Abstract
In this study we investigated the effects of Marburg virus and Ebola virus (species Zaire and Reston) infections on freshly isolated suspended monocytes in comparison to adherent macrophages under culture conditions. Our data showed that monocytes are permissive for both filoviruses. As is the case in macrophages, infection resulted in the activation of monocytes which was largely independent of virus replication. The activation was triggered similarly by Marburg and Ebola viruses, species Zaire and Reston, as indicated by the release of the proinflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor α, and IL-6 as well as the chemokines IL-8 and gro-α. Our data suggest that infected monocytes may play an important role in the spread of filoviruses and in the pathogenesis of filoviral hemorrhagic disease.
Marburg virus (MBGV) and Ebola virus (EBOV), family Filoviridae, cause severe hemorrhagic fevers in humans and nonhuman primates. Outbreaks such as the latest ones in the Democratic Republic of the Congo (MBGV) and Uganda (EBOV) are unpredictable and a matter of considerable public health concern to the affected as well as neighboring countries.
The Zaire species of EBOV shows the highest mortality in humans, whereas the Reston species may be apathogenic, based on very limited data. The clinical syndrome of MBGV and EBOV hemorrhagic fever is characterized by generalized fluid distribution problems, hypotension, coagulation disorders, variable degrees of hemorrhage, and widespread focal tissue destruction (6, 18, 23, 26, 28, 29). Morphological studies on postmortem material indicate that mononuclear phagocytic cells are the primary targets for filovirus replication (10, 11, 30, 39, 40). Cultured human macrophages are highly susceptible to infection with MBGV, the prototype filovirus, resulting in activation, massive virus production, and finally cell lysis (5). Endothelial cells are considered as secondary targets.
Filoviruses replicate in human umbilical cord vein endothelial cells, causing endothelial cell lysis (32). This effect may in part be associated with the cytotoxicity of the viral transmembrane glycoprotein (3, 38), which mediates virus attachment to target cells (33, 37). Postmortem observations on humans have demonstrated viral antigen in endothelial cells of several organs (39, 40), but widespread destruction of endothelial cells is not always obvious in the final stages of the disease in experimentally infected nonhuman primates (10, 11, 30).
The mechanisms of disease development during filovirus infections are still poorly understood. Following infection via small lesions, virus particles enter lymph vessels or the vascular system directly. The primary organ tropism (lymph nodes, spleen, and liver) may be explained by the direct access of viral particles to sessile macrophages without penetration of the cell or the tissue barrier (31). The pantropism of filovirus infections, typically occurring during late disease stages, is mechanistically unexplained. Extravasation of infected circulating cells, such as monocytes, might be a mechanism for the spread of the virus and the occurrence of focal tissue destruction. This requires the activation of the extravasating cells and the endothelium that is normally mediated through cytokines and chemokines (15, 21). However, neither infection nor activation of monocytes by filoviruses has been experimentally demonstrated yet.
In this study we compared the effects of filovirus infections on freshly isolated suspended monocytes and adherent macrophages under culture conditions. Monocytes could be established as permissive cells for MBGV and EBOV. Both cell subsets, the suspended monocytes and the adherent macrophages, became activated upon infection, as evidenced by the release of cytokines and chemokines. The activation seemed to be largely independent of viral replication. Based on our data, the infected monocytes may play a key role in the spread of the virus and therefore sufficiently explain the pantropism and the associated focal tissue destruction. Furthermore, the virus-induced cytokine and chemokine release from activated monocytes may trigger an instability of the endothelium in the infected host.
MATERIALS AND METHODS
Viruses and cell lines.
In this study we used the Musoke strain of MBGV, the Mayinga strain of the Zaire species of EBOV (EBOV-Zaire), and the Reston strain of the Reston species of EBOV (EBOV-Reston). All virus stocks were kindly provided by the Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, Ga. Virus stocks were freshly prepared in Vero E6 cells (ATCC 1568). Harvesting was performed when no obvious cytopathic effect was seen. Mock-infected Vero E6 cells were treated the same way in order to prepare a control (mock stock). Vero E6 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL, Karlsruhe, Germany) supplemented with 10% fetal calf serum (Biochrom, Berlin, Germany), penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM). For virus propagation, DMEM with 2% fetal calf serum was used.
Endotoxin test.
Prior to use, all virus stocks and media were analyzed for endotoxin presence using the Limulus amebocyte lysate test (QCL-1000; BioWhittaker, Walkersville, Md.). All compounds and media used in this study contained less than 0.3 EU/ml, which was less or equivalent to the amounts found in the mock stock used as controls for the experiments.
Inactivation of virus stocks.
Virus stocks were inactivated by exposure to UV light for 1 h. Proper inactivation was controlled by the incubation of Vero E6 cells with the inactivated virus particles and subsequent screening for the presence of viral proteins (immunofluorescence) and viral RNA (reverse transcription [RT]-PCR targeting virus-specific transcripts). The UV-inactivated stocks were used at the same dilutions as the noninactivated stocks.
Isolation of PBMC.
Peripheral blood mononuclear cells (PBMC) consisting of monocytes and lymphocytes were separated from the buffy coats of healthy blood donors using Ficoll-Hypaque density gradient centrifugation (nonpooled).
(i) Monocytes.
Blood monocytes were isolated by countercurrent centrifugal elutriation of PBMC-rich fractions using a JE-6B rotor (Beckman).
(ii) Macrophages.
Freshly prepared PBMC were seeded into 24-well culture plates (Primaria; Falcon), and the monocytes were allowed to adhere. After an adsorption period of 1 h, the monolayers were washed extensively to remove any nonadherent cells. The cells were incubated at 37°C in a humidified (95%) 5% CO2–air atmosphere for 7 days prior to infection. The monocytes and macrophages were cultured in RPMI 1640 (Linaris, Wertheim-Bettingen, Germany) containing 5% human AB serum, penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mM), nonessential amino acids (2 mM), and pyruvate (2 mM).
Infection of mononuclear phagocytic cells. (i) Monocytes.
A total of 106 fresh mononuclear cells were infected in suspension (test tube) using a multiplicity of infection (MOI) of 10. Subsequently, the cells were washed with phosphate-buffered saline (PBS), seeded into 24-well culture plates, and incubated at 37°C in a CO2 incubator (humidity 95%) for the appropriate time (up to 7 days postinfection).
(ii) Macrophages.
The infection was performed on day 7 postseeding at an MOI of 10. After an adsorption period of 1 h, the inoculum was replaced by new medium (RPMI containing 5% human AB serum), and the cultures were incubated for various times at 37°C in a CO2 incubator (humidity 95%).
Antibody treatment.
Macrophages were infected as described above. Following inoculation, fresh medium containing anti-human tumor necrosis factor alpha (TNF-α) antibody (5 μg/ml) (R&D Systems, Weisbaden, Germany) was added.
Immuno-Plaque assay.
Confluent Vero E6 cells grown on cover slips were infected using a 10-fold dilution series. Following virus adsorption for 45 min at 37°C, the cells were washed three times with PBS. The infected cells were overlaid with DMEM containing 0.4% low-melting-point agarose and 2% fetal calf serum. At 48 h postinfection, the cells were fixed with 2% formaldehyde in PBS overnight, washed with PBS, and permeabilized with 0.1% Triton X-100 in PBS for 15 min. Subsequently, the cells were incubated for 1 h at room temperature with the desired antibody (anti-MBGV NP, 1:1,000; anti-EBOV, 1:200) diluted in PBS. The samples were washed three times with PBS and incubated for another hour with the appropriate indocarbocyanine (Cy3)- or fluorescein isothiocyanate (FITC)-conjugated secondary antibody. Following washing (three times), cover slips were mounted with Supermount (BioGenex, Hamburg, Germany), and the immunostained plaques were counted.
Detection of cytokines.
The supernatants from the infected or mock-infected cell cultures were removed, clarified from cell debris by centrifugation (8,000 × g, 4°C, 10 min), and stored at −80°C. All samples were tested in duplicate for the presence of cytokines after being thawed only once using commercial enzyme-linked immunosorbent assay (ELISA) systems (human interleukin-6 [IL-6], TNF-α ELISA kit, and human IL-8 enzyme immunoassay [EIA] from Promocell, Heidelberg, Germany; human gro-α ELISA from R&D Systems). Since the samples were not inactivated, the analyses were conducted in a biosafety level 4 containment laboratory.
RT-PCR for cytokine-specific transcripts.
The supernatants from the infected or mock-infected cells were removed, and the cells were lysed using a guanidinium isothiocyanate-based buffer system (RNeasy kit; Qiagen, Hilden, Germany). At this point the samples were taken out of the containment laboratory, and RNA isolation was performed using the Qiagen kit. The RNA concentrations were adjusted to 100 ng/μl prior to use in RT-PCR. Reverse transcription and PCR were carried out in the same tube using the OneStep RT-PCR kit (Qiagen) according to the manufacturer's guidelines. RT-PCR was performed with a Perkin Elmer cycler (model 2400) using the following protocol: 1 cycle at 50°C for 30 min and 95°C for 15 min, followed by 19 to 32 cycles at 94°C for 30 s, 65°C for 30 s, and 72°C for 1 min. The cycle numbers were adjusted to allow semiquantitative analysis of the amplification products after ethidium bromide staining. Table 1 shows the sequences of the oligonucleotides and the cycle numbers used for the different target mRNAs.
TABLE 1.
Oligonucleotide sequences
| Gene | Primer sequence (5′ to 3′)a | Cycle no. | |
|---|---|---|---|
| β-Actin | GTG GGG CGC CCC AGG CAC CA | S | 19 |
| CTC CTT AAT GTC ACG CAC GAT TTC | AS | ||
| TNF-α | AGC ATG ATC CGG GAC GTG GAG | S | 25 |
| CCC AGA CTC GGC AAA GTC GAG | AS | ||
| IL-1β | TGG CAG AAG TAC CTG AGC TCG | S | 25 |
| TTA GGA AGA CAC AAA TTG CAT GGT G | AS | ||
| IL-6 | CTC CTT CTC CAC AAG CGC CTT CG | S | 28 |
| GAG CCC TCA GGC TGG ACT GCA GG | AS | ||
| IL-8 | TTC TGC AGC TCT GTG TGA AGG TAA G | S | 32 |
| GGA TCC TGG CTA GCA GAC TAG | AS | ||
| gro-α | CAG TGC TTG CAG ACC CTG | S | 26 |
| CAT GTT GCA GGC TCC TCA G | AS | ||
S, sense; AS, antisense.
RESULTS
Replication of filoviruses in monocytes .
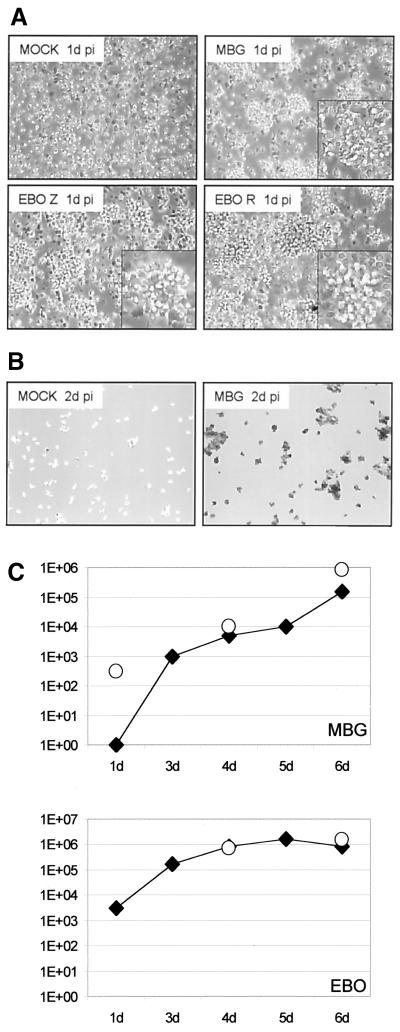
Human peripheral blood monocytes were infected with MBGV and EBOV-Zaire at an MOI of 10. Within several hours, the infection of the monocytes resulted in the formation of cell clumps indicative of activation (Fig. 1A). Immunostaining done at day 2 postinfection with a virus-specific antiserum demonstrated the infection of the monocytes (Fig. 1B). The supernatants were collected at different days postinfection, and the virus titers were determined by plaque assay in Vero E6 cells (Fig. 1C). No obvious difference in infection of suspended monocytes and adherent macrophages between the viruses was noted. The data clearly demonstrated that the circulating monocyte was a permissive target cell for filoviruses and that the infection did not require cell adhesion to the substrates.
FIG. 1.
Filovirus replication in mononuclear phagocytic cells. (A and B) Infection of monocytes. Monocytes were infected with MBGV (MBG) and EBOV-Zaire (EBO Z) and -Reston (EBO R) at an MOI of 10. A mock infection served as a control. Cultures were checked by light microscopy (A) and immunostaining (B). On day 1 postinfection (1d pi), activation could be demonstrated by clumping of monocytes (A). Immunostaining was performed on day 2 postinfection, as shown here for MBGV using an anti-MBGV antiserum (B). (C) Kinetic of virus growth. Virus growth was determined by immunoplaque assay. The supernatants of infected cultures were 10-fold diluted, and confluent monolayers of Vero E6 cells were infected. On day 2 postinfection, cells were stained with specific antisera, and the titer was determined. Open circles, infected macrophages; solid squares, infected monocytes.
Activation of mononuclear phagocytic cells.
Suspended monocytes and adherent macrophages were either infected with MBGV and EBOV-Zaire at an MOI of 10 or incubated with the same dilution of UV-inactivated virus stocks. The activation was measured on two levels, transcription and protein expression. Treated cells were harvested at 24 h postinfection, and RNA was isolated for RT-PCR analysis. RT-PCR was performed using the OneStep RT-PCR kit (Qiagen) with primers specifically designed to target TNF-α, IL-1β, IL-6, IL-8, gro-α, and β-actin (housekeeping gene) mRNAs (see Materials and Methods and Table 1). Transcriptional activation of the monocytes and the macrophages could be demonstrated for all the above-mentioned proinflammatory cytokines and chemokines (Fig. 2A; an example shown here for macrophages).
FIG. 2.
Mode of activation. Activation of suspended monocytes and adherent macrophages was determined on the level of transcription and expression. Cells were incubated with the same dilutions of UV-treated or untreated MBGV (MOI of 10), EBOV-Zaire (MOI of 10), or mock stocks (negative control). (A) Transcriptional activation. Cells were harvested 24 h postinfection, and RNA was isolated using a commercial kit. RT-PCR was performed with specific oligonucleotides for the indicated proinflammatory cytokines and chemokines. β-Actin was used as a housekeeping gene in order to verify that equal amounts of RNA were used in the RT-PCR assays (an example shown here for macrophages). To get semiquantitative results, the number of cycles was adjusted to 19 to 32 cycles. Products were run on a 2% agarose gel and visualized following ethidium bromide staining. (B) Activation of expression. Supernatants were harvested 24 h postinfection and assayed by ELISA for the presence of mediators. The amount of mediators released is given in picograms per milliliter. (C) Inactivation of virus stock. In order to prove proper inactivation of virus stocks by UV treatment, Vero E6 cells were incubated with the same dilutions of UV-treated and untreated MBGV (MOI of 5) and EBOV-Zaire (MOI of 5). Cells were prepared for antigen detection by fixation and inactivation with 2% paraformaldehyde on days 2 (upper and middle panel) and 14 (bottom panel) postinfection. For indirect immunofluorescence, cells were subsequently permeabilized with 0.1% Triton X-100 and incubated with anti-MBGV and anti-EBOV antisera (1:1,000 and 1:200 dilution, respectively) followed by Cy3- or FITC-labeled secondary antibodies.
The secretion of the corresponding proteins was investigated using specific ELISAs. Secretion was significantly increased for all assayed expression products that had been analyzed on a transcriptional level (Fig. 2B). Interestingly, similar levels of transcriptional activation and increased product secretion were achieved by incubation of the cells with UV-treated viral particles. In order to exclude the infectivity of the UV-treated virus stocks, incubations of Vero E6 cells (MOI of 5) were performed using UV-treated and nontreated viruses. No virus replication was detected after 14 days following incubation with UV-treated MBGV and EBOV-Zaire particles, whereas monolayers infected with nontreated virus were uniformly antigen positive 2 days postinfection (Fig. 2C). In addition, RT-PCR targeting virus-specific transcripts confirmed complete inactivation of UV-treated virus stocks (data not shown). The data suggest that activation is largely independent of virus replication.
TNF-α by itself can induce expression of mediators in mononuclear phagocytic cells (24, 34). To exclude the possible influence of TNF-α on the production of IL-6, IL-1β, IL-8, and gro-α, we infected macrophages in the absence or presence of a neutralizing anti-human TNF-α antibody. The supernatants were collected 24 h postinfection and assayed for the different mediators. Under these conditions, virus-mediated induction of IL-6, IL-8, and gro-α (Fig. 3) was only slightly affected, indicating that cytokine and chemokine expression is indeed largely induced by virus infection and not triggered by the production of TNF-α.
FIG. 3.
Mode of activation. Macrophages were infected with MBGV at an MOI of 10 with (+) or without (−) a neutralizing anti-TNF-α monoclonal antibody (5 μg/ml). A mock infection was performed as a negative control. Supernatants were harvested 24 h postinfection and assayed by ELISA for the indicated mediators (picograms per milliliter).
Time course of activation.
In general, MBGV and EBOV-Zaire showed a similar activation profile in both subsets of mononuclear phagocytic cells. Therefore, the kinetics of virus-induced activation was studied using MBGV. Monocytes and differentiated macrophages were infected at an MOI of 10. The supernatants and cells were harvested at different time points postinfection. RT-PCR was performed as described previously, but the number of cycles was adjusted to 19 to 32 in order to be semiquantitative. All target genes (TNF-α, IL-1β, IL-6, IL-8, and gro-α) were transcriptionally activated beginning at 3 h postinfection. TNF-α reached its maximum at about 3 to 6 h postinfection, whereas gro-α showed a very weak increase at 3 h postinfection and increased to peak values within 24 h. IL-6 and IL-8 remained at an increased level for a longer period and showed a slight decrease at 24 h postinfection (Fig. 4A; an example shown here for macrophages). The slower response in activation of gro-α can also be seen from the level of protein secretion, which showed an increased release beginning at 6 h postinfection, in contrast to TNF-α, which was detected after only 3 h (Fig. 4B). Interestingly, IL-6 and, to a lesser extent, IL-8 secretion was delayed compared to the transcriptional activation of the corresponding genes. The IL-8 background was high but significantly lower on the transcript and protein levels in the mock-infected compared to the virus-infected cells (Fig. 4).
FIG. 4.
Kinetics of activation. Suspended monocytes and adherent macrophages were infected with MBGV at an MOI of 10. A mock infection served as a control. (A) Transcriptional activation. Cells were harvested at the indicated time points postinfection, and RNA was isolated using a commercial kit. RT-PCR and product analyses were performed as described in the legend to Fig. 2 (an example shown here for macrophages). (B) Activation of expression. Supernatants were harvested at the indicated times and assayed by ELISA for the presence of the indicated mediators. The amount of products released is given in picograms per milliliter.
The data from the time course studies compared favorably with the results with the UV-inactivated virus (Fig. 2). Activation seemed to start earlier than virus replication, indicating that replication is not needed to trigger this event. In order to confirm this conclusion, we performed RT-PCR using primers that specifically target virus-specific RNA transcripts. At 3 h postinfection, we could not detect transcripts of viral genes, which supported the conclusion that replication (and most likely transcription) was not necessary for target cell activation (data not shown).
Infection of mononuclear phagocytic cells with EBOV- Reston.
Monocytes and macrophages were infected with MBGV, EBOV-Zaire, and EBOV-Reston at an MOI of 10. As demonstrated for MBGV and EBOV-Zaire previously (Fig. 1 to 4), EBOV-Reston infection also resulted in the activation of both subsets of mononuclear phagocytic cells (Fig. 5). In general, the levels of activation did not differ significantly from those activated by MBGV and EBOV-Zaire infections.
FIG. 5.
Infection of mononuclear phagocytic cells with EBOV-Reston. Suspended monocytes and adherent macrophages were infected with MBGV, EBOV-Zaire, and EBOV-Reston at an MOI of 10. A mock infection served as a control. (A) Transcriptional activation. Cells were harvested 24 h postinfection, and RNA was isolated using a commercial kit. RT-PCR and product analyses were performed as described in the legend to Fig. 2 (an example shown here for macrophages). (B) Activation of expression. Supernatants were harvested 24 h postinfection and assayed by ELISA for the indicated mediators (picograms per milliliter).
DISCUSSION
As hypothesized previously, filoviruses may enter through minor lesions on the skin and/or mucous membranes and subsequently access the blood directly or via the lymphatic system (31). Studies on experimentally infected animals have demonstrated that filovirus infections follow a specific order, starting with the blood or lymph fluid, the interstitium, and the parenchyma, with the crucial pathogenic events taking place in the bloodstream (30). On the basis of in vivo studies, mononuclear phagocytic cells, especially macrophages, seem to be the primary target cells for filoviruses (10, 11, 30, 39, 40). The vast majority of mononuclear phagocytic cells in the bloodstream are monocytes. Given the fulminant course of a filovirus hemorrhagic fever, virus production in this subset of mononuclear phagocytic cells could be the major source of the high viremia (up to 108 PFU/ml) in naturally and experimentally infected hosts during the critical early stages of the infection. High viremia may subsequently allow the direct infection of important secondary target cells, such as endothelial cells (10, 11, 30, 32, 39).
Filovirus infection leads to activation of monocytes, as demonstrated by clump formation (Fig. 1A) and the release of mediators (Fig. 2 to 4). In addition, cytokines (e.g., TNF-α and IL-1β) and other proinflammatory mediators are known to increase the expression of various cell adhesion molecules on endothelial cells (ICAM-1, VCAM-1, E-selectin, and P-selectin) that are involved in diapedesis of leukocytes (2, 15). The C-X-C chemokines IL-8 and gro-α are not the only chemoattractants for neutrophils. IL-8 also triggers firm adhesion of monocytes to activated vascular endothelium, suggesting a potential role in monocyte recruitment (12, 20). Therefore, extravasation of activated, infected monocytes may be a mechanism for MBGV and EBOV to spread from the bloodstream into organ tissues during the second stage of infection. This could explain the pantropism associated with severe filovirus infections, and this would not necessarily depend on damage to the endothelium, a feature that is not observed in all infected hosts (10, 11, 29, 30). A similar role for monocytes has been discussed for the spread of other virus infections, such as human immunodeficiency virus (25) and visna virus (27).
Clumping of monocytes (Fig. 1A) may be an important observation for the pathogenesis of filovirus hemorrhagic fever. Monocyte clumps in vessels in vivo could dramatically influence the rheology of the bloodstream, especially in small venules, which could lead to thrombus formation, as has been observed in infected patients. Intravascular clump formation could also activate coagulation, a general phenomenon observed in many clinical cases as well as in experimentally infected monkeys (6, 18, 23, 26, 28, 29). Alterations of the coagulation pathways, particularly the intrinsic one, were observed in infected monkeys (8, 9).
The release of cytokines as a result of monocyte and macrophage activation (Fig. 2 to 4) is also involved in the development of shock (1). Shock can be caused by many things, including bacterial (e.g., endotoxin) and viral infection (4). It has been shown that supernatants of filovirus-infected macrophages increased paraendothelial permeability in endothelial monolayers, an effect that was mainly driven by TNF-α (5). Increased paraendothelial permeability is one of the most important events during shock development (13, 22), and shock is known to be the major cause of death in filovirus hemorrhagic fever (6, 18, 23, 26, 28, 29). Elevated levels of mediators, including TNF-α, were detected in the acute-phase sera of several EBOV-infected patients (35) and in infected animals (17). Increased TNF-α values were found to correlate with the severity of Junin virus infections causing Argentine hemorrhagic fever (16). Therefore, it seems reasonable to assume that massive cytokine release triggered by filovirus-induced activation of mononuclear phagocytic cells may induce a systemic inflammatory response syndrome, which can be provoked by cytokine treatment and can be observed during endotoxin-induced shock (1, 4).
As mentioned above, virus-induced activation of monocytes will most likely result in the release of other proinflammatory mediators (e.g., histamine and serotonin), proteases (e.g., elastase), and peroxide (H2O2). These factors might be of increasing significance at the final stages of the disease, when infected monocytes/macrophages undergo cell lysis (5). Recently it was postulated that cell detachment and cytotoxicity were caused by the viral transmembrane glycoprotein and may play a crucial role in EBOV pathogenesis (3, 38). Although filoviruses do replicate cytolytically in target cells, it remains open as to what extent the cytolysis of target cells contributes to disease development. However, a cytotoxic effect on mononuclear phagocytic cells could further promote a pathological release of cytokines and other mediators and interfere with effective host immune responses.
The initial activation of monocytes and macrophages seems to be largely independent of virus replication (Fig. 2). However, a sustained release of mediators seems to require ongoing viral replication (14) or, as in our experiments, the continuous presence of inactivated virus. Therefore, replication may not be contributing directly to the early critical events in pathogenesis. If this is true, specific viral products may be important factors in the activation by binding to cell surface receptors, which subsequently trigger signal cascades. The different forms of the viral glycoproteins expressed by EBOV and MBGV are candidates for such products. The functions of the soluble glycoproteins have not been finally determined, whereas the viral transmembrane glycoprotein functions in receptor binding and fusion (7, 19). These recently described soluble products should be investigated in future work with respect to target cell activation.
MBGV and EBOV-Zaire are human pathogenic filoviruses, whereas EBOV-Reston may be apathogenic for humans, based on very limited data (6, 29). Therefore, propagation and activation of human mononuclear phagocytic cells by EBOV- Reston were not necessarily expected (Fig. 5). Similar mechanisms triggered by EBOV-Reston underline the importance of infection and activation of monocytes by filoviruses in pathogenesis. Differences in susceptibility of target cells among species as well as the lower cleavability of the EBOV-Reston virus transmembrane glycoprotein in human-derived cell lines (36) may contribute to lower pathogenicity in humans compared to nonhuman primates (7, 19).
In conclusion, it is reasonable to assume that mononuclear phagocytic cells, especially monocytes, play a central role in the pathogenesis of filovirus hemorrhagic fever by mediating virus spread through extravasation and by triggering a cascade of interrelated pathological host responses through virus-induced activation. This may lead to an impairment of the immune system, homeostasis, and the barrier function of the endothelium, finally leading to shock and death. Neutralizing antibodies directed against key inflammatory cytokines and chemokines might be able to help the host to overcome the early events in infection and thus allow time for the specific immune response needed to clear the virus.
ACKNOWLEDGMENTS
We thank Daryl Dick for editorial comments.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 535; Schn 430/1-1, Kl 238/7-1), the Canadian Institutes of Health Research (MOP-43921), and the European Community (INCO-grant ERBIC 18 CT9803832). Ute Ströher performed this work in partial fulfillment of the requirements for a Ph.D. degree from the Philipps-Universität, Marburg, Germany.
REFERENCES
- 1.Brouckaert P, Fiers W. Tumor necrosis factor and the systemic inflammatory response syndrome. Curr Top Microbiol Immunol. 1996;216:167–187. doi: 10.1007/978-3-642-80186-0_8. [DOI] [PubMed] [Google Scholar]
- 2.Butcher E C. Specificity of leukocyte-endothelial interactions and diapedesis: physiologic and therapeutic implications of an active decision process. Res Immunol. 1993;144:695–698. doi: 10.1016/s0923-2494(93)80053-2. [DOI] [PubMed] [Google Scholar]
- 3.Chan S Y, Ma M C, Goldsmith M A. Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J Gen Virol. 2000;81:2155–2159. doi: 10.1099/0022-1317-81-9-2155. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello C A. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–165. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann H, Bugany H, Mahner F, Klenk H D, Drenckhahn D, Schnittler H J. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol. 1996;70:2208–2214. doi: 10.1128/jvi.70.4.2208-2214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldmann H, Sanchez A, Klenk H D. Filoviruses. In: Collier L H, Balows A, Sussman M, editors. Topley and Wilson's microbiology and microbial infections. 9th ed. London, England: Arnold; 1998. pp. 651–664. [Google Scholar]
- 7.Feldmann H, Volchkov V E, Volchkova V A, Klenk H D. The glycoproteins of Marburg and Ebola virus and their potential roles in pathogenesis. Arch Virol Suppl. 1999;15:159–169. doi: 10.1007/978-3-7091-6425-9_11. [DOI] [PubMed] [Google Scholar]
- 8.Fisher-Hoch S P, Platt G S, Lloyd G, Simpson D I, Neild G H, Barrett A J. Haematological and biochemical monitoring of Ebola infection in rhesus monkeys: implications for patient management. Lancet. 1983;ii:1055–1058. doi: 10.1016/s0140-6736(83)91041-3. [DOI] [PubMed] [Google Scholar]
- 9.Fisher-Hoch S P, Platt G S, Neild G H, Southee T, Baskerville A, Raymond R T, Lloyd G, Simpson D I. Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola) J Infect Dis. 1985;152:887–894. doi: 10.1093/infdis/152.5.887. [DOI] [PubMed] [Google Scholar]
- 10.Geisbert T W, Hensley L E, Gibb T R, Steele K E, Jaax N K, Jahrling P B. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Investig. 2000;80:171–186. doi: 10.1038/labinvest.3780021. [DOI] [PubMed] [Google Scholar]
- 11.Geisbert T W, Jahrling P B, Hanes M A, Zack P M. Association of Ebola-related Reston virus particles and antigen with tissue lesions of monkeys imported to the United States. J Comp Pathol. 1992;106:137–152. doi: 10.1016/0021-9975(92)90043-t. [DOI] [PubMed] [Google Scholar]
- 12.Gerszten R E, Garcia-Zepeda E A, Lim Y C, Yoshida M, Ding H A, Gimbrone M A, Jr, Luster A D, Luscinskas F W, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 13.Glauser M P. The inflammatory cytokines. New developments in the pathophysiology and treatment of septic shock. Drugs. 1996;52:9–17. doi: 10.2165/00003495-199600522-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gupta M, Mahanty S, Ahmed R, Rollin P E. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology. 2001;284:20–25. doi: 10.1006/viro.2001.0836. [DOI] [PubMed] [Google Scholar]
- 15.Haraldsen G, Kvale D, Lien B, Farstad I N, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol. 1996;156:2558–2565. [PubMed] [Google Scholar]
- 16.Heller M V, Saavedra M C, Falcoff R, Maiztegui J I, Molinas F C. Increased tumor necrosis factor-alpha levels in Argentine hemorrhagic fever. J Infect Dis. 1992;166:1203–1204. doi: 10.1093/infdis/166.5.1203. [DOI] [PubMed] [Google Scholar]
- 17.Ignatyev G M. Immune response to filovirus infections. Curr Top Microbiol Immunol. 1999;235:205–217. doi: 10.1007/978-3-642-59949-1_11. [DOI] [PubMed] [Google Scholar]
- 18.Klenk H D, editor. Marburg and Ebola viruses. Curr Top Microbiol Immunol. 1999;235:1–225. [Google Scholar]
- 19.Klenk H D, Volchkov V E, Volchkova V A, Feldmann H. The polymorphism of the Ebola virus glycoprotein and its potential role in pathogenesis. Nova Acta Leopoldina. 1999;307:141–149. [Google Scholar]
- 20.Luscinskas F W, Gerszten R E, Garcia-Zepeda E A, Lim Y C, Yoshida M, Ding H A, Gimbrone M A, Jr, Luster A D, Rosenzweig A. C-C and C-X-C chemokines trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Ann N Y Acad Sci. 2000;902:288–293. doi: 10.1111/j.1749-6632.2000.tb06324.x. [DOI] [PubMed] [Google Scholar]
- 21.Luster A D. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 22.Maier R V, Bulger E M. Endothelial changes after shock and injury. New Horiz. 1996;4:211–223. [PubMed] [Google Scholar]
- 23.Martini G A, Siegert R. Marburg virus disease. 1st ed. Berlin, Germany: Springer-Verlag; 1971. [Google Scholar]
- 24.Netea M G, Selzman C H, Kullberg B J, Galama J M, Weinberg A, Stalenhoef A F, Van der Meer J W, Dinarello C A. Acellular components of Chlamydia pneumoniae stimulate cytokine production in human blood mononuclear cells. Eur J Immunol. 2000;30:541–549. doi: 10.1002/1521-4141(200002)30:2<541::AID-IMMU541>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Orenstein J M, Meltzer M S, Phipps T, Gendelman H E. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J Virol. 1988;62:2578–2586. doi: 10.1128/jvi.62.8.2578-2586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattyn S R. Ebola virus hemorrhagic fever. 1st ed. Amsterdam, The Netherlands: Elsevier/North-Holland; 1978. [Google Scholar]
- 27.Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- 28.Peters C J, LeDuc J W. Ebola: the virus and the disease. J Infect Dis. 1999;179(Suppl. 1):S1–S288. doi: 10.1086/514322. [DOI] [PubMed] [Google Scholar]
- 29.Peters C J, Sanchez A, Rollin P E, Ksiazek T G, Murphy F A. Filoviridae: Marburg and Ebola viruses. In: Fields B N, Knipe D M, et al., editors. Virology. 3rd ed. Philadelphia, Pa: Raven Press; 1996. pp. 1161–1176. [Google Scholar]
- 30.Ryabchikova E I, Kolesnikova L V, Luchko S V. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J Infect Dis. 1999;179(Suppl. 1):S199–S202. doi: 10.1086/514293. [DOI] [PubMed] [Google Scholar]
- 31.Schnittler H J, Feldmann H. Marburg and Ebola hemorrhagic fevers: does the primary course of infection depend on the accessibility of organ-specific macrophages? Clin Infect Dis. 1998;27:404–406. doi: 10.1086/517704. [DOI] [PubMed] [Google Scholar]
- 32.Schnittler H J, Mahner F, Drenckhahn D, Klenk H D, Feldmann H. Replication of Marburg virus in human endothelial cells. A possible mechanism for the development of viral hemorrhagic disease. J Clin Investig. 1993;91:1301–1309. doi: 10.1172/JCI116329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takada A, Robison C, Goto H, Sanchez A, Murti K G, Whitt M A, Kawaoka Y. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci USA. 1997;94:14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasilescu C, Berger D, Buttenschon K, Seidelmann M, Beger H G. Endotoxin-induced release of interleukin 6 and interleukin 1 beta in human blood is independent of tumor necrosis factor alpha. Eur Surg Res. 1996;28:55–62. doi: 10.1159/000129440. [DOI] [PubMed] [Google Scholar]
- 35.Villinger F, Rollin P E, Brar S S, Chikkala N F, Winter J, Sundstrom J B, Zaki S R, Swanepoel R, Ansari A A, Peters C J. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2: IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(Suppl. 1):S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 36.Volchkov V E, Feldmann H, Volchkova V A, Klenk H D. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc Natl Acad Sci USA. 1998;95:5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, Delgado R, Xu L, Todd R F, Nabel E G, Sanchez A, Nabel G J. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z Y, Duckers H J, Sullivan N J, Sanchez A, Nabel E G, Nabel G J. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med. 2000;6:886–889. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 39.Zaki S R, Goldsmith C S. Pathologic features of filovirus infections in humans. Curr Top Microbiol Immunol. 1999;235:97–116. doi: 10.1007/978-3-642-59949-1_7. [DOI] [PubMed] [Google Scholar]
- 40.Zaki S R, Peters C J. Viral hemorrhagic fevers. In: Connor D H, editor. Pathology of infectious diseases. Stamford, Conn: Appleton and Lange; 1997. pp. 347–364. [Google Scholar]