Abstract
Immune checkpoint inhibitors (ICIs) have transformed cancer treatment, providing significant benefit to patients across various tumour types, including melanoma. However, around 40% of melanoma patients do not benefit from ICI treatment, and accurately predicting ICI response remains challenging. We now describe a novel and simple approach that integrates immune-associated transcriptome signatures and tumour volume burden to better predict ICI response in melanoma patients. RNA sequencing was performed on pre-treatment (PRE) tumour specimens derived from 32 patients with advanced melanoma treated with combination PD1 and CTLA4 inhibitors. Of these 32 patients, 11 also had early during treatment (EDT, 5–15 days after treatment start) tumour samples. Tumour volume was assessed at PRE for all 32 patients, and at first computed tomography (CT) imaging for the 11 patients with EDT samples. Analysis of the Hallmark IFNγ gene set revealed no association with ICI response at PRE (AUC ROC curve = 0.6404, p = 0.24, 63% sensitivity, 71% specificity). When IFNg activity was evaluated with tumour volume (ratio of gene set expression to tumour volume) using logistic regression to predict ICI response, we observed high discriminative power in separating ICI responders from non-responders (AUC = 0.7760, p = 0.02, 88% sensitivity, 67% specificity); this approach was reproduced with other immune-associated transcriptomic gene sets. These findings were further replicated in an independent cohort of 23 melanoma patients treated with PD1 inhibitor. Hence, integrating tumour volume with immune-associated transcriptomic signatures improves the prediction of ICI response, and suggest that higher levels of immune activation relative to tumour burden are required for durable ICI response.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-024-02146-0.
Keywords: Melanoma, Immune checkpoint blockade, Transcriptomic analysis, PD1, CTLA4
To the editor
Immune checkpoint inhibitors (ICIs) have significantly extended the survival of patients with advanced melanoma. However, approximately 40% of melanoma patients will have minimal benefit from ICIs and more than 50% will progress on therapy [1]. There is an urgent need to identify robust biomarkers predictive of ICI response to optimize outcomes for each patient. Multiple studies have considered clinicopathological correlates, genomic features and transcriptomic signatures [2–4] but these often fail to predict ICI response in independent cohorts. For instance, the IPRES transcriptome signature [3] was not predictive of ICI response in multiple melanoma cohorts [5, 6]. Tumour mutation burden and PD-L1 expression are commonly used ICI response biomarkers but show predictive value only in select cancer types such as non-small cell lung cancer and head and neck squamous cell carcinoma (reviewed in [7]). Attempts to integrate multiomic features can improve ICI predictive accuracy [6], but these approaches can be costly and are difficult to replicate and interpret.
Currently, immune-associated transcriptomic signatures (e.g. IFNγ, IMPRES, TIDE) remain the most cited and utilized predictive biomarkers of ICI response [8–10]. Interestingly, despite preclinical and clinical studies demonstrating that lower tumour volume is associated with better response to ICIs [11], tumour volume is not commonly considered when evaluating predictive signatures of ICI response.
Integrating tumour volume into transcriptomic signatures improve predictive performance
In this study, we performed RNA sequencing on pre-treatment (PRE, 1-1162 days before treatment start) melanoma specimens from 32 patients treated with combination PD1 and CTLA4 inhibitors to identify transcriptomic features associated with ICI response. Eleven patients (11/32, 34%) also had early during treatment (EDT, 5–15 days after treatment start) tumour samples (Supplementary Table 1). Tumour volume was calculated by measuring the longest diameter of all measurable lesions and presented as either total tumour volume (sum of tumour diameters) or average tumour volume (total tumour volume divided by the number of metastases). Tumour volume was determined at PRE for all 32 patients, and at first progress computed tomography (CT) imaging for the 11 patients with EDT samples (Supplementary Table 2). Of the 32 patients, 24 were categorized as responders (irRECIST complete response (CR, n = 3), partial response (PR, n = 20), and stable disease > 6 months (SD, n = 1)) and eight were considered non-responders (irRECIST progressive disease (PD, n = 6) and PR with progression free survival (PFS) of < 6 months, n = 2).
Predictably, total PRE tumour volumes correlated with average PRE tumour volumes and the number of metastases (Supplementary Fig. 1A). Total PRE tumour volume was also associated with LDH status but not ICI response (Supplementary Fig. 1B-C).
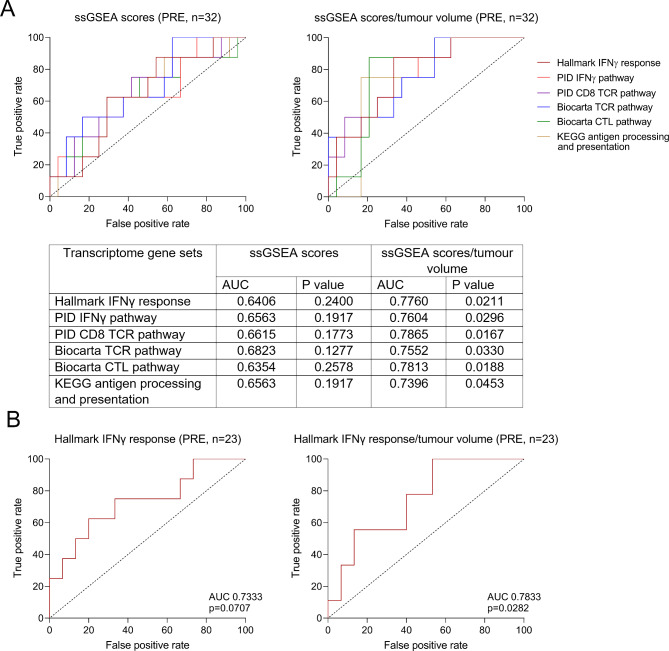
At PRE, analysis of the Hallmark Interferon Gamma (IFNγ) Response gene set (single sample Gene Set Enrichment Analysis (ssGSEA) score) revealed no significant difference between responders and non-responders (AUC ROC curve = 0.6406, p = 0.2400, 63% sensitivity, 71% specificity; Fig. 1A). However, when IFNγ signalling was considered along with baseline total tumour volume (ssGSEA score divided by the total tumour volume) as predictors in a logistic model with ICI response as dependent variable, the model demonstrated a clearer ability to discriminate responders from non-responders (AUC = 0.7760, p = 0.0211, 88% sensitivity, 67% specificity; Fig. 1A).
Fig. 1.
Tumour volume-normalised immune gene sets predict ICI response. A) Receiver operator characteristic (ROC) curves measuring the ICI predictive performance of each indicated immune-associated transcriptome gene sets with and without tumour volume normalisation (ssGSEA scores divided by total tumour volume) in melanoma tumours prior to treatment with combination ICIs (n = 32). B) ROC curves of Hallmark Interferon Gamma (IFNγ) Response gene set with and without tumour volume normalisation in melanoma tumours prior to treatment with PD-1 ICI (n = 23).
The improved predictive performance of tumour volume-normalised IFNγ response gene set score was reproduced for other immune-related transcriptome gene sets derived from the Molecular Signatures Database [12], including PID IFNγ pathway, PID CD8 TCR pathway, Biocarta TCR pathway, Biocarta CTL pathway, and KEGG antigen processing and presentation (Fig. 1A). Importantly, we validated the superior predictive performance of tumour volume-normalised IFNγ response gene set score (AUC = 0.7833, p = 0.0282, 88% sensitivity, 60% specificity) in a separate cohort of 23 melanoma patients prior to treatment with PD1 inhibitor [6] (Fig. 1B, Supplementary Table 3), confirming that this approach is generalizable to other melanoma cohorts, including those treated with anti-PD1 based monotherapy.
Furthermore, the model underwent internal validation within each cohort using bootstrapping techniques to assess the potential for overfitting and to measure optimism in the estimated performance metrics [13]. The differences between the observed AUC for each cohort and the corresponding average AUCs from 500 bootstrap replications were calculated. The bootstrapping result (Supplementary Fig. 2) also supported the integration of tumour volume and immune-associated transcriptomic signatures as predictors for ICI response. The mean differences between the observed AUC and the corresponding average AUCs from bootstrap replications were 0.003 (95% CI, -0.173 to 0.159) for the initial combination immunotherapy-treated cohort (n = 32) and 0.002 (95% CI, -0.184 to 0.163) for the PD1 inhibitor-treated cohort (n = 23).
Early during treatment biopsies better inform response to ICIs
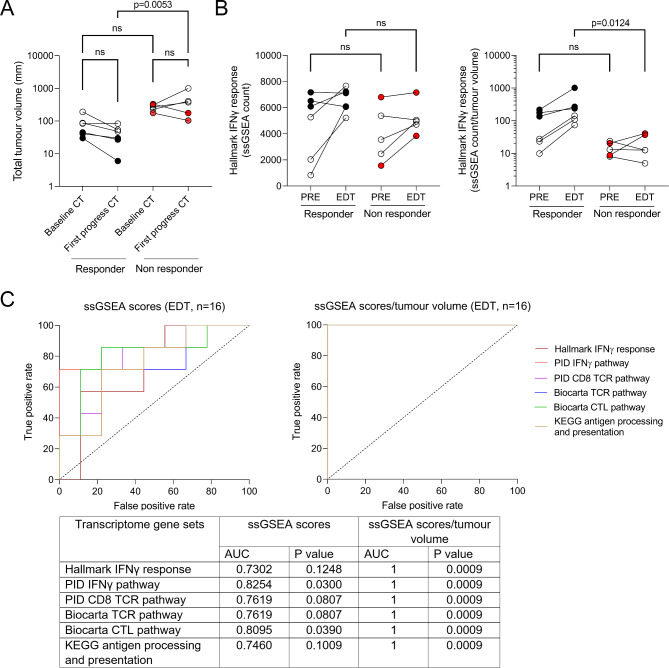
We also examined tumour volume and Hallmark Interferon Gamma (IFNγ) Response gene set in the 11 PRE-EDT matched tumour samples (six responders, irRECIST PR, n = 5 and SD of > 6 months, n = 1, and five non-responders, irRECIST PD, n = 3 and PR with PFS of < 6 months, n = 2). Tumour volumes in responding versus non-responding patients did not differ at PRE, but on first progress imaging, as expected, tumour volumes were significantly smaller in the responding patients (p = 0.0053, Kruskal-Wallis test with Dunn’s multiple comparison, Fig. 2A).
Fig. 2.
Comparison of tumour volume and transcriptomic signatures in 11 matched PRE-EDT tumour biopsies. A) Change in total tumour volume from baseline and first progress CT imaging in responders and non-responders. Black coloured circles indicate tumours with < 50 mm in sum diameter at baseline, red coloured circles indicate the two non-responders with initial PR but PFS < 6 months, Kruskal-Wallis test with Dunn’s multiple comparisons, p = 0.0053. B) Change in expression of the Hallmark IFNγ response gene set (left) and the tumour-normalised Hallmark IFNγ response gene set (right) in matched PRE-EDT samples (n = 11, n = 6 responders, n = 5 non-responders). Black coloured circles indicate tumours with < 50 mm in sum diameter at baseline, red coloured circles indicate the two non-responders with initial PR but PFS < 6 months, Kruskal-Wallis test with Dunn’s multiple comparisons, p = 0.0124. C) ROC curve analysis of IFNγ-related and immune-associated transcriptomic signatures with and without tumour volume normalisation (ssGSEA scores divided by total tumour volume) in melanoma tumours early during treatment (EDT) with combination ICIs (n = 16)
The expression of the Hallmark IFNγ gene set was upregulated, from PRE to EDT, in 8/11 patients (4/6, 73% responders and 4/5, 80% non-responders). This increase was not associated with ICI response or with change in tumour volume. For example, three responding patients with small PRE tumour volumes (< 50 mm in sum diameter) showed minimal change in IFNγ signature expression from PRE to EDT (mean fold change of 1.04, range 0.93–1.18, compared to an overall mean fold change of 2.02, range 0.93–7.33, Fig. 2B), and these tumours also showed high baseline IFNγ activity. Comparison of the IFNγ signature at EDT did not separate responders from non-responders, and the two groups were only stratified when IFNγ signature at EDT was normalised to tumour volume on first progress imaging (p = 0.0124, Kruskal-Wallis test with Dunn’s multiple comparison, Fig. 2B). Notably, all non-responding patients including the two patients who had initial PR but PFS < 6 months, had significantly lower expression of the IFNγ signature normalised to tumour volume compared to responders at EDT (Fig. 2B). We included five additional EDT samples that had no patient-matched PRE tumour, and ROC curve analysis of the 16 EDT samples with tumour volume data at first progress imaging confirmed superior predictive performance when tumour volume was integrated with immune-associated transcriptomic signatures at EDT (n = 16, Fig. 2C).
Conclusion
Baseline tumour volume is negatively correlated with ICI efficacy and this may reflect an immunosuppressive local and systemic immune landscape that is associated with larger tumours (reviewed in [11]). Indeed, an increased ratio of activated CD8 T cells (PD1+ Ki67+) to tumour burden post ICI treatment is associated with better clinical outcomes [14]. While markers of immune activation enable the selection of patients who are likely to benefit from ICI therapy, 20% of stage III melanoma patients with IFNγ-high tumours did not achieve a major pathological response to neoadjuvant combination ICIs [15]. We now present a straightforward approach that integrates these two opposing ICI biomarkers to enhance predictive accuracy. This explorative study was conducted on a small melanoma cohort (n = 32) and these findings need to be further confirmed in a larger cohort with proper model validation and subgroup analysis to explore sample heterogeneity. However, the replication of the results in an independent cohort of melanoma patients (n = 23) supports the predictive power of integrating tumour volume with immune-associated transcriptomic signatures for ICI response. Importantly, as tumour volume assessment is standardised and routinely performed for clinical trials, our predictive ratio is technically practical and easy to implement.
Importantly, tumour-normalised immune activity is predictive prior to combination and monotherapy ICI treatment and provides definitive predictive value early during treatment (i.e. within two weeks of treatment initiation). This is anticipated, as treatment response will amplify the difference between the numerator (immune activity) and denominator (tumour volume) of this predictive calculation. Accurate prediction early during treatment is particularly valuable in the neoadjuvant ICI setting, where definitive response prediction can guide surgical decisions and determine the need for adjuvant treatment versus surveillance. These findings underscore the value of re-examining new and existing biomarkers to refine predictive accuracy and emphasize the need for additional research to evaluate this approach in other cancers treated with ICI therapy, and especially in early-stage cancers in the neoadjuvant setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
IPdS is is supported by a CINSW Early Career Fellowship (ECF1376). AMM is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant. JHL is supported by a NHMRC Investigator Grant. RAS is supported by a NHMRC Investigator Grant (2022/GNT2018514). Support from The Cameron Family Foundation well as from colleagues at Melanoma Institute Australia and Royal Prince Alfred Hospital is also gratefully acknowledged. GVL is supported by a NHMRC Investigator Grant (2021/GNT2007839) and by the University of Sydney Medical Foundation. This work was supported by Macquarie University, Melanoma Institute Australia, and the National Health Medical Research Council of Australia project grants (grant 2012860, 2028055).
Author contributions
SYL and HR designed the study and wrote the manuscript. SYL analysed the data. NAA and SNL provided statistical analysis and support. IPdS, AMM, MC, RAS, GVL and JL provided clinical data and input. All authors read, revised and approved the manuscript.
Data availability
The dataset generated and analyzed in the current study are not yet publicly available but are available from the corresponding author on reasonable request, and will be made available upon publication.
Declarations
Ethics approval and consent to participate
Written consent was obtained from all patients included in this study (Human Research ethics approval from Royal Prince Alfred Hospital - Protocol X15-0454 & HREC/11/RPAH/444).
Consent for publication
Not applicable.
Competing interests
IPdS is on the advisory board of Merck Sharp & Dohme; has received fees for professional services from Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, and Pierre Fabre; and has had travel support from Bristol Myers Squibb and Merck Sharp & Dohme. MC has served on advisory boards or as a consultant for Amgen, BMS, Eisai, Ideaya, Merck, Sharp & Dohme (MSD), Nektar, Novartis, Oncosec, Pierre-Fabre, Qbiotics, Regeneron, Roche, Merck, Moderna and Sanofi and received honoraria from BMS, MSD and Novartis. AMM is a consultant advisor for Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Roche, Pierre-Fabre and QBiotics. SNL has received fees for professional services from SkylineDx and a stipend for editorial services at the British Journal of Dermatology. JHL has received honorarium from Merck, Sharp & Dohme, Bristol-Myers Squibb, Sanofi, Novartis, AstraZeneca, Roche. JHL has received conference support from Novartis and Merck, Sharp & Dohme. RAS has received fees for professional services from MetaOptima Technology Inc., F. Hoffmann-La Roche Ltd, Evaxion, Provectus Biopharmaceuticals Australia, Qbiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN Inc., Bristol-Myers Squibb, Myriad Genetics, GlaxoSmithKline. GVL is consultant advisor for Agenus, Amgen, Array Biopharma, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Immunocore, Innovent Biologics USA, Merck Sharpe & Dohme, Novartis, PHMR Ltd, Pierre Fabre, Provectus, Qbiotics, Regeneron. All other authors declare no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jenny Lee and Helen Rizos Co-senior authors.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with Nivolumab Plus Ipilimumab or Nivolumab alone Versus Ipilimumab in patients with Advanced Melanoma. J Clin Oncol. 2022;40(2):127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma. Cell. 2017;168(3):542. [DOI] [PubMed] [Google Scholar]
- 4.Pires da Silva I, Ahmed T, McQuade JL, Nebhan CA, Park JJ, Versluis JM, et al. Clinical models to define response and survival with Anti-PD-1 antibodies alone or combined with Ipilimumab in Metastatic Melanoma. J Clin Oncol. 2022;40(10):1068–80. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Shklovskaya E, Lim SY, Carlino MS, Menzies AM, Stewart A, et al. Transcriptional downregulation of MHC class I and melanoma de- differentiation in resistance to PD-1 inhibition. Nat Commun. 2020;11(1):1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newell F, Pires da Silva I, Johansson PA, Menzies AM, Wilmott JS, Addala V, et al. Multiomic profiling of checkpoint inhibitor-treated melanoma: identifying predictors of response and resistance, and markers of biological discordance. Cancer Cell. 2022;40(1):88–102. e7. [DOI] [PubMed] [Google Scholar]
- 7.Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019;4(6). [DOI] [PMC free article] [PubMed]
- 8.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auslander N, Zhang G, Lee JS, Frederick DT, Miao B, Moll T, et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med. 2018;24(10):1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SI, Cassella CR, Byrne KT. Tumor Burden and Immunotherapy: impact on Immune Infiltration and therapeutic outcomes. Front Immunol. 2020;11:629722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moons KG, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, Grobbee DE. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98(9):683–90. [DOI] [PubMed] [Google Scholar]
- 14.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reijers ILM, Rao D, Versluis JM, Menzies AM, Dimitriadis P, Wouters MW et al. IFN-gamma signature enables selection of neoadjuvant treatment in patients with stage III melanoma. J Exp Med. 2023;220(5). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated and analyzed in the current study are not yet publicly available but are available from the corresponding author on reasonable request, and will be made available upon publication.