Abstract
Background
The phenomenon of intercellular mitochondrial transfer from mesenchymal stromal cells (MSCs) has shown promise for improving tissue healing after injury and has potential for treating degenerative diseases like osteoarthritis (OA). Recently MSC to chondrocyte mitochondrial transfer has been documented, but the mechanism of transfer is unknown. Full-length connexin 43 (Cx43, encoded by GJA1) and the truncated, internally translated isoform GJA1-20k have been implicated in mitochondrial transfer between highly oxidative cells, but have not been explored in orthopaedic tissues. Here, our goal was to investigate the role of Cx43 in MSC to chondrocyte mitochondrial transfer. In this study, we tested the hypotheses that (a) mitochondrial transfer from MSCs to chondrocytes is increased when chondrocytes are under oxidative stress and (b) MSC Cx43 expression mediates mitochondrial transfer to chondrocytes.
Methods
Oxidative stress was induced in immortalized human chondrocytes using tert-Butyl hydroperoxide (t-BHP) and cells were evaluated for mitochondrial membrane depolarization and reactive oxygen species (ROS) production. Human bone-marrow derived MSCs were transduced for mitochondrial fluorescence using lentiviral vectors. MSC Cx43 expression was knocked down using siRNA or overexpressed (GJA1 + and GJA1-20k+) using lentiviral transduction. Chondrocytes and MSCs were co-cultured for 24 h in direct contact or separated using transwells. Mitochondrial transfer was quantified using flow cytometry. Co-cultures were fixed and stained for actin and Cx43 to visualize cell-cell interactions during transfer.
Results
Mitochondrial transfer was significantly higher in t-BHP-stressed chondrocytes. Contact co-cultures had significantly higher mitochondrial transfer compared to transwell co-cultures. Confocal images showed direct cell contacts between MSCs and chondrocytes where Cx43 staining was enriched at the terminal ends of actin cellular extensions containing mitochondria in MSCs. MSC Cx43 expression was associated with the magnitude of mitochondrial transfer to chondrocytes; knocking down Cx43 significantly decreased transfer while Cx43 overexpression significantly increased transfer. Interestingly, GJA1-20k expression was highly correlated with incidence of mitochondrial transfer from MSCs to chondrocytes.
Conclusions
Overexpression of GJA1-20k in MSCs increases mitochondrial transfer to chondrocytes, highlighting GJA1-20k as a potential target for promoting mitochondrial transfer from MSCs as a regenerative therapy for cartilage tissue repair in OA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-03932-9.
Keywords: Cx43, GJA1, GJA1-20k, Gap junctions, Osteoarthritis, Arthritis, Regenerative medicine, MSCs
Background
Osteoarthritis (OA) is a degenerative joint disease and the leading cause of disability in older adults, affecting > 300 million people globally each year [1]. OA is characterized by the degradation of articular cartilage, a highly specialized soft tissue that provides load support and a highly lubricated surface for joint articulation. Cartilage is composed of chondrocytes embedded within a dense extracellular matrix (ECM) and has little capacity for self-repair, in part due to the avascularity of the tissue. Despite decades of research, no available therapies prevent OA progression after cartilage injury [2, 3], and therefore, strategies to promote cartilage health and regeneration hold promise toward the pressing clinical need for effective OA treatments.
Mesenchymal stromal cell (MSC)-based therapies, including extracellular vesicles (EVs) derived from MSCs (EVs-MSCs), have recently emerged as a potential regenerative therapy for OA [4]. Injections of MSCs, or EVs-MSCs, into joints after a traumatic injury have been reported to attenuate cartilage degradation and joint degeneration in pre-clinical models [5–7]. Additionally, there has been significant clinical investigation of stem cell therapies for OA, with over 70 clinical trials completed and more than 40 currently underway [8]. Of note, MSC injections into human OA knee joints resulted in decreased pain and cartilage catabolic biomarkers one year after injection [9, 10]. While a growing body of evidence has identified therapeutic effects of MSCs including recruitment of endogenous stem cells and immunomodulation [11–13], the exact mechanism(s) underlying the beneficial effects of MSCs on cartilage repair remains unclear. One potential mechanism is the donation of whole-organelle mitochondria from MSCs to chondrocytes. This process of intercellular mitochondrial transfer has been shown to increase when cells are stressed, and transfer can rescue injured/stressed cells by restoring cellular bioenergetics, preserving cell viability, reducing oxidative stress, and improving tissue healing across multiple cell types including the lung, heart, and brain [14–24]. Recently, in orthopaedic tissues, mitochondrial transfer has been shown to improve tendon healing in vivo [25] and to occur from MSCs to chondrocytes [26–28].
Mitochondrial dysfunction is one of the earliest cellular responses to traumatic injury in cartilage tissue [29], and mitochondrial-targeted therapies have effectively decreased mitochondrial dysfunction and cartilage degeneration while preserving chondrocyte viability after injury in vitro [30, 31]. While these studies point to the therapeutic potential of mitochondrial transfer for cartilage repair, the mechanisms of mitochondrial transfer from MSCs to chondrocytes remain unknown. Mitochondrial transfer has been shown to occur through direct cell-cell contacts (i.e. tunneling nanotubules (TNTs)) or through microvesicles (including EVs) containing mitochondria from donor cells [22]. Notably, the gap junction protein connexin 43 (Cx43) has been identified as a critical regulator of both EV- and TNT-mediated mitochondrial transfer in multiple cell types [14, 32–36]. In the context of cartilage, both pharmacologic- (carbenoxolone disodium) and Cx43-mimetic peptide- (Gap 27) mediated inhibition of gap junctions were found to decrease mitochondrial transfer between MSCs and murine chondrocytes [26].
Cx43 is a transmembrane protein (gene name GJA1) that forms pores at the cell membrane (hemichannels) which can communicate with the extracellular space and/or dock with channels on opposing cells to form gap junctions effecting direct intercellular communication. Recently, GJA1 was identified to undergo alternative translation to create multiple N-terminally truncated isoforms [37]. Of the truncated isoforms, GJA1-20k has been implicated in mitochondrial transfer as it aids in mitochondrial motility by mobilizing mitochondria along microtubules [38], recruits actin to organize cell trafficking pathways [39], and overexpression of GJA1-20k increased mitochondrial transfer from astrocytes to neurons in vitro [40]. Additionally, GJA1-20k is critical for trafficking Cx43 hemichannels from the Golgi apparatus to the cell membrane and could therefore support the Cx43-channel role of mitochondrial transfer as discussed above [14, 32, 37, 41]. These studies highlight the potential multi-faceted role of GJA1-20k in mitochondrial transfer, but GJA1-20k has not been investigated in mitochondrial transfer involving MSCs or chondrocytes.
The objectives of this study were to determine the effect of oxidative stress on intercellular mitochondrial transfer from MSCs to chondrocytes, and to investigate the subcellular mechanisms of transfer, specifically the role of Cx43 and GJA1-20k. We hypothesized that (a) MSC-chondrocyte mitochondrial transfer would increase when chondrocytes undergo oxidative stress, (b) mitochondrial transfer from MSCs to chondrocytes will occur predominantly through direct cell-cell contacts, (c) the magnitude of mitochondrial transfer will be dependent on Cx43 in MSCs, where MSC Cx43 knockdown will decrease mitochondrial transfer and overexpression will increase transfer, and (d) expression of the GJA1-20k isoform in MSCs will be more strongly correlated with the rate of mitochondrial transfer than full-length Cx43.
Methods
Ethics statement
The immortalized human chondrocyte cell line (T/C-28a2) was kindly provided by Dr. Miguel Otero from the Hospital for Special Surgery, New York, NY. Human MSCs were purchased from RoosterBio.
Human cell culture
Human bone marrow-derived MSCs were purchased from RoosterBio (MSC-003) at passage 2. MSCs were cultured in MSC media (RoosterNourish, RoosterBio, KT-001) and incubated at 37 °C and 5% CO2. MSCs of passage 4–6 were used in experiments. To study the role of Cx43 in mitochondrial transfer from MSCs to chondrocytes, an immortalized human chondrocyte line was used as a demonstrated model for the study of Cx43 and gap junctions [42]. Chondrocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM, no glucose, L-Glutamine, and sodium pyruvate; VWR, 11966025) with 10% fetal bovine serum (R&D Systems, S11550), 1% Penicillin/Streptomycin (VWR, 100X, 97063-708), 1% sodium pyruvate (Thermo Fischer, 200 mM, 11360070), 2% L-Glutamine (Thermo Fisher, 200 mM, 25030081), 45 mg/100 mL of D-Glucose (VWR, BDH9230). Chondrocytes of passage 4–8 were used in experiments.
Mitochondrial membrane polarization
JC-10 was used to quantify mitochondrial membrane potential as this stain exhibits mitochondrial potential-dependent accumulation in the mitochondria as previously described [43]. Chondrocytes were seeded onto a black, flat-bottomed 96-well plate at 20,000 cells per well and allowed to culture overnight. Cells were stimulated with tert-Butyl hydroperoxide (t-BHP: 0, 1, 12, 30, or 60 µM, Thermo Fisher Scientific, #180345000) in 1X OptiMEM for 24 h. FCCP is a potent mitochondrial oxidative phosphorylation uncoupler and was used as a positive control (20 µM, 20 min incubation at room temperature, Sigma Aldrich, C2920). All cells were rinsed with PBS followed by incubation with JC-10 (10 µM, Enzo Life Sciences, ENZ-52305) for 45 min at room temperature in the dark. Following incubation, cells were read on a plate reader with an excitation of 490 nm and 540 nm. The ratio of emission at 525/590 was calculated for each well as the ratio of depolarized/polarized mitochondria within the cells.
Measurement of ROS
Chondrocyte ROS production was measured using the CellROX Green Flow Cytometry Assay Kit according to the manufacturer’s instructions (Invitrogen, C10492). After 24 h of +/- t-BHP stimulation, chondrocytes were rinsed with PBS, lifted, and incubated with CellROX Green (500 nM) for 30 min at room temperature in the dark. After incubation, cells were immediately analyzed using a Thermo Fisher Attune NxT flow cytometer. Unstained chondrocytes were used to set up gates.
GJA1 knockdown cell lines
siRNA was used to knockdown GJA1 expression in human MSCs based on previous work in other cell types [44]. GJA1 siRNA (Thermo Fisher Scientific, ID HSS178257) was used to knockdown GJA1 expression and Stealth TM RNAi (Thermo Fisher Scientific, ID 12935112) was used as a negative control. Human MSCs were plated onto 6-well plates and cultures until 60% confluent. Cells were then incubated in 1X OptiMEM (Thermo Fisher Scientific, ID 31985070) containing 100 pM of either GJA1 siRNA or Stealth RNAi and 2 µL of Lipofectamine (Invitrogen, STEM00001) per well for 24 h. After incubation, media was replaced with MSC media and cells were cultured for 4 days until cells were lysed for confirmation of knockdown using western blotting or used in co-culture experiments as described below.
Cx43 and GJA1-20k overexpression cell lines
Lentiviral transduction was performed to create MSCs that overexpress GJA1 (GJA1 + MSCs) and GJA1-20k (GJA1-20k + MSCs) as previously described [41]. Briefly, lentivirus was created from pLenti6.3-LacZ (control), pLenti6.3-hGJA1, pLenti6.3-GJA1-20k according to manufacturer instructions (Thermo Scientific, ViraPower Lentiviral Expression System). Viruses were titered and used to infect MSCs on 6-well plates in RoosterGEM (RoosterBio, M40200). After 24 h, fresh MSC media replaced the lentivirus media and cells were cultured for an additional 24 h. Transfected cells were selected using 10 µg/ml blasticidin added to the medium, then expanded and screened for overexpression by western blotting and immunofluorescence.
Fluorescent cell labeling
MSCs and chondrocytes were fluorescently labeled to distinguish between cell types during imaging and to identify mitochondrial transfer events. Lentiviral transduction was used to fluorescently label each cell type instead of using exogenous stains, which can be transferred between cells non-specifically. MSCs and chondrocytes were fluorescently labeled using mitochondrial or cytoplasm-targeted lentiviruses driven by an EF1α promoter for co-cultures (Takara Bio USA, Inc.). Mitochondria were labeled with GFP (mtGFP) or mCherry (mtMCherry) (0017VCT, 0024VCT) and chondrocyte cytoplasm was labeled with mCherry (0037VCT). Chondrocytes and MSCs were transduced for 24 h in RoosterGEM as described above.
Western blotting
Cells were lysed in RIPA buffer (Pierce, 89900) supplemented with the HALT Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, 87786). Western blotting was performed as previously described [41]. Briefly, cells were scraped into the RIPA buffer + HALT cocktail and centrifuged at 10,000 x g for 20 min at 4 °C. Supernatant was collected and stored at -80 °C. Protein concentration was quantified using the Bio-Rad DC Protein Assays with 10–15 µg of protein loaded for each sample. NuPage 4X Sample Buffer (Thermo Scientific) supplemented with dithiothreitol (DTT, 400 mM) was added and samples were heated for 10 min at 70 °C before SDS-PAGE. Protein was transferred to a PVDF membrane using the iBlot 2 system (Thermo Fisher, IB21001). Western blotting was performed with rabbit anti-Cx43 (1:5000; Sigma, C6219) and mouse anti–α-tubulin (1:5000; Abcam, ab7291) as the primary antibodies. The anti-Cx43 antibody targets the C-terminal domain and therefore targets both full-length Cx43 and GJA1-20k. Goat secondary antibodies conjugated to Alexa Fluor 647 and 555 (1:2500; Thermo Scientific) and imaged on a VersaDoc 5000 MP (Bio-Rad).
Immunofluorescence
Cells were cultured on chambered coverglass slides (Nunc™ Lab-Tek™ II Chamber Slides™, Thermo Fisher, 154526), fixed in 4% paraformaldehyde at 37 °C for 20 min, and then stored in PBS at 4 °C until staining. Cells were permeabilized with 0.2% Triton X-100 in PBS for 10 min then blocked with 5% goat serum in PBST (0.1% Tween-20 in PBS) for 1 h. Rabbit anti-Cx43 (1:400, Sigma, C6219) was used as a primary antibody with a goat anti-rabbit secondary antibody conjugated to either Alexa Fluor 488 or 633 (1:500, Thermo Scientific). The anti-Cx43 antibody targets the C-terminal domain and therefore targets both full-length Cx43 and GJA1-20k. Samples were mounted with cover glass using Pro-Long Glass Antifade Mountant with NucBlue (Invitrogen, P36983) and imaged on a Zeiss LSM 710 Confocal Microscope using a 63x oil immersion objective.
MSC and chondrocyte co-cultures
Contact Co-Cultures: Chondrocytes with mCherry cytoplasmic fluorescence were seeded onto 12-well plates for transfer quantification or on 2-well slides for imaging at a density of 35,000 and 40,000 cells, respectively, and cultured overnight in chondrocyte media (detailed above). MSCs with GFP mitochondrial fluorescence (mtGFP MSCs) were added in a 1:2 MSC:chondrocyte ratio for transfer quantification or 1:10 ratio for imaging and co-cultured in 1X OptiMEM for 24 h. After 24 h, cells were processed for transfer quantification using flow cytometry (see below) or imaging. For imaging, cells were fixed in 4% PFA for 20 min, mounted with cover glass using the ProLong Glass Antifade Mountant with NucBlue Stain (Thermo Fisher, P36983), and imaged on a Zeiss LSM 710 Confocal Microscope using a 63x oil immersion objective. Contact vs. Transwell Co-Cultures: mtGFP Chondrocytes were seeded on 12-well plates at a density of 100,000 cells/well and cultured overnight in chondrocyte media. mtMCherry MSCs were then seeded for co-cultures. For contact co-culture, 50,000 or 75,000 MSCs were added directly to the wells. For transwell co-cultures, 50,000 or 75,000 mtMCherry MSCs were seeded onto transwell inserts (1 μm pore size, Corning, 353103) and placed into wells containing mtGFP chondrocytes. Both co-cultures were performed for 24 h in a 1:1 ratio of chondrocyte and MSC media and were then processed for flow cytometry to quantify transfer events.
Mitochondrial transfer quantification
After co-culture, cells were rinsed, lifted, and fixed at 37 °C in 4% PFA for 20 min in the dark. Cells were re-suspended in 4 °C FCM buffer (PBS with 0.5% BSA and 2mM EDTA) and stored at 4 °C until analyzed. Cells were filtered (40 μm, Corning, 352235) and analyzed on a Thermo Fisher Attune NxT flow cytometer. Unstained cells and single-color control cells were used to set gates for identifying transfer events. For contact co-cultures, quadrant gates were used to quantify MSC to chondrocyte transfer events as cells that had both mCherry (chondrocyte cytoplasm) and GFP (MSC mitochondria). For experiments comparing contact versus transwell co-cultures, an oval gate was used to identify MSC-chondrocyte mitochondrial transfer events, defined as cell-sized events positively staining for both GFP and mCherry.
Normalization and correlation of GJA1 expression and mitochondrial transfer
To investigate the relationship between MSC GJA1-43k (full length Cx43) and GJA1-20k expression with mitochondrial transfer, western blot data and mitochondrial transfer data were normalized to their respective controls. Specifically, the GJA1 siRNA treated group was normalized to Stealth RNAi control, and the GJA1 + and GJA1-20k + overexpression groups were normalized to the LacZ control group. Error propagation was calculated for each normalized value from standard deviations of the group. A linear correlation was performed with normalized GJA1-43k or GJA1-20k as the independent variable and the normalized percentage of mitochondrial transfer as the dependent variable.
Statistics
Statistical analyses were performed using GraphPad Prism. Significant differences were analyzed by unpaired Student’s t-tests for comparisons between two groups. Mitochondrial transfer percentage was compared between transwell and contact co-culture groups using a two-way ANOVA. For all other comparisons between more than two groups, a one-way ANOVA was used. For both one-way and two-way ANOVAs, a Tukey post-hoc test was performed for multiple comparisons. A p-value < 0.05 was considered significant. Significance is denoted with asterisks or with letters where groups not sharing a letter are significantly different.
Results
Oxidative stress increases MSC to chondrocyte mitochondrial transfer
Mitochondria are the most important source of ROS in chondrocytes [45], and mitochondrial dysfunction in other cell types has been shown to elicit mitochondrial transfer from MSCs [25, 26, 46]. Therefore, to determine the effect of oxidative stress on MSC-chondrocyte mitochondrial transfer, chondrocytes were treated with t-BHP for 24 h (Fig. 1Ai). We found that t-BHP stimulation induced mitochondrial depolarization and increased ROS production in chondrocytes (Fig. 1B-D). Chondrocyte mitochondria were depolarized in a dose-dependent manner by t-BHP stimulation, where concentrations greater than 12 µM significantly increased the ratio of depolarized to polarized mitochondria compared to unstimulated controls (p < 0.05, Fig. 1B). Similarly, 12 µM and 30 µM t-BHP increased ROS in chondrocytes compared to unstimulated controls (p < 0.05, Fig. 1CD). MCherry Chondrocytes (cytoplasmic fluorescence) were co-cultured with mtGFP-MSCs (mitochondrial fluorescence) in 2D contact co-cultures for 24 h (Fig. 1A). Confocal imaging confirmed mitochondrial transfer events with Z-stacks identifying the presence of GFP fluorescence within chondrocyte cell bodies (Fig. 1E). Flow cytometry was used to quantify the percentage of mitochondrial transfer events. Single color controls were used to establish quadrant gates and identify co-staining events (cells that were both mCherry + and GFP+) as transfer events (Fig. 1F). Chondrocytes with t-BHP-induced oxidative stress had significantly more mitochondrial transfer events compared to unstimulated chondrocytes (p < 0.001, Fig. 1G-I).
Fig. 1.
t-BHP-induced oxidative stress in chondrocytes increases incidence of mitochondrial transfer from MSCs. (A) Chondrocytes were cultured alone with or without t-BHP (12 µM, 24 h) to quantify mitochondrial polarization and ROS production (i) and prior to co-culture with MSCs (ii). (B) Ratio of depolarized to polarized mitochondria in chondrocytes after t-BHP or FCCP stimulation (n = 5–6). (C) Histograms represent ROS quantification for chondrocytes stimulated with t-BHP (pooled across n = 5 replicates). (D) Quantification of percentage of chondrocytes that were positive for CellROX green staining on flow cytometry (n = 5). (E) Z-projection of co-culture with chondrocytes and MSCs (red: chondrocytes; green: MSC mitochondria; blue: nuclei). Quadrant gate showing representative flow data for single color controls (F), co-cultures with unstimulated chondrocytes (G), and co-cultures with t-BHP stimulated chondrocytes (H). (I) Quantification of mitochondrial transfer from co-cultures (n = 7). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
MSC-chondrocyte mitochondrial transfer occurs predominantly through actin-positive cellular extensions
To determine the importance of direct cell contacts in MSC-chondrocyte mitochondrial transfer, co-cultures were performed for 24 h in direct contact (i.e. cell types seeded in the same culture wells) or without direct contact (i.e. cell types separated by transwell inserts; Fig. 2A). These experiments utilized lentiviral-labeled mtGFP chondrocytes and mtMCherry MSC. Contact co-cultures had a significantly higher incidence of transfer events compared to transwell co-cultures for MSC seeding densities (p < 0.0001). After 24 h, contact co-cultures resulted in approximately 10% transfer events, whereas few (< 2%) transfer events were observed in the transwell co-cultures (Fig. 2B-D). Notably, flow quantification revealed minimal mCherry + only events (Fig. 2D; lower right quadrant), confirming MSCs did not migrate across the transwell insert. Contact co-cultures with a higher MSC seeding density had a higher incidence of mitochondrial transfer (p < 0.01), but MSC seeding density had no effect on transfer in the transwell co-culture groups (p = 0.99).
Fig. 2.
Direct cell-cell contacts are critical for MSC-Chondrocyte mitochondrial transfer. (A) Chondrocytes (GFP mitochondrial fluorescence) and MSCs (mCherry mitochondrial fluorescence) were co-cultured for 24 h in either 2D contact on cell culture plates or with no direct contact using transwell inserts. (B) Single color controls were used to establish gating strategy for identifying transfer events. Representative data for co-cultures from contact (C) and transwell (D) co-cultures. (E) Quantification of mitochondrial transfer from flow cytometry (n = 3, groups not sharing a letter are significantly different, p < 0.01). (F-L) Z-projections from 2D contact co-cultures captured using confocal imaging with MSCs (GFP mitochondria) and unlabeled chondrocytes (green: MSC mitochondria; grey: actin; blue: nuclei). MSC actin-positive filapodial extensions contain mitochondria (white asterisks) and MSC mitochondria localized in adjacent chondrocytes (white arrowheads)
To further examine cell-cell interactions during mitochondrial transfer, mtGFP-MSCs and unlabeled chondrocytes were directly co-cultured for 24 h, then fixed and stained with phalloidin to visualize the F-actin cytoskeleton for both cell types. Confocal imaging revealed actin-positive filapodial extensions in contact with adjacent chondrocytes that contained mtGFP fluorescence, indicating mitochondrial transfer had occurred (Fig. 2F-L). MSC filapodial extensions ranged in length, spanning up to 90 μm to reach an adjacent chondrocyte (Fig. 2F), and also ranged in diameter, measuring less than 1 μm (Fig. 2G, J). Notably, MSC mitochondria were located within these actin cellular extensions (Fig. 2G, J).
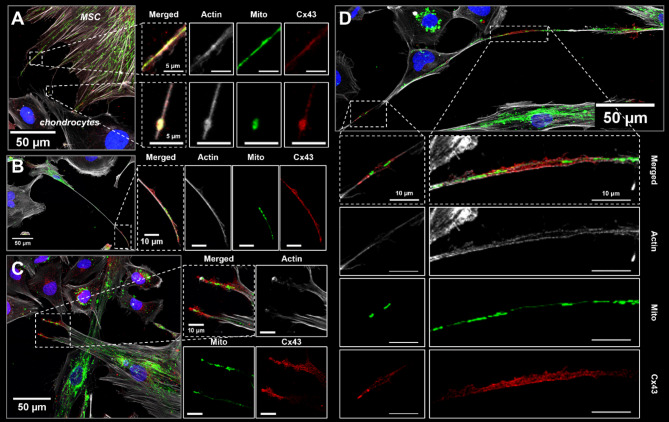
Cx43 staining is enriched in MSCs along actin-positive cellular extensions containing mitochondria
To investigate the role of Cx43 in mitochondrial transfer between MSCs and chondrocytes, Cx43 immunofluorescence was performed after 24 h of contact co-culture. Confocal imaging revealed abundant actin-positive cellular extensions from MSCs which ranged in size, some measuring over 150 μm in length from the cell body (Fig. 3). There were spatial variations in Cx43 staining where the distal extent of the cellular processes contained mitochondria and had enriched Cx43 staining (Fig. 3). Additionally, cell-cell junctions had enriched Cx43 staining (Fig. 3D). Cx43 staining at the actin cellular extensions was diffuse and not punctate, suggesting the presence of truncated Cx43 isoforms and not sites of connexon formation [37].
Fig. 3.
Cx43 staining is enriched along actin cellular extensions containing mitochondria in MSCs. MSC actin cellular extensions were observed in contact co-cultures with chondrocytes after 24 h (mtGFP MSCs; unstained chondrocytes). Cells were stained for Cx43 and actin (phalloidin). Merged z-projections and individual channels are shown (green: MSC mitochondria; red: Cx43; grey: actin; blue: nuclei). (A) MSC mitochondria and Cx43 staining are found within MSC filapodial extensions. (B, C) Cx43 staining is enriched at the terminal end of MSC filapodial extensions that are actin-positive, and (D) Cx43 localizes to apparent cell-cell junction between a MSC and chondrocyte
Cx43 knockdown in MSCs decreases mitochondrial transfer to chondrocytes
To evaluate the role of Cx43 expression in mediating mitochondrial transfer between MSCs and chondrocytes, siRNA was used to knockdown Cx43 expression in MSCs prior to co-culture. The knockdown of Cx43 in MSCs was confirmed using western blot and normalized to α-tubulin (Fig. 4A). Western blot quantification showed a significant decrease in full-length Cx43 and the truncated isoform GJA1-20k (n = 3; p < 0.0001 and p < 0.05, respectively). Additionally, GJA1 siRNA treatment increased the relative expression of the 20k isoform to the full-length protein (GJA1-20k/Cx43, p < 0.01). Immunofluorescence confirmed the knockdown of Cx43 in MSCs where there was a decrease in the punctate and diffuse staining within the cells (Fig. 4E, F). GJA1 siRNA treated and control (Stealth RNAi treated) mtGFP MSCs were co-cultured with mCherry chondrocytes for 24 h. Chondrocytes co-cultured with GJA1 siRNA-treated MSCs had fewer mitochondrial transfer events compared to control MSCs (p < 0.01, Fig. 4G-I). Notably, knocking down Cx43 in MSCs did not completely suppress mitochondrial transfer to chondrocytes.
Fig. 4.
GJA1 siRNA knocks down Cx43 expression in MSCs and decreases incidence of mitochondrial transfer. (A) Western blot analysis of full length Cx43 (GJA1-43k) and the truncated isoform GJA1-20k in MSCs after GJA1 siRNA treatment. Cx43 (B) and GJA1-20k (C) expression were normalized to α-tubulin (n = 3). (D) Relative isoform expression was calculated as the ratio of GJA1-20k to Cx43 (n = 3). (E-F) Representative images of Cx43 immunofluorescence for control (Stealth RNAi) and GJA1 siRNA treated MSCs. (G-H) Representative data from flow cytometry analyses of co-cultures. (I) Quantification of mitochondrial transfer events from co-cultures using flow cytometry (n = 8). Full-length western blot for quantification is presented in Supplementary Fig. 2. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
MSC GJA1-20k expression mediates mitochondrial transfer to chondrocytes
To investigate the roles of full-length Cx43 (GJA1-43k) and GJA1-20k in mediating MSC-chondrocyte mitochondrial transfer, lentiviral transduction was performed to overexpress Cx43 (GJA1+; all isoforms) or GJA1-20k (GJA1-20k+) in MSCs. Overexpression of Cx43 and GJA1-20k was confirmed using western blot and immunofluorescence (Fig. 5A-G). Western blot quantification confirmed a significant increase in full-length Cx43 in GJA1 + MSCs compared to LacZ (control) MSCs (p < 0.05), and a significant increase in GJA1-20k in GJA1 + and GJA1-20k + MSCs compared to LacZ (control) MSCs (p < 0.05 and p < 0.01, respectively). The ratio of GJA1-20k to full-length Cx43 was significantly higher in the GJA1-20k + MSCs compared to both GJA1 + and LacZ MSCs, indicating an increased expression of the 20k isoform relative to the full-length Cx43 expression (p < 0.01). From confocal imaging, GJA1 + MSCs had enriched diffuse and punctate Cx43 staining compared to LacZ MSCs. GJA1-20k + MSCs had enriched diffuse Cx43 staining throughout the cell body compared to both GJA1 + and LacZ MSCs, but no apparent difference in punctate staining compared to LacZ (Fig. 5E-G).
Fig. 5.
GJA1-20k overexpression in MSCs increases mitochondrial transfer and MSC GJA1-20k expression correlates with transfer incidence. Western blot analysis of full length Cx43 (GJA1-43k) and the truncated isoform GJA1-20k in MSCs after lentiviral transduction (LacZ (control), GJA1+, GJA1-20k+). Cx43 (B) and GJA1-20k (C) expression were normalized to α-tubulin (n = 3). (D) Relative isoform expression was calculated as the ratio of GJA1-20k to Cx43 (n = 3). (E-G) Representative images of Cx43 immunofluorescence for LacZ (control), GJA1+, and GJA1-20k + MSCs. (H-J) Representative data from flow cytometry analyses of co-cultures. (K) Quantification of mitochondrial transfer events from co-cultures using flow cytometry (n = 8). (L-M) Linear correlations for normalized protein expression of full-length Cx43 (GJA1-43k) and GJA1-20k with normalized incidence of mitochondrial transfer (groups normalized to their respective controls). Groups not sharing a letter are significantly different (p < 0.05). Full-length western blot for quantification is presented in Supplementary Fig. 3. Uncropped western blot in (A) can be found in Supplementary Figs. 8 and 9
To investigate the effect of Cx43 overexpression on MSC-chondrocyte intercellular mitochondrial transfer, GJA1 + and GJA1-20k + MSCs were co-cultured with chondrocytes for 24 h and transfer events were quantified using flow cytometry. GJA1 + MSCs had an increased incidence of mitochondrial transfer compared to LacZ controls (p < 0.05). Interestingly, GJA1-20k + MSC cocultures had the highest incidence of mitochondrial transfer; significantly more than GJA1 + MSCs, and 2-fold that of LacZ controls (p < 0.001 and p < 0.0001, respectively; Fig. 5K). To further understand the relationship between Cx43 isoform expression and incidence of MSC-chondrocyte mitochondrial transfer, both MSC siRNA and Cx43 overexpression data were normalized with respect to control protein expression and mitochondrial transfer incidence (stealth RNAi and LacZ for siRNA and Cx43 overexpression, respectively), and linear correlations were performed with normalized values. These analyses revealed no correlation between the expression of full-length Cx43 in MSCs and the incidence of mitochondrial transfer to chondrocytes (R2 = 0.00, Fig. 5L). In contrast, we found a strong positive correlation between MSC GJA1-20k expression and the incidence of mitochondrial transfer (R2 = 0.93, Fig. 5M).
Discussion
The objectives of this study were to investigate the role of oxidative stress-induced mitochondrial dysfunction on MSC-chondrocyte mitochondrial transfer and to examine the role of Cx43 in the mechanism of transfer. Here we show that t-BHP-induced oxidative stress in chondrocytes increases mitochondrial transfer from MSCs to chondrocytes, and that GJA1-20k is a key mediator of mitochondrial transfer through direct cell-cell contacts. These data agree with evidence in chondrocytes and other cell types that mitochondrial dysfunction and oxidative stress in injured cells initiates mitochondrial donation by MSCs [14, 25, 26]. Recently, our group reported that chondrocytes treated with the mitochondrial stressors rotenone and antimycin had increased mitochondrial transfer from MSCs [26]. Rotenone and antimycin inhibit complex I and III of the electron transport chain, respectively, and results in the inhibition of ATP production and the subsequent increase in ROS due to the incomplete transfer of electrons through ATP synthase. In tendon and in the data presented here in chondrocytes, cells were directly stimulated with an oxidant that led to increased ROS production and increased mitochondrial transfer [25]. Altogether, these data directly link cellular oxidative stress and mitochondrial transfer incidence.
Full-length Cx43 has been implicated in mitochondrial transfer through multiple mechanisms including gap junction communication [14, 26, 32] and gap junction internalization [34]. Gap junction signaling through Cx43 was found to stimulate the formation of TNTs and microvesicles in MSCs [14], which are two mechanisms of mitochondrial transfer that have been previously identified for MSCs to chondrocytes [26, 47]. In the current study, transwell co-cultures resulted in significantly lower levels of mitochondrial transfer compared to contact co-cultures, suggesting direct cell-contacts are the primary route of transfer in this model system. Microvesicle-mediated mitochondrial transfer can occur from MSCs to chondrocyte but has been reported at low efficiencies (< 1%) [47]. Notably, here we found enriched Cx43 staining at cell-cell junctions between MSCs and chondrocytes, and it has previously been shown that Cx43 is enriched at chondrocyte cell junctions [42, 48, 49]. While there are likely multiple mechanisms of mitochondrial transfer occurring between MSCs and chondrocytes, these data highlight the role of Cx43 in increasing transfer events.
Recently, over-expression of the truncated isoform GJA1-20k in astrocytes was found to significantly increase mitochondrial transfer to neurons in vitro [40], but, to the author’s knowledge, GJA1-20k has not been evaluated in MSCs. Here, we report that MSCs express GJA1-20k and that MSC expression of GJA1-20k strongly correlates with the incidence of mitochondrial transfer. GJA1-20k has been identified as a regulator of mitochondrial motility and dynamics through interactions with microtubules and the actin cytoskeleton [38, 44, 50, 51]. Additionally, GJA1-20k is known to stabilize actin filaments in cardiac intercalated discs and is critical for trafficking Cx43 hemichannels from the Golgi apparatus to the cell membrane where gap junctions can form [37, 41]. Previous reports have identified the importance of Cx43 channel function in mitochondrial transfer [14, 26, 32, 52], and therefore elevated levels of GJA1-20k may increase transfer through augmented trafficking of GJA1-43k hemichannels to the cell membrane. Notably, expression of GJA1-20k is regulated by multiple cell signaling pathways including Smad3, ERK, P13K/Akt/mTOR, and MNK1/2, and has been implicated as a means for cells to control Cx43 hemichannel and gap junction formations post-transcriptionally [37, 41, 53, 54]. These data suggest that GJA1-20k aids in the transport of mitochondria from MSCs to adjacent cells and may assist through mobilization of mitochondria through TNTs, packaging of mitochondria within microvesicles (EVs), trafficking of full-length Cx43 hemichannels to the cell membrane, or through the formation of actin cellular extensions (Fig. 6). Future studies are warranted to further investigate these mechanisms confirm the hypotheses proposed in Fig. 6.
Fig. 6.

Hypothesized role of Cx43 and GJA1-20k in MSC to chondrocyte mitochondrial transfer. Our previous work implicated gap junction communication in MSC to chondrocyte mitochondrial transfer (i, Fahey + Scientific Reports 2022). Results presented here confirm the importance of Cx43 in mediating this phenomenon and reveal distinct roles for the full-length protein and the N-terminal truncated isoform (GJA1-20k). Given our imaging data and previous evidence that GJA1-20k increases trafficking of mitochondria along actin cytoskeletal elements (ii, iii) and recruits actin to organize cell trafficking pathways (iv), we propose that GJA1-20k likely aids in mitochondrial transfer by facilitating transport of mitochondria from MSCs to adjacent cells through mobilization of mitochondria via TNTs (ii), the formation of actin cellular extensions (iv), and/or packaging of mitochondria within microvesicles (iii). Future studies are warranted to further investigate these mechanisms
Here, ectopic alteration of Cx43 expression in the MSC population caused significant changes in the incidence of mitochondrial transfer; whereby knocking down Cx43 decreased transfer events and overexpression of full-length Cx43 and the 20k truncated isoform increased transfer events. While these data suggest Cx43 expression and isoform regulation is important for MSCs to donate mitochondria, this study did not explore the effect of Cx43 expression in chondrocytes. Here, we focused on MSCs, as there is mounting evidence from clinical trials on the use of MSCs for treating clinical OA [4, 9]. Further, since MSCs are generally harvested and expanded in culture, they represent a more readily manipulated therapy. However, it is likely that chondrocyte Cx43 expression could also influence mitochondrial transfer between these cell type with important potential clinical implications; evidence suggests that while healthy chondrocytes have low levels of Cx43, in clinical OA, chondrocytes have over a 3-fold increased expression of Cx43 [48]. Therefore, overexpression of Cx43 in chondrocytes, like that seen in OA, may lead to increased mitochondrial transfer events from MSCs, as increased Cx43 gap junction communication was found to increase TNT and microvesicle release from MSCs [14]. t-BHP was used in this study to induce oxidative stress and resulted in an increase in transfer events but did not affect Cx43 or GJA1-20k expression in chondrocytes (Supplementary Fig. 1). These data suggest oxidative stress in chondrocytes may drive increased mitochondrial transfer through pathways independent of changes in Cx43 protein expression.
To investigate the role of Cx43 and GJA1-20k in mitochondrial transfer, we used molecular methods to knockdown or overexpress the protein in MSCs using siRNA and lentiviral transduction, respectively. Interestingly, GJA1 siRNA increased the relative expression of the 20k isoform the full-length protein (GJA1-20k/Cx43, p < 0.01). As both Cx43 and GJA1-20k arise from the same mRNA, this may be due to GJA1-20k potentially having a longer half-life. Immunostaining was performed to confirm overexpression of Cx43 protein following lentiviral transduction in the MSCs. These images revealed differences between the GJA1 + and GJA1-20k + MSCs; GJA1-20k + MSCs had enriched diffuse staining throughout the cell body compared to both GJA1 + and LacZ (control), but there was no apparent difference in punctate Cx43 staining compared to LacZ. This is consistent with prior work demonstrating localization of GJA1-20k to the cytoplasm within the vesicular transport pathway and mitochondria [37, 41, 50].
While our findings shed light on the mechanisms mediating MSC-chondrocyte mitochondrial transfer and this work is the first to explore the role of GJA1-20k in MSCs, this study has limitations. First, we chose to study MSC-chondrocytes mitochondrial transfer in monolayer cell culture. This model does not account for the 3D tissue niche chondrocytes inhabit in vivo, namely a dense, highly charged, avascular ECM. Our recent work demonstrated that MSCs can transfer mitochondria to chondrocytes in impact-injured cartilage tissue explants [26]. Specifically, MSCs homed to areas of microcracking on the articular surface of cartilage and transferred their mitochondria to chondrocytes within cracks (up to 70 μm from the articular surface) and up to 100 μm from the ECM defect [26]. There is promising evidence that MSC injections preserve cartilage tissue after injury in the short-term [9, 10]. Mitochondrial transfer could be a driving cause of the observed therapeutic benefit, as the transfer of mitochondria from MSCs to stressed cells has been shown to be protective against injury in vivo in lung epithelial and tendon cells [14, 25]. Given the current findings that Cx43-20k overexpression increases mitochondrial donation by MSCs, further in situ and in vivo studies are warranted to determine the effect on chondrocytes in their native tissue environment. Additionally, while full-length Cx43 is upregulated in OA [48], it is unknown if chondrocyte GJA1-20k expression is altered in disease. Future studies investigating changes in chondrocyte GJA1-20k expression with disease would shed further light on the potential shift in mitochondrial transfer incidence from MSCs to chondrocytes in OA. Knocking down Cx43 in the MSC population did not fully suppress MSC-chondrocyte mitochondrial transfer, indicating the involvement of Cx43-independent mechanisms. In addition to Cx43, other proteins have been identified as regulators of mitochondrial transfer between MSCs and other cell types, such as Miro1 [20, 22, 23]. While multiple proteins are likely involved in regulating mitochondrial transfer, the data presented here identified Cx43 as a critical mediator of transfer between MSCs and chondrocytes. Additionally, our work incorporates the impact of GJA1-20k to reveal a key role for this internally translated Cx43 isoform, for the first time, in mitochondrial transfer between MSCs and chondrocytes.
Conclusions
The data presented in this study highlights a role for the truncated Cx43 isoform GJA1-20k in MSC to chondrocyte mitochondrial transfer and is the first to evaluate the role of GJA1-20k in MSC mitochondrial donation. In this experimental model, we found direct cell-cell contacts to be the primary route of MSC-chondrocyte mitochondrial transfer, with GJA1-20k serving as a key mediator in this process. Collectively, these data suggest new strategies for promoting mitochondrial transfer as a regenerative therapy for cartilage repair, including increasing cell-cell contacts and increasing GJA1-20k expression in MSCs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Imaging and flow cytometry data were acquired through the Cornell Institute of Biotechnology’s BRC Imaging and Flow Cytometry Facilities (RRID: SCR_021741 and RRID: SCR_021740, respectively). Figures 1 and 2, and 6 were created using BioRender.com.
Abbreviations
- Cx43
Connexin 43
- DMEM
Dulbecco’s modified Eagle’s medium
- DTT
Dithiothreitol
- ECM
Extracellular matrix
- FCCP
Carbonyl cyanide p-trifluoro-methoxyphenyl hydrazone
- GFP
Green fluorescence protein
- GJA1
Gap junction protein alpha 1 (gene name for Cx43)
- GJA1-43k
Full length 43k isoform of GJA1
- GJA1-20k
Truncated 20k isoform of GJA1
- LacZ
Gene encoding β-galactosidase
- MSC
Mesenchymal stromal cell
- OA
Osteoarthritis
- PBS
Phosphate-buffered saline
- PFA
Paraformaldehyde
- RNA
Ribonucleic acid
- ROS
Reactive oxygen species
- siRNA
Short interfering RNA
- t-BHP
tert-Butyl hydroperoxide
- TNT
Tunneling nanotubules
Author contributions
RMI contributed to the study design, data collection, data analysis, data interpretation, and drafting of the paper. MAT and MJF contributed to data collection, data analysis, data interpretation, and editing of the paper. MDM, JWS, and MLD participated in the study design, data interpretation, and editing of the paper. All authors have read and approved the current version of the manuscript.
Funding
This study was funded by the Harry M. Zweig Fund for Equine Research, the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (K08AR068470, R03AR075929), The Stem Cell Program at Cornell University, and a grant from Stryker administered by the Orthopaedic Research Society (ORS).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This manuscript includes the use of an immortalized human chondrocyte line and human MSCs that are commercially available. The creation of the immortalized chondrocyte cell line has been previously published [55], and initial ethical approval and informed consent was obtained for the RoosterBio human MSCs. The Delco lab was granted institutional approval to work with human and animal cells and tissues on 10/11/2022 by the Cornell University Institutional Biosafety Committee (Title: The study of mitochondria in orthopedic disease; MUA Approval Number: 16342-2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet [Internet]. 2018 Nov 10 [cited 2021 Sep 15];392(10159):1789. Available from: /pmc/articles/PMC6227754/. [DOI] [PMC free article] [PubMed]
- 2.DS C, PM R [Internet]. CJ V. Pharmaceutical therapy for osteoarthritis. 2012 May [cited 2021 Sep 15];4(5 Suppl). https://pubmed.ncbi.nlm.nih.gov/22632707/ [DOI] [PubMed]
- 3.B G, FP T, MI H, RP G, MG C. KB F. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med [Internet]. 2015 Mar 17 [cited 2021 Sep 15];43(3):734–44. https://pubmed.ncbi.nlm.nih.gov/24866892/ [DOI] [PubMed]
- 4.Wei P, Bao R. Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. Int J Mol Sci [Internet]. 2022 Jan 1 [cited 2024 Apr 24];24(1). https://pubmed.ncbi.nlm.nih.gov/36613502/ [DOI] [PMC free article] [PubMed]
- 5.Delco ML, Goodale M, Talts JF, Pownder SL, Koff MF, Miller AD, et al. Integrin α10β1-Selected mesenchymal stem cells mitigate the progression of Osteoarthritis in an equine Talar Impact Model. Am J Sports Med. 2020;48(3):612–23. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Xu M, Zhu H, Dong C, Ji J, Liu Y et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J Cell Mol Med [Internet]. 2021 Oct 1 [cited 2023 Dec 18];25(19):9281. /pmc/articles/PMC8500984/ [DOI] [PMC free article] [PubMed]
- 8.clinicaltrial.gov. 2024.
- 9.Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med [Internet]. 2019 Mar 1 [cited 2023 Oct 16];8(3):215–24. https://pubmed.ncbi.nlm.nih.gov/30592390/ [DOI] [PMC free article] [PubMed]
- 10.Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl Med [Internet]. 2019 Aug 1 [cited 2023 Oct 16];8(8):746–57. https://pubmed.ncbi.nlm.nih.gov/30964245/ [DOI] [PMC free article] [PubMed]
- 11.Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther [Internet]. 2023 [cited 2024 Jan 7];14:66. http://creativecommons.org/licenses/by/4.0/.TheCreativeCommonsPublicDomainDedicationwaiver [DOI] [PMC free article] [PubMed]
- 12.Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential HHS Public Access. Trends Pharmacol Sci. 2020;41(9):653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liesveld JL, Sharma N, Aljitawi OS. Stem cell homing: From physiology to therapeutics. 2020 [cited 2024 Jan 7]; https://academic.oup.com/stmcls/article/38/10/1241/6430514 [DOI] [PubMed]
- 14.MN I, SR D et al. MT E, M W, L S, K W,. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med [Internet]. 2012 May [cited 2021 Sep 15];18(5):759–65. https://pubmed.ncbi.nlm.nih.gov/22504485/ [DOI] [PMC free article] [PubMed]
- 15.Wang J, Li H, Yao Y, Zhao T, Chen YY, Shen YL et al. Stem cell-derived mitochondria transplantation: a novel strategy and the challenges for the treatment of tissue injury. Stem Cell Res Ther. 2018;9(1):1–10. [DOI] [PMC free article] [PubMed]
- 16.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med [Internet]. 2017 Nov 15 [cited 2023 Oct 15];196(10):1275–86. Available from: www.atsjournals.org. [DOI] [PMC free article] [PubMed]
- 17.Jackson MV, Morrison TJ, Doherty DF, Mcauley DF, Matthay MA, Kissenpfennig A et al. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS SIGNIFICANCE STATEMENT. Stem Cells [Internet]. 2016 [cited 2023 Oct 15];34:2210–23. Available from: www.listlabs.com. [DOI] [PMC free article] [PubMed]
- 18.Acquistapace A, Bru T, Ois Lesault Pfranç, Figeac F, Lie Coudert AE, Coz OLE et al. Human Mesenchymal Stem Cells Reprogram Adult Cardiomyocytes Toward a Progenitor-Like State Through Partial Cell Fusion and Mitochondria Transfer. Stem Cells [Internet]. 2011 [cited 2023 Oct 15];29:812–24. Available from: www.abcam. [DOI] [PMC free article] [PubMed]
- 19.Babenko VA, Silachev DN, Zorova LD, Pevzner IB, Khutornenko AA, Plotnikov EY et al. Improving the Post-Stroke Therapeutic Potency of Mesenchymal Multipotent Stromal Cells by Cocultivation With Cortical Neurons: The Role of Crosstalk Between Cells. Stem Cells Transl Med [Internet]. 2015 Sep 1 [cited 2023 Oct 15];4(9):1011–20. 10.5966/sctm.2015-0010 [DOI] [PMC free article] [PubMed]
- 20.Babenko VA, Silachev DN, Popkov VA, Zorova LD, Pevzner IB, Plotnikov EY et al. Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules [Internet]. 2018 [cited 2023 Oct 16];23(3). https://pubmed.ncbi.nlm.nih.gov/29562677/ [DOI] [PMC free article] [PubMed]
- 21.Shen J, Zhang JH, Xiao H, Wu JM, He KM, Lv ZZ et al. Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis. Cell Death Dis [Internet]. 2018 Feb 1 [cited 2023 Oct 16];9(2). https://pubmed.ncbi.nlm.nih.gov/29362447/ [DOI] [PMC free article] [PubMed]
- 22.Liu D, Gao Y, Liu J, Huang Y, Yin J, Feng Y et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther. 2021;6(1):65. [DOI] [PMC free article] [PubMed]
- 23.Zhang Y, Yu Z, Jiang D, Liang X, Liao S, Zhang Z et al. iPSC-MSCs with High Intrinsic MIRO1 and Sensitivity to TNF-α Yield Efficacious Mitochondrial Transfer to Rescue Anthracycline-Induced Cardiomyopathy. Stem Cell Reports [Internet]. 2016 Oct 11 [cited 2023 Oct 16];7(4):749–63. https://pubmed.ncbi.nlm.nih.gov/27641650/ [DOI] [PMC free article] [PubMed]
- 24.Konari N, Nagaishi K, Kikuchi S, Fujimiya M. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci Rep. 2019;9(1):5184. [DOI] [PMC free article] [PubMed]
- 25.Wei B, Ji M, Lin Y, Wang S, Liu Y, Geng R et al. Mitochondrial transfer from bone mesenchymal stem cells protects against tendinopathy both in vitro and in vivo. Stem Cell Res Ther. 2023;14(1):104. [DOI] [PMC free article] [PubMed]
- 26.Fahey M, Bennett M, Thomas M, Montney K, Vivancos-Koopman I, Pugliese B et al. Mesenchymal stromal cells donate mitochondria to articular chondrocytes exposed to mitochondrinvironmental, and mechanical stress. Sci Rep [Internet]. 2022 Dec 1 [cited 2023 Oct 15];12(1). https://pubmed.ncbi.nlm.nih.gov/36513773/ [DOI] [PMC free article] [PubMed]
- 27.Korpershoek JV, Rikkers M, Wallis FSA, Dijkstra K, te Raa M, de Knijff P, et al. Mitochondrial Transport from mesenchymal stromal cells to chondrocytes increases DNA content and Proteoglycan Deposition in Vitro in 3D cultures. Cartilage. 2022;13(4):133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R, Maimaitijuma T, Ma YY, Jiao Y, Cao YP. Mitochondrial transfer from bone-marrow-derived mesenchymal stromal cells to chondrocytes protects against cartilage degenerative mitochondrial dysfunction in rats chondrocytes. Chin Med J (Engl) [Internet]. 2020 Jan 20 [cited 2022 Apr 3];134(2):212–8. https://pubmed.ncbi.nlm.nih.gov/32858593/ [DOI] [PMC free article] [PubMed]
- 29.Delco ML, Bonnevie ED, Bonassar LJ, Fortier LA. Mitochondrial dysfunction is an acute response of articular chondrocytes to mechanical injury. Journal of Orthopaedic Research [Internet]. 2018 Feb 1 [cited 2020 Feb 26];36(2):739–50. 10.1002/jor.23651 [DOI] [PMC free article] [PubMed]
- 30.Delco ML, Bonnevie ED, Szeto HS, Bonassar LJ, Fortier LA. Mitoprotective therapy preserves chondrocyte viability and prevents cartilage degeneration in an ex vivo model of posttraumatic osteoarthritis. J Orthop Res. 2018;36(8):2147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LR B, LA F, LJ B. HH S, I C, ML D. Mitoprotective therapy prevents rapid, strain-dependent mitochondrial dysfunction after articular cartilage injury. J Orthop Res [Internet]. 2020 Jun 1 [cited 2021 Sep 15];38(6):1257–67. https://pubmed.ncbi.nlm.nih.gov/31840828/ [DOI] [PMC free article] [PubMed]
- 32.XL YY, ZB FDJYZXL. X, Connexin 43-Mediated Mitochondrial Transfer of iPSC-MSCs Alleviates Asthma Inflammation. Stem Cell Reports [Internet]. 2018 Nov 13 [cited 2021 Sep 15];11(5):1120–35. https://pubmed.ncbi.nlm.nih.gov/30344008/ [DOI] [PMC free article] [PubMed]
- 33.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J et al. Brain tumour cells interconnect to a functional and resistant network. Nature [Internet]. 2015 Dec 3 [cited 2023 Oct 16];528(7580):93–8. https://pubmed.ncbi.nlm.nih.gov/26536111/ [DOI] [PubMed]
- 34.Norris RP, Rachael Norris CP. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic [Internet]. 2021 [cited 2023 Mar 14];22:174–9. https://onlinelibrary.wiley.com/doi/10.1111/tra.12786 [DOI] [PubMed]
- 35.Golan K, Singh AK, Kollet O, Bertagna M, Althoff MJ, Khatib-Massalha E et al. Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma. Blood [Internet]. 2020 Dec 3 [cited 2023 Oct 16];136(23):2607–19. https://pubmed.ncbi.nlm.nih.gov/32929449/ [DOI] [PMC free article] [PubMed]
- 36.Yang J, Liu L, Oda Y, Wada K, Ago M, Matsuda S et al. Extracellular Vesicles and Cx43-Gap Junction Channels Are the Main Routes for Mitochondrial Transfer from Ultra-Purified Mesenchymal Stem Cells, RECs. Int J Mol Sci [Internet]. 2023 Jun 1 [cited 2023 Oct 16];24(12). https://pubmed.ncbi.nlm.nih.gov/37373439/ [DOI] [PMC free article] [PubMed]
- 37.Smyth JW, Shaw RM. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep [Internet]. 2013 [cited 2022 Jan 10];5(3):611–8. https://pubmed.ncbi.nlm.nih.gov/24210816/ [DOI] [PMC free article] [PubMed]
- 38.SS YF. Z, S X, WA B, R B, I E, Cx43 Isoform GJA1-20k Promotes Microtubule Dependent Mitochondrial Transport. Front Physiol [Internet]. 2017 Nov 7 [cited 2021 Sep 15];8(NOV). https://pubmed.ncbi.nlm.nih.gov/29163229/ [DOI] [PMC free article] [PubMed]
- 39.Basheer WA, Xiao S, Epifantseva I, Fu Y, Kleber AG, Hong TT, et al. GJA1-20k arranges actin to Guide Cx43 delivery to Cardiac Intercalated discs. Circ Res. 2017;121(9):1069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren D, Zheng P, Zou S, Gong Y, Wang Y, Duan J et al. GJA1-20K Enhances Mitochondria Transfer from Astrocytes to Neurons via Cx43-TnTs After Traumatic Brain Injury. Cell Mol Neurobiol [Internet]. 2021; 10.1007/s10571-021-01070-x [DOI] [PMC free article] [PubMed]
- 41.James CC, Zeitz MJ, Calhoun PJ, Lamouille S, Smyth JW. Altered translation initiation of Gja1 limits gap junction formation during epithelial-mesenchymal transition. Mol Biol Cell [Internet]. 2018 Apr 1 [cited 2022 Sep 29];29(7):797–808. https://pubmed.ncbi.nlm.nih.gov/29467255/ [DOI] [PMC free article] [PubMed]
- 42.R GF PCF, MB G, PR B. MD M, FJ B. Biochemical evidence for gap junctions and Cx43 expression in immortalized human chondrocyte cell line: a potential model in the study of cell communication in human chondrocytes. Osteoarthritis Cartilage [Internet]. 2014 [cited 2021 Sep 22];22(4):586–90. https://pubmed.ncbi.nlm.nih.gov/24530659/ [DOI] [PubMed]
- 43.Yang J, Liu L, Oda Y, Wada K, Ago M, Matsuda S et al. Highly-purified rapidly expanding clones, RECs, are superior for functional-mitochondrial transfer. Stem Cell Res Ther [Internet]. 2023 Dec 1 [cited 2023 Oct 19];14(1):1–22. https://stemcellres.biomedcentral.com/articles/10.1186/s13287-023-03274-y [DOI] [PMC free article] [PubMed]
- 44.Shimura D, Nuebel E, Baum R, Valdez SE, Xiao S, Warren JS et al. Protective mitochondrial fission induced by stress-responsive protein GJA1-20k. Elife. 2021;10:e69207. [DOI] [PMC free article] [PubMed]
- 45.Wolff KJ, Ramakrishnan PS, Brouillette MJ, Journot BJ, McKinley TO, Buckwalter JA et al. Mechanical stress and ATP synthesis are coupled by mitochondrial oxidants in articular cartilage. Journal of Orthopaedic Research [Internet]. 2013 Feb 1 [cited 2024 Apr 24];31(2):191–6. https://onlinelibrary.wiley.com/doi/full/10.1002/jor.22223 [DOI] [PMC free article] [PubMed]
- 46.Yang F, Zhang Y, Liu S, Xiao J, He Y, Shao Z et al. Tunneling Nanotube-Mediated Mitochondrial Transfer Rescues Nucleus Pulposus Cells from Mitochondrial Dysfunction and Apoptosis. Oxid Med Cell Longev. 2022;2022. [DOI] [PMC free article] [PubMed]
- 47.Thomas MA, Fahey MJ, Pugliese BR, Irwin RM, Antonyak MA, Delco ML. Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles. Front Bioeng Biotechnol [Internet]. 2022 Aug 19 [cited 2022 Dec 12];10:870193. http://www.ncbi.nlm.nih.gov/pubmed/36082164 [DOI] [PMC free article] [PubMed]
- 48.MD M, R GF PCF, O M de I, HZ W et al. V V,. Human articular chondrocytes express multiple gap junction proteins: differential expression of connexins in normal and osteoarthritic cartilage. Am J Pathol [Internet]. 2013 Apr [cited 2021 Sep 15];182(4):1337–46. https://pubmed.ncbi.nlm.nih.gov/23416160/ [DOI] [PMC free article] [PubMed]
- 49.Mayan MD, Gago-Fuentes R, Carpintero-Fernandez P, Fernandez-Puente P, Filgueira-Fernandez P, Goyanes N et al. Articular chondrocyte network mediated by gap junctions: role in metabolic cartilage homeostasis. Ann Rheum Dis [Internet]. 2015 Jan 1 [cited 2022 Jul 16];74(1):275. /pmc/articles/PMC5500216/ [DOI] [PMC free article] [PubMed]
- 50.Basheer WA, Fu Y, Shimura D, Xiao S, Agvanian S, Hernandez DM et al. Stress response protein GJA1-20k promotes mitochondrial biogenesis, metabolic quiescence, and cardioprotection against ischemia/reperfusion injury. JCI Insight. 2018;3(20):1–17. [DOI] [PMC free article] [PubMed]
- 51.Fu YL, Tao L, Peng FH, Zheng NZ, Lin Q, Cai SY et al. GJA1-20k attenuates Ang II-induced pathological cardiac hypertrophy by regulating gap junction formation and mitochondrial function. Acta Pharmacol Sin [Internet]. 2020; 10.1038/s41401-020-0459-6 [DOI] [PMC free article] [PubMed]
- 52.Mistry JJ, Marlein CR, Moore JA, Hellmich C, Wojtowicz EE, Smith JGW, S A [Internet]. ROS-mediated PI3K activation drives mitochondrial transfer from stromal cells to hematopoietic stem cells in response to infection. Proc Natl Acad Sci U. 2019 Dec 3 [cited 2024 Apr 24];116(49):24610–9. https://www.pnas.org/doi/abs/10.1073/pnas.1913278116 [DOI] [PMC free article] [PubMed]
- 53.Zeitz MJ, Calhoun PJ, James CC, Taetzsch T, George KK, Robel S, et al. Dynamic UTR usage regulates alternative translation to Modulate Gap Junction formation during stress and aging. Cell Rep. 2019;27(9):2737–e27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salat-Canela C, Sesé M, Peula C, Ramón Y, Cajal S, Aasen T. Internal translation of the connexin 43 transcript. Cell Commun Signal [Internet]. 2014 May 8 [cited 2023 Dec 20];12(1):31. /pmc/articles/PMC4108066/ [DOI] [PMC free article] [PubMed]
- 55.Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, et al. Interleukin-1 beta-modulated gene expression in Immortalized Human chondrocytes. J Clin Invest. 1994;94:2307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.