Abstract
As interest in deep space travel grows exponentially, understanding human adaptation in becoming an interplanetary species is crucial. This includes the prospect of reproduction. This review summarizes recent updates and innovations in assisted reproductive technologies (ART) on Earth, while also discussing current challenges and areas for improvement in adapting ART studies to the space environment. We discuss the critical components of ART - gamete handling and preparation, fertilization, embryo culture, and cryopreservation - from the daily practice perspective of clinical embryologists and reproductive endocrinologists and lay out the complicated path ahead.
In vitro embryo development in low Earth orbit and beyond remains questionable due to synergetic effects of microgravity and radiation-induced damage observed in simulated and actual in-space mammalian studies. Cryopreservation and long-term storage of frozen samples face substantial obstacles - temperature limitations, lack of trained personnel, and absence of adapted cosmic engineering options. We touch on recent innovations, which may offer potential solutions, such as microfluidic devices and automated systems. Lastly, we stress the necessity for intensive studies and the importance of an interdisciplinary approach to address numerous practical challenges in advancing reproductive medicine in space, with possible implications for both space exploration and terrestrial fertility treatments.
Keywords: Assisted Reproductive technologies (ART), Spaceflight, Oocyte, Spermatozoa, Embryo, In Vitro fertilization (IVF), Microgravity
Background
Space exploration has led to human habitation in space while also raising questions about how to reproduce beyond the realm of Earth. The ambitious prospect of reproduction in space has spurred a new frontier in reproductive technology and innovation, bringing focus on this field to the forefront of future missions. Although animals have been sent to space since the 1940s [1], it is essential to emphasize an entire reproductive cycle (i.e., gametogenesis, fertilization, pregnancy, parturition) in a mammalian species has not yet occurred in space. Investigations into the possibility of reproduction in space can and have utilized approaches already developed for the same goals on Earth. The field of ART, which includes in vitro fertilization (IVF), provides many tools to approach the question of whether successful reproduction can occur in space.
Here on Earth, gamete handling, embryo culture, and cryopreservation methods have dramatically transformed since the first IVF baby was born in 1978. To comprehend the impact of space on human reproduction, these advanced technologies are now being utilized to function in space’s demanding environmental conditions while striving to overcome various biological and technical challenges. The space environment is filled with unique challenges such as radiation, gravitational forces, immune dysregulation, circadian rhythm changes, isolation, stressors, and magnetic fields.
Previous studies have investigated the impact of spaceflight under simulated conditions on amphibian and rodent gametes and embryos, but few studies exist on the effect of spaceflight on human gametes [2]. We will discuss human gamete manipulation techniques, embryo culture conditions, and cutting-edge advancements in cryogenic technology and how they may be applied in orbit. In addition to providing the models and methods to study the effects of space on reproduction, developing a successful IVF approach in space may also open the door to using IVF in space programs as humans become an interplanetary species and attempt colonization off Earth. ART will be crucial to understanding how biological specimens can be handled and preserved over long-duration space flights. Developing an IVF system in space requires a multidisciplinary focus on technological innovation, biological safety, and ethical considerations and will be highly challenging.
IVF Laboratory on Earth today: current practices
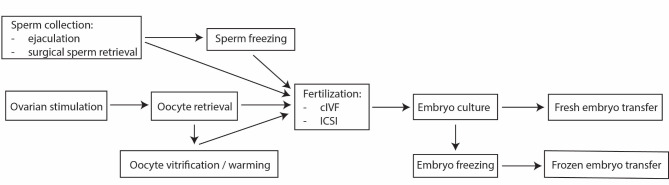
A modern clinical IVF laboratory is a highly specialized environment designed to support the delicate processes of gamete fusion and human preimplantation development. An IVF procedure also consists of multiple steps (Fig. 1).
Fig. 1.
Schematic representation of the main steps of IVF
IVF relies on four essential elements for consistency and expected success. The first element is standardization and protocol optimization. It is provided through detailed standard operating procedures and maintained by regular protocol reviews. The second element is precision in environmental stability. Human embryos are created and maintained in environments controlled for optimal temperature, humidity, pH, and osmolarity. In addition, the laboratory must provide high air quality and avoid contamination. Technological advancements in the last decade in culture media, incubation systems, and customized IVF laboratory equipment have led to the desired precision of controlling the culture and gamete/embryo handling conditions. The third element is a quality control, assurance, and management program that provides consistently high clinical success rates. The final element is personnel [3]. Handling and manipulating precious gametes and embryos require extraordinary hand-eye coordination, attention to detail, and focused care. Clinical embryologists possess a unique skill set and go through vigorous training to achieve the desired success. It typically requires at least three years of training to perform all aspects of procedures in the IVF laboratory with acceptable precision and accuracy [4, 5]. Further, the quality and monitoring of IVF labs are regularly assessed by key performance indicators (KPIs) [6].
Gamete retrieval and handling: oocytes and sperm
Retrieval and processing of oocytes and sperm on Earth
Typically, oocyte retrieval for IVF is preceded by controlled ovarian hyperstimulation to harvest multiple oocytes [7]. The medical management of stimulation relies on regular ultrasound evaluations and monitoring of hormone levels to track follicle development. With the current technology, these steps are challenging to apply in a space environment.
Retrieval of oocytes from follicles is typically performed transvaginally using an ultrasound-guided needle under general sedation. In some cases, oocyte retrieval can be done transabdominally. Follicular fluid is aspirated from punctured follicles into tubes and passed to the embryologist for search under a stereomicroscope for the presence of cumulus-oocyte complexes. After the oocytes surrounded by cumulus cells are identified, they are transferred into a culture dish for further processing. As an invasive procedure, oocyte retrieval demands stringent sterility, complications prevention, and specialized medical training for personnel. Given these complexities, retrieving fresh oocytes for subsequent fertilization or preservation in space currently is not feasible. In addition, the manipulation of liquids under microgravity conditions is quite challenging. Fluids cannot be easily transferred from one container to another as they tend to form sphere droplets and float. Under simulated microgravity conditions during paraboloid flight, simple, widely used techniques like pipetting and fluid aliquoting were proven to be feasible [8, 9]. However, multi-step manipulations are required to handle gametes in IVF. These manipulations are quite challenging and will be difficult to implement in space. Moreover, due to the surface tension of the test tube containing the fluid, it may be impossible to retrieve all the contents for analysis. These challenges may be avoided using closed, automated microfluidic systems currently in development.
Sperm samples for IVF are typically collected via ejaculation through masturbation, which is physiologically possible in space [10]. Raw ejaculate is subjected to specific processing steps to initiate capacitation and maximize sperm fertilization potential before IVF. The two standard methods for sperm preparation are swim-up or density gradient [11]. Both require the transfer of semen from collection containers to tubes with subsequent selection, washing, and concentration steps. Both methods are centrifuge-dependent and will be difficult to perform due to the challenging nature of multi-step fluid manipulations in microgravity (µg). Commercially available sperm-sorting microfluidic devices can be a solution because they aim to separate spermatozoa without centrifugation and require minimal intervention [12]. These devices rely on sperm movement through a channel or thin, porous membrane. After incubation of samples at 37℃, the best-performing spermatozoa gradually move from the initial load section to a media-filled compartment and are collected for subsequent usage. Though effectiveness of sperm sorting devices in microgravity environment has not been tested yet, this approach with some adaptations may be a promising tool for sperm handling in space conditions.
Studies on mammalian gamete performance in simulated space conditions
Exposure of gametes to space conditions introduces risks of multiple stress factors, including gravitational changes, cosmic radiation, vibrations, and magnetic fields. Disruptions in the hormonal milieu could also impair the gametogenesis. Cell-free RNA samples from astronauts demonstrated changes in estrogen receptor gene expressions after spaceflight [13]. These risks may intercalate, creating a synergetic effect that is difficult to address [14]. In vitro simulations enable scientists to study the individual impact of known stress factors of space flight with convenience and reduced cost [15]. To investigate the impact of microgravity on human sperm motility, 18 washed semen samples from healthy donors were treated with clinostat rotation and parabolic flights to imitate space conditions [16]. Spermatozoa subjected to microgravity (µg) conditions had lower motility and altered linear movement, though this could be an adaptive reaction to change in gravity. This potential explanation is supported by a study reporting that microgravity does not affect fertilization in mice [17]. In a parabolic flight study, human frozen sperm samples were exposed to microgravity for 8.5 s in each parabola, and they exhibited similar sperm functions compared to ground controls [18]. But it is important to note that the exposure to microgravity in parabolic flights is significantly shorter than the exposure during long-term spaceflight.
Gravitational changes and space radiation may negatively affect the fertilization capacity of spermatozoa, which are highly specialized cells with reduced damage repair potential and temporarily terminated transcription and translation [19]. Tail suspension model and carbon ion beam irradiation were used to evaluate the effects of these negative factors on apoptosis, proliferation, and cell DNA damage levels in Swiss Webster mice testis [20]. Simultaneous exposure to microgravity conditions and irradiation decreased epididymal sperm count and viability, increased spermatogenic cell apoptosis, and sperm DNA fragmentation indices reflecting disrupted spermatogenesis. A recent review on sperm functions under both simulated conditions and actual spaceflight reported decreased sperm motility and increased sperm DNA fragmentation in samples exposed to microgravity and ionizing radiation [20, 21].
In the human oocyte, cytoskeletal components, and cellular organelles, crucial to oocyte maturation and competence, are sensitive to changes in gravity conditions [22, 23]. A recent experiment involving 24 h incubation of mature human oocytes in a 3D-clinostat with subsequent light and electron microscopy observations of their semithin sections revealed significant ultrastructural changes induced by microgravity exposure. In particular, studied oocytes tended to have unevenly expanded perivitelline space, thicker zona pellucida, and irregularly aligned cumulus cells [24]. These structural changes may impact the fertilization of oocytes. Microgravity also affected the smooth endoplasmic reticulum shape with accumulation of large complexes of mitochondria and excessive fission of these organelles, indicating the presence of metabolic distress leading to cellular regression. The shifting of spatial mitochondria distribution from aggregates to complexes and the abundance of hooded, swollen, or irregular mitochondria in oocytes exposed to microgravity resembles the changes attributable to oocyte aging [24]. Though alterations of mitochondrial structure possibly represent a common adaptive mechanism observed in other cell types [25, 26], these altered organelles are directly inherited by the embryo and may compromise subsequent development.
Murine oocytes displayed induced autophagy with an accumulation of large peripheral vacuoles in simulated microgravity [27]. Abnormalities of intercellular communicative structures, like oocyte microvilli and granulosa cell projections, led to decreased quality of murine oocytes if ovarian follicles were exposed to microgravity. The culture of follicles in a rotating system caused the downregulation of actin-associated genes and an increase of intracellular reactive oxygen species in oocytes [28]. Exposure of mural immature oocytes to microgravity conditions severely disrupted their maturation due to cytoplasmic blebbing and 𝝲-tubulin-dependent spindle organization disturbance. Moreover, during in vitro maturation, microtubules and chromosomes could not assemble a functional spindle under microgravity conditions, rendering meiosis I completion almost impossible [23]. After 6 h in simulated microgravity, murine oocytes preserved stable levels of actin and acetylated alpha-tubulin. Still, cytoskeleton molecules tended to redistribute from the cortical to the central part of the oocyte [29]. Despite oocyte chromosome migration to the periphery and formation of actin cortical caps, gametes failed to extrude the first polar body. It may be assumed that microtubules primarily suffer from microgravity, while microfilaments remain functional in space conditions [23].
Ionizing radiation is a concern for gametes and oocytes may also suffer from the addition of radiation-induced mutagenesis and organelle damage. Murine oocytes are susceptible to Krypton-78 and ultraviolet-B irradiation with massive caspase activation leading to necrosis, while bovine oocytes adapt by rapid activation of DNA repair mechanisms [30]. Mammalian germ cell response to low-dose cosmic radiation likely varies across species, underscoring the importance of investigating the response of the human gamete to the influences of such hazards.
Fertilization: what is done in the IVF laboratories on Earth and what was achieved in space
In clinical IVF, the two standard methods of oocyte fertilization are conventional insemination (cIVF) and intracytoplasmic sperm injection (ICSI). cIVF mimics natural fertilization by adding processed capacitated sperm to oocyte-cumulus complexes in a dish. This method is effective with normozoospermic samples and presents a less technologically demanding option that still offers substantial efficacy in suitable cases without the bulky and sensitive micromanipulation equipment required for ICSI. The latter, designed to address male infertility, requires injection of a single selected and manually immobilized spermatozoon into the cytoplasm of a mature oocyte using a micromanipulation device operated by a trained embryologist. This fertilization method has become a cornerstone in fertility treatments, being used in approximately 70% of all IVF cycles regardless of the underlying infertility issue [31]. ICSI requires stripping of cumulus cells surrounding oocytes through enzymatic and mechanical treatments to identify those displaying the first polar body and are eligible for injection. However, ICSI necessitates additional equipment (an inverted microscope with micromanipulators) and highly skilled personnel. The success of this fertilization method relies heavily on the expertise of embryologists, as their skill in performing this delicate procedure can significantly impact the outcome [32]. The rigorous multistep nature of ICSI is particularly challenging under microgravity conditions encountered in space.
Several studies indicated microgravity and spaceflight conditions have no detrimental effects on fertilization and embryo development in fish, amphibians, sea urchins, Drosophila melanogaster, and birds [33]. Unfortunately, mammalian fertilization in microgravity is very complicated and specialized compared to non-mammalian animals. Fertilization rates achieved when murine oocytes were incubated with sperm in a rotating clinostat were comparable to controls, and retrieved zygotes contributed to normal pregnancies and the birth of healthy offspring when transferred to foster mothers [17]. On the contrary, pregnancies were not achieved during the pioneer 18.5-day spaceflight experiment Cosmos 1129 [34], where five female and two male rats were sent into orbit and allowed to mate. Similarly, none of the inseminated bovine oocytes subjected to simulated microgravity in the High Aspect Ratio Vessel device were fertilized [35].
Successful in vitro fertilization by cIVF or ICSI in space requires human sperm to maintain motility and capacitate in the microgravity environment. NASA’s Micro-11 experiment is one of the first studies investigating human sperm performance on the International Space Station (ISS). Frozen donor sperm samples were thawed in a special box and compared to ground control samples. The experiment examined the capacitation of human sperm through time-dependent activation. Results showed that sperm motility was significantly reduced in microgravity compared to Earth controls. Additionally, sperm capacitation was affected through inhibition of necessary biochemical changes for fertilization [36]. Based on these findings, we may expect impairment of fertilization in the space environment.
Embryo culture and handling: today and tomorrow
Acknowledging the difficulties of oocyte retrieval, gamete manipulation, and fertilization, research focusing on more advanced stages of embryo development might be more achievable. The current state of the art of in vitro human embryo culture mimics the intrauterine environment through use of specifically composed media often overlaid with oil. Non-toxic, non-pyrogenic plastic dishes are used to culture fertilized oocytes for 5–7 days in incubators, which maintain specific temperature and gas levels but require reliable long-term electricity and - what is quite challenging in space conditions – gas supply. Current standards of care feature uninterrupted stable conditions fulfilled by a single-step medium comprised of a wide range of necessary components used by the embryo according to its stage-specific needs: lactate, pyruvate, and glucose as energy sources, essential and non-essential amino acids, protein, vitamins, and microelements in buffer solutions. Media composition for embryo culture is well-established and proven to support success. However, these media have been optimized for Earth’s atmosphere and operate under the assumption of gas availability to provide appropriate pH. Maintaining proper conditions for maximum embryo performance in the resource-limited environment of the ISS is challenging, primarily due to the required uninterrupted gas supply for incubators. Modern embryo culture depends on bicarbonate-based media which maintain optimal pH of 7.2–7.4 through the formation of carbonic acid under regulated CO2 level [37]. Alternative buffer systems, such as HEPES and MOPS, which maintain pH in atmospheric air, are only suitable for short-term manipulations and can negatively affect embryos with prolonged exposure. Additionally, low oxygen culture conditions, achieved by reducing atmospheric oxygen to intrauterine levels (typically 5%) by nitrogen supply to culture chambers, are a significant limiting factor for implementing established embryo culture techniques during space travel. Establishing specific conditions to attain acceptable outcomes of embryo development, including air-purification of volatile chemical compounds and maintenance of sterility to prevent bacterial and fungal contamination of samples, pose additional challenges.
Apart from the difficulties of simulating optimal conditions for in vitro embryo culture, space possesses additional hazards, such as radiation and gravitational changes, that may adversely influence human embryo development. Due to the ethical limitations of studying human embryos, we must rely on mammalian models to predict preimplantation human development in space conditions, and these predictions are dismaying. For example, of the 49 mice embryos launched into space on the Columbia STS-80 mission, 0 progressed in development, and all degenerated over the study period [22, 38]. Developmental capacity of murine 4-cell embryos and blastocysts in space was studied during the SJ-8 Satellite launch into orbit. As samples were not destined to return to Earth, their growth in a sealed embryo incubator was tracked with micrography for 72 h. 4-cell samples did not progress in space, while the control group of embryos on the ground successfully reached the hatching blastocyst stage, suggesting space flight factored into embryo degeneration [39]. The mission of the SJ-10 recoverable satellite also investigated the patterns of murine preimplantation development on 3400 2-cell embryos cultured in sealed incubators and regularly filmed or fixed in designated units after 64 h of culture. Though blastocyst formation and morphology were significantly compromised, this experiment provided conditions to support embryo development in space [40]. The cellular differentiation pattern of blastocysts formed in orbit was altered, and severe DNA damage and global hypomethylation of the genome were evident. These adverse effects on embryo development were mainly attributed to space radiation. Still, they may be due to the synergetic influence of low-dose prolonged irradiation and microgravity inducing gene expression or methylation alterations [40].
Studies utilizing simulated microgravity conditions showed a significant decrease in morula and blastocyst formation rates for murine embryos cultured for 96 h [17]. Similarly, after prolonged culture under 3D rotational conditions, murine blastocysts displayed slower development and decreased number of trophectoderm cells with preserved polarization patterns compared to controls [41]. Furthermore, long-term incubation of embryos under microgravity led to a significant decrease in live birth rate compared to control samples (5% vs. 21%). The decreased implantation potential of study blastocysts could be attributable to impaired trophectoderm differentiation due to delayed Oct4 downregulation [41]. Bovine zygotes and cleavage-stage embryos placed in a Rotating Cell Culture System bioreactor failed to develop into morulae or blastocysts [35]. Still, it should be noted the microgravity simulation process itself may generate shear stress on embryos, affecting predominantly outer embryo cells destined to become trophectoderm [42].
Like other cells [26, 43], human embryos are gravity-dependent, and their proliferation and differentiation patterns could be modulated under microgravity conditions. The underlying mechanism behind these changes is still unknown. Cytokine production and signal transduction may be the best candidates for evaluation as these systems are crucial for early mammalian development [17]. Also, microgravity may alter epigenetic modification of genes and chromatin structure [40, 42], activate stress response through protein kinase activity [44], and change NO and oxidants balance [45]. Although simulated microgravity does not impair cell proliferation in murine embryonic stem cells, a decreased adhesion rate and delayed DNA repair have been demonstrated, which may be exacerbated under synergetic influence of cosmic radiation [46]. In microgravity conditions, altered cell and adjacent fluid-filled cavity (blastocele) buoyancy and abnormalities of fluid convention within the embryo may become excess stress factors [47]. The cytoskeleton and organelles may also be susceptible to displacements under microgravity and shear forces, causing cells to rotate and compensate for the mild effects of µg. This adaptive mechanism may be disrupted during implantation, and shear stress may become an additional factor interfering embryo with implantation [42].
Cryopreservation and storage
Cryopreservation of oocytes, sperm and embryos - current practice
Even the most advanced culture conditions in IVF laboratories cannot support embryo development beyond day 7, necessitating either the transfer of embryos to the uterus or their cryopreservation. Slow freezing, once common in the early days of IVF, has mainly been replaced by vitrification, an efficient method for cryopreserving microscopic multicellular structures like embryos. During vitrification, samples are equilibrated in a highly concentrated solution of cryoprotectants for dehydration and placed on specialized devices in a minimal amount of accompanying solution with subsequent direct depositing into liquid nitrogen. This enables cooling of living cells to cryogenic temperatures in the absence of ice formation eliminating the risk of mechanical injury to cell membranes and organelles [48]. The warming process reverses the procedure through a rapid plunge of the sample into a 37 °C 1 M solution of non-permeating cryoprotectant. This rapid action allows the sample to avoid ice formation and osmotic shock and is followed by washing steps to remove cryoprotective agents before settling in culture conditions. Vitrification is a robust procedure which provides high survival rates, exceeding 99% for embryos and 95% for oocytes [6, 49]. The advancements in gamete cryopreservation offer a potential alternative involving fertilizing warmed oocytes with fresh or frozen sperm with comparable to fresh oocyte success rates [50, 51].
Manipulation with low-volume droplets of viscous solutions during vitrification and warming demands extensive training and may be feasible to accomplish without trained personnel on the ISS. Under microgravity conditions, droplets of a media with embryos would float, making typical operations used on the ground impossible in space. It poses the necessity to invent a completely closed system with gradual medium exchange for embryo equilibration with cryoprotectants during preparation for vitrification and slow cryoprotectant removal in the warming cycle, which is currently unavailable. In addition, liquid convection is absent in weightlessness. Therefore, culture and vitrification/warming media exchange is complicated and requires supplementary manipulations, e.g., shaking or stirring [52]. Automated vitrification systems could potentially be adapted for the whole cycle of sample cryopreservation and warming in a closed environment.
Furthermore, vitrified samples must remain stored under stable conditions; even short-term temperature increases above − 130 °C can lead to specimen loss [48]. In IVF clinics, vitrified oocytes and embryos are preserved in liquid nitrogen or its vapors in cryotanks under constant monitoring of laboratory staff and alarm systems. Due to pressure change concerns, sealed containers with liquid nitrogen are unsafe on the ISS and the coldest available freezer can only maintain − 95 °C [54]. An uninterrupted liquid nitrogen supply is crucial for safe storage on Earth and could be an important limiting factor for long-term embryo transportation in space. Also, space radiation may induce mutation accumulation in transported samples whose repair systems are inactive in the frozen state [53], limiting the continuance of space travels with embryos or gametes on board.
Yet perhaps the most practical application of ART in the space program will be the development of optimal vitrification and warming protocols in space conditions and, most importantly, the implementation of new, preferably fully automated, cryostorage systems. These technologies are crucial to develop in support of long-range space travel. Humans may one day populate extraterrestrial systems and will age as they undergo these enormous travel distances and time frames. A fertility preservation program will be integral to population growth. With this in mind, early steps have been taken to establish a freezing program in the space environment and engineer potential storage technologies. These technologies will be particularly important beyond low Earth orbit (LEO) in planets with extreme temperatures and exposure to deep space radiation.
Results of attempted warming of frozen mammal samples in ISS conditions
Wakayama et al. [54] have developed an embryo thawing and culturing unit (ETC), representing a significant advancement in the cryopreservation of embryos in space. This new protocol and device were essential to overcome current challenges, allowing for the successful thawing, washing, and culturing of mouse 2-cell embryos in the unique environment of the ISS. During the testing of equipment after one month of storage at -80 °C, 32.7% of the embryos progressed to the blastocyst stage. The ETC employs a high osmolarity vitrification method [55], enabling the preservation of embryos at -80 °C for several months without liquid nitrogen. In their trial experiments on the ground, researchers demonstrated embryos could be retrieved and developed into blastocysts with a recovery rate ranging from 24 to 78% and development rates reaching up to 100%. This success paves the way for further reproductive experiments in space and has potential applications in clinical infertility and animal biotechnology on Earth. The ETC’s ability to be used by untrained personnel, such as astronauts, marks a significant improvement in the feasibility of conducting complex biological experiments in space.
An invented device was used during an experiment involving 720 two-cell mice embryos cultured for four days in microgravity and artificial 1 g conditions aboard the ISS in September 2021 [52]. Though embryo recovery rates for microgravity and artificial 1 g were 20% and 16.9%, which were significantly lower than those achieved in the ground laboratory control (37.2%), untrained astronauts could retrieve enough blastocysts to conduct subsequent genetic and cellular studies of the launched samples. From the recovered embryos, 23.6% developed into blastocysts under microgravity at ISS. The study revealed murine embryos may form blastocysts when cultured in space. Moreover, cell numbers, trophectoderm, and ICM developmental patterns with respect to Nanog and CDX2 antibody binding, as well as gene expression profiles, were comparable for orbit- and ground-cultured samples. Interestingly, continuous rotation used to generate artificial gravity and simulate Earth conditions on the ISS affected embryo development and led to a decreased blastocyst rate and less cellular embryos [52]. As sterile conditions cannot be appropriately maintained in space, all the used thawing and culturing devices displayed signs of bacterial contamination, posing an additional threat when the possibility of human embryo culture during spaceflight is considered.
Advancements in oocyte freezing have enabled the option of donor egg banking and present a reasonable scenario for delayed parenthood. On Earth, planned oocyte vitrification is an option to preserve future fertility. However, oocyte cryopreservation tends to be more challenging than blastocysts, with lower survival rates due to the low surface-volume ratio leading to complicated equilibration of these large cells. Vitrification of oocytes may lead to spindle disassembly, subcellular abnormalities, subsequent fertilization failure, or altered embryo development [56–58]. Thus, oocyte freezing and warming are a matter of concern on Earth due to their sensitivity, and handling in orbit will be a challenge for untrained personnel of the ISS.
Luckily, freezing and warming of sperm samples is efficient and straightforward. Male gametes are abundant and more easily accessible subjects to study the effect of space conditions. These cells have a decreased cytoplasmic volume, specific composition and fluidity of membranes, rendering them less prone to intracellular crystallization and cryodamage and even tolerant of freeze-drying. The Wakayama team studied the impact of cosmic radiation on DNA integrity and fertilization capacity of freeze-dried mice spermatozoa in 2013–2014. Samples stored on ISS for nine months at -95 °C were used for fresh oocyte fertilization after landing. Though these sperm samples had significantly higher DNA fragmentation levels, embryos developed into blastocysts that successfully implanted and produced genetically normal and fertile offspring [59]. The authors surmised that acquired irradiation will not affect the quality of mammalian spermatozoa and their offspring’s health, even if freeze-fried spermatozoa are stored for more than 200 years [60]. They also hypothesize the oocytes could repair DNA breaks acquired by irradiated sperm during the first cell division cycle [59]. However, Yoshida et al. reported changes in mouse testes’ transcription factors and alterations in spermatozoa microRNA expression profiles. Additionally, space-flown sperm produced F1 progeny, but epigenetic changes were observed in liver gene expressions in the next generations [61]. Although there are morphological and physiological differences between murine and human sperm, the studied effects of microgravity in the mouse testes and spermatozoa may potentially be observed in human. Further studies using human samples will be necessary to elucidate effects.
New updates on automation and robotics solutions on the ground and possible utilization in space
The repetitive and high-precision nature of IVF laboratory tasks lends itself to developing automated systems. Unsurprisingly, automation has become a recent focus of clinical IVF research [62, 63]. Microfluidic devices and lab-on-a-chip technologies utilize small channels and automated mechanisms to manipulate small volumes of liquid accurately, which may provide precise and reliable liquid handling in space. These solutions help maintain the stability and reliability needed for different experimental approaches and procedures in microgravity.
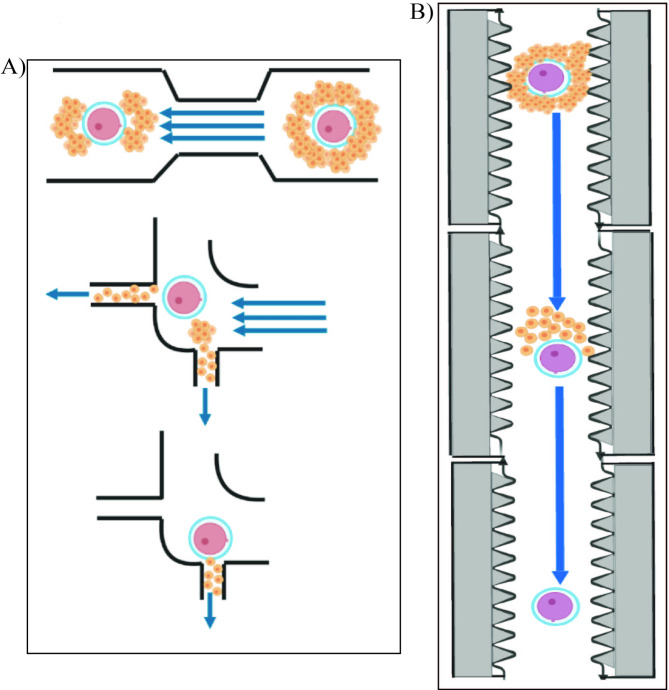
Microfluidic devices developed to automate oocyte retrieval may become a tool in a microgravity environment and are currently under development. One example of an effort to mechanize IVF procedures is an oocyte collection device which is intended to isolate gametes from the follicular fluid during a retrieval. It is also designed to denude oocytes and assess their maturity before ICSI in cases of male-factor infertility [63]. Under microgravity conditions, this operation sequence is challenging, even with the most experienced embryologist. Automated or semi-automated microfluidic systems for oocyte retrieval may provide solutions for future microgravity problems. Microfluidic devices were specifically designed to perform cumulus cell removal as described by Zeringue et al. [64] and Weng et al. [65] (Fig. 2).
Fig. 2.
The denudation devices proposed by: (A) Zeringue et al. (2001) and (B) Weng et al. (2018)
(A) Device created from polydimethylsiloxane with channels of oocyte-cumulus complex size (400 μm) is implemented for tracking and denudation of the oocytes. Two narrow regions of the microchip reorient the cumulus into a doughnut shape, while two ports (channels significantly narrower than the ovum) are used to remove the cumulus. The device uses pressure-driven flow to properly position and denude the oocytes. (B) The microfluidic device implicates denudation of enzyme-treated cumulus–oocyte complexes by flow-guided passage through a series of jagged-surface constriction microchannels of optimized geometries. The jagged inner wall of the channels is stripping off cumulus–corona cell mass. Reprint with permission from “Automation in Clinical Embryology Laboratories – What is Next?” by Halicigil C., Ogut M.G., Demirci U. © 2023, World Scientific
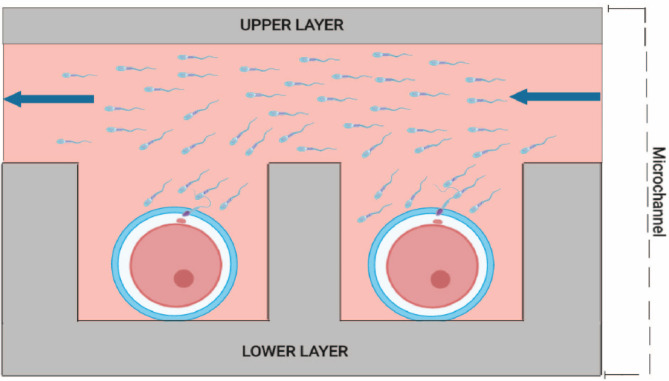
Similarly, conventional microdroplet embryo culture and gamete handling are complicated to implement in space if possible. Several designs employing a traditional IVF approach with microfluidic models have been developed on Earth. These designs typically utilize a system stabilizing oocytes using microsieves or microwells positioned along channels. This setup allows sperm to flow through narrow pipelines towards the oocyte, increasing fertilization chances even with lower sperm concentrations than conventional insemination. One of the most comprehensive examples of a microfluidic approach is the microwell array that allows fertilization and embryo culture processes in an integrated fashion [66] (Fig. 3). This approach may solve the pitfalls of microgravity and handling during conventional insemination.
Fig. 3.
Integration of fertilization and development in a microwell array proposed by Han et al. (2010).
Reprint with permission from “Automation in Clinical Embryology Laboratories – What is Next?” by Halicigil C., Ogut M.G., Demirci U. © 2023, World Scientific
The proposed device consists of microchannel in the upper layer and a microwell array in the lower layer, with inlet and outlet connected to the microchannel. Square-shaped microwells are located in shifted rows to trap oocytes efficiently. During the loading of the device, oocytes are randomly trapped in the microwells with subsequent addition of the sperm suspension. It is implied that debris and spermatozoa may be removed from wells with the flow generated by syringe pump with simultaneous change of the fertilization medium to culture medium after incubation. Obtained embryos may be cultured in the device for 5–6 days, individually monitored and tracked, and washed out of the microwells by pipetting for further manipulation.
The foundational work of Lu et al. [67] on automated robotic ICSI (ICSIA) significantly contributed to advancements that led to the thriving live birth of the first babies conceived through automated ICSI in 2023 [68], showcasing the practical application and potential of automated reproductive technologies. This robotic system automates critical steps of the ICSI procedure, such as alignment and penetration of the oocyte by the injection pipette using piezo pulses, which are controlled by advanced artificial intelligence (AI) algorithms [68]. Although some steps, like sperm immobilization and release, still require manual intervention, the ICSIA system reduces the variability and potential damage to oocytes, thereby enhancing the consistency of the procedure. Regarding performance metrics, the ICSIA system has demonstrated fertilization and blastocyst formation rates similar to manual ICSI. For instance, in clinical trials, the fertilization rate for oocytes injected with ICSIA was 92.5%, and the blastocyst formation rate was comparable to manual methods [68]. Thus, it may be a powerful instrument to obtain fertilization ICSI in space, even if performed by untrained personnel.
Furthermore, technological advancements like robotic and Internet of Things (IoT) devices may help resolve the problem with remote IVF. At the Alpha conference in Lisbon (2024), the first remote ICSI was presented as an embryologist performing the procedure remotely using IoT technology connected to a micromanipulator. This approach may resolve the hurdle of trained personnel availability in orbit as there may be the possibility of performing the manipulations remotely with automation solutions in the future. Many modern startups focused on robotic and AI models that streamline various aspects of the IVF process are developing innovative products and prototypes to automate IVF labs. These models are designed to handle and culture embryos with high precision, thereby reducing the manual nature of traditional IVF procedures [67].
Conclusion
Transferring current IVF practices to space presents significant challenges. The ethical limitations of using human subjects in space-based IVF research and the extremely low efficiency of existing IVF techniques in microgravity make this endeavor highly impractical. Artificial gravity in space may tackle the issues stemming from microgravity; however, its impact on cells and tissues needs to be investigated further. Besides gravity, the impact of space radiation on human gametes, fertilization and embryo development also needs to be explored. We also need to understand the impact of space-related factors not only on the quantity but also on the quality of gametes and embryos. Identifying the optimal cryostorage environment in space is essential for preserving biological integrity and ensuring the viability of cells and tissues under extraterrestrial conditions.
Currently, most manipulations in clinical embryology are operator dependent. Fully automated systems are in development, but not yet widely implemented in clinical practice. Moreover, even basic laboratory procedures must be optimized for low Earth orbit and beyond, which poses another obstacle to performing IVF in space. Therefore, there is still a long way to go in managing gamete handling, fertilization, embryo culture, and utilization in space.
Numerous simulated and actual space studies of preimplantation embryo development are necessary to understand and address the cellular mechanisms contributing to embryo development in microgravity [22]. Space-based mammalian, and particularly human reproduction research, is crucial for understanding human fertility to even begin the discussion of long-term space flights and other planets’ colonization.
In conclusion, gamete retrieval, fertilization, embryo culture, and manipulation under space conditions present significant challenges. Advances in culture conditions, procedure automation, and rigorous environmental and manipulation control are critical for future reproductive research in space travel. Despite these obstacles, the optimal path forward is adapting current IVF technologies for space research. By doing so, we can gain invaluable insights into human reproduction in space and on Earth. This knowledge could lead to advancements in reproductive medicine, ultimately enhancing our understanding of fertility and aiding in developing more effective reproductive technologies for all environments.
Abbreviations
- µg
Microgravity
- AI
Artificial Intelligence
- ART
Assisted Reproductive Technologies
- cIVF
Conventional IVF
- DNA
Deoxyribonucleic Acid
- ICSI
Intracytoplasmic Sperm Injection
- ICSIA
Automated Intracytoplasmic Sperm Injection
- IoT
Internet of Things
- ISS
International Space Station
- IVF
In Vitro Fertilization
- KPI
Key Performance Indicator
- LEO
Low Earth orbit
- NASA
National Aeronautics and Space Administration
Author contributions
Conceptualization – BAM, CH. Original draft: OC, SNB, BAM, CH. Review and editing: OC, BAM, SNB, MB, CH. OC and CH prepared the figures.
Funding
No external funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Begum Aydogan Mathyk, Email: abegum@usf.edu.
Cihan Halicigil, Email: cihan.halicigil@yale.edu.
References
- 1.Gray T. Brief History of Animals in Space 1998 [ https://www.nasa.gov/history/a-brief-history-of-animals-in-space/
- 2.Proshchina A, Gulimova V, Kharlamova A, Krivova Y, Besova N, Berdiev R et al. Reproduction and the Early Development of vertebrates in Space: problems, results, opportunities. Life (Basel). 2021;11(2). [DOI] [PMC free article] [PubMed]
- 3.Sciorio R, Aiello R, Janssens R. Considerations on staffing levels for a modern assisted reproductive laboratory. JBRA Assist Reprod. 2023;27(1):120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eshre GG, De los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K et al. Revised guidelines for good practice in IVF laboratories (2015). Hum Reprod. 2016;31(4):685-6. [DOI] [PubMed]
- 5.Practice Committees of the American Society for Reproductive M. The Society for Reproductive B, technologists. Electronic address aao. Comprehensive guidance for human embryology, andrology, and endocrinology laboratories: management and operations: a committee opinion. Fertil Steril. 2022;117(6):1183–202. [DOI] [PubMed] [Google Scholar]
- 6.Eshre SIGoE. The Vienna consensus: report of an expert meeting on the development of art laboratory performance indicators. Hum Reprod Open. 2017;2017(2):hox011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho JR, Paulson RJ. Modified natural cycle in in vitro fertilization. Fertil Steril. 2017;108(4):572–6. [DOI] [PubMed] [Google Scholar]
- 8.McIntyre ABR, Rizzardi L, Yu AM, Alexander N, Rosen GL, Botkin DJ, et al. Nanopore sequencing in microgravity. NPJ Microgravity. 2016;2:16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzardi LF, Kunz H, Rubins K, Chouker A, Quiriarte H, Sams C, et al. Evaluation of techniques for performing cellular isolation and preservation during microgravity conditions. NPJ Microgravity. 2016;2:16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronca AE, Baker ES, Bavendam TG, Beck KD, Miller VM, Tash JS, et al. Effects of sex and gender on adaptations to space: reproductive health. J Womens Health (Larchmt). 2014;23(11):967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 6 ed. Geneva2021. 276 p.
- 12.Zhang X, Khimji I, Gurkan UA, Safaee H, Catalano PN, Keles HO, et al. Lensless imaging for simultaneous microfluidic sperm monitoring and sorting. Lab Chip. 2011;11(15):2535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathyk BA, Tabetah M, Karim R, Zaksas V, Kim J, Anu RI, et al. Spaceflight induces changes in gene expression profiles linked to insulin and estrogen. Commun Biol. 2024;7(1):692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathyk B, Imudia AN, Quaas AM, Halicigil C, Karouia F, Avci P, et al. Understanding how space travel affects the female reproductive system to the Moon and beyond. Npj Women’s Health. 2024;2(1):20. [Google Scholar]
- 15.Rydze R, Schutt A, Gibbons W, Nodler J. Gravity and embryo development. Curr Obstet Gynecol Rep. 2017;6(1):51–4. [Google Scholar]
- 16.Ikeuchi T, Sasaki S, Umemoto Y, Kubota Y, Kubota H, Kaneko T, et al. Human sperm motility in a microgravity environment. Reprod Med Biol. 2005;4(2):161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima Y, Sasaki S, Kubota Y, Ikeuchi T, Hayashi Y, Kohri K. Effects of simulated microgravity on mammalian fertilization and preimplantation embryonic development in vitro. Fertil Steril. 2000;74(6):1142–7. [DOI] [PubMed] [Google Scholar]
- 18.Boada M, Perez-Poch A, Ballester M, Garcia-Monclus S, Gonzalez DV, Garcia S, et al. Microgravity effects on frozen human sperm samples. J Assist Reprod Genet. 2020;37(9):2249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Marin C, Gosalvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13(11):14026–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li HY, Zhang H, Miao GY, Xie Y, Sun C, Di CX, et al. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm DNA damage. Biomed Environ Sci. 2013;26(9):726–34. [DOI] [PubMed] [Google Scholar]
- 21.Ahrari K, Omolaoye TS, Goswami N, Alsuwaidi H, du Plessis SS. Effects of space flight on sperm function and integrity: a systematic review. Front Physiol. 2022;13:904375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford-Young SJ. Effects of microgravity on cell cytoskeleton and embryogenesis. Int J Dev Biol. 2006;50(2–3):183–91. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Guo X, Wang F, Li X, Tian XC, Li L, et al. Simulated microgravity compromises mouse oocyte maturation by disrupting meiotic spindle organization and inducing cytoplasmic blebbing. PLoS ONE. 2011;6(7):e22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miglietta S, Cristiano L, Espinola MSB, Masiello MG, Micara G, Battaglione E et al. Effects of simulated Microgravity in Vitro on Human Metaphase II oocytes: an Electron Microscopy-based study. Cells. 2023;12(10). [DOI] [PMC free article] [PubMed]
- 25.Michaletti A, Gioia M, Tarantino U, Zolla L. Effects of microgravity on osteoblast mitochondria: a proteomic and metabolomics profile. Sci Rep. 2017;7(1):15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang M, Wang H, Liu Z, Lin L, Wang L, Xie M, et al. Endoplasmic reticulum stress-dependent activation of iNOS/NO-NF-kappaB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity. FASEB J. 2020;34(8):10835–49. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Zheng D, Wu Y, Lin W, Chen Z, Meng L, et al. Simulated microgravity using a Rotary Culture System compromises the in Vitro Development of Mouse Preantral follicles. PLoS ONE. 2016;11(3):e0151062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng K, Feng X, Yang C, Ma C, Niu S, Jia L, et al. Simulated microgravity reduces quality of ovarian follicles and oocytes by disrupting communications of follicle cells. NPJ Microgravity. 2023;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sventitskaya MA, Ogneva IV. Reorganization of the mouse oocyte’ cytoskeleton after cultivation under simulated weightlessness. Life Sci Space Res (Amst). 2024;40:8–18. [DOI] [PubMed] [Google Scholar]
- 30.Kujjo LL, Ronningen R, Ross P, Pereira RJ, Rodriguez R, Beyhan Z, et al. RAD51 plays a crucial role in halting cell death program induced by ionizing radiation in bovine oocytes. Biol Reprod. 2012;86(3):76. [DOI] [PubMed] [Google Scholar]
- 31.Bosch E, Espinos JJ, Fabregues F, Fontes J, Garcia-Velasco J, Llacer J, et al. ALWAYS ICSI? A SWOT analysis. J Assist Reprod Genet. 2020;37(9):2081–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alikani MER. The success of ICSIA and the tough road to automation. Reprod Biomed Online. 2023;47(3):103244. [DOI] [PubMed] [Google Scholar]
- 33.Lei X, Cao Y, Zhang Y, Duan E. Advances of mammalian Reproduction and Embryonic Development under Microgravity. In: Duan E, Long M, editors. Life Science in Space: experiments on Board the SJ-10 Recoverable Satellite. Singapore: Springer Singapore; 2019. pp. 281–315. [Google Scholar]
- 34.Serova LV, Denisova LA. The effect of weightlessness on the reproductive function of mammals. Physiologist. 1982;25(6):S9–12. [PubMed] [Google Scholar]
- 35.Jung S, Bowers SD, Willarda ST. Simulated microgravity influences bovine oocyte in vitro fertilization and preimplantation embryo development. J Anim Veterinary Adv. 2009;8:1807–14. [Google Scholar]
- 36.Turner JL. Micro-11. Spaceflight-Altered Motility Activation and Fertility-Dependent Responses in Sperm 2023 [ https://www.nasa.gov/mission/station/research-explorer/investigation/?#id=1922
- 37.Swain JE. Is there an optimal pH for culture media used in clinical IVF? Hum Reprod Update. 2012;18(3):333–9. [DOI] [PubMed] [Google Scholar]
- 38.Schenker E, Forkheim K. Mammalian mice embryo early development in weightlessness environment on STS 80 space flight. Isr Aerosp Med Inst Rep. 1998;5.
- 39.Ma B-H, Cao Y-J, Zheng W-B, Lu J-R, Kuang H-b, Lei X-H, et al. Real-time micrography of mouse preimplantation embryos in an Orbit Module on SJ-8 Satellite. Microgravity Sci Technol. 2008;20(2):127–36. [Google Scholar]
- 40.Lei X, Cao Y, Ma B, Zhang Y, Ning L, Qian J, et al. Development of mouse preimplantation embryos in space. Natl Sci Rev. 2020;7(9):1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakayama S, Kawahara Y, Li C, Yamagata K, Yuge L, Wakayama T. Detrimental effects of microgravity on mouse preimplantation development in vitro. PLoS ONE. 2009;4(8):e6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruden DM, Bolnick A, Awonuga A, Abdulhasan M, Perez G, Puscheck EE, et al. Effects of gravity, Microgravity or Microgravity Simulation on early mammalian development. Stem Cells Dev. 2018;27(18):1230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feger BJ, Thompson JW, Dubois LG, Kommaddi RP, Foster MW, Mishra R, et al. Microgravity induces proteomics changes involved in endoplasmic reticulum stress and mitochondrial protection. Sci Rep. 2016;6:34091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Xie Y, Wygle D, Shen HH, Puscheck EE, Rappolee DA. A major effect of simulated microgravity on several stages of preimplantation mouse development is lethality associated with elevated phosphorylated SAPK/JNK. Reprod Sci. 2009;16(10):947–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li F, Ye Y, Lei X, Zhang W. Effects of Microgravity on Early Embryonic Development and embryonic stem cell differentiation: phenotypic characterization and potential mechanisms. Front Cell Dev Biol. 2021;9:797167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, An L, Jiang Y, Hang H. Effects of simulated microgravity on embryonic stem cells. PLoS ONE. 2011;6(12):e29214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreazzoli M, Angeloni D, Broccoli V, Demontis GC, Microgravity. Stem cells, and Embryonic Development: challenges and opportunities for 3D tissue generation. Front Astronomy Space Sci. 2017;4.
- 48.Fahy GM, Wowk B. Principles of ice-free cryopreservation by vitrification. Methods Mol Biol. 2021;2180:27–97. [DOI] [PubMed] [Google Scholar]
- 49.Alpha Scientists In Reproductive Medicine A. The alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reprod Biomed Online. 2012;25(2):146–67. [DOI] [PubMed]
- 50.Trokoudes KM, Pavlides C, Zhang X. Comparison outcome of fresh and vitrified donor oocytes in an egg-sharing donation program. Fertil Steril. 2011;95(6):1996–2000. [DOI] [PubMed] [Google Scholar]
- 51.Karagianni M, Papadopoulou MI, Oraiopoulou C, Christoforidis N, Papatheodorou A, Chatziparasidou A. Embryos from vitrified vs. fresh oocytes in an oocyte donation program: a comparative morphokinetic analysis. F S Sci. 2024;5(2):174–81. [DOI] [PubMed] [Google Scholar]
- 52.Wakayama S, Kikuchi Y, Soejima M, Hayashi E, Ushigome N, Yamazaki C, et al. Effect of microgravity on mammalian embryo development evaluated at the International Space Station. iScience. 2023;26(11):108177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yatagai F, Honma M, Takahashi A, Omori K, Suzuki H, Shimazu T, et al. Frozen human cells can record radiation damage accumulated during space flight: mutation induction and radioadaptation. Radiat Environ Biophys. 2011;50(1):125–34. [DOI] [PubMed] [Google Scholar]
- 54.Wakayama S, Soejima M, Kikuchi Y, Hayashi E, Ushigome N, Hasegawa A, et al. Development of a new device for manipulating frozen mouse 2-cell embryos on the International Space Station. PLoS ONE. 2022;17(10):e0270781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mochida K, Hasegawa A, Li MW, Fray MD, Kito S, Vallelunga JM, et al. High osmolality vitrification: a new method for the simple and temperature-permissive cryopreservation of mouse embryos. PLoS ONE. 2013;8(1):e49316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonte D, Thys V, De Sutter P, Boel A, Leybaert L, Heindryckx B. Vitrification negatively affects the ca(2+)-releasing and activation potential of mouse oocytes, but vitrified oocytes are potentially useful for diagnostic purposes. Reprod Biomed Online. 2020;40(1):13–25. [DOI] [PubMed] [Google Scholar]
- 57.Lei T, Guo N, Liu JQ, Tan MH, Li YF. Vitrification of in vitro matured oocytes: effects on meiotic spindle configuration and mitochondrial function. Int J Clin Exp Pathol. 2014;7(3):1159–65. [PMC free article] [PubMed] [Google Scholar]
- 58.Iussig B, Maggiulli R, Fabozzi G, Bertelle S, Vaiarelli A, Cimadomo D, et al. A brief history of oocyte cryopreservation: arguments and facts. Acta Obstet Gynecol Scand. 2019;98(5):550–8. [DOI] [PubMed] [Google Scholar]
- 59.Wakayama S, Kamada Y, Yamanaka K, Kohda T, Suzuki H, Shimazu T, et al. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc Natl Acad Sci U S A. 2017;114(23):5988–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakayama S, Ito D, Kamada Y, Shimazu T, Suzuki T, Nagamatsu A et al. Evaluating the long-term effect of space radiation on the reproductive normality of mammalian sperm preserved on the International Space Station. Sci Adv. 2021;7(24). [DOI] [PMC free article] [PubMed]
- 61.Yoshida K, Fujita SI, Isotani A, Kudo T, Takahashi S, Ikawa M, et al. Intergenerational effect of short-term spaceflight in mice. iScience. 2021;24(7):102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casciani V, Galliano D, Franasiak JM, Mariani G, Meseguer M. Are we approaching automated assisted reproductive technology? Embryo culture, metabolomics, and cryopreservation. F&S Reviews. 2021;2(4):251–64. [Google Scholar]
- 63.Halicigil C, Ogut MG, Demirci U. Automation in Clinical Embryology Laboratories — What is Next? Emerging Technologies in Biophysical Sciences: A World Scientific Reference. pp. 105 – 26.
- 64.Zeringue HC, Beebe DJ, Wheeler MB. Removal of Cumulus from mammalian zygotes using microfluidic techniques. Biomed Microdevices. 2001;3(3):219–24. [Google Scholar]
- 65.Weng L, Lee GY, Liu J, Kapur R, Toth TL, Toner M. On-chip oocyte denudation from cumulus-oocyte complexes for assisted reproductive therapy. Lab Chip. 2018;18(24):3892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han C, Zhang Q, Ma R, Xie L, Qiu T, Wang L, et al. Integration of single oocyte trapping, in vitro fertilization and embryo culture in a microwell-structured microfluidic device. Lab Chip. 2010;10(21):2848–54. [DOI] [PubMed] [Google Scholar]
- 67.Lu Z, Zhang X, Leung C, Esfandiari N, Casper RF, Sun Y. Robotic ICSI (intracytoplasmic sperm injection). IEEE Trans Biomed Eng. 2011;58(7):2102–8. [DOI] [PubMed] [Google Scholar]
- 68.Costa-Borges N, Munne S, Albo E, Mas S, Castello C, Giralt G, et al. First babies conceived with automated intracytoplasmic sperm injection. Reprod Biomed Online. 2023;47(3):103237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.