Abstract
Background
Individuals using methamphetamine (METH) may experience psychosis, which usually requires aggressive treatment. Studies of the neural correlates of METH-associated psychosis (MAP) have focused predominantly on the default mode network (DMN) and cognitive control networks. We hypothesize that METH use alters global functional connections in resting-state brain networks and that certain cross-network connections could be associated with psychosis.
Methods
We recruited 24 healthy controls (CRL) and 54 men with METH use disorder (MUD) who were then divided into 25 without psychosis (MNP) and 29 with MAP. Psychotic symptom severity was assessed using the Positive and Negative Syndrome Scale (PANSS), evaluating (1) large-scale alterations in regional-wise resting-state functional connectivity (rsFC) across 11 brain networks and (2) associations between rsFC and psychotic symptom severity.
Results
The MUD group exhibited greater rsFC between the salience network (SN)-DMN, and subcortical network (SCN)-DMN compared to the CRL group. The MAP group exhibited decreased rsFC in the sensory/somatomotor network (SMN)-dorsal attention network (DAN), SMN-ventral attention network (VAN), SMN-SN, and SMN-auditory network (AN), whereas the MNP group exhibited increased rsFC in the SMN-DMN and the frontoparietal network (FPN)-DMN compared to CRL. Additionally, the MAP group exhibited decreased rsFC strength between the SMN-DMN, SMN-AN, SMN-FPN, and DMN-VAN compared to the MNP group. Furthermore, across the entire MUD group, the PANSS-Positive subscale was negatively correlated with the DMN-FPN and FPN-SMN, while the PANSS-Negative subscale was negatively correlated with the DMN-AN and SMN-SMN.
Conclusion
MUD is associated with altered global functional connectivity. In addition, the MAP group exhibits a different brain functional network compared to the MNP group.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06112-4.
Keywords: Brain, Functional magnetic resonance imaging (fMRI), Methamphetamine, Neurotransmitter, Psychosis
Background
Methamphetamine (METH) is a highly addictive psychostimulant that principally affects brain neurotransmitter systems, particularly dopamine, and produces an increase in alertness, energy, and euphoria [1]. With the increasing prevalence of METH exposure globally, converging evidence indicates that repeated METH exposure causes a variety of deleterious physical and psychological consequences [2–4]. Among them, METH-associated psychosis (MAP) has been of particular concern because of its association with an increased risk of suicide and violence, poorer cognitive function, and increased health service utilization [5, 6]. Compared to the general population, individuals who use METH are 11 times more likely to develop psychotic symptoms [7]. Additionally, most experience “positive” psychotic symptoms during intoxication, typically lasting no more than one week [8]. Treating MAP is particularly important because the affected population is at a very high risk of experiencing symptom exacerbation, and it is associated with a poorer prognosis [9]. Understanding how the neurobiological features of MAP are distinct and different from METH without psychosis (MNP) will ultimately optimize therapeutic strategies and reduce the risks of unfavorable outcomes.
Current evidence suggests that the difference between health and disease, or between the presence and absence of nominally psychiatric symptoms, is not defined by sharp or discontinuous neurobiological boundaries [10]. In light of this, some reports indicate that psychotic symptoms are a consequence of disordered connectivity within and between distributed brain networks [11, 12]. In the context of METH use, both animal and human studies have shown that differences in METH-related neuropsychiatric functions could be reflected in variability across the collective set of neural systems [2, 13]. Studies using task-based functional magnetic resonance imaging (fMRI) have demonstrated the impact of METH on various brain networks that underlie multiple neurocognitive domains such as reward learning [14], decision making [15], and adaptive cognitive control [16]. Although there have been an increasing number of studies linking brain network functioning with neuropsychiatric disturbances following METH administration, we are far from understanding how brain networks might explain the differences between MAP and MNP.
Beyond focusing on alterations of task-driven brain regions or networks, resting-state functional connectivity (rsFC) MRI technique has been widely used to investigate alterations in large-scale functional organizations of brain networks under neuropsychological conditions when people are not engaged in a specific task [17, 18]. To date, only a few human neuroimaging studies have focused on exploring functional network disruptions between MAP and MNP [19]. For example, using the rsFC MRI technique, Ipser et al. identified abnormal connectivity between the default mode network (DMN) and the cognitive control network (CCN) in these two groups of patients [20]. Furthermore, connectivity between these two networks was found to be inversely associated with the duration of antipsychotic treatment among MAP patients, indicating a relationship between connectivity and psychotic severity [20]. Similarly, a more recent study found that, compared to controls, the connectivity distinctions of MAP involved several networks such as attention, memory, and self-processing; however, a negative correlation between connectivity in the frontal gyrus and psychotic illness was reported [21]. Despite the literature describing regional and cross-network rsFC abnormalities in the population with MAP, a consensus has not yet been reached as to how connectome disturbances associated with psychotic severity are systematically manifested.
Given that individuals with MNP can benefit from substance use treatment, whereas those with MAP could require additional antipsychotic treatment [22], exploring the neuroimaging characteristics associated with MAP could provide insights into the appropriate placement of clinical treatment. In this study, we investigated rsFC alterations using pairwise comparisons between control participants and individuals with METH use disorder (MUD), including both MNP and MAP patients, to unravel the METH-induced psychotic effect from METH exposure based on large-scale brain functional networks. Furthermore, we examined the association between functional connections and the severity of psychotic symptoms. Identifying these altered networks could facilitate future classification approaches for METH users with and without psychoses.
Methods
All participants were given a full explanation of the study procedure and were asked to provide written informed consent as per the guidelines of the Research Ethics Committee (IRB No. TCHIRB-10902009). Treatment-seeking individuals who used METH were recruited from the Department of Addiction Sciences at the Taipei City Psychiatric Centre. Neuroimaging was performed at the Department of Radiology at Wang Fang Hospital in Taipei.
Participants
MUD group
The inclusion criteria were: (1) Men aged between 20 and 45 years; (2) fulfilling the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) for MUD as verified by two board-certified psychiatrists (coauthors M.C.H. and C.H.C.); (3) positive urine toxicology test for METH when treatment was administered; and (4) no MRI incompatibilities or known claustrophobia. Exclusion criteria were: (1) history of any other substance use disorder except for nicotine; (2) history of schizophrenia, bipolar disorder, or major depressive disorder; (3) history of major systemic illness such as hypertension, cardiovascular disease, diabetes mellitus, or thyroid, renal, or liver disease; (4) history of epileptic seizures, head injury, loss of consciousness, or neurological disorders; and (5) use of benzodiazepines one week prior to MRI examination. We have implemented the Chinese version of Diagnostic Interview of Genetic Studies (DIGS) [23, 24], a semi-structured diagnostic interview for psychiatric disorders, to collect sociodemographic data and METH use variables, and to screen for psychotic symptoms, including delusions and hallucinations. These symptoms were further confirmed by M.C.H. and C.H.C., who followed the participants for a month to verify the presence of METH-induced psychotic symptoms, such as delusions, hallucinations, suspiciousness, and unusual thought content, as suggested previously [7, 8]. Accordingly, those with psychotic symptoms were classified as the MAP subgroup, and those without psychotic symptoms were classified as the MNP subgroup.
Healthy control group
Healthy sex-, age-, and education-matched volunteers were recruited from the community through advertisements. Recruitment criteria were: (1) aged between 20 and 45 years; (2) no history of any substance use disorders except nicotine; (3) no history of major psychiatric, systemic, or neurological diseases, as described above; and (4) no MRI incompatibilities or known claustrophobia. In addition, the handedness of the healthy controls and MUD groups was recorded.
Psychological assessments
Because METH withdrawal lasts for 2–4 weeks [25], clinical data were evaluated after 3–4 weeks, when the participant was clear of withdrawal symptoms. A trained research assistant conducted face-to-face interviews, employing the Hollingshead Four-Factor Index of socioeconomic status (SES) scale [26–28] and the Chinese version of DIGS [23, 24]. The severity of psychotic symptoms was assessed using the Chinese version of the Positive and Negative Syndrome Scale (PANSS) (M.C.H.) [29]. Depressive and anxiety symptoms were evaluated using the Chinese versions of the 21-item Beck Depression Inventory (BDI) [30] and Beck Anxiety Inventory (BAI) [31].
MRI data acquisition
All datasets were acquired using a 3-T scanner (Discovery MR750w; GE Healthcare, Milwaukee, USA) with a 24-channel brain coil at Wan Fang Hospital. The protocol included rs-fMRI scans using a T2* weighted gradient echo-planar imaging sequence (repetition time/echo time, 2500 ms/30 ms; flip angle, 80°; 43 slices at a thickness of 3 mm with no gap; in-plane resolution, 3 × 3 mm2; 210 volumes), followed by a three-dimensional T1 weighted scan using a brain volume gradient echo sequence (FSPGR-BRAVO) (repetition time, 8.5 ms; echo time, 3.248 ms; time to inversion, 450 ms; flip angle, 12°; field of view matrix size, 256 × 256; voxel size, 0.94 × 0.94 × 1.2 mm3). The total MRI scan time was approximately 40 min. During the resting-state scan, participants were asked to keep their eyes closed, to remain stationary but avoid falling asleep, and not to think about anything in particular during the 6-minute acquisition period of 210 volumes, of which the first ten were discarded.
Functional MRI analysis
All resting-state functional MRI (rs-fMRI) data preprocessing and subsequent computation of the whole-brain functional connectome were performed using the open-access IClinfMRI [32] and AFNI software [33], respectively. Data preprocessing involved correcting each rs-fMRI volume for head movement using a rigid-body spatial transformation, applying nonlinear normalization to the standard Montreal Neurological Institute space, and then resampling to 2-mm isotropic voxels. Following normalization, high-motion spikes and third-order trends presented in the rs-fMRI data were removed through volume censoring (3dDespike) and detrending (3dDetrend) methods to reduce voxel-wise effects of head movement and drifting artifacts. Multiple linear regression was then employed to eliminate the nuisance effects from motion profiles and physiological fluctuations from white matter and cerebrospinal fluid. Subsequently, band-pass filtering of rs-fMRI fluctuations within a range of 0.01–0.08 Hz was applied in order to preserve neural frequencies while reducing motion-induced frequencies, followed by smoothing at 4-mm full-width at half-maximum. To address potential negative influence attributed to head motion during data acquisition, we implemented a 5-stage retrospective strategy as recommended [34]. The first four stages were performed in individual preprocessing, including realignment, volume censoring, nuisance regressor (individual-level generalized linear model, GLM), and band-pass filtering. The last stage involved regressing out the individual mean framewise displacement (FD) during group analysis. After the above preprocessing, the whole-brain functional connectome was then examined, reflecting inter-regional communications within a predefined framework of eleven functional networks [35]: dorsal attention network (DAN), ventral attention network (VAN), subcortical network (SCN), salience network (SN), frontoparietal network (FPN), visual network (VN), default mode network (DMN), auditory network (AN), cingulo-opercular network (CON), sensory/somatomotor network (SMN), and memory networks (MN). The construction of the whole-brain functional connectome was based on a set of region of interests (ROIs) defined with this functional network framework, where each ROI was represented by a sphere with a 5-mm radius. To comprehensively analyze the functionality disruptions in terms of the functional network framework following METH administration, 245 ROIs derived from three sources were integrated. The sources were: (1) 229 ROIs summarized from previous task-evoked fMRI and rs-fMRI studies in the meta-analytic literature [36]; (2) 12 ROIs from an rs-fMRI study that thoroughly modeled the basal ganglia circuitry [37]; and (3) 4 ROIs (2 amygdala and 2 hippocampus) to complement the SCN, sourced from large-scale automated syntheses using “emotion” and “memory” terms, respectively, spanning 1037 and 2744 studies, respectively, in Neurosynth [38]. The detailed coordinates of each ROI are shown in Supplementary Table 1. Correlation coefficients between the preprocessed time series of each ROI pair in the functional network framework were computed using a Pearson correlation, as implemented in the AFNI 3dNetCorr method [39]. For each participant, a 245 × 245 correlation matrix was produced and subsequently transformed into a z-transformed matrix using Fisher’s r-to-z transformation. Specifically, these connectivity matrices include links for both the intra- and inter-network connections among the 11 large-scale functional networks.
Motion analysis of rs-fMRI data
For each participant, the motion profiles of the six motion parameters, encompassing translations and rotation along a three-dimensional coordinate system, were summarized as the mean frame displacement (FD) metric.
Statistical analysis
To analyze demographic and clinical characteristics, as well as motion profiles of rs-fMRI data, one-way ANOVA and chi-square tests were conducted to examine the main effects using SPSS software (version 22, IBM, Chicago, IL). For data distribution violating the normality assumption, as determined by the Shapiro–Wilk test, the non-parametric Kruskal–Wallis test was used instead. Beyond the main effect, post-hoc pairwise comparisons with Bonferroni corrections were applied to examine significance between groups. The statistical threshold was set at p < 0.05, two-tailed.
In this study, two primary statistical analyses of the whole-brain functional connectome were performed using the Network-Based Statistic method [40]. Link-based statistical tests were applied to identify significant changes in pairwise functional matrices within and between the 11 large-scale functional networks. These changes were examined across all possible pairings of the three participant groups: CRL, MNP, and MAP. To mitigate the effects of head movement at the population level, the mean FD for each participant was included as a nuisance regressor. Additional nuisance regressors accounted for potential confounding factors, including age and education, which may interact with METH dependence. Besides, we controlled for depression (BDI) and anxiety (BAI) scores to disentangle the effects of psychosis from those of depression and anxiety states. All nuisance regressors were centered on their mean during statistical testing [41].
In addition to examining group differences among the three groups, the relationship between connectivity and the severity of psychotic symptoms within the MUD group (n = 54) was examined using regression analysis. To account for multiple comparisons in both the group comparison and the regression analysis, the significance level was corrected using a false discovery rate, p < 0.05, and 5,000 permutations.
Results
Sociodemographic, clinical characteristics, and rs-fMRI motion profiles
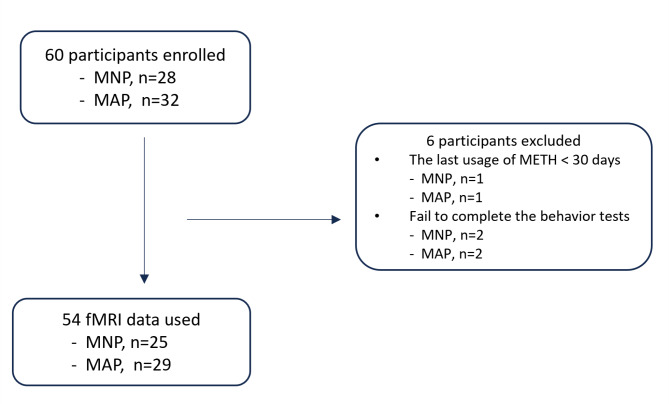
Of the 60 men enrolled in the MUD group, 6 were excluded due to recent (within 30 days) exposure to METH (n = 2) or incomplete psychiatric assessment results (n = 4). The remaining 54 participants were classified into the MAP subgroup (n = 25) or the MNP subgroup (n = 29) (Fig. 1). Table 1 summarizes the demographic and clinical characteristics of each participant group. No significant differences were observed between the three groups regarding age, years of education, socioeconomic status, or handedness. Furthermore, the MAP and MNP subgroups did not differ in age at initiation of METH use, duration of use, or severity of psychotic symptoms (PANSS-Positive [PANSS-P], PANSS-Negative [PANSS-N], PANSS-General Psychopathology [PANSS-GP], and PANSS-Total [PANSS-T]). In post-hoc analysis, the MAP subgroup exhibited significantly higher scores on the BAI compared to the MNP subgroup (p < 0.05 after Bonferroni correction). However, there was no significant difference in the BDI between these two subgroups. Significant differences in both BDI and BAI scores were observed between the CRL and MAP groups (p < 0.05 after Bonferroni correction). Additionally, years of smoking and pack years showed significant differences between the CRL group and both the MNP and the MAP subgroups (p < 0.05 after Bonferroni correction). Regarding head-motion characteristics during the rs-fMRI scan, no participants exhibited maximum head movement (displacements ≥ 4.0 mm or rotations ≥ 3.0° in any direction). The Kruskal-Wallis H test showed no significant difference in mean FD across the three groups, χ2(2) = 1.46, p > 0.48.
Fig. 1.
Flow diagram of study participants
Table 1.
Demographic and clinical variables between healthy controls and MUD group
| CRL | MUD patients | Main effect | Post-hoc pairwise comparisons | ||||
|---|---|---|---|---|---|---|---|
| MNP | MAP | CRL vs. MNP | CRL vs. MAP | MNP vs. MAP | |||
| Variables | n = 24 | n = 25 | n = 29 | ||||
| Age, years old, | 32.1 ± 5.1 | 35.1 ± 8.0 | 32.7 ± 6.0 | 0.24k | |||
| Education (years) | 13.6 ± 2.3 | 14.0 ± 2.3 | 14.3 ± 2.7 | 0.61k | |||
| SES (range) |
35.1 ± 10.3 (range 13–52) |
34.5 ± 12.7 (range 16–61) |
29.2 ± 10.3 (range 11–51) |
0.11k | |||
| Smokers, n (%) | 3(12.5%) | 13(52%) | 18(62%) | < 0.001 c | 0.001 # | 0.521 | 0.056 |
| Years of Smoking | 2.4 ± 6.7 | 10.2 ± 12.3 | 9.5 ± 9.7 | < 0.005 k | 0.012* | 0.004 # | 1.000 |
| Average package of smoking per day | 0.08 ± 0.24 | 0.3 ± 0.36 | 0.6 ± 1.04 | 0.002 k | 0.032* | 0.001# | 1.000 |
| Pack Years | 1.7± 5.5 | 5.4 ± 7.1 | 10.4± 18.9 | < 0.001 k | 0.019* | 0.002# | 1.000 |
| Age of first use of METH, years old | N/A | 30.4 ± 9.7 | 26.1 ± 6.6 | 0.06 m | |||
| Duration of METH use, years | N/A | 3.7 ± 4.5 | 4.5 ± 4.3 | 0.10k | |||
| Handedness | 0.98c | ||||||
| Right | 22 | 23 | 27 | ||||
| Left | 2 | 2 | 2 | ||||
| Psychological assessment | |||||||
| BDI (0–63) | 5.2 ± 6.3 | 9.3 ± 8.9 | 14.0 ± 10.9 | < 0.001 k | 0.247 | 0.001 # | 0.151 |
| BAI (0–63) | 2.8± 4.7 | 3.4± 5.5 | 9.7 ± 10.5 | < 0.005 k | 1.000 | 0.005 # | 0.021 * |
| PANSS Total | 37.9 ± 5.2 | 39.4 ± 5.1 | 0.20m | ||||
| PANSS-Positive | 8.3 ± 1.6 | 8.9 ± 2.2 | 0.36m | ||||
| PANSS-Negative | 8.9 ± 1.7 | 9.0 ± 2.3 | 0.62m | ||||
| PANSS-GP. | 20.7± 3.4 | 21.6 ± 3.0 | 0.23m | ||||
k: Kruskal Wallis test, m: Mann–Whitney U test, c: Chi–square test
*: p < 0.05 after Bonferroni correction; #: p < 0.01 after Bonferroni correction. The p-values in bold represent those p < 0.05
Abbreviations: CRL: healthy controls; MUD: methamphetamine use disorder; MNP: methamphetamine users with no psychosis; MAP: methamphetamine-associated psychosis; N/A: not applicable; SES: Hollingshead Four-Factor Index of SocioEconomic status scale; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; PANSS: Positive and Negative Syndrome Scale; GP: General Psychopathology
Group differences in inter-network and intra-network functional connectivity
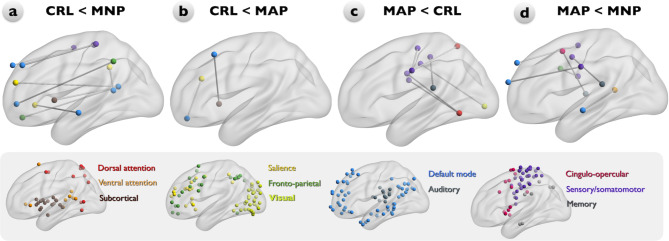
Compared to the CRL group, the MNP subgroup showed enhanced rsFC between the FPN-DMN, SN-DMN, SMN-DMN, and SCN-DMN (Fig. 2a). The MAP subgroup also showed enhanced rsFC between the SN-DMN and the SCN-DMN compared to the CRL group (Fig. 2b) but decreased rsFC between the SMN-DAN, SMN-VAN, SMN-SN, SMN-AN (Fig. 2c). Additionally, compared to the MNP subgroup, the MAP subgroup exhibited a lower rsFC between the SMN-DMN, SMN-AN, SMN-FPN, DMN-VAN, and CON-AN (Fig. 2d). In intra-network analysis, decreased rsFC within SMN was observed in the MAP subgroup compared to the CRL group (Fig. 2c). After controlling for the nuisance factors of BDI and BAI, further link disruptions were observed between the DAN-FPN, FPN-VN, and SMN in the MAP subgroup compared to the MNP subgroup (Supplementary Fig. 1d). Compared to the CRL group, the MAP subgroup showed decreased rsFC only in the SMN-AN (Supplementary Fig. 1c). Additionally, the MNP subgroup exhibited increased rsFC between the VAN-SCN and SN-SMN, and within the FPN (Supplementary Fig. 1a).
Fig. 2.
Significant group differences in large scale resting-state functional connectivity (rsFC) among the CRL, MNP, and MAP participant groups. Node color represents the network and corresponding distributions. a: Compared to the CRL group, the MNP subgroup shows enhanced rsFC between the FPN-DMN, SN-DMN, SMN-DMN, and SCN-DMN. b-c: The MAP group shows increased strength of rsFC between the SN-DMN and the SCN-DMN but decreased strength of rsFC between the SMN-DAN, SMN-VAN, SMN-SN, SMN-AN, and within SMN compared to the CRL group. d: Compared with the MNP subgroup, the MAP subgroup shows decreased strength of rsFC between the SMN-DMN, SMN-AN, SMN-FPN, DMN-VAN, and CON-AN. Abbreviations: CRL: healthy controls; MNP: methamphetamine users with no psychosis; MAP: methamphetamine-associated psychosis; DAN: dorsal attention network (red); VAN: ventral attention network (orange); SCN: subcortical network (maroon); SN: salience network (yellow); FPN: frontoparietal network (sea green); VN: visual network (lawn green); DMN: default mode network (dodger blue); AN: auditory network (slate grey); CON: cingulo-opercular network (deep pink); SMN: sensory/somatomotor network (dark violate); MN: memory network (grey)
Relationships between functional connectivity and PANSS scores in individuals with MUD
A correlation analysis was conducted between both the inter-network and intra-network functional matrices of the two MUD subgroups and their corresponding psychotic severities, assessed using the PANSS-Total (PANSS-T), PANSS-Positive (PANSS-P), PANSS-Negative (PANSS-N) and PANSS-General Psychopathology (PANSS-GP) subscales. Every subscale showed a significant negative correlation with the rsFC of the corresponding site (Fig. 3). Specifically, PANSS-P was negatively correlated with the DMN-FPN, FPN-SMN (Fig. 3a), PANSS-N was negatively correlated with the DMN-AN and within SMN (Fig. 3b), PANSS-GP was negatively correlated with the DAN-SN, SMN-CON, SMN-SN (Fig. 3c), and PANSS-T was negatively correlated with the VN-FPN, AN-FPN, AN-SMN, SMN-CON and within the SMN (Fig. 3d). After adjusting for BDI and BAI, we found an additional negative correlation between SMN-AN connection and PANSS-P (Supplementary Fig. 2a). Additionally, the PANSS-GP exhibited negative correlations with both the FPN-AN and SCN-AN connections but showed no correlation with the DAN-SN and SMN-SN connections (Supplementary Fig. 2c). Moreover, an additional negative correlation between the PANSS-T and the SCN-DMN connection was identified (Supplementary Fig. 2d).
Fig. 3.
Relationships between and within the functional connectivity of the resting-state (rsFC) network and PANSS scores in the MAP and MNP subgroups. The PANSS-Total (PANSS-T), PANSS-Positive (PANSS-P), PANSS-Negative (PANSS-N), and PANSS-General Psychopathology (PANSS-GP) subscales were assessed, yielding a negative correlation with rsFC at corresponding sites. a: The PANSS-P is negatively correlated with the DMN-FPN and FPN-SMN. b: The PANSS-N is negatively correlated with the DMN-AN and within the SMN. c: The PANSS-GP is negatively correlated with the DAN-SN, SMN-CON, and SMN-SN. d: The PANSS-T is negatively correlated with the VN-FPN, AN-FPN, AN-SMN, SMN-CON, and within the SMN. Abbreviations: PANSS: Positive and Negative Syndrome Scale; DMN: default mode network (dodger blue); FPN: frontoparietal network (sea green); SMN: sensory/somatomotor network (dark violate); AN: auditory network (slate grey); DAN: dorsal attention network (red); SN: salience network (yellow); CON: cingulo-opercular network (deep pink); VN: visual network (lawn green)
Discussion
This study investigated differences in both inter-network and intra-network connectomes between the MNP and MAP subgroups and healthy controls. Our results showed that: (1) both of the MUD subgroups exhibited increases in rsFC between the SN-DMN and SCN-DMN compared to the CRL group; (2) the MNP subgroup exhibited an increase in rsFC strength between the FPN-DMN and SMN-DMN compared to the CRL group; (3) the MAP subgroup exhibited a decrease in rsFC strength between the SMN-DAN, SMN-VAN, SMN-SN, SMN-AN and within the SMN compared to the CRL group; (4) compared to the MNP subgroup, the MAP subgroup exhibited a lower rsFC between the SMN-DMN, SMN-AN, SMN-FPN, DMN-VAN, and CON-AN; and (5) PANSS was negatively correlated with the rsFC in both MUD subgroups. While PANSS-P was negatively correlated with the DMN-FPN and FPN-SMN, PANSS-N was negatively correlated with the DMN-AN and within the SMN. These results lend support to the hypothesis that certain network dysfunctions represent a core neural deficit underlying psychosis development in individuals with MUD.
Inter-network connectivity in METH users: Alterations in DMN-SN-FPN coupling
The findings of this study appear consistent with the model of disrupted DMN–SN–FPN coupling in substance use disorders [20, 42–44]. The FPN is related to cognitive control and executive function, especially for external stimuli, while the DMN is associated with self-referential cognition [20]. Cooperation between the DMN and FPN produces an internal train of thought [45]. Furthermore, the FPN regulates the two anticorrelated behaviors between the DMN and the CCN-DAN. It coordinates dynamic and task-dependent switching between the CCN-DAN and DMN in response to various task demands, given its anatomical location in the parietal lobe and its highest degree of internetwork temporal variability [20, 46, 47]. Our results show increased rsFC between the FPN and DMN in the MNP subgroup compared to controls. This finding is consistent with the result found in the previous literature [20], which suggests that chronic METH exposure is related to a disability of the FPN in coactivation of temporal variability and a switch between the DMN and the CCN-DAN, resulting in hyperconnectivity of the FPN-DMN. However, we did not find hyperconnectivity in the FPN-DMN in the MAP subgroup compared to the CRL group. This disparity could be the result of heterogeneity in the duration of psychotic symptoms among members of the MAP subgroup in this study.
Increased rsFC strength between the SN-DMN was found in both MUD subgroups compared to the CRL group, supporting several other studies [42–44]. Briefly, the SN, consisting of the anterior insula and the dorsal anterior cingulate cortex, detects significant events and facilitates other networks, modulating autonomic reactivity in response to significant stimuli [48]. Li et al. showed increased functional connectivity between the posterior cingulate cortex (PCC) and the insula in chronic heroin users compared to healthy controls, and they showed a positive correlation with the duration of heroin use [43]. Similarly, Wetherill et al. demonstrated enhanced functional connectivity of the PCC-right anterior insula in cannabis-dependent individuals compared to healthy controls [44]. Naqvi and Bechara addressed the role of the insula in the process of interoceptive effects of drug use and drug urges [49, 50]. Zhang and Volkow found that altered SN-DMN connectivity might enhance salience toward drug signals [42]. Collectively, these studies suggest that altered rsFC in the SN-DMN could be a factor in the increased relevance of METH use, and it could ultimately contribute to METH addiction and relapse.
Aberrant inter-network rsFC of SMN and DMN in the MNP and MAP subgroups
We demonstrated a disrupted rsFC in functional networks that interact with the SMN in the MUD group. The MNP subgroup showed enhanced rsFC in the SMN-DMN compared to the CRL group. The MAP subgroup, however, showed decreased rsFC in inter-SMN networks (e.g., SMN-DAN, SMN-VAN, SMN-SN, and SMN-AN) compared to the CRL group. In addition, the MAP subgroup showed lower rsFC in the SMN-DMN compared to the MNP subgroup. Aberrant inter-network rsFC of the SMN in METH users has been described in the literature. For example, Li et al. reported lower rsFC between the SMN and the cerebellum in individuals with MUD compared to healthy controls [51] and suggested that these impaired networks were related to drug compulsivity and the inability to control habitual drug consumption.
The DMN can be divided into anterior and posterior regions. The anterior DMN plays a role in personal and emotional regulation, and the posterior DMN directs attention to internal thoughts [42]. The two regions coordinate with each other, with the posterior DMN directing attention to internal/external stimuli and transferring the stimuli to the anterior DMN, which monitors the internal states and regulates emotional and personal relevance [42, 52–54]. A review of the literature on DMN dysfunction in addiction found that rsFC in addicted individuals decreased in the anterior DMN and increased in the posterior DMN [42]. Additionally, dysfunction of the ventromedial prefrontal cortex, which is part of the anterior DMN, is correlated with compromised self-awareness in drug addiction, including self-reported behavior dissociation and inappropriate social behavior [54].
Taken together, our results potentially imply that MAP is distinct from MNP in terms of aberrant inter-network rsFC of the SMN, and we suggest that those with MAP are more vulnerable to drug-related neurobiological deficits and subsequent psychological consequences such as greater compulsivity and preserving less self-awareness relative to those with MNP. Notably, all METH users were scanned after discontinuation of METH use for at least 3–4 weeks, by which time the psychotic symptoms had generally subsided. The distinct connectome profiles between the MNP and MAP subgroups suggest that MAP is associated with more specific neurobiological pathways in susceptibility to psychosis development among individuals exposed to METH. Identification of these altered networks could aid in future classification approaches.
Relationships between functional connectivity and PANSS scores in individuals with MUD
This study shows that the severity of positive psychotic symptoms, as measured by the PANSS-P, is negatively correlated with the strength of functional connectivity in the FPN-DMN among the MNP and MAP. The FPN plays a vital role in cognitive control and executive function and acts as a flexible hub in adaptive implementations of task demands [55]. Cognitive deficits or impairments are common in psychotic disorders, and altered FPN has been associated with psychotic disorders [56]. Lewandowski et al. showed an additional reduction of FPN connectivity in psychotic patients with impaired cognition compared to psychotic patients with intact cognition [57]. Notably, recent studies have shown damage to the FPN-DMN in substance use disorders. Li et al. reported that hypoconnectivity between the left executive control network (ECN) and the DMN is associated with heroin relapse behavior [58]. Liang et al. showed that cocaine addiction is associated with disrupted connectivity between the PCC and the ECN [59]. In particular, our result is supported by Gong et al. who demonstrated a decrease of FPN-DMN in METH abstainers [60]. Taken together, our findings suggest that hypoconnectivity between the FPN-DMN is positively related to the severity of positive symptoms.
Our results also demonstrate that PANSS-N is negatively correlated with rsFC in the DMN-AN and intra-SMN. The control of emotions and behaviors is regulated by the anterior DMN, whereas the AN interacts with the external environment and further processes auditory information and cognitive tasks such as language [61]. The negative symptoms of PANSS include blunted affect, emotional withdrawal, poor rapport, passive/apathetic social withdrawal, difficulty in abstract thinking, lack of spontaneity and flow of conversation, and stereotyped thinking. Our findings suggest that these negative symptoms are related to decreased activity caused by external or environmental stimuli. Correspondingly, the observed hypo-rsFC between the DMN-AN could indicate a slower reaction or a delayed response in the attentional processing of auditory information and social cognition. Subsequently, these negative symptoms may exert an influence on the SMN domain.
In this study, both the MNP and MAP subgroups exhibited a negative correlation between PANSS-GP and the rsFC of the CON-SMN, DAN-SN, and SMN-SN. In addition to FPN, the CON regulates top-down control of executive functioning. On the other hand, the DAN is thought to be involved in attending to external stimuli and engaging in an active attention process such as visuospatial attention during a task [62]. The DAN is also suggested to involve top-down attention to sensory inputs [62]. Therefore, decreased rsFC involving the DAN and the CON could explain executive dysfunction in MNP and MAP.
Limitations
This study has several limitations. Firstly, its cross-sectional design does not allow investigation of changes in the functional connectome over time, for example, from initial recreational use of METH to dependence, or to assess reversibility potential after long-term abstinence. In other words, the single-observation nature of the study precludes the possibility of confirming a causal relationship. Secondly, our MAP subgroup exhibited more severe depression and anxiety than our MNP subgroup. This is in line with previous evidence indicating that METH users with symptoms of anxiety and depression had a significantly greater risk of experiencing psychosis [63]. Our study, which focused on a single psychiatric phenomenon (psychosis), could conceal the existence of significant overlap in the distributions of connectome functioning related to subthreshold depression and anxiety symptoms. This could consequently create an illusion of group specificity for psychosis based on our connectome finding when, in reality, there could be shared and coordinated functional neural networks among complex psychiatric conditions [10, 64]. However, after controlling for BDI and BAI factors, we observed more disrupted inter-network rsFC between the MAP and MNP subgroups. These results further highlighted the credibility of the different neurobiological pathways associated with MAP compared to MNP, as proposed in our study. The third limitation of our study is that all recruited participants were from a single center, with a small sample size consisting exclusively of men; therefore, our results cannot represent all individuals who use METH and, specifically, it cannot represent the female population. Given the relatively infrequent occurrence of MAP compared to other substance use disorders, conduct of a formal sample size calculation was not feasible. Therefore, all eligible patients who were identified during the study period were recruited. A larger dataset from a gender-balanced population is warranted in future studies. A fourth limitation is that the potential effects of nicotine on altered network connections cannot be entirely excluded. Previous studies have shown that nicotine decreases activity within the DMN during the resting state [65] and that chronic nicotine exposure decreases the effectiveness of the functional network, while acute nicotine exposure enhances connectivity of particular limbic circuits [66]. Fifth, we performed rs-MRI after the participants in the MUD group were free from withdrawal symptoms, which limits our ability to generalize the data to those in an acute psychotic state, which usually subsides within one week after METH use [7, 8].
Conclusions
In summary, this study shows that exposure to METH alters global functional connectivity in the DMN, FPN, SN, and SMN. In addition, the severity of psychotic symptoms is inversely correlated with the rsFC of the corresponding sites, and the PANSS-P score is negatively correlated with the DMN-FPN and FPN-SMN. Moreover, the MAP subgroup exhibited more disrupted rsFC in the DMN and SMN than the MNP subgroup, suggesting that MAP involves greater drug compulsivity and less self-awareness. Our results suggest that MAP involves specific neurobiological pathways that determine the greater vulnerability of some METH users to developing psychosis. Identifying these modified networks could be beneficial for future classification strategies aimed at distinguishing individuals with and without psychoses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Fig. 1: The upper panels showed significant group differences in resting-state functional connectivity (rsFC) between the CRL, MNP, and MAP groups without controlling for BDI and BAI factors. The lower panels demonstrated various rsFC after additional controlling for both BDI and BAI factors. a: Compared to the CRL group, the MNP subgroup exhibited additionally increased rsFC between the VAN-SCN, SN-SMN, and within the FPN-FPN. b: The MAP subgroup exhibited no changes in enhanced rsFC of SN-DMN and SCN-DMN compared to the CRL group. c: the MAP subgroup only showed decreased rsFC between the SMN-AN compared to the CRL group. d: More disrupted rsFC between the DAN-FPN, FPN-VN, and within the SMN-SMN in the MAP subgroup compared to the MNP subgroup. Abbreviations: CRL: healthy controls; FD: frame displacement; MNP: methamphetamine users with no psychosis; MAP: methamphetamine-associated psychosis; DAN: dorsal attention network (red); VAN: ventral attention network (orange); SCN: subcortical network (maroon); SN: salience network (yellow); FPN: frontoparietal network (sea green); VN: visual network (lawn green); DMN: default mode network (dodger blue); AN: auditory network (slate grey); CON: cingulo-opercular network (deep pink); SMN: sensory/somatomotor network (dark violate); MN: memory network (grey). Fig. 2: The upper panels showed the relationships between and within the functional connectivity of the resting-state (rsFC) network and PANSS scores in the MAP and MNP subgroups without controlling for BDI and BAI factors. The lower panel demonstrated various rsFC after adjusting for BDI and BAI. a: An additional negative correlation between the SMN-AN and the PANSS-P score. b: No changes in the negative correlation for the PANSS-N and rsFC of DMN-AN and within SMN-SMN. c: Additionally, the PANSS-GP was negatively correlated with the FPN-AN and SCN-AN, but showed no correlation with the DAN-SN and SMN-SN. d: An additional negative correlation between the PANSS-T and the SCN-DMN connection was identified. Abbreviations: FD: frame displacement; MAP: methamphetamine-associated psychosis; MNP: methamphetamine users with no psychosis; PANSS: Positive and Negative Syndrome Scale; DMN: default mode network (dodger blue); FPN: frontoparietal network (sea green); SMN: sensory/somatomotor network (dark violate); AN: auditory network (slate grey); DAN: dorsal attention network (red); SN: salience network (yellow); CON: cingulo-opercular network (deep pink); VN: visual network (lawn green). Table 1: The MNI coordinates and suggested networks of 245 ROIs.
Acknowledgements
The authors would like to thank the participants for their time and efforts.
Author contributions
C.-H. C., M.-C.H.: Conceptualization, funding acquisition, project administration. Z.-A.H., C.-W.L., W.P. C.: Methodology. A.-L.H, C.-W.L., M.-C.H.: Data curation and formal analysis. Z.-A.H., C.W. W., W.P. C.: Investigation. Z.-A.H., A.-L.H: Investigation, literature search, figures, data analysis, data interpretation, writing-original draft. W.P.C., M.-C.H.: Supervision. All authors: Writing- Reviewing and Editing.
Funding
This work was supported by the Ministry of Science and Technology (MOST) of Taiwan (grant number MOST109-2314-B-532-004 [MCH]), (grant number MOST110-2314-B-532-005-MY3 [MCH]), the National Science Council (grant number NSC111-2314-B-038-062-MY2 [CHC]), and the Taipei City Government (grant number TPECH 11101-62-029 [MCH]) and (grant number 11301-62-016 [MCH]).
Data availability
Data supporting our findings in this study are available upon request from the corresponding author. Data are not publicly available because of privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
This study has been approved by the guidelines of the Research Ethics Committee (IRB No. TCHIRB-10902009) of Taipei City Psychiatric Centre. Informed consents were obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
Zhen-An Hwang, Ai-Ling Hsu, Changwei W. Wu, Chun-Hsin Chen, Chun-Hsin Chen, Ming-Chyi Huang, and Wing P. Chan declare no competing or potential conflicts of interest. One author, Chia-Wei Li, is an employee of GE Healthcare in Taiwan. No funding was received from GE Healthcare for publication activities.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhen-An Hwang and Ai-Ling Hsu contributed equally to this work.
Contributor Information
Wing P. Chan, Email: wingchan@tmu.edu.tw
Ming-Chyi Huang, Email: mch@tpech.gov.tw.
References
- 1.Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129(3):167–79. [DOI] [PubMed] [Google Scholar]
- 2.Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38(2):259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang MC, Yang SY, Lin SK, Chen KY, Chen YY, Kuo CJ, Hung YN. Risk of Cardiovascular diseases and stroke events in methamphetamine users: a 10-Year Follow-Up study. J Clin Psychiatry. 2016;77(10):1396–403. [DOI] [PubMed] [Google Scholar]
- 5.Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R. Methamphetamine Treatment Project Corporate A: clinical course and outcomes of methamphetamine-dependent adults with psychosis. J Subst Abuse Treat. 2008;35(4):445–50. [DOI] [PubMed] [Google Scholar]
- 6.McKetin R, Lubman DI, Najman JM, Dawe S, Butterworth P, Baker AL. Does methamphetamine use increase violent behaviour? Evidence from a prospective longitudinal study. Addiction. 2014;109(5):798–806. [DOI] [PubMed] [Google Scholar]
- 7.McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473–8. [DOI] [PubMed] [Google Scholar]
- 8.McKetin R. Methamphetamine psychosis: insights from the past. Addiction. 2018;113(8):1522–7. [DOI] [PubMed] [Google Scholar]
- 9.Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;185:196–204. [DOI] [PubMed] [Google Scholar]
- 10.Baker JT, Dillon DG, Patrick LM, Roffman JL, Brady RO Jr., Pizzagalli DA, Öngür D, Holmes AJ. Functional connectomics of affective and psychotic pathology. Proc Natl Acad Sci USA. 2019;116(18):9050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Constable RT. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinen JM, Chén OY, Hutchison RM, Yeo BTT, Anderson KM, Sabuncu MR, Öngür D, Roffman JL, Smoller JW, Baker JT, et al. The human cortex possesses a reconfigurable dynamic network architecture that is disrupted in psychosis. Nat Commun. 2018;9(1):1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13(3):141–54. [DOI] [PubMed] [Google Scholar]
- 14.Bernacer J, Corlett PR, Ramachandra P, McFarlane B, Turner DC, Clark L, Robbins TW, Fletcher PC, Murray GK. Methamphetamine-induced disruption of frontostriatal reward learning signals: relation to psychotic symptoms. Am J Psychiatry. 2013;170(11):1326–34. [DOI] [PubMed] [Google Scholar]
- 15.Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky decision making, Prefrontal Cortex, and Mesocorticolimbic Functional Connectivity in Methamphetamine Dependence. JAMA Psychiatry. 2014;71(7):812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65(8):706–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zalesky A, Fornito A, Cocchi L, Gollo LL, Breakspear M. Time-resolved resting-state brain networks. Proc Natl Acad Sci USA. 2014;111(28):10341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Hsu FC, Li CW, Huang MC. Structural, functional, and neurochemical neuroimaging of methamphetamine-associated psychosis: a systematic review. Psychiatry Res Neuroimaging. 2019;292:23–31. [DOI] [PubMed] [Google Scholar]
- 20.Ipser JC, Uhlmann A, Taylor P, Harvey BH, Wilson D, Stein DJ. Distinct intrinsic functional brain network abnormalities in methamphetamine-dependent patients with and without a history of psychosis. Addict Biol. 2018;23(1):347–58. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Hu Q, Tang T, Liu C, Li C, Zang YY, Cai WX. Changes in Gray Matter Density, Regional Homogeneity, and functional connectivity in Methamphetamine-Associated psychosis: a resting-state functional magnetic resonance imaging (fMRI) study. Med Sci Monit. 2018;24:4020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKetin R, Voce A, Burns R, Ali R, Lubman DI, Baker AL, Castle DJ. Latent psychotic symptom profiles Amongst people who Use Methamphetamine: what do they tell us about existing diagnostic categories? Front Psychiatry. 2018;9:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry. 1998;155(9):1214–20. [DOI] [PubMed] [Google Scholar]
- 24.Nurnberger JI Jr., Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–59. discussion 863 – 844. [DOI] [PubMed] [Google Scholar]
- 25.Shoptaw SJ, Kao U, Heinzerling K, Ling W. Treatment for amphetamine withdrawal. Cochrane Database Syst Rev. 2009;2009(2):Cd003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai T-o, Yi C-C, Liu C-H. Early marriage in Taiwan: evidence from Panel Data. J Fam Issues. 2019;40(14):1989–2014. [Google Scholar]
- 27.Chen AS-y, Lin G-h, Yang H-w. Staying connected: effects of social connectedness, cultural intelligence, and socioeconomic status on overseas students’ life satisfaction. Int J Intercultural Relations. 2021;83:151–62. [Google Scholar]
- 28.Hollingshead AB. Four factor index of social status. In.: New Haven, CT; 1975.
- 29.Lane HY, Lee CC, Chang YC, Lu CT, Huang CH, Chang WH. Effects of dopamine D2 receptor Ser311Cys polymorphism and clinical factors on risperidone efficacy for positive and negative symptoms and social function. Int J Neuropsychopharmacol. 2004;7(4):461–70. [DOI] [PubMed] [Google Scholar]
- 30.Lu M-L, Che HH, Chang S, Shen WW. Reliability and validity of the Chinese Version of the Beck Depression Inventory-II. Taiwan J Psychiatry. 2002;16:301–10. [Google Scholar]
- 31.Lee DT, Yip AS, Chiu HF, Leung TY, Chung TK. Screening for postnatal depression: are specific instruments mandatory? J Affect Disord. 2001;63(1–3):233–8. [DOI] [PubMed] [Google Scholar]
- 32.Hsu AL, Hou P, Johnson JM, Wu CW, Noll KR, Prabhu SS, Ferguson SD, Kumar VA, Schomer DF, Hazle JD, et al. IClinfMRI Software for integrating functional MRI techniques in Presurgical Mapping and Clinical studies. Front Neuroinformatics. 2018;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. [DOI] [PubMed] [Google Scholar]
- 34.Goto M, Abe O, Miyati T, Yamasue H, Gomi T, Takeda T. Head Motion and correction methods in resting-state functional MRI. Magn Reson Med Sci. 2016;15(2):178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cerebral cortex (New York, NY: 1991) 2008, 18(12):2735–2747. [DOI] [PubMed]
- 38.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor PA, Saad ZS. FATCAT: (an efficient) functional and Tractographic Connectivity Analysis Toolbox. Brain Connect. 2013;3(5):523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53(4):1197–207. [DOI] [PubMed] [Google Scholar]
- 41.Scott JCC. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 2007, 17(3). [DOI] [PubMed]
- 42.Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. NeuroImage. 2019;200:313–31. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Yang WC, Wang YR, Huang YF, Li W, Zhu J, Zhang Y, Zhao LY, Qin W, Yuan K, et al. Abnormal function of the posterior cingulate cortex in heroin addicted users during resting-state and drug-cue stimulation task. Chin Med J (Engl). 2013;126(4):734–9. [PubMed] [Google Scholar]
- 44.Wetherill RR, Fang Z, Jagannathan K, Childress AR, Rao H, Franklin TR. Cannabis, cigarettes, and their co-occurring use: disentangling differences in default mode network functional connectivity. Drug Alcohol Depend. 2015;153:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012;1428:60–70. [DOI] [PubMed] [Google Scholar]
- 46.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. 2012;33(1):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214(5–6):435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Su H, Zhong N, Chen T, Du J, Xiao K, Xu D, Song W, Jiang H, Zhao M. Aberrant resting-state cerebellar-cerebral functional connectivity in methamphetamine-dependent individuals after six months abstinence. Front Psychiatry. 2020;11:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abraham A. The world according to me: personal relevance and the medial prefrontal cortex. Front Hum Neurosci. 2013;7:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316(1):29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18(12):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16(9):1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cole MW, Repovš G, Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist: Rev J Bringing Neurobiol Neurol Psychiatry. 2014;20(6):652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewandowski KE, McCarthy JM, Öngür D, Norris LA, Liu GZ, Juelich RJ, Baker JT. Functional connectivity in distinct cognitive subtypes in psychosis. Schizophr Res. 2019;204:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Q, Liu J, Wang W, Wang Y, Li W, Chen J, Zhu J, Yan X, Li Y, Li Z, et al. Disrupted coupling of large-scale networks is associated with relapse behaviour in heroin-dependent men. J Psychiatry Neurosci. 2018;43(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Interactions between the salience and default-mode networks are disrupted in cocaine addiction. J Neuroscience: Official J Soc Neurosci. 2015;35(21):8081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong M, Shen Y, Liang W, Zhang Z, He C, Lou M, Xu Z. Impairments in the default Mode and executive networks in methamphetamine users during short-term abstinence. Int J Gen Med. 2022;15:6073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morillon B, Lehongre K, Frackowiak RS, Ducorps A, Kleinschmidt A, Poeppel D, Giraud AL. Neurophysiological origin of human brain asymmetry for speech and language. Proc Natl Acad Sci USA. 2010;107(43):18688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su MF, Liu MX, Li JQ, Lappin JM, Li SX, Wu P, Liu ZM, Shi J, Lu L, Bao Y. Epidemiological characteristics and risk factors of Methamphetamine-Associated psychotic symptoms. Front Psychiatry. 2018;9:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, Ugurbil K, Barch DM, Van Essen DC, Miller KL. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18(11):1565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanabe J, Nyberg E, Martin LF, Martin J, Cordes D, Kronberg E, Tregellas JR. Nicotine effects on default mode network during resting state. Psychopharmacology. 2011;216(2):287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci. 2015;1349(1):64–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Fig. 1: The upper panels showed significant group differences in resting-state functional connectivity (rsFC) between the CRL, MNP, and MAP groups without controlling for BDI and BAI factors. The lower panels demonstrated various rsFC after additional controlling for both BDI and BAI factors. a: Compared to the CRL group, the MNP subgroup exhibited additionally increased rsFC between the VAN-SCN, SN-SMN, and within the FPN-FPN. b: The MAP subgroup exhibited no changes in enhanced rsFC of SN-DMN and SCN-DMN compared to the CRL group. c: the MAP subgroup only showed decreased rsFC between the SMN-AN compared to the CRL group. d: More disrupted rsFC between the DAN-FPN, FPN-VN, and within the SMN-SMN in the MAP subgroup compared to the MNP subgroup. Abbreviations: CRL: healthy controls; FD: frame displacement; MNP: methamphetamine users with no psychosis; MAP: methamphetamine-associated psychosis; DAN: dorsal attention network (red); VAN: ventral attention network (orange); SCN: subcortical network (maroon); SN: salience network (yellow); FPN: frontoparietal network (sea green); VN: visual network (lawn green); DMN: default mode network (dodger blue); AN: auditory network (slate grey); CON: cingulo-opercular network (deep pink); SMN: sensory/somatomotor network (dark violate); MN: memory network (grey). Fig. 2: The upper panels showed the relationships between and within the functional connectivity of the resting-state (rsFC) network and PANSS scores in the MAP and MNP subgroups without controlling for BDI and BAI factors. The lower panel demonstrated various rsFC after adjusting for BDI and BAI. a: An additional negative correlation between the SMN-AN and the PANSS-P score. b: No changes in the negative correlation for the PANSS-N and rsFC of DMN-AN and within SMN-SMN. c: Additionally, the PANSS-GP was negatively correlated with the FPN-AN and SCN-AN, but showed no correlation with the DAN-SN and SMN-SN. d: An additional negative correlation between the PANSS-T and the SCN-DMN connection was identified. Abbreviations: FD: frame displacement; MAP: methamphetamine-associated psychosis; MNP: methamphetamine users with no psychosis; PANSS: Positive and Negative Syndrome Scale; DMN: default mode network (dodger blue); FPN: frontoparietal network (sea green); SMN: sensory/somatomotor network (dark violate); AN: auditory network (slate grey); DAN: dorsal attention network (red); SN: salience network (yellow); CON: cingulo-opercular network (deep pink); VN: visual network (lawn green). Table 1: The MNI coordinates and suggested networks of 245 ROIs.
Data Availability Statement
Data supporting our findings in this study are available upon request from the corresponding author. Data are not publicly available because of privacy or ethical restrictions.