biodegradability are still needed. The combined use of different noninvasive morphological and functional imaging modalities, such as magnetic resonance imaging and positron emission tomography, which are particularly predestined for intraindividual long‐term monitoring, should be explored.
The development of tailor‐made DCHs for biomedical applications is still in the early stages. In addition to the scientific and technological challenges of biomaterials, the integration of microelectronics and the biocompatibility of the resulting complex systems will also become a future research focus. This would advance the development of DCH‐based biomedical devices and pave the way to transfer these multifunctional materials to clinical uses, for example, to couple electrical stimulation with cell‐based regenerative therapy.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Acknowledgements
Y.X. and Y.Z. are grateful to the Bundesministerium für Bildung und Forschung (BMBF) for supporting this work (OptiZeD, BMBF 03Z22E511). The authors thank the Helmholtz Association for funding this work by the Helmholtz Cross‐Programme Initiative “Technology and Medicine – Adaptive Systems.” D.V. was supported by the EU COST Action ENBA (CA 15216).
Open access funding enabled and organized by Projekt DEAL.
Biographies
Yong Xu received his Ph.D. degree in chemistry from the Technische Universität Dresden (TU‐Dresden, Germany) in 2019. He is currently a postdoctoral researcher in B CUBE – Center for Molecular Bioengineering, TU‐Dresden. His research interests are focused on self‐assembled biomaterials, bioadhesives, and bio‐based polymeric conductive materials for biomedical applications, including tissue engineering, 3D bioprinting, and controlled drug delivery.

Jens Pietzsch is a biochemist/toxicologist and received his Ph.D./M.D. degree in pathological biochemistry and internal medicine in 1996 from the Technische Universität Dresden (Germany). Currently, he is the head of the Department of Radiopharmaceutical and Chemical Biology at the Institute of Radiopharmaceutical Cancer Research, Helmholtz‐Zentrum Dresden‐Rossendorf, and holds a position as professor of pathological biochemistry at the Faculty of Chemistry and Food Chemistry, School of Science, Technische Universität Dresden. His research interest focuses on targeted radionuclide‐based theranostics of therapy‐resistant malignant tumors including intelligent biomaterial approaches for targeted drug delivery.

Yixin Zhang is a professor at the Technische Universität Dresden, Germany. He studied organic chemistry in Shanghai, China and did his Ph.D. on immunosuppressive drug and cis/trans isomerase with Gunter Fischer in Halle, Germany. After his postdoc with Dario Neri in ETH Zürich, he joined B CUBE, Center for Molecular Bioengineering in 2009 as group leader, and became professor of biomolecular interaction in 2016. His research interests include self‐assembled biomaterials, drug screening with molecule array and DNA‐encoded chemical library, drug design, and peptide bond cis/trans isomerase.

Xu Y., Patino M. Gaillez, Rothe R., Hauser S., Voigt D., Pietzsch J., Zhang Y., Conductive Hydrogels with Dynamic Reversible Networks for Biomedical Applications. Adv. Healthcare Mater. 2021, 10, 2100012. 10.1002/adhm.202100012
References
- 1. Mouw J. K., Ou G., Weaver V. M., Nat. Rev. Mol. Cell Biol. 2014, 15, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theocharis A. D., Skandalis S. S., Gialeli C., Karamanos N. K., Adv. Drug Delivery Rev. 2016, 97, 4. [DOI] [PubMed] [Google Scholar]
- 3. Frantz C., Stewart K. M., Weaver V. M., J. Cell Sci. 2010, 123, 4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raeburn J., Cardoso A. Z., Adams D. J., Chem. Soc. Rev. 2013, 42, 5143. [DOI] [PubMed] [Google Scholar]
- 5. Kharkar P. M., Kiick K. L., Kloxin A. M., Chem. Soc. Rev. 2013, 42, 7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang G., Li F., Zhao X., Ma Y., Li Y., Lin M., Jin G., Lu T. J., Genin G. M., Xu F., Chem. Rev. 2017, 117, 12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher O. Z., Khademhosseini A., Langer R., Peppas N. A., Acc. Chem. Res. 2010, 43, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobolak J., Dinnyes A., Memic A., Khademhosseini A., Mobasheri A., Methods 2016, 99, 62. [DOI] [PubMed] [Google Scholar]
- 9. Bazgir B., Fathi R., Rezazadeh Valojerdi M., Mozdziak P., Asgari A., Cell J. 2017, 18, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liao C., Zhang M., Yao M. Y., Hua T., Li L., Yan F., Adv. Mater. 2015, 27, 7493. [DOI] [PubMed] [Google Scholar]
- 11. Balint R., Cassidy N. J., Cartmell S. H., Acta Biomater. 2014, 10, 2341. [DOI] [PubMed] [Google Scholar]
- 12. Liu K., Zhang J. J., Cheng F. F., Zheng T. T., Wang C., Zhu J. J., J. Mater. Chem. 2011, 21, 12034. [Google Scholar]
- 13. Kopeček J., Biomaterials 2007, 28, 5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Slaughter B. V., Khurshid S. S., Fisher O. Z., Khademhosseini A., Peppas N. A., Adv. Mater. 2009, 21, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoare T. R., Kohane D. S., Polymer 2008, 49, 1993. [Google Scholar]
- 16. Peppas N. A., Hilt J. Z., Khademhosseini A., Langer R., Adv. Mater. 2006, 18, 1345. [Google Scholar]
- 17. Yuk H., Lu B., Zhao X., Chem. Soc. Rev. 2019, 48, 1642. [DOI] [PubMed] [Google Scholar]
- 18. Qiu Y., Park K., Adv. Drug Delivery Rev. 2001, 53, 321. [DOI] [PubMed] [Google Scholar]
- 19. Jeong B., Kim S. W., Bae Y. H., Adv. Drug Delivery Rev. 2012, 64, 154. [Google Scholar]
- 20. Seliktar D., Science 2012, 336, 1124. [DOI] [PubMed] [Google Scholar]
- 21. Calvert P., Adv. Mater. 2009, 21, 743. [Google Scholar]
- 22. Inal S., Rivnay J., Suiu A. O., Malliaras G. G., McCulloch I., Acc. Chem. Res. 2018, 51, 1368. [DOI] [PubMed] [Google Scholar]
- 23. Rivnay J., Owens R. M., Malliaras G. G., Chem. Mater. 2014, 26, 679. [Google Scholar]
- 24. Karunakaran C., Bhargava K., Benjamin R., Biosensors and Bioelectronics, Elsevier, Amsterdam, The Netherlands: 2015. [Google Scholar]
- 25. Shi Z., Gao X., Ullah M. W., Li S., Wang Q., Yang G., Biomaterials 2016, 111, 40. [DOI] [PubMed] [Google Scholar]
- 26. Rong Q., Lei W., Liu M., Chemistry 2018, 24, 16930. [DOI] [PubMed] [Google Scholar]
- 27. Guiseppi‐Elie A., Biomaterials 2010, 31, 2701. [DOI] [PubMed] [Google Scholar]
- 28. Talebian S., Mehrali M., Taebnia N., Pennisi C. P., Kadumudi F. B., Foroughi J., Hasany M., Nikkhah M., Akbari M., Orive G., Dolatshahi‐Pirouz A., Adv. Sci. 2019, 6, 1801664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor D. L., in het Panhuis M., Adv. Mater. 2016, 28, 9060. [DOI] [PubMed] [Google Scholar]
- 30. Phadke A., Zhang C., Arman B., Hsu C. C., Mashelkar R. A., Lele A. K., Tauber M. J., Arya G., Varghese S., Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hennink W. E., van Nostrum C. F., Adv. Drug Delivery Rev. 2012, 64, 223. [DOI] [PubMed] [Google Scholar]
- 32. Appel E. A., Forster R. A., Rowland M. J., Scherman O. A., Biomaterials 2014, 35, 9897. [DOI] [PubMed] [Google Scholar]
- 33. Thompson M. S., Tsurkan M. V., Chwalek K., Bornhauser M., Schlierf M., Werner C., Zhang Y., Chem. – Eur. J. 2015, 21, 3178. [DOI] [PubMed] [Google Scholar]
- 34. Madl C. M., Lesavage B. L., Dewi R. E., Dinh C. B., Stowers R. S., Khariton M., Lampe K. J., Nguyen D., Chaudhuri O., Enejder A., Heilshorn S. C., Nat. Mater. 2017, 16, 1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai P., Hu B., Leow W. R., Wang X., Loh X. J., Wu Y. L., Chen X., Adv. Mater. 2018, 30, 1800572. [DOI] [PubMed] [Google Scholar]
- 36. Zhao X., Huebsch N., Mooney D. J., Suo Z., J. Appl. Phys. 2010, 107, 3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chaudhuri O., Gu L., Darnell M., Klumpers D., Bencherif S. A., Weaver J. C., Huebsch N., Mooney D. J., Nat. Commun. 2015, 6, 6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S. A., Weaver J. C., Huebsch N., Lee H. P., Lippens E., Duda G. N., Mooney D. J., Nat. Mater. 2016, 15, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maceri A., Theory of Elasticity, Springer, Berlin: 2010. [Google Scholar]
- 40. Cacopardo L., Guazzelli N., Nossa R., Mattei G., Ahluwalia A., J. Mech. Behav. Biomed. Mater. 2019, 89, 162. [DOI] [PubMed] [Google Scholar]
- 41. Bartnikowski M., Wellard R. M., Woodruff M., Klein T., Polymers 2015, 7, 2650. [Google Scholar]
- 42. Cui K., Ye Y. N., Yu C., Li X., Kurokawa T., Gong J. P., ACS Macro Lett. 2020, 9, 1582. [DOI] [PubMed] [Google Scholar]
- 43. Guvendiren M., Lu H. D., Burdick J. A., Soft Matter 2012, 8, 260. [Google Scholar]
- 44. Highley C. B., Rodell C. B., Burdick J. A., Adv. Mater. 2015, 27, 5075. [DOI] [PubMed] [Google Scholar]
- 45. Guo K., Zhang D. L., Zhang X. M., Zhang J., Ding L. S., Li B. J., Zhang S., Angew. Chem., Int. Ed. 2015, 54, 12127. [DOI] [PubMed] [Google Scholar]
- 46. Shi Y., Wang M., Ma C., Wang Y., Li X., Yu G., Nano Lett. 2015, 15, 6276. [DOI] [PubMed] [Google Scholar]
- 47. Gabriel C., Peyman A., Grant E. H., Phys. Med. Biol. 2009, 54, 4863. [DOI] [PubMed] [Google Scholar]
- 48. Dymond A. M., IEEE Trans. Biomed. Eng. 1976, 23, 274. [DOI] [PubMed] [Google Scholar]
- 49. Rutten W. L. C., Annu. Rev. Biomed. Eng. 2002, 4, 407. [DOI] [PubMed] [Google Scholar]
- 50. Tandon N., Cannizzaro C., Figallo E., Voldman J., Vunjak‐Novakovic G., in Annual Int. Conf. IEEE Engineering in Medicine and Biology Society, IEEE, Piscataway, NJ: 2006, p. 845. [DOI] [PubMed] [Google Scholar]
- 51. Thakral G., LaFontaine J., Najafi B., Talal T. K., Kim P., Lavery L. A., Diabetic Foot Ankle 2013, 4, 22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ghasemi‐Mobarakeh L., Prabhakaran M. P., Morshed M., Nasr‐Esfahani M. H., Baharvand H., Kiani S., Al‐Deyab S. S., Ramakrishna S., J. Tissue Eng. Regener. Med. 2011, 5, e17. [DOI] [PubMed] [Google Scholar]
- 53. Kloth L. C., Adv. Wound Care 2014, 3, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kloth L. C., McCulloch J. M., Adv. Wound Care 1996, 9, 42. [PubMed] [Google Scholar]
- 55. Williams D. F., Biomaterials 2008, 29, 2941. [DOI] [PubMed] [Google Scholar]
- 56. Anderson J. M., Regener. Biomater. 2016, 3, 73. [Google Scholar]
- 57. Tondera C., Wieduwild R., Röder E., Werner C., Zhang Y., Pietzsch J., Adv. Funct. Mater. 2017, 27, 1605189. [Google Scholar]
- 58. Ullm S., Krüger A., Tondera C., Gebauer T. P., Neffe A. T., Lendlein A., Jung F., Pietzsch J., Biomaterials 2014, 35, 9755. [DOI] [PubMed] [Google Scholar]
- 59. Tondera C., Hauser S., Krüger‐Genge A., Jung F., Neffe A. T., Lendlein A., Klopfleisch R., Steinbach J., Neuber C., Pietzsch J., Theranostics 2016, 6, 2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaur G., Adhikari R., Cass P., Bown M., Gunatillake P., RSC Adv. 2015, 5, 37553. [Google Scholar]
- 61. Nambiar S., Yeow J. T. W., Biosens. Bioelectron. 2011, 26, 1825. [DOI] [PubMed] [Google Scholar]
- 62. Kayser L. V., Lipomi D. J., Adv. Mater. 2019, 31, 1806133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang J. C., Mun J., Kwon S. Y., Park S., Bao Z., Park S., Adv. Mater. 2019, 31, 1904765. [DOI] [PubMed] [Google Scholar]
- 64. Liu Y., Li J., Song S., Kang J., Tsao Y., Chen S., Mottini V., McConnell K., Xu W., Zheng Y. Q., Tok J. B. H., George P. M., Bao Z., Nat. Biotechnol. 2020, 38, 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu J., Kim Y. S., Richardson C. E., Tom A., Ramakrishnan C., Birey F., Katsumata T., Chen S., Wang C., Wang X., Joubert L.‐M., Jiang Y., Wang H., Fenno L. E., Tok J. B. H., Pașca S. P., Shen K., Bao Z., Deisseroth K., Science 2020, 367, 1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. AL‐Oqla F. M., Sapuan S. M., Anwer T., Jawaid M., Hoque M. E., Synth. Met. 2015, 206, 42. [Google Scholar]
- 67. Liu Y., Turner A. P. F., Zhao M., Mak W. C., Biosens. Bioelectron. 2018, 100, 374. [DOI] [PubMed] [Google Scholar]
- 68. Dong R., Ma P. X., Guo B., Biomaterials 2020, 229, 119584. [DOI] [PubMed] [Google Scholar]
- 69. Guo B., Ma P. X., Biomacromolecules 2018, 19, 1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vegas A. J., Veiseh O., Doloff J. C., Ma M., Tam H. H., Bratlie K., Li J., Bader A. R., Langan E., Olejnik K., Fenton P., Kang J. W., Hollister‐Locke J., Bochenek M. A., Chiu A., Siebert S., Tang K., Jhunjhunwala S., Aresta‐Dasilva S., Dholakia N., Thakrar R., Vietti T., Chen M., Cohen J., Siniakowicz K., Qi M., McGarrigle J., Lyle S., Harlan D. M., Greiner D. L., et al., Nat. Biotechnol. 2016, 34, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xu Y., Yang X., Thomas A. K., Patsis P. A., Kurth T., Kräter M., Eckert K., Bornhäuser M., Zhang Y., ACS Appl. Mater. Interfaces 2018, 10, 14418. [DOI] [PubMed] [Google Scholar]
- 72. Yu C., Wang C., Liu X., Jia X., Naficy S., Shu K., Forsyth M., Wallace G. G., Adv. Mater. 2016, 28, 9349. [DOI] [PubMed] [Google Scholar]
- 73. Si Y., Wang L., Wang X., Tang N., Yu J., Ding B., Adv. Mater. 2017, 29, 1700339. [DOI] [PubMed] [Google Scholar]
- 74. Roshanbinfar K., Vogt L., Greber B., Diecke S., Boccaccini A. R., Scheibel T., Engel F. B., Adv. Funct. Mater. 2018, 28, 1803951. [Google Scholar]
- 75. Deng Z., Guo Y., Zhao X., Ma P. X., Guo B., Chem. Mater. 2018, 30, 1729. [Google Scholar]
- 76. Xu Y., Cui M., Patsis P. A., Günther M., Yang X., Eckert K., Zhang Y., ACS Appl. Mater. Interfaces 2019, 11, 7715. [DOI] [PubMed] [Google Scholar]
- 77. Zhu Y., Liu S., Shi X., Han D., Liang F., Mater. Chem. Front. 2018, 2, 2212. [Google Scholar]
- 78. Zhang S., Chen Y., Liu H., Wang Z., Ling H., Wang C., Ni J., Çelebi‐Saltik B., Wang X., Meng X., Kim H. J., Baidya A., Ahadian S., Ashammakhi N., Dokmeci M. R., Travas‐Sejdic J., Khademhosseini A., Adv. Mater. 2020, 32, 1904752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu B., Yuk H., Lin S., Jian N., Qu K., Xu J., Zhao X., Nat. Commun. 2019, 10, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yuk H., Lu B., Lin S., Qu K., Xu J., Luo J., Zhao X., Nat. Commun. 2020, 11, 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li S., Wang L., Zheng W., Yang G., Jiang X., Adv. Funct. Mater. 2020, 30, 2002370. [Google Scholar]
- 82. Chen J., Peng Q., Thundat T., Zeng H., Chem. Mater. 2019, 31, 4553. [Google Scholar]
- 83. He P., Wu J., Pan X., Chen L., Liu K., Gao H., Wu H., Cao S., Huang L., Ni Y., J. Mater. Chem. A 2020, 8, 3109. [Google Scholar]
- 84. Ryplida B., Lee K. D., In I., Park S. Y., Adv. Funct. Mater. 2019, 29, 1903209. [Google Scholar]
- 85. Shi H., Liu C., Jiang Q., Xu J., Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar]
- 86. Kurkov S. V., Loftsson T., Cyclodextrins. Int. J. Pharm. 2013, 453, 167. [DOI] [PubMed] [Google Scholar]
- 87. Del Valle E. M. M., Process Biochem. 2004, 39, 1033. [Google Scholar]
- 88. Loftsson T., Duchene D., Int. J. Pharm. 2007, 329, 1. [DOI] [PubMed] [Google Scholar]
- 89. Brewster M. E., Loftsson T., Adv. Drug Delivery Rev. 2007, 59, 645. [DOI] [PubMed] [Google Scholar]
- 90. Wu L., Xu J., Lu L., Yang T., Gao Y., Colloids Surf., A 2015, 482, 203. [Google Scholar]
- 91. Ha W., Zhao X. B., Jiang K., Kang Y., Chen J., Li B. J., Shi Y. P., Chem. Commun. 2016, 52, 14384. [DOI] [PubMed] [Google Scholar]
- 92. Yao B., Wang H., Zhou Q., Wu M., Zhang M., Li C., Shi G., Adv. Mater. 2017, 29, 1700974. [DOI] [PubMed] [Google Scholar]
- 93. Buckingham A. D., Del Bene J. E., McDowell S. A. C., Chem. Phys. Lett. 2008, 463, 1. [Google Scholar]
- 94. Zhu Y., Murali S., Cai W., Li X., Suk J. W., Potts J. R., Ruoff R. S., Adv. Mater. 2010, 22, 3906. [DOI] [PubMed] [Google Scholar]
- 95. Paul A., Hasan A., Al Kindi H., Gaharwar A. K., Rao V. T. S., Nikkhah M., Shin S. R., Krafft D., Dokmeci M. R., Shum‐Tim D., Khademhosseini A., ACS Nano 2014, 8, 8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xu Y., Wu Q., Sun Y., Bai H., Shi G., ACS Nano 2010, 4, 7358. [DOI] [PubMed] [Google Scholar]
- 97. Luan V. H., Tien H. N., Hoa L. T., Hien N. T. M., Oh E. S., Chung J., Kim E. J., Choi W. M., Kong B. S., Hur S. H., J. Mater. Chem. A 2013, 1, 208. [Google Scholar]
- 98. Dong R., Zhao X., Guo B., Ma P. X., ACS Appl. Mater. Interfaces 2016, 8, 17138. [DOI] [PubMed] [Google Scholar]
- 99. He J., Shi M., Liang Y., Guo B., Chem. Eng. J. 2020, 394. [Google Scholar]
- 100. Liu S., Kang M., Li K., Yao F., Oderinde O., Fu G., Xu L., Chem. Eng. J. 2018, 334, 2222. [Google Scholar]
- 101. Zhao X., Wu H., Guo B., Dong R., Qiu Y., Ma P. X., Biomaterials 2017, 122, 34. [DOI] [PubMed] [Google Scholar]
- 102. Qu J., Zhao X., Ma P. X., Guo B., Acta Biomater. 2018, 72, 55. [DOI] [PubMed] [Google Scholar]
- 103. Liang W., Chen J., Li L., Li M., Wei X., Tan B., Shang Y., Fan G., Wang W., Liu W., ACS Appl. Mater. Interfaces 2019, 11, 14619. [DOI] [PubMed] [Google Scholar]
- 104. Dang C., Wang M., Yu J., Chen Y., Zhou S., Feng X., Liu D., Qi H., Adv. Funct. Mater. 2019, 29, 1902467. [Google Scholar]
- 105. Shang Y. Y., Liang W., Tan B. Y., Xiao M., Zou Y., Liu W. G., Wang W., Sci. China Technol. Sci. 2019, 62, 1747. [Google Scholar]
- 106. Xin Y., Peng H., Xu J., Zhang J., Adv. Funct. Mater. 2019, 29, 1808989. [Google Scholar]
- 107. Tran V. T., Mredha M. T. I., Na J. Y., Seon J. K., Cui J., Jeon I., Chem. Eng. J. 2020, 394, 124941. [Google Scholar]
- 108. Cai G., Wang J., Qian K., Chen J., Li S., Lee P. S., Adv. Sci. 2017, 4, 1600190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhong R., Tang Q., Wang S., Zhang H., Zhang F., Xiao M., Man T., Qu X., Li L., Zhang W., Pei H., Adv. Mater. 2018, 30, 1706887. [DOI] [PubMed] [Google Scholar]
- 110. Ding Q., Xu X., Yue Y., Mei C., Huang C., Jiang S., Wu Q., Han J., ACS Appl. Mater. Interfaces 2018, 10, 27987. [DOI] [PubMed] [Google Scholar]
- 111. Wang Z., Tao F., Pan Q., J. Mater. Chem. A 2016, 4, 17732. [Google Scholar]
- 112. He J., Shi M., Liang Y., Guo B., Chem. Eng. J. 2020, 394, 124888. [Google Scholar]
- 113. Xu J., Liu Y., hui Hsu S., Molecules 2019, 24, 3005.31430954 [Google Scholar]
- 114. Choh S. Y., Cross D., Wang C., Biomacromolecules 2011, 12, 1126. [DOI] [PubMed] [Google Scholar]
- 115. Shu X. Z., Liu Y., Luo Y., Roberts M. C., Prestwich G. D., Biomacromolecules 2002, 3, 1304. [DOI] [PubMed] [Google Scholar]
- 116. Shu X. Z., Liu Y., Palumbo F., Prestwich G. D., Biomaterials 2003, 24, 3825. [DOI] [PubMed] [Google Scholar]
- 117. Öztürk E., Arlov Ø., Aksel S., Ling L., Ornitz D. M., Skjåk‐Bræk G., Zenobi‐Wong M., Adv. Funct. Mater. 2016, 26, 3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ji F., Li J., Zhang G., Lan W., Sun R., Wong C. P., Polymer 2019, 184, 121882. [Google Scholar]
- 119. Chen Y., Qian W., Chen R., Zhang H., Li X., Shi D., Dong W., Chen M., Zhao Y., ACS Macro Lett. 2017, 6, 1129. [DOI] [PubMed] [Google Scholar]
- 120. Li Y., Yang L., Zeng Y., Wu Y., Wei Y., Tao L., Chem. Mater. 2019, 31, 5576. [Google Scholar]
- 121. Aeridou E., Díaz Díaz D., Alemán C., Pérez‐Madrigal M. M., Biomacromolecules 2020, 21, 3984. [DOI] [PubMed] [Google Scholar]
- 122. Li W., Gao F., Wang X., Zhang N., Ma M., Angew. Chem., – Int. Ed. 2016, 55, 9196. [DOI] [PubMed] [Google Scholar]
- 123. Lin F., Wang Z., Shen Y., Tang L., Zhang P., Wang Y., Chen Y., Huang B., Lu B., J. Mater. Chem. A 2019, 7, 26442. [Google Scholar]
- 124. Zhang Y. S., Khademhosseini A., Science 2017, 356, eaaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sun J. Y., Zhao X., Illeperuma W. R. K., Chaudhuri O., Oh K. H., Mooney D. J., Vlassak J. J., Suo Z., Nature 2012, 489, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen Q., Zhu L., Zhao C., Wang Q., Zheng J., Adv. Mater. 2013, 25, 4171. [DOI] [PubMed] [Google Scholar]
- 127. Lin P., Ma S., Wang X., Zhou F., Adv. Mater. 2015, 27, 2054. [DOI] [PubMed] [Google Scholar]
- 128. Hirai H., Maru Y., Hagiwara K., Nishida J., Takaku F., Science 1987, 238, 1717. [DOI] [PubMed] [Google Scholar]
- 129. Darabi M. A., Khosrozadeh A., Mbeleck R., Liu Y., Chang Q., Jiang J., Cai J., Wang Q., Luo G., Xing M., Adv. Mater. 2017, 29, 1700533. [DOI] [PubMed] [Google Scholar]
- 130. Cooke M. E., Jones S. W., ter Horst B., Moiemen N., Snow M., Chouhan G., Hill L. J., Esmaeli M., Moakes R. J. A., Holton J., Nandra R., Williams R. L., Smith A. M., Grover L. M., Adv. Mater. 2018, 30, 1705013. [DOI] [PubMed] [Google Scholar]
- 131. Wang L., Chen D., Jiang K., Shen G., Chem. Soc. Rev. 2017, 46, 6764. [DOI] [PubMed] [Google Scholar]
- 132. He L., Xiao Q., Zhao Y., Li J., Reddy S., Shi X., Su X., Chiu K., Ramakrishna S., ACS Appl. Mater. Interfaces 2020, 12, 53150. [DOI] [PubMed] [Google Scholar]
- 133. Spencer A. R., Shirzaei Sani E., Soucy J. R., Corbet C. C., Primbetova A., Koppes R. A., Annabi N., ACS Appl. Mater. Interfaces 2019, 11, 30518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Xie C., Wang X., He H., Ding Y., Lu X., Adv. Funct. Mater. 2020, 30, 1909954. [Google Scholar]
- 135. Pinnaratip R., Bhuiyan M. S. A., Meyers K., Rajachar R. M., Lee B. P., Adv. Healthcare Mater. 2019, 8, 1801568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Saiz‐Poseu J., Mancebo‐Aracil J., Nador F., Busqué F., Ruiz‐Molina D., Angew. Chem., Int. Ed. 2019, 58, 696. [DOI] [PubMed] [Google Scholar]
- 137. Zhang W., Wang R., Sun Z., Zhu X., Zhao Q., Zhang T., Cholewinski A., (Kuo) Yang F., Zhao B., Pinnaratip R., Forooshani P. K., Lee B. P., Chem. Soc. Rev. 2020, 49, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Han L., Lu X., Wang M., Gan D., Deng W., Wang K., Fang L., Liu K., Chan C. W., Tang Y., Weng L.‐T., Yuan H., Small 2017, 13, 1601916. [DOI] [PubMed] [Google Scholar]
- 139. Xu Y., Patsis P. A., Hauser S., Voigt D., Rothe R., Günther M., Cui M., Yang X., Wieduwild R., Eckert K., Neinhuis C., Akbar T. F., Minev I. R., Pietzsch J., Zhang Y., Adv. Sci. 2019, 6, 1802077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gan D., Xing W., Jiang L., Fang J., Zhao C., Ren F., Fang L., Wang K., Lu X., Nat. Commun. 2019, 10, 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Jia Z., Zeng Y., Tang P., Gan D., Xing W., Hou Y., Wang K., Xie C., Lu X., Chem. Mater. 2019, 31, 5625. [Google Scholar]
- 142. Gan D., Wang Z., Xie C., Wang X., Xing W., Ge X., Yuan H., Wang K., Tan H., Lu X., Adv. Healthcare Mater. 2019, 8, 1901103. [DOI] [PubMed] [Google Scholar]
- 143. Gan D., Huang Z., Wang X., Jiang L., Wang C., Zhu M., Ren F., Fang L., Wang K., Xie C., Lu X., Adv. Funct. Mater. 2020, 30, 1907678. [Google Scholar]
- 144. Shin M., Song K. H., Burrell J. C., Cullen D. K., Burdick J. A., Adv. Sci. 2019, 6, 1901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Deng J., Yuk H., Wu J., Varela C. E., Chen X., Roche E. T., Guo C. F., Zhao X., Nat. Mater. 2021, 20, 229. [DOI] [PubMed] [Google Scholar]
- 146. Miyata T., Uragami T., Nakamae K., Adv. Drug Delivery Rev. 2002, 54, 79. [DOI] [PubMed] [Google Scholar]
- 147. Peppas N. A., Van Blarcom D. S., J. Controlled Release 2016, 240, 142. [DOI] [PubMed] [Google Scholar]
- 148. Bayer C. L., Peppas N. A., J. Controlled Release 2008, 132, 216. [DOI] [PubMed] [Google Scholar]
- 149. Li J., Mooney D. J., Nat. Rev. Mater. 2016, 1, 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Paradee N., Sirivat A., J. Phys. Chem. B 2014, 118, 9263. [DOI] [PubMed] [Google Scholar]
- 151. Ge J., Neofytou E., Cahill T. J., Beygui R. E., Zare R. N., ACS Nano 2012, 6, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhao Y., Li Z., Song S., Yang K., Liu H., Yang Z., Wang J., Yang B., Lin Q., Adv. Funct. Mater. 2019, 29, 1901474. [Google Scholar]
- 153. Alabas O. A., Jernberg T., Pujades‐Rodriguez M., Rutherford M. J., West R. M., Hall M., Timmis A., Lindahl B., Fox K. A. A., Hemingway H., Gale C. P., Cardiovasc. Res. 2020, 116, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Frangogiannis N. G., Compr. Physiol. 2015, 5, 1841. [DOI] [PubMed] [Google Scholar]
- 155. Lu L., Liu M., Sun R. R., Zheng Y., Zhang P., Cell Biochem. Biophys. 2015, 72, 865. [DOI] [PubMed] [Google Scholar]
- 156. Reed G. W., Rossi J. E., Cannon C. P., Lancet 2017, 389, 197. [DOI] [PubMed] [Google Scholar]
- 157. Rabimmkulovna S. G., Asrorovna R. A., Urokovich N. B., Mirkhamidovich S. A., Int. J. Curr. Res. Rev. 2020, 12, 91. [Google Scholar]
- 158. Mawad D., Mansfield C., Lauto A., Perbellini F., Nelson G. W., Tonkin J., Bello S. O., Carrad D. J., Micolich A. P., Mahat M. M., Furman J., Payne D. J., Lyon A. R., Gooding J. J., Harding S. E., Terracciano C. M., Stevens M. M., Sci. Adv. 2016, 2, e1601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Liang S., Zhang Y., Wang H., Xu Z., Chen J., Bao R., Tan B., Cui Y., Fan G., Wang W., Wang W., Liu W., Adv. Mater. 2018, 30, 1704235. [DOI] [PubMed] [Google Scholar]
- 160. Zhou L., Fan L., Yi X., Zhou Z., Liu C., Fu R., Dai C., Wang Z., Chen X., Yu P., Chen D., Tan G., Wang Q., Ning C., ACS Nano 2018, 12, 10957. [DOI] [PubMed] [Google Scholar]
- 161. Jellinger K. A., Eur. J. Neurol. 2004, 11, 503. [Google Scholar]
- 162. Scheib J., Höke A., Nat. Rev. Neurol. 2013, 9, 668. [DOI] [PubMed] [Google Scholar]
- 163. Faroni A., Mobasseri S. A., Kingham P. J., Reid A. J., Adv. Drug Delivery Rev. 2015, 82–83, 160. [DOI] [PubMed] [Google Scholar]
- 164. Schmidt C. E., Leach J. B., Annu. Rev. Biomed. Eng. 2003, 5, 293. [DOI] [PubMed] [Google Scholar]
- 165. Kellomäki M., Niiranen H., Puumanen K., Ashammakhi N., Waris T., Törmälä P., Biomaterials 2000, 21, 2495. [DOI] [PubMed] [Google Scholar]
- 166. Rajabi A. H., Jaffe M., Arinzeh T. L., Acta Biomater. 2015, 24, 12. [DOI] [PubMed] [Google Scholar]
- 167. Griffin M., Bayat A., Eplasty 2011, 11, e34. [PMC free article] [PubMed] [Google Scholar]
- 168. Mollon B., da Silva V., Busse J. W., Einhorn T. A., Bhandari M., J. Bone Jt. Surg., Am. Vol. 2008, 90, 2322. [DOI] [PubMed] [Google Scholar]
- 169. Lu J., Cheng C., He Y.‐S., Lyu C., Wang Y., Yu J., Qiu L., Zou D., Li D., Adv. Mater. 2016, 28, 4025. [DOI] [PubMed] [Google Scholar]
- 170. Liu X., George M. N., Li L., Gamble D., Miller A. L. II, Gaihre B., Waletzki B. E., Lu L., ACS Biomater. Sci. Eng. 2020, 6, 4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


