Abstract
Background
Major psychotic disorders (MPD), including schizophrenia (SCZ) and schizoaffective disorder (SAD), are severe neuropsychiatric conditions with unclear causes. Understanding their pathophysiology is essential for better diagnosis, treatment, and prognosis. Recent research highlights the role of inflammation and the immune system, particularly the Interleukin 17 (IL-17) family, in these disorders. Elevated IL-17 levels have been found in MPD, and human IL-17 A antibodies are available. Changes in chemokine levels, such as CCL20, are also noted in SCZ. This study investigates the relationship between serum levels of IL-17 A and CCL20 in MPD patients and their clinical characteristics.
Method
We conducted a case-control study at the Ibn Sina Psychiatric Hospital (Mashhad, Iran) in 2023. The study involved 101 participants, of which 71 were MPD patients and 30 were healthy controls (HC). The Positive and Negative Symptom Scale (PANSS) was utilized to assess the symptoms of MPD patients. Serum levels of CCL20 and IL-17 A were measured using Enzyme-Linked Immunosorbent Assay (ELISA) kits. We also gathered data on lipid profiles and Fasting Blood Glucose (FBS).
Results
The mean age of patients was 41.04 ± 9.93 years. The median serum levels of CCL20 and IL-17 A were significantly elevated in MPD patients compared to HC (5.8 (4.1–15.3) pg/mL and 4.2 (3–5) pg/mL, respectively; p < 0.001). Furthermore, CCL20 and IL-17 A levels showed a positive correlation with the severity of MPD. MPD patients also had significantly higher FBS, cholesterol, and Low-Density Lipoprotein (LDL) levels, and lower High-Density Lipoprotein (HDL) levels compared to HC. No significant relationship was found between PANSS components and blood levels of IL17 and CCL20.
Conclusion
The current study revealed that the serum levels of IL-17 A and CCL20 in schizophrenia patients are higher than those in the control group. Metabolic factors such as FBS, cholesterol, HDL, and LDL also showed significant differences between MPD and HC. In conclusion, the findings suggest that these two inflammatory factors could serve as potential therapeutic targets and prognostic biomarkers for schizophrenia.
Keywords: Major psychotic disorders, Schizophrenia, Schizoaffective disorder, CCL20, IL-17A, PANSS
Background
Major Psychotic Disorders (MPD) are a group of chronic and severe mental disorders, such as schizophrenia (SCZ) and schizoaffective disorder (SCA), in which patients are at high risk of premature death. Comorbidities of MPD are a significant public health challenge affecting more than 20 million people worldwide and require ongoing research, education, and treatment [1]. The global prevalence of schizophrenia is 1% of the population [2]and the prevalence of psychotic disorders in the Iranian population is 0.89% [3].The issue of the involvement of inflammatory and autoimmune processes in the pathogenesis of schizophrenia has become an important issue in recent years and is still not fully understood [4]. One theory suggests that there may be a link between neuroinflammation and the development of this disorder. The lack of a clear understanding of the mechanisms that underlie MPD has made it challenging to develop effective treatments for this disorder. However, recent advances in neuroscience research, including studies of brain structure and function, neurotransmitter systems, and inflammatory processes, are providing new insights into the complex nature of MPD [5, 6]There is increasing evidence that immune system dysfunction may play a role in the development and progression of MPD [7]. Several studies have shown that individuals with MPD exhibit elevated levels of inflammatory markers [8, 9].Recent studies have shown cytokine changes in both early SCZ patients with MPD and those at very high risk [10]. Research showed that increased circulating cytokine concentration is associated with positive regulation of Th17 cells in patients with MPD. Th17-related upregulation factors were significantly higher in MPD deficits than in healthy controls [11].Another study also identified Th17-related regulated factors as strong predictors of health-related quality of life and disability [12].In addition, a study reported a significant decrease in regulatory T cells and a significant increase in Th1, Th2, and Th17 levels in paranoid SCZ with cognitive impairment, suggesting a potential role of inflammatory factors in the development of MPD [4]. Th17 leads to the production of IL-17 [13].The interleukin 17 (IL-17) family is the newest subclass of cytokines. To date, six ligands of the IL-17 family [IL-17 A, IL-17B, IL-17 C, IL-17D, IL-17E (IL-25), and IL-17 F] and five receptors (IL-17RA, IL-17RB/IL-25R, IL-17RC, IL-17RD/SEF, and IL-17RE) have been identified [14, 15].IL-17 was originally thought to be produced exclusively by T cells, but is now known to be produced by a variety of innate cells including macrophages, dendritic cells (DC), natural killer, natural killer T, and tissue inducers. Lymphocytes secrete it [16, 17].IL-17 mediates its immunoregulatory function mainly by promoting the production of proinflammatory cytokines and chemokines that lead to the recruitment of neutrophils and macrophages to the site of inflammation [18].IL-17 is a proinflammatory cytokine that has been reported to be involved in tissue damage in the central nervous system [19]. Drugs such as Secukinumab, Ixekizumab, and Brodalumab show anti-IL17 activity [20, 21]. Several studies have reported high levels of IL-17 in psychiatric disorders [22, 23].
Inflammatory cytokines and their regulatory factors are relatively large molecules that cannot freely cross the blood-brain barrier under normal physiological conditions [24]. Chemokines attract peripheral leukocytes to the site of chronic inflammation. CCL20 is abundant in the choroid plexus and subarachnoid space and creates a link between the environment and the brain, and CCL20 probably easily enters the brain from the systemic circulation [25].
IL-17 A leads to the upregulation of several chemokines, including CCL20 [18]. It should also be mentioned that CCL20 is also one of the most important absorbers of TH17 lymphocytes [26]. Research shows that IL-17 A is involved in stimulating the expression of CCL20. Notably, the CCL20 receptor (CCR6) is selectively expressed by TH17 cells, creating a cycle that promotes IL-17 and directs IL-17-producing cells, such as TH-17, to inflammatory sites [24, 27, 28].
Inflammatory cytokines and their regulatory factors are relatively large molecules that cannot freely cross the blood-brain barrier under normal physiological conditions [29].Chemokines attract peripheral leukocytes to the site of chronic inflammation. CCL20 is a chemokine involved in chemotaxis and inflammatory response and has been implicated in several inflammation-related diseases such as rheumatoid arthritis. Inflammatory mediators can induce CCL20 production in microglia and can alter T cell behavior, suggesting a potential role for CCL20 in neuroinflammation and neuroprogressive processes in psychiatric disorders [30]. CCL20 is overexpressed in the choroid plexus and subarachnoid space, providing a link between the environment and the brain, and CCL20 likely enters the brain easily from the systemic circulation. In inflammatory conditions, CCL20 is upregulated, and under experimental conditions, various cytokines have been found to induce CCL20 expression [25].The finding that opposing cell subsets (TH17 and Treg cells) express and respond to CCL20 points to a potential regulatory role between immune activation and suppression [30].
On the other hand, the complex interplay between metabolic factors and chemokines has attracted considerable attention in recent years due to its implications for metabolic homeostasis and immune system regulation. Metabolic factors, including fasting blood glucose (FBS) and lipid profile components, serve as vital indicators of the physiological state of an individual’s energy metabolism [2].The prevalence of metabolic syndrome in Iranian patients with schizophrenia was reported as 23.9%. In other words, about one fourth of Iranian patients with schizophrenia suffered from metabolic syndrome [31].
Prevalence rates varied widely among studies. This probably reflects the epidemiological diversity of the patient groups studied, and factors such as age, sex, ethnicity, medication status, smoking, disease duration, and country of origin affect the final result [32].The lowest reported prevalence rate was 3.9%, derived from an Indian population with chronic schizophrenia [33].The highest prevalence was reported, 68%, from a New Zealand rehabilitation setting [34].
Given the current focus on cytokines in the field of study and the need to better understand the pathophysiology and identify new therapeutic methods to improve care and prognosis, we compared serum levels of CCL20 and IL17A in patients with schizophrenia to those in controls.
Materials and methods
Study design
This control-study was conducted in 2023 at Ibn Sina Psychiatric Hospital in Mashhad-Iran. The patients from the hospital and the control group were invited to the study through posters and social media from all over the city. The purpose of this study was fully explained to all participants, both patients and control group volunteers. After understanding the objectives of the research, written and informed consent was obtained from all participants. The inclusion and exclusion criteria were checked, and out of 83 primary patients with MPD and 34 volunteers of the control group, we finally included 71 patients and 30 healthy controls (HC).
Both MPD and control groups were investigated by a psychiatrist and diagnosis was based on DSM-V criteria. Both groups were matched in age, sex, and BMI.
Inclusion and exclusion criteria were examined with an structural interview with participants in the study and their companions. People who met the entry criteria and did not have any exclusion criteria were included in this study [35].
Inclusion criteria for the study were as follows:
The age range of 18 years and older.
Diagnosis of schizophrenia for patients and lack of major psychiatric disorder based on DSM-5 criteria.
Providing consent to participate in the study.
Exclusion criteria subjects:
Disruptive behavior by participants at each stage of the study.
The presence of autoimmune and inflammatory diseases (such as rheumatism).
Use of corticosteroids, stimulants, or immunosuppressive drugs at the time of the study.
Pregnancy or lactation at the time of the study.
The presence of active infection at the time of conducting the study.
The scale of positive and negative symptoms (PANSS) was used to evaluate the symptoms of MPD patients. This questionnaire contains 30 questions that the subject answers each question on a 5-choice scale (not at all, sometimes, average, high, and very high). A study by the creators of the questionnaire reported a Cronbach’s alpha of 83% for the questionnaire, and the correlation of this scale was obtained with the positive and negative signals of Andreasen at 58%. Its validity is also reported using an acceptable factor analysis [36–38].
Data collection and assessments
Demographic data were collected via a questionnaire. Participants abstained from caffeine and exercise for 30 min before blood collection. Blood samples (3 ml) were collected and the serum was separated and stored at -80 °C. Serum concentrations of CCL20 and IL-17 A were measured using Enzyme-linked immunosorbent assay (ELISA) (kits: CCL20 catalog number: 441404 from Biolegend company and IL-17 A catalog number:3520 from Mabtech company). Levels of metabolic factors (Cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, and fasting blood sugar (FBS) were measured by Automatic biochemistry analyzer.
Continuous variables were presented as Mean ± Standard Deviation (SD) or Median (inter-quartile range), and categorical variables were expressed as frequency (percent). The comparison of each risk factor between the two groups was assessed by applying the nonparametric Mann-Whitney U test for continuous variables without normal distribution. Correlation between cytokines and metabolic factors in MPD patients was also examined. Data were analyzed using SPSS v24. The significance level was set at 0.05 (α = 0.05).
Results
Clinical characteristics of all study participants
The clinical characteristics of all study participants are shown in Table 1. A total of 101 participants were included in the analysis, comprising 71 patients with MPD and 30 healthy individuals. Patients received antipsychotic drugs, whereas the control group did not. The MPD group consisted of 52 (73.2%) male and 19 (26.8%) female subjects with a mean age of 41.04 ± 9.93 (20–65) years and the mean BMI was 24.58 ± 4.78 kg/m2. There was no significant difference between men and women in the cytokine factors. However, a significant difference was found between men and women in the lipid markers, such as cholesterol (P-value = 0.04), LDL (P-value = 0.04), and HDL (P-value = 0.004).
Table 1.
Demographic, clinical, and behavioral characteristics of MPD patients
| Yes | No | |
|---|---|---|
| Academic education | 8 (11.3%) | 63 (88.7%) |
| ECT | 41 (57.7%) | 30 (42.3%) |
| Status of Job | 29 (40.8%) | 42 (59.2%) |
| Smoking | 33 (46.5%) | 38 (53.5%) |
| Opium usage | 27 (38.0%) | 44 (62.0%) |
| Hospitalization history | 58 (81.7%) | 13 (18.3%) |
| Thoughts of suicide | 6 (8.5%) | 65 (91.5%) |
| Obesity and Overweight (BMI ≥ 25) | 25 (35.21%) | 46 (64.79%) |
Abbreviations ECT: electroconvulsive therapy
Inflammatory and metabolic alterations between MPD and HC groups
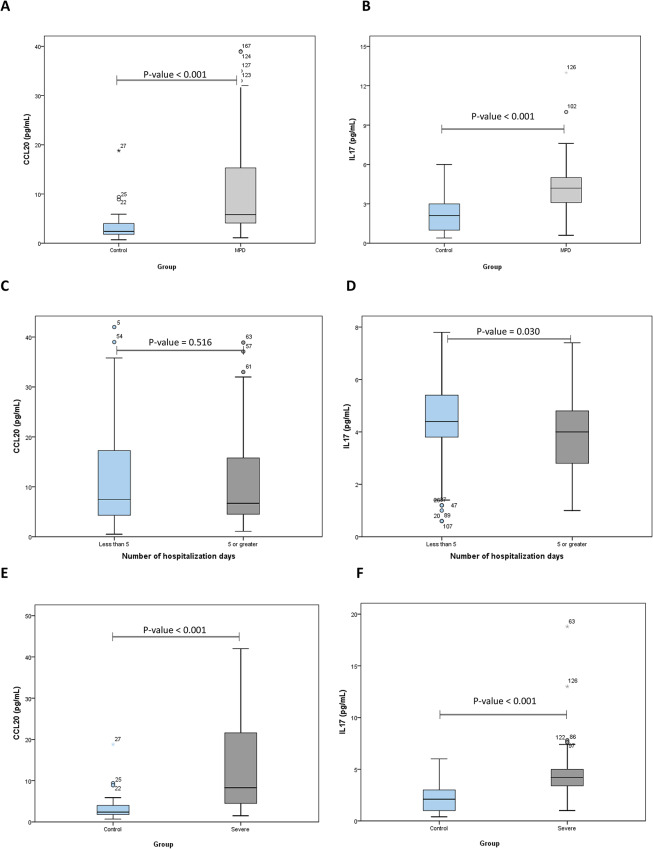
The circulating levels of CCL20 and IL-17 A analytes were found to be significantly different between patients with Major Psychotic Disorders (MPD) and Healthy Controls (HC), as shown in Fig. 1(A) and 1(B). Additionally, significant differences were observed in the levels of Fasting Blood Sugar (FBS), cholesterol, High-Density Lipoprotein (HDL), and Low-Density Lipoprotein (LDL) between MPD and HC. The detailed results are presented in Table 2.
Fig. 1.
CCL20 (A) and IL-17 (B) concentrations between patients with MPD (Major psychotic disorders) and Control, CCL20 (C) and IL-17 (D) concentrations between patients having less than 5 times hospitalization and hospitalization above 5 times, and Comparison between Control and severe MPD in terms of CCL20 (E) and IL-17 (F)
Table 2.
Blood parameters variations between patients with MPD and HC
| Variables | MPD Median (inter-quartile) |
Control Median (inter-quartile) |
P-value |
|---|---|---|---|
| CCL20 | 5.8 (4.1–15.3) | 2.4 (1.8–4.3) | < 0.001 |
| IL-17 A | 4.2 (3.0–5.0) | 2.1 (1-3.1) | < 0.001 |
| FBS | 103 (93–112) | 80.5 (71.8–91.00) | < 0.001 |
| TG | 113 (76–150) | 104.5 (73.8-143.8) | 0.792 |
| Chol | 172 (149–197) | 151 (134.5–176) | 0.009 |
| LDL | 96 (85–107) | 82 (72.8–92.3) | < 0.001 |
| HDL | 43 (35–49) | 35 (30-38.3) | < 0.001 |
Abbreviations MPD, major psychotic disorders; CCL20, chemokine ligand 20; IL-, interleukin-; EBI3, FBS, fasting blood sugar; FBS, fasting blood sugar; TG, triglyceride; Chol, cholesterol; LDL, low density lipoprotein; HDL, high density lipoprotein
These findings suggest a potential role of these inflammatory markers and metabolic factors in the pathophysiology of Major Psychotic Disorders.
Association of IL-17 A and CCL20 with metabolic factors in MPD patients
The mean circulating levels of CCL20 and IL-17 A among patients were found to be 18.31 ± 32.24 and 4.18 ± 2.02, respectively. Despite the observed variation in these levels among patients, this variation was not statistically significant (P-value = 0.254). This suggests that while there is a difference in the levels of these inflammatory markers among patients, this difference is not enough to be considered statistically significant.The results of the examination of the relationship between these inflammatory factors (CCL20 and IL-17 A) and metabolic factors are detailed in Table 3.
Table 3.
Correlation between cytokines and metabolic factors in MPD patients
| Metabolic factors | Mean ± SD | CCL20 | IL-17 A |
|---|---|---|---|
| FBS | 115.65 ± 50.89 | 0.699 | 0.080 |
| Triglycerides | 128.42 ± 73.90 | 0.091 | 0.311 |
| Cholesterol | 175.44 ± 41.52 | 0.404 | 0.028* |
| LDL | 98.22 ± 21.43 | 0.119 | 0.003* |
| HDL | 43.46 ± 9.78 | 0.304 | 0.192 |
*P < 0.05
CCL20, chemokine ligand 20; IL-17 A, interleukin-17 A; FBS, fasting blood sugar; TG, triglyceride; Chol, cholesterol; LDL, low density lipoprotein; HDL, high density lipoprotein
The effect of the duration of the disease and the number of hospitalizations on blood levels of inflammatory factors
58 (81.7%) MPD patients have a history of hospitalization, among which 35 people have a history of hospitalization more than 5 times (≥ 5). The duration of MPD disease in 50 (70.42%) was more than ten years (≥ 10). Also, the serum level of CCL20 in MPD patients who have been diagnosed for more than 10 years is higher than the serum level of CCL20 in patients with a disease duration of less than 10 years. However, this increase did not determine a significant relationship between the serum levels of this chemokine and the duration of MPD. In MPD patients who have been diagnosed for more than 10 years (≥ 10), the serum level of IL-17 was 4.4 (5.1–2.25), and the serum level of IL-17 in patients with a disease duration of less than 10 years was 4.4 (3.7-5). It was reported that the relationship between these two groups was not significant (p-value = 0.363). Also, the serum level of CCL20 in MPD patients who have been diagnosed for more than 10 years (≥ 10) was 6 (4.05–17.87), which is higher than the serum level of CCL20 in patients with a duration of illness of less than 10 years, but this increase in serum level was not reported (p-value = 0.42).
The serum level of IL-17 in the group of MPD patients who had been hospitalized more than 5 times (≥5) was 4 (2.8–4.4) and 4.8 (3.4–5.55) among patients with a history of less than 5 times, which has a significant correlation between these two groups. It was reported (p-value = 0.03). Also, the serum level of CCL20 in the group of MPD patients who had been hospitalized more than 5 times (≥5) was 5.5 (3.8–15.1) and among patients with a history of less than 5 times it was 6.95 (16.2–4.12). No significant correlation was found in the study (p-value = 0.516) (Fig. 1C-F).
All patients had used antipsychotics during the treatment period, but the other drug categories used were not the same.
In this study, a subgroup analysis of metabolic factors was performed based on the severity and duration of Major Psychotic Disorders (MPD) in patients with Schizophrenia (SCZ). The findings revealed that cholesterol and Low-Density Lipoprotein (LDL) levels were significantly higher in patients with severe SCZ compared to those with moderate SCZ (P = 0.03 and P = 0.01, respectively). Furthermore, Fasting Blood Sugar (FBS) levels were significantly higher in patients who had MPD for more than 10 years compared to those with a shorter duration of MPD (P = 0.035). These results suggest that MPD may have a negative impact on metabolic health in patients with SCZ, indicating a potential area for further research and intervention.
Association of PANSS with interleukin 17 and ccl20
The Positive and Negative Syndrome Scale (PANSS) items were divided into five domains: positive factor, negative factor, disordered factor, excited factor, and depressed factor [39] The relationship between these PANSS components and blood levels of Interleukin 17 (IL-17) and Chemokine Ligand 20 (CCL20) was investigated using Spearman’s non-parametric correlation coefficient. The results showed very weak relationships, and no significant relationship was found (as detailed in Table 4).
Table 4.
Correlation between cytokines and PANSS in MPD patients
| CCL20 | IL17 | |||
|---|---|---|---|---|
| Spearman’s rho | NPANSS | Correlation Coefficient | 0.066 | − 0.096 |
| Sig. (2-tailed) | 0.582 | 0.428 | ||
| N | 71 | 71 | ||
| PPANSS | Correlation Coefficient | − 0.034 | 0.010 | |
| Sig. (2-tailed) | 0.775 | 0.934 | ||
| N | 71 | 71 | ||
| DISORGANIZED | Correlation Coefficient | − 0.133 | − 0.110 | |
| Sig. (2-tailed) | 0.268 | 0.360 | ||
| N | 71 | 71 | ||
| EPANSS | Correlation Coefficient | 0.024 | − 0.075 | |
| Sig. (2-tailed) | 0.845 | 0.532 | ||
| N | 71 | 71 | ||
| DPANSS | Correlation Coefficient | − 0.057 | − 0.041 | |
| Sig. (2-tailed) | 0.636 | 0.735 | ||
| N | 71 | 71 | ||
| CCL20 | Correlation Coefficient | 1.000 | 0.098 | |
| Sig. (2-tailed) | . | 0.416 | ||
| N | 71 | 71 | ||
| IL17 | Correlation Coefficient | 0.098 | 1.000 | |
| Sig. (2-tailed) | 0.416 | . | ||
| N | 71 | 71 |
This suggests that while IL-17 and CCL20 levels may be elevated in patients with Major Psychotic Disorders, they may not directly correlate with the severity of symptoms as measured by the PANSS.
Discussion
Our results showed that patients with Major Psychotic Disorders (MPD) had higher levels of IL-17 A and CCL20 in their blood than Healthy Controls (HC), and these cytokines were even higher in patients with severe MPD. We also found significant differences in metabolic factors, such as Fasting Blood Sugar (FBS), cholesterol, High-Density Lipoprotein (HDL), and Low-Density Lipoprotein (LDL), between MPD and HC groups. The relationship between these PANSS components and blood levels of Interleukin 17 (IL-17) and Chemokine Ligand 20 (CCL20) was investigated. The results showed very weak relationships, and no significant relationship was found. This suggests that while IL-17 and CCL20 levels may be elevated in patients with Major Psychotic Disorders, they may not directly correlate with the severity of symptoms as measured by the PANSS.
These findings suggest that MPD is associated with both immune and metabolic dysregulation. Previous studies have reported that MPD is linked to altered cytokine profiles and immune cell functions [40]. It has been proposed by previous investigators that an altered state of neurotransmitters like dopamine and γ-aminobutyric acid (GABA) can stimulate the secretion of proinflammatory cytokines like IL-17, which in turn causes structural and functional neuronal changes in schizophrenia [41, 42].
A genome-wide association study of MPD identified the MHC1 locus, which contains many immune genes, as a potential risk factor for the disorder [4]. Moreover, evidence from clinical and basic research indicates that the brain’s immune system interacts with the peripheral immune system and influences behavior [43].A meta-analysis of cytokine changes in MPD patients with acute relapse or first-episode psychosis revealed that MPD exacerbations are related to immune imbalance, independently of antipsychotic treatment [40].
Therefore, our results support the hypothesis that MPD involves chronic inflammation and metabolic disturbances, which may contribute to the pathophysiology and clinical manifestations of the disorder.
Correlation of IL17A with MPD
Patients with schizophrenia had a significant decrease in central memory T-regulator levels, and an increase in Th1 and Th2 subsets, «double-positive» and «classic» Th17, Tfh2, «classic» Tfh17, and Tfh17.1 [44]. Th17 cells are a subset of CD4 + T lymphocytes that secrete cytokines such as IL-17 A, IL-17 F, IL-22, IL-26, and CCL20 in humans [45, 46]. They play a key role in defending against pathogens such as bacteria and fungi [46–48]. However, they can also cause tissue damage during inflammation, implying their contribution to chronic inflammatory diseases. The IL-23/IL-17 axis is essential for the development and persistence of such diseases [49].The IL-17 cytokine family has a vital role in the inflammatory response of the immune system and its members have been implicated in various diseases [50, 51].Targeting the IL-17 A pathway is a promising therapeutic strategy for many chronic inflammatory diseases, and further research in this field may lead to more effective treatments for these disorders [52].
In 2023, a study showed that first-episode psychosis (FEP) patients have a higher proportion of the pro-inflammatory Th1/Th17 subset [53]. Saeki et al. found that an excessive amount of Raftlin increases the Th17-mediated response by causing excessive IL-17 production [54]. In 2022, a study showed increased NOX1 and Raftlin serum levels in patients with chronic schizophrenia compared to the control group. In addition, a significant positive relationship between NOX1 and Raftlin levels was identified regarding the clinical symptoms of patients based on PANSS [55]. Ding et al. showed a positive correlation between plasma levels of IL-17 and Th17 cells and the severity of all clinical symptoms in patients with SCZ [56].
Mazrakhondi et al. (2022) measured circulating IL-17 A levels in SCZ patients and healthy controls before and after treatment. They found no significant changes in IL-17 A levels in patients with MPD before or after treatment [57]A meta-analysis conducted in 2021 that included three studies with 130 patients showed a significant effect size of 0.72 (95% CI 0.07–1.37) for IL-17 levels in FEP patients. They also reported that medicated FEP patients had significantly higher IL-17 levels, indicating an altered inflammatory response in patients with early stages of psychosis [58].
In 2023, a study compared the levels of immune markers in acute and transient psychotic disorders (ATPDs) with healthy subjects and patients with schizophrenia in the recovery period. In the follow-up, re-evaluation of the levels of immune markers in the ATPD group was done after ensuring the recovery status. Patients with ATPDs had elevated levels of the pro-inflammatory cytokines IL-6 and IL-17 and low levels of IL-8 in the acute phase and low levels of IL-6 and elevated levels of IL-8 during the remission phase. Compared to patients with SZ in remission, patients with ATPD in remission had low levels of all the three pro-inflammatory cytokines (with significantly low IL-6 levels and non-significant, yet low levels of IL-8 and IL-17) and had significantly low and high levels of IL-6 and IL-8 respectively than healthy controls [59].
Ouyang et al. (2021) investigated the possible associations between cytokines and the risk of transition from high-risk individuals to clinical cases. They observed that IL-17 levels were significantly higher in the group of very high-risk individuals who later transitioned to MPD than in those who did not transition [10].These findings, along with ours, suggest that IL-17 could serve as a predictive biomarker for identifying high-risk individuals who may transition to MPD in the future.
CCL20 was an immune-related factor for MPD
The chemokine family consists of a large number of ligands and receptors that can participate in the development of the immune system and inflammatory responses [60]. Previous studies have shown that chemokines are involved in psychiatric disorders [61]. Akkouh et al. (2020) investigated the inflammatory changes in SCZ patients using human induced pluripotent stem cell (iPSC)-derived astrocytes from SCZ subjects. They found significant differences in CCL20 gene expression [62]. CCL20 interacts with its chemokine receptor CCR6 and the CCL20-CCR6 axis can regulate T-B cell immunobiology [63]. Duan et al. (2023) reported significantly higher levels of CCL20 during the remission phase compared to the acute episode in MPD and bipolar patients, suggesting the potential role of CCL20 as a therapeutic target in psychosis [64].Furthermore, another study showed that MPD patients had significantly higher levels of both CCL20 and high-sensitivity C-reactive protein (hsCRP) than healthy controls. Network analysis of several inflammatory factors also revealed independent and strong associations between MPD and CCL20 and hsCRP [65]. There seems to be a correlation between cytokine levels and both disease duration and symptom severity. Patients with higher cytokine levels had longer disease duration and hospital stays [66–68]. Consistent with these findings, we observed higher levels of CCL20 in patients with chronic MPD (more than 10 years) than in patients with shorter disease duration, although the results were not statistically significant.
Inflammatory and metabolic factors in patients with MPD
It is noteworthy that metabolic disorders increase as the duration of MPD progresses [69, 70]. Studies have shown that second-generation antipsychotics can cause abdominal obesity, weight gain, dyslipidemia, abnormal glucose metabolism, and insulin resistance [71]. Recent research suggests that metabolic changes occur in MPD patients even without medication or sustained behavioral changes, indicating that the disorder itself may be a factor. It has been demonstrated that glucose homeostasis is impaired from the onset of MPD, even without the use of antipsychotic medication [72]. In line with these findings, we observed that the lipid profile (except for triglycerides) and FBS were significantly different between the MPD and HC groups. Moreover, FBS levels were significantly higher in patients with more than 10 years of MPD than in those with less than 10 years of MPD. Furthermore, our results showed that FBS, LDL, and HDL levels were significantly different between the severe MPD and HC groups. Various mechanisms have been proposed to explain these outcomes, such as the effect of pro-inflammatory cytokines like TNF-α on adipokine levels, reduced insulin receptor activity [73], increased liver lipolysis [74], and enhanced adiponectin secretion [75]. Additionally, antipsychotic drugs and the patient’s unhealthy lifestyle, characterized by low physical activity, overeating, and smoking, may also play a role [74–76]. A review in 2024 showed that early identification and management of metabolic syndrome are crucial to mitigate the long-term cardio-metabolic toll in patients with Psychosis-spectrum disorders. Interventions should focus on a healthy lifestyle and appropriate pharmacological and behavioral interventions [75]. Also, in the same year, another study stated that persons with severe mental illness experience high rates of obesity associated with cardio-metabolic disorders and reduced life expectancy. The lifestyle intervention program was effective and there was a significant improvement in cholesterol, LDL, FBS, weight, and BMI. The exploration of the facilitators and barriers in following a healthy lifestyle in persons with schizophrenia and their caregivers identified that a greater contribution is needed from family and friends, mental health [77]. The specific contribution of these mechanisms to our results is unclear, but they can be explored in future studies.
Levels of inflammatory factors compared to PANSS
To date in literature, there is a paucity of data about IL-17 levels with an increase in PANSS score in schizophrenia patients. In the present study, the relationship between PANSS components and blood levels of IL17 and blood levels of CCL20 was investigated, and the relationships were shown to be very weak. In a 2020 study by Chenniappan et al., in line with our results, there was no relationship with negative symptoms, but they found a significant relationship with other components [78] At the same time, Borovcanin et al. showed that there was no correlation between IL-17 serum levels and PANSS [79]. Contrary to our results, Dimitrov et al. correlated IL-17 levels with PANSS (85). Interestingly, in the study showing decreased IL-17 levels in SCZ patients, a positive correlation was reported between PANSS scores and cytokines representing the IL-17 pathway [21].
Limitations
This study has several limitations that should be considered in future research. The first limitation is the small sample size, which was limited by the time frame of the study. This may affect the generalizability of the findings. The second limitation is the lack of information about the specific antipsychotic drugs used by the MPD patients. This makes it difficult to assess the potential impact of the medication on the inflammatory and metabolic outcomes. The third limitation is the uncertainty about the effect of the number and duration of SCZ episodes on the factors measured in this study.
Conclusion
The current study revealed that the serum levels of IL-17 A and CCL20 in schizophrenia patients are higher than those in the control group. Metabolic factors such as Fasting Blood Sugar (FBS), cholesterol, High-Density Lipoprotein (HDL), and Low-Density Lipoprotein (LDL) also showed significant differences between Major Psychotic Disorders (MPD) and Healthy Controls (HC). In conclusion, the findings suggest that these two inflammatory factors could serve as potential therapeutic targets and prognostic biomarkers for schizophrenia. Together with our results, this suggests that IL-17 has the potential to serve as a predictive biomarker to identify individuals at risk for schizophrenia. It should be noted that the relationships observed in several studies between IL-17 and psychological diseases such as schizophrenia can be a strong point for the development of new treatments for this disease. This is not the first time that treatments affecting interleukin 17 have been proposed in diseases. Drugs such as secukinumab, ixekizumab, and brodalumab, which show anti-IL17 activities, have been introduced for the treatment of psoriasis and provide valuable insights for formulating similar therapies [80, 81]. Taken together with our results and other studies, it appears that IL-17 has the potential to serve as a predictive biomarker to identify individuals at risk for developing schizophrenia. Overall, targeting the IL-17 pathway is a promising therapeutic strategy for many chronic inflammatory diseases, including psychiatric disorders, and continued research in this area is likely to lead to the development of more effective treatments for these disorders [80, 81].
According to the results of this study and other studies, the triangle of T-helper 17 (TH-17), CCL20, and IL-17 A seems to have a potential role in the pathophysiology of psychiatric diseases such as MPD.
Acknowledgements
The authors extend their heartfelt gratitude to Golestan University of Medical Sciences and Professor Mohammad Al Uzri at Leicestershire Partnership NHS Trust for their invaluable support. We also deeply appreciate the voluntary participation and cooperation of all the patients involved in this study.
Author contributions
Parisa ghasemi noghabi: writing the article, data collection. Yasser Bagheri: revise discussion and laboratory analysis.zenireh Salimi: design and supervision on writing and writing introduction, Somayeh Ghorbani: statistical analysis.firoozeh derakhshanpour and Najmeh Shahini: study concept and design, final approval of the article, and literature review.
Funding
The research was conducted at Mashhad University of Medical Sciences without any organizational financial support.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.MEDICAL.REC.1402.033). Informed written consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yasser Bagheri, Email: bagheri_yasser@yahoo.com.
Firoozeh Derakhshanpour, Email: dr.derakhshanpoor@gmail.com.
References
- 1.James SL, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shojaeimotlagh V, et al. Prevalence of metabolic syndrome in Iranian patients with schizophrenia: a systematic review and meta-analysis. Volume 13. Diabetes & Metabolic Syndrome: Clinical Research & Reviews; 2019. pp. 143–7. 1. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadi M-R, et al. An epidemiological survey of psychiatric disorders in Iran. Clin Pract Epidemiol Mental Health. 2005;1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrova N, et al. Clinical and immunological profile of patients with schizophrenia. Eur Psychiatry. 2022;65(S1):S105–6. [Google Scholar]
- 5.Lang FU, et al. Subtyping schizophrenia: a comparison of positive/negative and system-specific approaches. Compr Psychiatr. 2015;61:115–21. [DOI] [PubMed] [Google Scholar]
- 6.Bosia M, et al. From cognitive and clinical substrates to functional profiles: disentangling heterogeneity in schizophrenia. Psychiatry Res. 2019;271:446–53. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues-Amorim D, et al. Cytokines dysregulation in schizophrenia: a systematic review of psychoneuroimmune relationship. Schizophr Res. 2018;197:19–33. [DOI] [PubMed] [Google Scholar]
- 8.Roomruangwong C, et al. The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol. 2020;57:778–97. [DOI] [PubMed] [Google Scholar]
- 9.Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102–12. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang L, et al. IL-17 and TNF-β: predictive biomarkers for transition to psychosis in ultra-high risk individuals. Front Psychiatry. 2022;13:1072380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hakeim HK, et al. The interleukin-6/interleukin-23/T helper 17-axis as a driver of neuro-immune toxicity in the major neurocognitive psychosis or deficit schizophrenia: a precision nomothetic psychiatry analysis. PLoS ONE. 2022;17(10):e0275839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Musawi AF, et al. In schizophrenia, the effects of the IL-6/IL-23/Th17 axis on health-related quality of life and disabilities are partly mediated by generalized cognitive decline and the symptomatome. Int J Environ Res Public Health. 2022;19(22):15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toussirot É. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy-Drug Targets (Formerly Curr Drug Targets-Inflammation Allergy)(Discontinued). 2012;11(2):159–68. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71(1):1–8. [PubMed] [Google Scholar]
- 15.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korn T, et al. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 18.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann J, et al. CNS-targeted production of IL-17A induces glial activation, microvascular pathology and enhances the neuroinflammatory response to systemic endotoxemia. PLoS ONE. 2013;8(2):e57307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silfvast-Kaiser A, Paek SY, Menter A. Anti-IL17 therapies for psoriasis. Expert Opin Biol Ther. 2019;19(1):45–54. [DOI] [PubMed] [Google Scholar]
- 21.Chandrakumar SF, Yeung J. Interleukin-17 antagonists in the treatment of psoriasis. J Cutan Med Surg. 2015;19(2):109–14. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, HO RCM, Mak A. The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. 2012;15(2):183–7. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, et al. Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry Res. 2011;188(2):224–30. [DOI] [PubMed] [Google Scholar]
- 24.Barbosa IG, et al. The immunology of bipolar disorder. Neuroimmunomodulation. 2014;21(2–3):117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Axtell RC, Steinman L. Gaining entry to an uninflamed brain. Nat Immunol. 2009;10(5):453–5. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki T, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181(12):8391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen F, Gaffen SL. Structure–function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41(2):92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogura H, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29(4):628–36. [DOI] [PubMed] [Google Scholar]
- 29.Lee AY, et al. CC chemokine ligand 20 and its cognate receptor CCR6 in mucosal T cell immunology and inflammatory bowel disease: odd couple or axis of evil? Front Immunol. 2013;4:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tandon P, et al. Metabolic regulation of inflammation and its resolution: current status, clinical needs, challenges, and opportunities. J Immunol. 2021;207(11):2625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papanastasiou E. The prevalence and mechanisms of metabolic syndrome in schizophrenia: a review. Therapeutic Adv Psychopharmacol. 2013;3(1):33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padmavati R, McCreadie RG, Tirupati S. Low prevalence of obesity and metabolic syndrome in never-treated chronic schizophrenia. Schizophr Res. 2010;121(1–3):199–202. [DOI] [PubMed] [Google Scholar]
- 33.Tirupati S, Chua L-E. Obesity and metabolic syndrome in a psychiatric rehabilitation service. Australian New Z J Psychiatry. 2007;41(7):606–10. [DOI] [PubMed] [Google Scholar]
- 34.Young RC, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–35. [DOI] [PubMed] [Google Scholar]
- 35.Shahini N, et al. Relationship of serum homocysteine and vitamin D with positive, negative, and extrapyramidal symptoms in schizophrenia: a case–control study in Iran. BMC Psychiatry. 2022;22(1):681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noto MN, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 2019;29(3):416–31. [DOI] [PubMed] [Google Scholar]
- 37.Wallwork R, et al. Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophr Res. 2012;137(1–3):246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller BJ, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Upthegrove R, Barnes NM. The immune system and schizophrenia: an update for clinicians. Adv Psychiatr Treat. 2014;20(2):83–91. [Google Scholar]
- 40.Smith R, Maes M. The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses. 1995;45(2):135–41. [DOI] [PubMed] [Google Scholar]
- 41.Pantelis C, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett F, Molofsky A. The immune system and psychiatric disease: a basic science perspective. Clin Experimental Immunol. 2019;197(3):294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17–producing helper T cells. Nat Immunol. 2007;8(9):950–7. [DOI] [PubMed] [Google Scholar]
- 44.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123(5):1004–11. [DOI] [PubMed] [Google Scholar]
- 45.Kelly MN, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma Gondii infection. Infect Immun. 2005;73(1):617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eyerich K, et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatology. 2008;128(11):2640–5. [DOI] [PubMed] [Google Scholar]
- 47.Romagnani S. Human Th17 cells. Arthritis Res Therapy. 2008;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milovanovic J, et al. Interleukin-17 in chronic inflammatory neurological diseases. Front Immunol. 2020;11:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y, et al. Advances in the study of IL-17 in neurological diseases and mental disorders. Front Neurol. 2023;14:1284304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mills KH. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. 2023;23(1):38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.León-Ortiz P, et al. Systemic inflammation and cortical neurochemistry in never-medicated first episode-psychosis individuals. Brain Behav Immun. 2023;111:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saeki K, et al. A major lipid raft protein raftlin modulates T cell receptor signaling and enhances th17-mediated autoimmune responses. J Immunol. 2009;182(10):5929–37. [DOI] [PubMed] [Google Scholar]
- 53.Hurşitoğlu O et al. Serum NOX1 and raftlin as new potential biomarkers of interest in schizophrenia: a preliminary study. Neuropsychiatr Dis Treat, 2022: p. 2519–27. [DOI] [PMC free article] [PubMed]
- 54.Ding M, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:78–82. [DOI] [PubMed] [Google Scholar]
- 55.Mazrakhondi SAM, Zare-Zardini H. Comparison of serum changes of Interleukin-17A and Interleukin-21 between schizophrenic patients and healthy individuals. Volume 11. Reports of Biochemistry & Molecular Biology; 2022. p. 465. 3. [DOI] [PMC free article] [PubMed]
- 56.Dunleavy C et al. Inflammation in first-episode psychosis. 2022.
- 57.Kale A, et al. Immune-mediated inflammatory markers in acute and transient psychotic disorders—comparison with schizophrenia: an exploratory comparative study. Early Interv Psychiat. 2023;17(2):183–91. [DOI] [PubMed] [Google Scholar]
- 58.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stuart M, Baune B. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehavioral Reviews. 2014;42:93–115. [DOI] [PubMed] [Google Scholar]
- 60.Akkouh IA, et al. Decreased IL-1β-induced CCL20 response in human iPSC-astrocytes in schizophrenia: potential attenuating effects on recruitment of regulatory T cells. Brain Behav Immun. 2020;87:634–44. [DOI] [PubMed] [Google Scholar]
- 61.Lee AY, Körner H. The CCR6-CCL20 axis in humoral immunity and TB cell immunobiology. Immunobiology. 2019;224(3):449–54. [DOI] [PubMed] [Google Scholar]
- 62.Duan F, et al. Changes of immune-related factors in the blood of schizophrenia and bipolar disorder patients receiving monotherapy. Translational Psychiatry. 2022;12(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cathomas F, et al. Increased random exploration in schizophrenia is associated with inflammation. Npj Schizophrenia. 2021;7(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dahan S, et al. The relationship between serum cytokine levels and degree of psychosis in patients with schizophrenia. Psychiatry Res. 2018;268:467–72. [DOI] [PubMed] [Google Scholar]
- 65.Szymona K, et al. Correlations of kynurenic acid, 3-hydroxykynurenine, sIL-2R, IFN-α, and IL-4 with clinical symptoms during acute relapse of schizophrenia. Neurotox Res. 2017;32:17–26. [DOI] [PubMed] [Google Scholar]
- 66.Momtazmanesh S, Zare-Shahabadi A, Rezaei N. Cytokine alterations in schizophrenia: an updated review. Front Psychiatry. 2019;10:496638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carney R, et al. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res. 2016;170(2–3):290–300. [DOI] [PubMed] [Google Scholar]
- 68.Penninx BW, Lange SM. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialog Clin Neurosci. 2018;20(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rojo LE, et al. Metabolic syndrome and obesity among users of second generation antipsychotics: a global challenge for modern psychopharmacology. Pharmacol Res. 2015;101:74–85. [DOI] [PubMed] [Google Scholar]
- 70.Pillinger T, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halle M, et al. Importance of TNF-alpha and leptin in obesity and insulin resistance: a hypothesis on the impact of physical exercise. Exerc Immunol Rev. 1998;4:77–94. [PubMed] [Google Scholar]
- 72.Leonard BE, Schwarz M, Myint AM. The metabolic syndrome in schizophrenia: is inflammation a contributing cause? J Psychopharmacol. 2012;26(5suppl):33–41. [DOI] [PubMed] [Google Scholar]
- 73.Jin H, et al. Impact of atypical antipsychotic therapy on leptin, ghrelin, and adiponectin. Schizophr Res. 2008;100(1–3):70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. 2004;66(1):51–7. [DOI] [PubMed] [Google Scholar]
- 75.Saccaro LF, et al. Shared and unique characteristics of metabolic syndrome in psychotic disorders: a review. Front Psychiatry. 2024;15:1343427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gurusamy J, et al. Effect of lifestyle modification intervention (LMI) on metabolic syndrome in schizophrenia in a residential mental health care setting–A mixed method study. Schizophr Res. 2024;266:75–84. [DOI] [PubMed] [Google Scholar]
- 77.Borovcanin MM, et al. Type 17 immune response facilitates progression of inflammation and correlates with cognition in stable schizophrenia. Diagnostics. 2020;10(11):926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chenniappan R, et al. Interleukin-17 and interleukin-10 association with disease progression in schizophrenia. Annals Neurosciences. 2020;27(1):24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dimitrov DH, et al. Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: potential role for IL-17 pathway. Schizophr Res. 2013;151(1–3):29–35. [DOI] [PubMed] [Google Scholar]
- 80.Göteson A, et al. A serum proteomic study of two case-control cohorts identifies novel biomarkers for bipolar disorder. Translational Psychiatry. 2022;12(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luger D, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205(4):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.