Abstract
Gastric cancer (GC) is a prevalent malignant tumor and ranks as the second leading cause of death among cancer patients worldwide. Due to its hidden nature and difficulty in detection, GC has a high incidence and poor prognosis. Traditional treatment methods such as systemic chemotherapy, radiotherapy, and surgical resection are commonly used, but they often fail to achieve satisfactory curative effects, resulting in a very low 5-year survival rate for GC patients. Currently, targeted therapy and immunotherapy are prominent areas of research both domestically and internationally. These methods hold promise for the treatment of GC. This article focuses on the signaling pathways associated with the development of GC, as well as the recent advancements and applications of targeted therapy and immunotherapy. The aim is to provide fresh insights for the clinical treatment of GC.
Keywords: gastric cancer, targeted therapy, immunotherapy, immune checkpoint inhibitors, signaling pathway
Introduction
GC is a worldwide malignant disease. Many risk factors for GC include dietary habits, smoking, drinking, ethnicity, and family inheritance. GC is the second most common cause of death worldwide. 1 Over the past few decades, many studies have been conducted on GC. GC occurs insidiously, with asymptomatic or no specific symptoms in the early stage, and it is difficult to detect the disease. 2 Most GCs are often diagnosed at an advanced stage, and the mortality rate of patients is very high. 3
Patients with advanced gastric cancer (AGC) are usually treated with traditional therapies such as chemotherapy, radiotherapy, and surgery, which not only affect the differentiation of normal cells in the body and lead to a decrease in the body's immunity but also bring about serious side effects, such as gastrointestinal maladaptation, alopecia, and even inhibition of certain organ function.4,5 Patient survival remains low after aggressive combination therapy. The antitumor mechanism of classical chemotherapeutic drugs has been explored more completely, and new areas, including immunotherapy and targeted therapy, have become new research hotspots for GC treatment. CAR-T-cell therapy in immunotherapy has caused a sensation, and the cancer vaccine has also become an emerging treatment method. Targeted therapy can specifically select abnormal proteins or genes in the cytomembrane or cytoplasm of tumor cells for binding, blocking the signaling related to tumor cell growth and proliferation, 6 and exerting anti-tumor effects, as well as alleviating drug resistance, 7 side effects, 8 it has been used in the treatment of various malignant tumors, like GC, prostate cancer, and breast cancer. This review will focus on the advances and findings in targeted therapy for GC.
Targets in GC
According to the Gene Expression Profiling Interactive Analysis (GEPIA) database, when NT5E (which encodes CD73) is highly expressed, it leads to shorter survival of patients with GC. 2 CD73, also known as a GPI-anchoring enzyme, catalyzes the conversion of adenosine monophosphate (AMP) to adenosine and inorganic phosphate, and adenosine plays an antitumor immune role by inhibiting CD8+ T-cells, as well as natural killer (NK) cell infiltration and function. CD73 has enzymatic properties, inhibits the host immune system, and promotes tumor progression. 9 Thus, CD73 is a potential therapeutic target. The tumor microenvironment (TME) is essential for tumor progression. Tumor-associated macrophages (TAM) are classified into M1 and M2. vascular endothelial growth factor(VEGF)is converted to M2 macrophages by Th2 cytokines (IL-4, IL-10, TGF-β1) and immune complexes. M2 macrophages overproduce VEGF, indoleamine-2, 3-dioxygenase (IDO), and transforming growth factor-β (TGF-β) to decrease T-cell activity and increase angiogenesis to promote GC progression. 10 The CSF-1/CSF-1 receptor (CSF-1R, which belongs to the platelet-derived growth factor receptor family) signaling pathway is critical for macrophage survival and the transition from TAM M1-type to TAM M2-type, and several monoclonal antibodies targeting CSF-1/CSF-1R are under clinical trials. 11 Alpha-fetoprotein-producing GC (AFPGC) is a rare subtype with a poorer prognosis and is more aggressive than the common GC. There is evidence that targeting CCNE1 and ERBB2 (HER-2) has achieved favorable benefits in treating AFPGC. In addition, targeting the immune checkpoint that inhibits members of the B7 family has brought new hope for the treatment of patients with GC. 12 The B7 family of molecules comprises of many members, among which programmed cell death ligands 1 (PD-L1) binds to programmed cell death protein-1 (PD-1), prevents T-cell activation, inhibits tumor immunity, and promotes tumor cell proliferation. Compared to PD-L1, the expression of PD-L2 is more restricted and less frequent, 13 but its affinity for PD-1 is 2–6 times higher. 14 Combining these two inhibitors inhibits TCR-mediated T-cell proliferation and associated cytokine production. 15 Currently, there are many clinically available antibodies against PD-L1 and PD-L2, including atezolizumab and tocilizumab. Circular RNAs (CircRNAs) are a class of non-coding RNAs with a large body of evidence indicating their involvement in various human diseases, including GC. CircRNAs are aberrantly expressed in GC and are involved in cytological processes, such as proliferation, migration, invasion, and apoptosis of GC cell.16,17 CircRNAs can be categorized as oncogenic or anticancer circRNAs, including ciRS-7, circHIPK3, circYAP1, and circLARP4. Thus, circRNAs may be potential therapeutic targets for GC. 17 In addition, given that circRNAs can be stably expressed in tissues, blood, and other bodily fluids, and are easily detected, they might also serve as biomarkers for the diagnosis of GC. 18 Like circRNAs, microRNAs (miRNAs) belong to a class of non-coding RNAs involved in the maintenance and renewal of cancer stem cells. They are also associated with tumor angiogenesis and metastasis. Oncogenic miRNAs cause cell cycle dysregulation and inhibit apoptosis through pathways involving GC cell carving, metastasis, and invasion.19,20 Thus, miRNAs may be novel targets for GC treatment. 21
Targeted Therapy in GC
HER-2 Targeted Therapy
HER-2 belongs to the human epidermal growth factor receptor family (EGFR); the positive rate of HER-2 overexpression in GC reported globally ranges from 7.3–20.2%, 22 and the amplification of HER-2 when functioning as an oncogene causes overexpression of proteins on the cellular membrane of the cells, transforming them into malignant T-cells. Several studies have reported that HER-2 protein expression is significantly higher in patients with AGC than in normal subjects, which is associated with poor patient prognosis.23,24 Clinical studies on targeted therapies for HER-2+ GC have been conducted. Trastuzumab is an anti-HER-2 monoclonal antibody that specifically targets HER-2 proteins by directly binding to the receptor's extracellular domain, blocking HER-2 mediated signaling and promoting antibody-dependent cytotoxicity, resulting in the death of HER-2 expressing cells. 6 Trastuzumab deruxtecan, a HER-2-targeted antibody conjugate drug, was evaluated for efficacy in patients with HER-2 positive AGC in the DESTINY-Gastric01 trial. Among 187 patients, 125 received trastuzumab and 62 received chemotherapy (55 with irinotecan and 7 with paclitaxel). Patients treated with trastuzumab deruxtecan had a longer overall survival (OS) compared to those on chemotherapy (median: 12.5 months vs 8.4 months), showing significant improvements in clinical benefit for HER-2-positive GC patients. 25 Furthermore, the DESTINY-Gastric02 study screened 79 patients with HER-2-positive unresectable or metastatic gastric or gastroesophageal junction cancer (GEJC) for trastuzumab treatment. Results indicated that 33 of these patients had objective responses (42%), with 4 cases achieving complete remission (5%) and 29 cases showing partial remission (37%). This study supports the use of trastuzumab deruxtecan as a second-line treatment for patients with HER-2-positive AGC or GEJC. 26 In patients with AGC or GEJC, trastuzumab in combination with platinum- and fluorouracil-based chemotherapy significantly improved OS compared to chemotherapy alone, and the efficacy of the combination regimen was better in patients with a high rate of HER-2-positive expression than in patients with low HER-2 expression. 27 Pembrolizumab is a PD-1 antibody which exhibits a high affinity for PD-1, pembrolizumab leads to the inhibition of PD-1 binding to PD-L1 and PD-L2 which enhances the anti-tumor effect of activated T-cells. When added to the above treatment regimen, pembrolizumab has shown a significant improvement in progression-free survival (PFS) for metastatic HER-2-positive GEJC patients, particularly those with a PD-L1 composite positive score of 1 or higher. 28
As an antibody targeting HER-2, pertuzumab is utilized in conjunction with trastuzumab and chemotherapy for the treatment of patients with HER-2-positive metastatic GC or GEJC. The addition of pertuzumab did not show a significant improvement in patient survival rates compared to the placebo regimen.29,30
With our increasing understanding of the mechanisms of GC, more drugs are being used in clinical studies. Margetuximab is an FC-engineered anti-HER-2 monoclonal antibody. By optimizing the structural domain of Fc, it increases binding to activated Fcγ receptor IIIA (CD16A) and decreases binding to inhibitory Fcγ receptor IIB (CD32B), which promotes killing of NK cells, and macrophages, and enhances the antibody-dependenT-cellular cytotoxicity (ADCC) to achieve antitumor effects.31,32 In a single-arm, phase 1b-2 trial, 33 Catenacci et al evaluated the efficacy of margetuximab + pembrolizumab in patients with HER-2-positive gastroesophageal adenocarcinoma (GEA) who had previously received trastuzumab + chemotherapy. The trial enrolled 95 patients older than 18 years, and the final results showed an objective response rate (ORR) of 18.48%, a disease control rate (DCR) of 53%, and a mPFS of 2.73 months; the mOS was 12.48 months. Margetuximab + Pembrolizumab are effective drugs for treating HER-2-positive GEAs. The new drug Zanidatamab is a bispecific antibody that simultaneously binds to two different HER-2 epitopes. 34 A trial enrolled 24 patients with HER-2-positive cancers, including ten patients with GC, five with colorectal cancer, and 9 with other malignancies, and achieved a final mPFS of 6.2 months, an ORR of 41%, and a DCR of 82%. These results were encouraging. An expanded cohort of patients with gastroesophageal cancer is being included, and a phase II trial is planned. 35
Lapatinib is an oral HER-2 antagonist that suppresses HER-2 signaling by inhibiting tyrosine kinase (TK) activity. 36 Lapatinib failed to show favorable efficacy in patients with HER-2-positive advanced or metastatic GC, esophageal cancer, or GEA treated with capecitabine and oxaliplatin. 37 The mOS in the lapatinib and placebo groups was 12.2 months and 10.5 months, respectively, with a statistically non-significant difference, and the mPFS was 6.0 and 5.4 months, suggesting that the addition of lapatinib to capecitabine versus oxaliplatin did not achieve the expected treatment effect. The ongoing clinical trials of targeted therapy in gastric cancer (Table 1).
Table 1.
Targets and Therapeutic Result.
| Targets | Drugs | Phase | Patients | Therapeutic regimen | Result |
|---|---|---|---|---|---|
| HER-2 | Trastuzumab de ruxtecan | phase 2 (DESTINY-Gastric01) | HER 2 positive advanced gastric cancer | trastuzumab deruxtecan versus irinotecan and paclitaxel | mOS:12.5 versus 8.4 months |
| Trastuzumab deruxtecan Margetuximab | phase 2 (DESTINY-Gastric02) phase 1b-2 | USA and Europe patients with HER 2 positive advanced gastric or gastroesophageal junction cancer HER 2-positive gastroesophageal adenocarcinoma (They had been treated with Trastuzumab and chemotherapy) | trastuzumab deruxtecan monotherapy Margetuximab + Pembrolizumab | ORR: 42% (5% complete responses and 37% partial responses) ORR:18.48% mPFS:2.73 months mOS:12.48 months | |
| Lapatinib | Phase 3 | HER 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma | Lapatinib + Capecitabine + Oxaliplatin | mOS:12. 2 versus 10.5months mPFS:6.0 versus 5.4 months |
Angiogenesis-Targeted Therapy
Nutrients required for tumor growth are supplied by blood vessels. Therefore, tumor development is often accompanied by neovascularization. In 1971, Folkman pointed out that in the absence of neovascularization, the diameter of solid tumors can stay at 2–3 mm, which is beneficial for patient treatment. The antitumor effect is improved if the tumor does not metastasize or metastasizes less. 38 Both the local infiltration and distant metastasis of tumors depend on angiogenesis, which plays an important role in tumorigenesis, development, and metastasis. Thus, anti-angiogenesis has become a new approach to cancer treatment. 39 Many studies have focused on anti-angiogenic therapeutic strategies, especially on VEGF and VEGFR, whose expression is higher in GC than in normal gastric tissues.
Ramucirumab is a fully human IgG 1 monoclonal antibody VEGFR-2 antagonist. Its efficacy as a monotherapy in the treatment of patients with AGC or gastroesophageal junction adenocarcinoma (GEJA) was evaluated in the REGARD study. 40 Ramucirumab treatment significantly improved the OS compared to the placebo group, with a mOS of 5.2 months in patients in the ramucirumab group compared to a mOS of 3.8 months in patients treated with placebo. The 6-month overall survival rates in the ramucirumab and placebo groups were 41.8% and 31.6%, respectively. Another RAINBOW trial, 41 which investigated the combination of ramucirumab and paclitaxel in the treatment of patients with AGC or GEJA, showed that the OS was significantly longer in the ramucirumab and paclitaxel group than in the placebo and paclitaxel group (9.6 months vs7.4 months); the overall survival rate in the combination group was also higher than that in the placebo group (at three months:72% vs57%, and at 12 months: 40% vs 30%). Both studies have demonstrated that ramucirumab has significant efficacy in patients with AGC and GEA, both as a single agent and in combination with chemotherapy. Another novel anti-angiogenic drug is apatinib, which selectively targets VEGFR-2 and binds to its intracellular adenosine triphosphate (ATP) binding site, thereby inhibiting phosphorylation and downstream signaling pathways (including the RAF/MEK/ERK signaling pathway) and suppressing tumor microvessel formation. 6 In addition, apatinib inhibits tumor growth by stimulating endoplasmic reticulum stress and protective autophagy. 42 It inhibits tumor growth and has been approved by the Chinese Food and Drug Administration (FDA) for the treatment of patients with GC. 43
Tyrosine Kinase Inhibitors (TKIs)
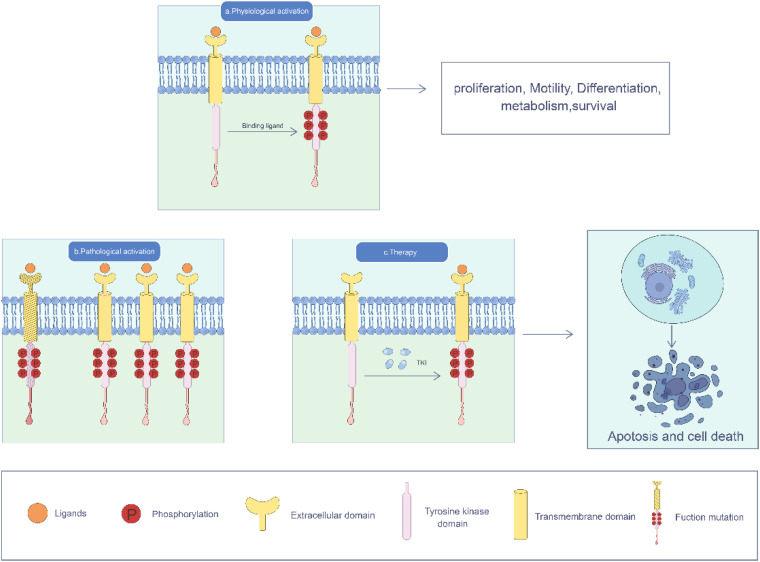
To date, 90 TKs have been identified, of which 58 are of the receptor type. 44 TK are a group of enzymes that transfer phosphate from ATP to tyrosine residues (serine/threonine) as the target amino acids. ATP is involved in cell proliferation, cell cycle, motility, differentiation, metabolism, survival, and programmed cell death by activating intracellular signaling pathways. 45 TKs are prototypes of oncogenes involved in the development of a wide range of human malignancies. 46 Receptor tyrosine kinases (RTKs) include the EGFR, VEGFR, and fibroblast growth factor receptor (FGFR). 47 Certain RTKs are necessary for maintaining normal cellular function; however, when dysregulated, they cause tumor angiogenesis, thus promoting tumor growth. Numerous small-molecule inhibitors have been developed to treat cancers caused by RTK mutations, as well as other diseases. In this review, we highlight the TKI associated with treating GC (Figure 1).
Figure 1.
Under physiological conditions, RTKs are activated upon binding to their ligands and undergo C-terminal phosphorylation. When functional mutations occur in RTK, RTK is overexpressed, the RTK gene is amplified, and the amount of RTK increases. TKIs target RTKs to prevent phosphorylation of the TK domain, interfere with cell proliferation, movement, differentiation, metabolism, survival and other processes, and induce cell death.
The HER family includes RTK encoded by the erbB oncogene, including EGFR (also known as HER1), HER-2, HER-3, and HER-4. After activation, EGFR participates in biological processes, such as cell proliferation, differentiation, migration, and apoptosis. EGFR signaling pathways are activated in many cancers.48,49 EGFR amplification has been detected in approximately 30%–70% of GC cases and is associated with poor prognosis in patients with GC50–52 . These results indicated the feasibility of targeting EGFR in the treatment of GC. Cetuximab, a chimeric (mouse/human) IgG 1 monoclonal antibody against EGFR, has been successfully used in the treatment of metastatic colorectal, head, and neck cancers. In a clinical trial, 35 patients with previously treated metastatic esophageal or gastric adenocarcinoma (GA) received cetuximab intravenously, and only one patient (3%) had a partial response. This patient had a primary GEJ tumor and received third-line therapy, demonstrating that cetuximab monotherapy has minimal clinical activity in patients with metastatic esophageal and GA. 53 To further assess the anticancer effectiveness of cetuximab, we conducted a review on the outcomes of First-line treatment with cetuximab, S-1, and cisplatin in 40 Japanese patients diagnosed with AGC, including GEJA. Out of the 40 patients, one case achieved a complete response (2.5%) while 15 cases showed a partial response (37.5%), resulting in an ORR of 40.0%. The patients generally tolerated the combination treatment of cetuximab with S-1 and cisplatin well, but, this study did not demonstrate any additional clinical benefits. 54 Unfortunately, cetuximab combined with docetaxel and oxaliplatin in the treatment of patients with metastatic GA and GEJA also failed to improve the OS and PFS of patients and did not produce the expected clinical benefit. 55 Cetuximab combined with chemotherapy failed to provide additional benefit to patients, possibly because cetuximab cannot interact with chemotherapy drugs.
Panitumumab is a human immunoglobulin G2 monoclonal antibody directed against the EGFR, 56 commonly used to treat metastatic colon cancer . A phase II trial assessed the combination of panitumumab with docetaxel and cisplatin in patients with AGC and GEJA. The study revealed an ORR of 27.3% in a cohort of 44 patients, with mPFS and OS of 5.0 and 7.2 months, respectively. The addition of panitumumab to standard chemotherapy as a first-line treatment for these patients did not yield improved outcomes. 57
Neovascularization is essential for tumor progression; it not only brings oxygen and nutrients to the tumor, but also serves as an important pathway for the tumor to excrete metabolites. Tumors can also obtain growth factors, such as insulin-like growth factor, platelet-derived growth factor (PDGF), and granulocyte-macrophage colony-stimulating factor (GM-CSF), through endovascular cells, playing a supportive role in tumors, 48 among which VEGF is an important key regulator of angiogenesis. It contains six members, the most widely distributed of which is VEGF-A. 58
The AVAGAST trial 59 included 774 patients with AGC who were administered bevacizumab + fluoropyrimidine-cisplatin or placebo + fluoropyrimidine-cisplatin. The main objective was to improve the mOS of patients after combined chemotherapy, from 10.0 months estimated with chemotherapy alone to 12.8 months, and reduce the risk of death by 22%. The most common grade 3–5 adverse events in the trial were neutropenia (experimental group,35%; control group,37%), anemia (10% vs 14%), and decreased appetite (8% vs 11%). Although the trial ultimately failed to meet its principal goal, adding bevacizumab to first-line treatment for AGC significantly improved patients’ PFS and overall efficiency (PFS:6.7 vs5.3 months). In this trial, regional differences in anti-angiogenic efficacy were observed, with a significant benefit from adding bevacizumab in North American and Latin American patients, but little benefit in Asian patients. This phenomenon may be attributed to the management of Asian patients and their higher risk of developing unpredictable diseases.
Sunitinib, a small-molecule RTK inhibitor that targets VEGFR, is highly effective in advanced renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal mesenchymal stromal tumors (GIST). 60 A phase I study demonstrated the feasibility and safety of sunitinib in combination with capecitabine and oxaliplatin chemotherapeutic agents in Korean patients with AGC. 61 Another phase I study 62 evaluated the safety of sunitinib in combination with S-1 and cisplatin for the treatment of advanced or metastatic GC in Japan. Compared with the previous average PFS of 6 months for AGC treated with sunitinib alone/S-1 + cisplatin, the PFS of 27 patients in this study was 12.5 months, suggesting that sunitinib in combination with chemotherapy has a promising antitumor activity. The most common grades 3 and 4 adverse reactions in the 27 patients were neutropenia and leukopenia, respectively. In 2011, sunitinib was approved as second-line monotherapy for patients with AGC who had previously received chemotherapy. Trastuzumab, which we previously described as a monoclonal antibody targeting HER-2, and, ramucirumab and apatinib, a monoclonal antibody and TKI targeting VEGFR-2, also have significant therapeutic effects in patients with GC.
Immune Checkpoint
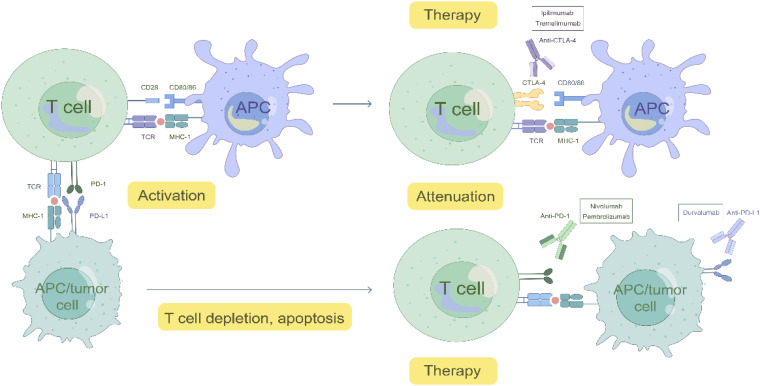
Immune checkpoints are vital for the stabilization of the immune microenvironment and avoidance of autoimmunity. Currently, the most comprehensively studied immune checkpoints are cytotoxic T-lymphocyte-associated molecule-4 (CTLA-4), PD-1, and PD-L1, 63 which exert inhibitory effects on immune cells in the tumor immune microenvironment through various pathways, thereby promoting immune evasion and facilitating tumor growth. 64 Immune checkpoint inhibitors (ICIs) that restore antitumor immunity by blocking co-inhibitory signaling pathways, have gradually replaced radiotherapy and chemotherapy as first-line treatments for many tumors (Figure 2).
Figure 2.
Mechanism of ICIs.
CTLA-4 is expressed on activated T-cells and regulatory T (Treg) cells, where it competes with CD28 for the binding ligand CD80/CD86, blocking afferent secondary co-stimulatory signals and impeding the activation of T-cells. 65 In 1996, the reduction in vivo tumor load in mice injected with an anti-CTLA-4 antibody after implantation of colon carcinoma or fibrosarcoma cells was the first study to demonstrate the antitumor effect produced by the inhibition of CTLA-4. 66 Ipilimumab was the first anti-CTLA-4 monoclonal antibody approved by the FDA for use in patients with advanced melanoma. 67 In one study, the ORR of patients with AGC treated with ipilimumab monotherapy was 14%. 68 Nivolumab is an anti-PD-1 monoclonal antibody with a precise complementary mechanism of action to that of ipilimumab. However, in a randomized global phase III trial (CheckMate-649), nivolumab in combination with ipilimumab failed to achieve the expected therapeutic effect of improving patients’ OS. 69
Tremelimumab, a human IgG 2 mAb inhibitor targeting CTLA-4, was used in a randomized, multicenter, phase II clinical study on the efficacy of tremelimumab alone or in combination with durvalumab, an anti-PD-L1 monoclonal antibody, in metastatic/recurrent GC or GEJCs, with unsatisfactory results. Patients with a mPFS of 1.7 months and a mOS of 7.7 months after tremelimumab monotherapy failed to show exciting activity. The ORR to combination therapy was also low. 70
PD-1 is a co-inhibitory molecule that is often expressed on activated T-cells. When combined with the ligand PD-L1, which is expressed on antigen-presenting and tumor cells, it causes T-cell depletion, apoptosis, and other endpoints, promoting immune evasion of tumors. 71 ICIs activate antitumor activity by blocking PD-1 and PD-L1 interactions. 68 The expression of PD tumor cells and immune cells can be detected by immunohistochemistry. PD-L1 is a biomarker that verifies the level of benefit in patients with various tumors treated with ICIs. 72 Existing studies have shown that ICI monotherapy is not clinically effective; combination of multiple approaches is usually used, including chemotherapy, targeted therapy, and radiation therapy. 68 A randomized, open-label, phase 3 clinical trial 73 showed that the combination of chemotherapy with nivolumab for AGC, GEJ/ esophageal adenocarcinoma(EA) resulted in a median follow-up OS of 6.7 months in the combination group of patients, compared with a median follow-up OS of 5.8 months for chemotherapy alone. Nivolumab, in combination with chemotherapy, significantly improved OS and PFS. Nivolumab, in combination with chemotherapy, is now approved in the United States as a new standard first-line treatment for patients with previously untreated AGC, GEJC, and EA.
Pembrolizumab is a humanized monoclonal IgG4-κ isotype antibody that binds to PD-1 and blocks the PD-L1/ PD-L2 interaction. 74 When pembrolizumab monotherapy was used to treat AGC and GEJCs, 75 the ORR was 11.6% in 259 patients, the complete remission rate (CRR) was 2.3%, and the median duration of remission was 8.4 months. Another 95 patients (42.4%) exhibited a reduction in tumor volume. ORR was higher in PD-L1-positive than in PD-L1-negative patients (15.5% vs 6.4%). On September 22, 2017, the FDA approved pembrolizumab for the treatment of recurrent locally advanced or metastatic adenocarcinoma of the stomach or GEJ expressing PD-L1. 76
However, despite the success of anti-CTLA-4 and anti-PD-1/PD-L-1 therapies in different types of tumor trials, only a small percentage of patients benefit from them (Table 2). In some of these, severe immune-related adverse events (irAEs) and local and systemic autoimmune responses, 77 such as rashes, colitis, pneumonitis, and adrenal or thyroid insufficiency, have been observed. 63 The challenge in the field of ICI antitumor therapy is to expand the beneficiary population by increasing the benefits without introducing new toxic effects. Fortunately, in addition to CTLA-4, PD-1/PD-L1, others such as LAG-3 (CD223), B7-H3, and BTLA (CD272) may be potential therapeutic targets, 63 and other immune-related targets such as CAR-T, TCR-T, lysosomal viruses, and neoantigen vaccines also have good therapeutic prospects. T-cells play an important role in cellular immunity. Immunotherapies targeting T-cells, including CAR-T and TCR-T immunotherapies, have achieved unprecedented efficacy in many malignant tumors. 78
Table 2.
ICIs-Related Targets and Treatment Options
| Item | Targets | Drugs | Phase | Patients | Therapeutic regimen | Result |
|---|---|---|---|---|---|---|
| ICIs | CTLA-4 PD-1 PD-L1 |
Ipilimumab Tremelimumab Nivolumab Pembrolizumab Durvalumab |
phase 1/2 phase 1b/2 phase 3 phase2 phase 1b/2 |
Advanced gastric cancer progressed on chemotherapy. Metastatic/recurrent gastric cancer or gastroesophageal junction cancer Advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma Advanced gastric cancer and gastroesophageal junction cancer Metastatic/recurrent gastric cancer or gastroesophageal junction cancer |
Monotherapy Monotherapy Nivolumab + chemotherapy Monotherapy Durvalumab + Tremelimumab |
ORR: 14% mPFS:1.7 months mOS:7.7months Significantly improved OS ORR:11.6% CRR: 2.3% median duration of remission: 8.4 months Low response rate |
CAR-T-cell therapy has demonstrated remarkable efficacy in patients with B-cell malignancies and multiple myeloma, yet its effectiveness in solid tumors remains suboptimal due to two primary reasons. Firstly, the TME enriched with Treg cell, TAMs, tumor-associated neutrophils (TAN), myeloid-derived suppressor cells (MDSCs), 79 among others, contributes to the establishment of an immunosuppressive TME, thus impeding the efficacy of CAR-T-cell therapy. Secondly, the scarcity of tumor-specific targets presents a challenge. Only a small fraction of solid tumors exhibits tumor-specific antigens suitable for CAR-T targets. The ideal CAR-T therapeutic target would be a membrane protein or glycolipid exclusively expressed on cancer cells’ surface without any expression on normal tissues. However, such a target is theoretically non-existent. Even in the case of successful CD19-CAR-T-cell therapy for B-cell malignancies, treated patients may develop B lymphopenia as normal B-cells also express CD19 on their surface. 80 Moreover, CAR-T treatment can lead to severe adverse events, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity (ICANS), primarily associated with IL-1 (Interleukin) and IL-6, impacting the therapeutic benefit for patients. 81
To overcome the immunosuppressive microenvironment, CAR-T-cells can be engineered as “armored” CAR-T-cells capable of secreting immunostimulatory cytokines like IL-12, IL-18, or IL-15. This helps reduce immunosuppression within the TME, enhancing the persistence and anti-tumor activity of CAR-T-cells.82,83 Furthermore, these modified CAR-T-cells can recruit endogenous immune cells, such as T memory cells and central memory T-cells, which are better suited for proliferation and long-term presence in the body. Treg, MDSCs and M2 macrophages with CAR-T-cells, or combining CAR-T-cells with chemotherapy, are also strategies that can enhance the persistence of CAR-T-cells. 84
One strategy to enhance the specificity of CAR-T-cell therapy involves engineering T-cells to express dual CARs. These dual CARs possess distinct signaling functions and extracellular antigen recognition regions, allowing them to target two different antigens present on the same cancer cell. This approach not only enhances the precision of tumor targeting but also minimizes off-target effects on normal tissue cells. 85 NK cells, a subset of innate lymphoid cells, possess the ability to spontaneously target and kill cells without the need for antigen sensitization, costimulatory signals, gene rearrangements, or Human leukocyte antigen (HLA) peptide complexes. Activated NK cells eliminate target T-cells through mechanisms such as releasing perforin and granzyme, inducing apoptosis by promoting FasL expression, and releasing tumour necrosis factor -α (TNF-α).86,87 CAR NK cell therapy exhibits superior tumor-specific targeting and cytotoxicity compared to CAR-T-cell therapy. CAR NK cells have a reduced secretion of inflammatory cytokines like IFN-γ and GM-CSF, while avoiding the secretion of pro-inflammatory cytokines like IL-1 and IL-6, thereby mitigating CRS risk. 88 Furthermore, NK cells can recognize cancer cells via various receptors such as natural cytotoxicity receptors, costimulatory receptors NKp46, NKp44, and NKp30, and NKG2D. Clinical samples of NK cells are readily available, making them an accessible resource for therapeutic applications. Considering these advantages, CAR NK therapy holds promise as an alternative approach to CAR-T-cell therapy. 84
In the treatment of solid tumors, TCR-T has demonstrated several advantages over CAR-T therapy. TCR-T-cells can recognize epitopes from both intracellular and cell membrane surface proteins, presented by the major histocompatibility complex (MHC), whereas CAR-T-cells are limited to epitopes on the cell surface. TCR-T has a broader epitope range compared to CAR-T. 89 Additionally, tumor antigens in cancer cells are predominantly intracellular, requiring lower epitope density for TCR-T-cell activation, enhancing tumor detection and killing capabilities. 90 Furthermore, the highest specificity antigens found in cancer cells can only bind to TCRs, not CARs. 91 CAR-T therapy can lead to supraphysiological T-cell activation, resulting in increased cytokine release and stronger CRS.
Claudins-Related Targets
Claudins are a new family of tight junction proteins involved in the interactions between epithelial and endothelial cells, connecting the most apical portions of adjacent basolateral cell membranes. 92 They prevent the movement of membrane proteins from the apical to basolateral end of the cell membrane, and maintain cell polarity. 93 Numerous studies have suggested that claudin promotes tumorigenesis through various signaling pathways and enzymes, and it is involved in tumor progression processes such as inflammation, survival, proliferation, and metastasis. 93 Targeting claudins for cancer treatment is a promising future strategy.
The most widely studied claudin family is claudin 18. Many studies have demonstrated that downregulation of claudin 18 expression is associated with increased invasiveness in early GC. 94 Compared to patients with GC having claudin 18 expression, patients lacking claudin 18 expression had shorter OS (5-year survival, 64.8% vs 90.5%); and poorer prognosis than patients with deletion of claudin7 expression.95,96 claudin 18.2, a splice variant of claudin 18, differentiates in the gastric mucosa. It is highly expressed in epithelial cells but is lacking in gastric stem cells. Claudin 18.2 is, however, present in malignant transformation and has an exposed extracellular loop; its targeting facilitates binding to monoclonal antibodies and reduces the chance of off-targeting.97,98 In the dose-escalation and safety trial by Qi et al, 31 of 37 patients (all with GEJC, pancreatic ductal adenocarcinoma, or other previously treated unexplained gastrointestinal malignancies) showed some tumor shrinkage. In all the patients, the ORR was 48.6% and the DCR was 73%. 99 This suggests that claudin 18.2 is a highly desirable therapeutic target for GC. In addition, claudin 18.2 is expressed in primary and metastatic GCs as well as pancreatic and ovarian cancers 100
Zolbetuximab is a chimeric human-mouse monoclonal antibody targeting claudin 18.2, which acts through complement activation and antibody-dependent cytotoxicity. 101 In the MONO study, 102 it was evaluated for its efficacy as monotherapy in patients with recurrent or refractory advanced GA as well as adenocarcinoma of the lower esophagus, all of whom showed high claudin 18.2 expression, and out of 43 patients with available antitumor data, four achieved partial remission, ORR 9% and six achieved stable disease (ORR 14%), with a clinical benefit rate of 23%. Common adverse reactions include nausea, vomiting, and fatigue. It has also been demonstrated that zolbetuximab enhances the efficacy of epirubicin + oxaliplatin + capecitabine chemotherapy in treating claudin18.2-positive GC and GEJCs. In this phase II study, 103 combining zolbetuximab with chemotherapy significantly improved the PFS and OS of patients (median 11.2 vs 9.9 months). These studies demonstrate the feasibility of claudin 18.2 as a target for treating GC with zolbetuximab.
CAR-T-cell immunotherapy is a new cancer treatment modality that has yielded encouraging results in hematologic malignancies and has caused a sensation. 104 CT041 is a CAR-T-cell expressing CLDN18.2 targeting. In a phase I trial, CT041 was used to treat patients with GC, and it was found that CT041 was not only well tolerated in patients with CLDN18.2-positive 105 cancers, but also that CAR-T-cells expanded more in ascites than in peripheral blood when treating patients with GC with multiple peritoneal metastases. Moreover, CT041 treatment in patients with GC was not inhibited by the expression status of PD-L1 in tumor cells or affected by anti-PD-1/PD-L1 inhibitors. CAR-T-cell therapy may be a breakthrough treatment for GC or other gastrointestinal cancers. Other members of the claudin family, such as claudin 4 and 7, are potential targets for the treatment of GC. The former is considered a marker for the development of GA precursor lesions, 106 and its overexpression not only inhibits the invasion and migration of GC cells but also enhances the function of the tight junction barrier. 107 The latter is highly expressed in GC, and one study showed that patients with claudin 7 expression had shorter OS than those without claudin 7 expression (5-year survival, 63.4% vs 79.0%). 95
Epigenetically Related Targets
Epigenetic regulation is indispensable for normal cell growth and development. If dysregulated, it drives aberrant transcriptional programs associated with tumorigenesis. Epigenetic modifications, including non-coding RNAs, miRNAs, DNA methylation, and histone modifications, are widespread in GC. DNA methylation and histone modifications are two of the most common and important epigenetic alterations. 108 DNA methylation is a common method of DNA modification in eukaryotic cells in vivo. Aberrant DNA hypomethylation leads to chromosomal instability, activation of oncogenes, and repression of transposons.109–111 This is the original driver of gastric carcinogenesis. 112 In addition, aberrant DNA methylation has been associated with chemotherapy resistance in patients with GC. 113 Therefore, DNA methylation is a promising therapeutic target for GC, and the homoterpene (3E)-4,8-dimethyl-13,7-nonatriene (DMNT) inhibitors have been developed to alter the DNA methylation status. Histone modifications include acetylation, methylation, phosphorylation, ubiquitination, and sumoylation. 114 Histone methylation is involved in the silencing of relevant tumor suppressor genes, 115 which are implicated in and play important role in many processes related to carcinogenesis, such as DNA repair, metabolism, cell adhesion, cell cycle control and apoptosis, and angiogenesis. 116 Changes in the methylation levels of certain histones are correlated with increased cancer recurrence and decreased survival. Fortunately, gene silencing is reversible and normal expression of tumor suppressor genes can be restored by targeting histone modifications.
Long-stranded non-coding RNAs (lncRNAs) are a class of non-coding RNAs that are more than 200 nucleotides in length and do not encode proteins but are involved in regulating a variety of genes and various physiological processes. 117 lncRNAs use polymerase II to induce changes in genes on different chromosomes in the trans and cis modes of regulation to cause chromosomal remodeling of genes on the same chromosome. 118 In addition to affecting oncogenes, lncRNAs play important roles in gastric carcinogenesis, cell proliferation, apoptosis, migration, invasion, metastasis, and angiogenesis. 119 Recently, an increasing number of studies have identified aberrant expression of lncRNAs in GC. 120 For example, lncRNA H19 promotes GC cell proliferation, migration, and invasion by activating the NF-κB signaling pathway and inhibits apoptosis in GC cells. 121 Particularly in HP-infected GC, the expression of H19 is significantly higher than that in HP-negative patients and normal subjects. 122 lncRNA AC093818.1 is also highly expressed in metastatic GC, promotes the expression of PDK-1 (involved in the invasion of GC cells in vivo and ex vivo, and in liver and lung metastasis), and closely correlates with gastric carcinogenesis. 123 Another lncRNA, SNHG17, facilitates cell proliferation and metastasis, and inhibits G0/G1 blockade and apoptosis. Moreover, it targets EZH2 and SUZ12 to inhibit the p15 and p57 pathways, all of which are involved in GC genesis and development. 124 In addition to the aforementioned lncRNAs, many lncRNAs related to GC are highly expressed in GC. They induce tumorigenesis and shorten survival in various ways, making them predictive biomarkers for patients with GC and promising targets for GC therapy. 125 MiRNAs are a class of endogenous nucleotides that range in length from 17 to 25 and are non-coding regulatory RNAs involved in many biological processes, such as cell proliferation, development, and invasion. Various miRNAs (miRNA-674, miRNA-181b, and miRNA-148a) are overexpressed in GC, and promote cell proliferation and migration and inhibit apoptosis, 19 miRNAs can be divided into two main categories according to their functions: oncogenic miRNAs, whose oncogenic functions can be blocked by miRNA inhibitors, and tumor-suppressor miRNAs, which use recombinant miRNA precursors to promote their functions. 126 Inhibiting the expression of the former and restoring the expression of the later are prospective therapeutic options for GC that can provide new choices for patients. CircRNAs have also been reported to play an important role in GC progression and may be potential therapeutic targets. 16 Epigenetic regulation is essential for the maintenance of normal functional activities in an organism, and when uncontrolled, it drives tumor formation. Along with the emergence of numerous epigenetic targets, there is new hope for our patients.
Other Signaling Pathway-Related Targets
Recently, an increasing number of studies have focused on signaling pathway-related targets. The pI3 K/AKT/mTOR signaling pathway plays an important role in the occurrence and development of GC. This molecular pathway consists of two crucial components: phosphatidylinositol 3-kinase (PI3 K) and protein kinase B (PKB/AKT). The activation of PI3 K is dependent on the binding of various growth factors, hormones, and cytokines to RTK or G- protein-coupled receptors (GPCR). Once PI3 K is activated, it triggers the activation of AKT and other protein kinases, including downstream activation of the mechanistic target of rapamycin (mTOR). 127 After activation of the PI3 K/AKT/mTOR signaling pathway, it promotes tumor cell survival, proliferation, adhesion and migration by inhibiting apoptosis-related genes and activating anti-apoptotic proteins. 128 The PI3 K/AKT/mTOR signaling pathway is also associated with resistance to lapatinib treatment in patients with HER-2-amplified GC. 129 Autophagy is a self-protection mechanism of cells, which means the T-cells promote the production of a variety of metabolic products by degrading their own macromolecules or organelles to provide energy for survival. 130 However, in GC, mTOR, an important component in the PI3 K/AKT/mTOR signaling pathway, is upregulated and inhibits protective autophagy. 131
The Notch signaling pathway is crucial for cell proliferation, differentiation, and apoptosis, playing a significant role in the self-renewal of stem cells and regulating cell fate in both embryonic and adult stages. 132 Dysregulation of this pathway is closely linked to cancer stem cell maintenance, tumor cell metabolism, angiogenesis, and tumor immunity. 133 Notably, activation of both Notch and mTOR pathways is frequently observed in human GA tissues. Experimental evidence suggests that activation of the Notch pathway enhances the activity of mouse LGR5+ gastric antral stem cells, leading to the formation of highly proliferative, undifferentiated gastric antral polyps. These polyps also exhibit activation of the mTOR signaling pathway, indicating a cooperative relationship between the Notch and mTOR pathways in driving excessive proliferation of gastric epithelial cells. 134
The mitogen-activated protein kinase (MAPK) signaling pathway is a crucial component of the eukaryotic signal transmission network, playing key roles in cell proliferation, differentiation, apoptosis, and stress response. Comprising four distinct pathways, the most well-known is the RAS/RAF/MEK/ERK signaling pathway. Upon activation of RAS it interacts with various downstream proteins, such as RAF. RAS-GTP recruits RAF protein to the plasma membrane, activating its kinase function. The activated RAF kinase then binds to MEK, leading to ERK activation.127,135 The RAF protein family includes BRAF, ARAF, and CRAF, with the BRAF gene mutation rate in cancer being 7%-10%. 136 Mutations in BRAF and RAS genes can aberrantly activate the MAPK signaling pathway and enhance PI3 K/AKT/mTOR pathway activity. 137 These pathways synergistically contribute to GC development by promoting tumor cell growth, survival, proliferation, angiogenesis, and metastasis.
The occurrence of GC is closely related to gene mutations. The most common mutated genes are ARID1A, CDH1, ERBB3, KRAS, PIK3CA and TP53. 138 A study found that PIK 3CA mutations affected the overall prognosis of patients with GC, patients with PIK 3CA amplification had significantly shorter survival time (516.0 months vs758.4 months on average) than those without PIK 3CA amplification. 139 It was closely related to tumor cells with abnormal proliferation and apoptosis.
The role of the TP53 signaling pathway in GC makes it a potential for targeted therapy. TP53 mainly regulates DNA repair and modulates the cell cycle, apoptosis, and differentiation. 140 It has been claimed that more than 75% of patients with GC have high levels of TP53 expression, and the mutation rate of the TP53 gene in all patients with GC can reach 30%.141,142 DDIT4 is a DNA damage-induced transcript that exhibits higher expression in GC tissues compared to normal tissues. It facilitates GC cell proliferation and tumorigenesis by activating the TP53 and MAPK signaling pathways.
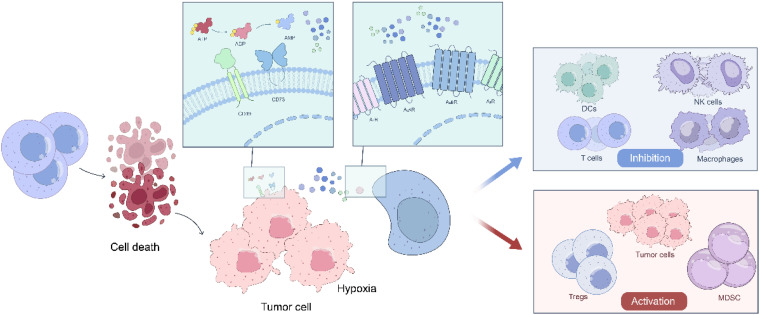
Adenosine is an energy metabolite that participates in a variety of processes such as inflammation, hypoxia, and the TME, and plays an important role in regulating tumor cell growth and apoptosis (Figure 3). In the TME, adenosine is converted to inosine through a series of pathways, and inosine inhibits the activation and function of CD8+ T-cells and NK cells, weakening the immune response and creating an immunosuppressive environment that promotes tumorigenesis. 9 In addition, the activation of adenosine receptors (A1, A2, A3) is associated with an increase in the release of anti-inflammatory cytokines and a decrease in the release of anti-inflammatory mediators. When there is damage to the tissues, such as occurs in inflammation and ischemia, the concentration of adenosine increases sharply with binding to receptors to inhibit the activation (such as Cytotoxic T lymphocytes and NK cells) and functions of the immune cells18,143; thus, suppressing the immune response. Adenosine receptor activation also induces tumor cell proliferation, leading to tumor growth and survival. 2 Given the special role of adenosine in tumor immunity, the adenosine pathway has been studied and researched. However, the targeted therapy of GC for the adenosine pathway is in its early stages and has not yet been popularized. It is usually believed that combination immunotherapy is the best way to treat patients with cancer, and several studies combining adenosine signaling pathway-targeted therapy with other therapies, such as immunotherapy, the MAPK signaling pathway, and immune checkpoint blockade, can improve clinical therapeutic benefits.
Figure 3.
Immunomodulatory effects of adenosine in hypoxic TME.
In hypoxic TME, adenosine is gradually generated from ATP under the action of CD39 and CD73. After binding to its receptors (A1R, A2AR, A2BR, and A3R), it inhibits the antigen presentation ability of dendritic cells (DCs), promotes the transformation of macrophages from M1 to M2, inhibits the maturation of NK cells, and weakens the activity of NK cells. Prevent the production of IL-2 by CD4+ T-cells and the proliferation and differentiation of CD8+ T-cells. In addition, adenosine promotes the differentiation of Treg cells, participates in the proliferation and migration of tumor cells, and promotes the immunosuppressive function of MDSCs
Novel Biomarkers for GC
GC is a prevalent malignant tumor globally, frequently diagnosed in late stages. Certain patients do not exhibit favorable responses to targeted therapy and immunotherapy. Hence, the identification of suitable biomarkers is crucial for assessing the effectiveness and prognosis of diverse treatments for GC.
PD-L1 is typically found on antigen-presenting cells and tumor cells, while PD-1 is expressed on activated T-cells. Their interaction leads to T-cell exhaustion and inactivation, allowing tumors to evade immune surveillance. ICIs can disrupt this interaction, exerting an anti-tumor effect. Therefore, the level of PD-L1 expression can indicate GC's response to ICIs, often assessed through immunohistochemistry.
lncRNA plays a crucial role in Helicobacter pylori infection, TME modulation, drug resistance, and related signaling pathways, influencing the development, metastasis, and progression of GC. 144 It can serve as both a treatment target and a potential biomarker for predicting response to GC immunotherapy. LncRNAs are linked to the upregulation of inhibitory immune checkpoints. For instance, the expression of lncRNA hypoxia-inducible factor 1 alpha-antisense RNA 2 (HIF1a-AS2) is elevated in GC tissues, where it negatively regulates miR-429. This miRNA (microRNA), in turn, binds to PD-L1, inhibiting its expression. HIF1a-AS2 promotes PD-L1 expression by sequestering miR-429, contributing to GC development. 145 LncRNAs also impact tumor metabolism through various mechanisms such as cis-regulation, antisense inhibition, and protein interactions. A study developed a GC risk model based on metabolic lncRNAs from The Cancer Genome Atlas (TCGA) database, reflecting tumor immune status and mutation burden. This model can independently predict the prognosis of GC patients, particularly those undergoing ICI treatment. 146
MiRNAs play various roles in gastric carcinogenesis, with examples including anti-angiogenic miR-218, pro-angiogenic miR-130a, and miR616–3p. Additionally, miR-92a, miR-433, miR-454, and miR-497 are implicated in regulating apoptosis and proliferation of GC cells. 147 Aberrant expression of numerous miRNAs, such as miR223, miR-233, miR-378, miR-421, miR-451, miR-4865p, and miR-199-3p, has been observed in the plasma and serum of GC patients. 148 These miRNAs can be detected in blood samples using reliable methods like reverse transcription quantitative real-time polymerase chain reaction and Next-generation sequencing (NGS). 149 Research by Zhu et al has demonstrated that combining miR-425-5p, miR-1180-3p, miR122-5p, miR-24-3p, and miR-4632-5p with gastroscopy significantly enhances early GC diagnosis. 150 Moreover, Zheng et al revealed that miR-196a-5p promotes the progression from chronic atrophic gastritis to GC by facilitating cell proliferation and migration while inhibiting apoptosis. This miRNA could serve as a potential biomarker for detecting the development of both conditions. 151
Liquid biopsy is a non-invasive detection technology that collects body fluid samples from tumor patients to detect free circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes in order to gather tumor-related information. CTCs are cells shed from primary tumors or metastases that enter the bloodstream. CTCs play a significant role in the diagnosis, prognosis, treatment response, and drug resistance mechanisms in various cancers. 152 For instance, a study found a link between CTCs and chemotherapy effectiveness. By monitoring CTCs levels before and after chemotherapy treatment, researchers observed that patients with AGC who had ≥4 CTCs had significantly shorter OS and PFS compared to those with <4 CTCs. 153 This suggests that measuring CTCs could serve as a diagnostic tool for AGC patients undergoing specific chemotherapy regimens.
On the other hand, ctDNA refers to DNA fragments released by tumor cells into the blood, which can be detected using highly sensitive methods. In a study by Jin et al, patients with AGC who underwent PD-1 antibody immunotherapy and genetic sequencing testing showed varying levels of ctDNA post-treatment, with a clear correlation between ctDNA levels and treatment response. Monitoring changes in ctDNA levels could potentially serve as a biomarker for assessing the response of AGC to immunotherapy with ICIs, with a decrease in ctDNA levels indicating a more favorable treatment response. 154
Discussion
This study delves into the various treatment modalities for GC, such as chemotherapy, targeted therapy, and immunotherapy, and conducts a comparative analysis of multiple trials to assess treatment efficacy. Specifically, findings show that trastuzumab monotherapy in targeted HER-2 therapy yielded better clinical outcomes for patients when compared to chemotherapy involving irinotecan and paclitaxel. Additionally, the combination of trastuzumab with platinum- and fluorouracil-based chemotherapy demonstrated a significant enhancement in overall patient survival rates for those with AGC or GEJC, particularly in cases with high HER-2-positive expression. Trastuzumab has emerged as the primary approved drug for targeted therapy in GC. However, the study found no substantial survival improvement in patients with HER-2-positive metastatic GC or GEJC when treated with pertuzumab alongside trastuzumab and chemotherapy, nor was there a significant difference in survival rates between lapatinib and capecitabine. Overall, limited efficacy was observed in patients with HER-2-positive advanced or metastatic GC, esophageal cancer, or GEA.
Tumors rely on angiogenesis for nutrient supply. In the REGARD study, ramucirumab, a VEGFR-2 antagonist, was assessed as a standalone treatment for patients with advanced or GEJA. The trial showed an OS of 5.2 months, compared to 3.8 months in the placebo group. In the RAINBOW trial, the combination of ramucirumab and paclitaxel was studied in similar patients, resulting in a significantly longer OS period compared to the placebo and paclitaxel groups (9.6 months vs7.4 months). Ramucirumab demonstrates promising anti-tumor effects in both monotherapy and combination therapy settings. In the evaluation of TKIs, cetuximab plus S-1 plus cisplatin was studied for patients with AGC, showing good tolerance but no additional clinical benefits. Similarly, the combination of anti-docetaxel and oxaliplatin did not show improved clinical benefits in patients with metastatic GA and GEJA, possibly due to cetuximab's limited interaction with chemotherapy drugs. Sunitinib, another VEGFR-targeting drug, has been approved as a second-line monotherapy for patients with AGC after chemotherapy.
In addition to targeted therapies, our research also delved into the development of immunotherapy for GC. We found that treating GC patients with nivolumab combined with chemotherapy versus nivolumab combined with ipilimumab resulted in improved clinical benefits for the patients. This combination was approved in the United States as a new treatment for patients with previously untreated AGC, GEJC, and EA as a standard first-line treatment. Pembrolizumab, which blocks the PD-L1/PD-L2 interaction by binding with PD-1 and inhibiting T-cell inactivation, has shown positive clinical outcomes in patients with AGC and gastroesophageal cancer when used alone. In 2017, the FDA approved pembrolizumab for the treatment of recurrent locally advanced or metastatic gastric or GEJA expressing PD-L1. It is crucial to make correct and reasonable treatment choices during GC therapy, as different options yield different therapeutic effects that directly impact a patient's prognosis and treatment outcome.
Conclusion
Cancer, as a significant cause of mortality, has always been a focal point in medical research. GC, in particular, poses a substantial threat to global health. Various treatment modalities have been developed for clinical use, including radiotherapy, chemotherapy, ICIs, cancer vaccines, and targeted therapies. The emergence of targeted therapy has brought optimism to GC treatment, offering new avenues for clinical management and expanding our understanding of cancer. Despite these advancements, challenges persist on the path of development. One of the major obstacles to targeted therapy is the development of drug resistance in patients, which significantly diminishes treatment efficacy. Moreover, treatment-related irAEs, such as cutaneous, gastrointestinal, hepatic, and endocrine toxicities, further complicate the treatment landscape. While CAR-T-cells have shown remarkable efficacy in hematological malignancies, their application in treating solid tumors remains limited due to challenges related to tumor antigen recognition and the tumor immune microenvironment. TCR-T therapy offers advantages over CAR-T in solid tumor treatment, but is also hindered by issues such as cytokine storm toxicity and the impact of the TME. The development of CAR-T and TCR-T therapies has introduced novel approaches to cancer treatment. As scientific advancements and knowledge of tumors progress, overcoming these obstacles appears promising. The future of GC treatment is poised to expand, with targeted therapy, immunotherapy, radiotherapy, chemotherapy, and combination therapy offering patients a wider array of treatment options. Specifically, targeting HER-2, VEGFR, ICIs (PD-1/PD-L1, CTLA-4), as well as emerging targets like CAR-T, TCR-T, and claudin, holds great promise for improving GC treatment.
The global burden of GC highlights the need for more effective treatment options. Emphasizing the exploration of novel treatment approaches, tailored therapies, and understanding drug resistance mechanisms can enhance treatment efficacy and minimize side effects. Additionally, the discovery of reliable biomarkers is essential for early detection, prognostic assessment, and treatment evaluation in GC.
Acknowledgement
None.
Abbreviation
- GC
gastric cancer
- AGC
advanced gastric cancer
- GA
gastric adenocarcinoma
- TME
tumor microenvironment
- TAM
tumor-associated macrophages
- VEGF
vascular endothelial growth factor
- TGF-β
transforming growth factor-β
- PD-L1
programmed cell death ligands 1
- PD-1
programmed cell death protein 1
- CircRNAs
circular RNAs
- MiRNAs
microRNAs
- EGFR
epidermal growth factor receptor family
- GEA
gastroesophageal adenocarcinoma
- GEJC
gastroesophageal gastroesophageal junction cancer
- GEJA
gastroesophageal junction adenocarcinoma
- EA
esophageal adenocarcinoma
- PFS
progression-free survival
- NK
natural killer
- ORR
objective response rate
- DCR
disease control rate
- OS
overall survival
- TK
tyrosine kinases
- TKIs
tyrosine kinase inhibitors
- RTKs
receptor tyrosine kinases
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- CTLA-4
cytotoxic T-lymphocyte-associated molecule-4
- ICIs
immune checkpoint inhibitors
- Treg cell
regulatory T cells
- CRR
complete remission rate
- TAN
tumor-associated neutrophils
- MDSCs
myeloid-derived suppressor cells
- CRS
cytokine release syndrome
- Interleukin
IL
- lncRNAs
long-stranded non-coding RNAs
- MAPK
mitogen-activated protein kinase
- TNF-α
tumour necrosis factor -α
Footnotes
Author Contributions: Mingfang Wu: Conceptualization, Supervision, Writing-original draft, Writing-review and editing. Shiman Yuan: Writing-original draft, Writing-review and editing. Kai Liu: Writing-original draft, Writing-review and editing. Chenyu Wang: Software, Writing-original draft. Feng Wen: Conceptualization, Supervision, Writing-review and editing.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: Our study did not require an ethical board approval because it did not contain human or animal trials.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Feng Wen https://orcid.org/0009-0009-6203-0559
References
- 1.Joshi S, Badgwell BJ. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264-279. doi: 10.3322/caac.21657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Du L, Chen XJ. Adenosine signaling: Optimal target for gastric cancer immunotherapy. Front Immunol. 2022;13:1027838. doi: 10.3389/fimmu.2022.1027838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet.. 2020;396(10251):635-648. doi: 10.1016/S0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- 4.Boku N. HER2-positive Gastric cancer. Gastric Cancer. 2014;17(1):1-12. doi: 10.1007/s10120-013-0252-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Q, Sun B, Xue T, et al. Advances in CAR T-cell therapy in bile duct, pancreatic, and gastric cancers. Front Immunol. 2022;13:1025608. doi: 10.3389/fimmu.2022.1025608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Xu J, Xie J, Yang WJ. Research progress in targeted therapy and immunotherapy for gastric cancer. Chin Med J (Engl). 2022;135(11):1299-1313. doi: 10.1097/CM9.0000000000002185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W, Yang Z, Lu N. Molecular targeted therapy for the treatment of gastric cancer. J Exp Clin Cancer Res. 2016;35:1. doi: 10.1186/s13046-015-0276-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedard P, Hyman D, Davids M, Siu LJL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395(10229):1078-1088. doi: 10.1016/S0140-6736(20)30164-1 [DOI] [PubMed] [Google Scholar]
- 9.Shi L, Yang L, Wu Z, Xu W, Song J, Guan W. Adenosine signaling: Next checkpoint for gastric cancer immunotherapy? Int Immunopharmacol 2018;63:58-65. doi: 10.1016/j.intimp.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 10.Kamath V. Cancer vaccines: An unkept promise? Drug Discov Today. 2021;26(6):1347-1352. doi: 10.1016/j.drudis.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 11.Gambardella V, Castillo J, Tarazona N, et al. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. 2020;86:102015. doi: 10.1016/j.ctrv.2020.102015 [DOI] [PubMed] [Google Scholar]
- 12.Bolandi N, Derakhshani A, Hemmat N, et al. The Positive and Negative Immunoregulatory Role of B7 Family: Promising Novel Targets in Gastric Cancer Treatment. Int J Mol Sci. 2021;22(19):10719. doi: 10.3390/ijms221910719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keir M, Butte M, Freeman G, Sharpe A. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. doi: 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao L, Guan X, Xiang M, et al. B7 family protein glycosylation: Promising novel targets in tumor treatment. Front Immunol. 2022;13:1088560. doi: 10.3389/fimmu.2022.1088560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latchman Y, Wood C, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261-268. doi: 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- 16.Shan C, Zhang Y, Hao X, Gao J, Chen X, Wang K. Biogenesis, functions and clinical significance of circRNAs in gastric cancer. Mol Cancer. 2019;18(1):136. doi: 10.1186/s12943-019-1069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Jiang J, Shi H, et al. CircRNA: A rising star in gastric cancer. Cell Mol Life Sci. 2020;77(9):1661-1680. doi: 10.1007/s00018-019-03345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan Y, Li Z, Shen Y, et al. Functions of circular RNAs and their potential applications in gastric cancer. Expert Rev Gastroenterol Hepatol. 2020;14(2):85-92. doi: 10.1080/17474124.2020.1715211 [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Shen J, Xiao Z, et al. An overview of the multifaceted roles of miRNAs in gastric cancer: Spotlight on novel biomarkers and therapeutic targets. Biochem Pharmacol. 2019;163:425-439. doi: 10.1016/j.bcp.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 20.Ahadi A. Dysregulation of miRNAs as a signature for diagnosis and prognosis of gastric cancer and their involvement in the mechanism underlying gastric carcinogenesis and progression. IUBMB Life. 2020;72(5):884-898. doi: 10.1002/iub.2259 [DOI] [PubMed] [Google Scholar]
- 21.Wu H, Lin W, Tsai K. Advances in molecular biomarkers for gastric cancer: MiRNAs as emerging novel cancer markers. Expert Rev Mol Med. 2014;16:e1. doi: 10.1017/erm.2013.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Zhang X, Li Y, et al. The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747-795. doi: 10.1002/cac2.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravalos C, Jimeno A. HER2 In gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523-1529. doi: 10.1093/annonc/mdn169 [DOI] [PubMed] [Google Scholar]
- 24.Lei Y, Huang J, Zhao Q, et al. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: A meta-analysis of literature. World J Surg Oncol. 2017;15(1):68. doi: 10.1186/s12957-017-1132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shitara K, Bang Y-J, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419-2430. doi: 10.1056/NEJMoa2004413 [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem E, di Bartolomeo M, Smyth E, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): Primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023;24(7):744-756. doi: 10.1016/S1470-2045(23)00215-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bang Y, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 28.Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402(10418):2197-2208. doi: 10.1016/S0140-6736(23)02033-0v [DOI] [PubMed] [Google Scholar]
- 29.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metasta tic gastric or gastro-oesophageal junction cancer (JACOB): Final analy sis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372-1384. doi: 10.1016/S1470-2045(18)30481-9 [DOI] [PubMed] [Google Scholar]
- 30.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab, trastuzumab, and chemotherapy in HER2-positive gastric/gas troesophageal junction cancer: End-of-study analysis of the JACOB phas e III randomized clinical trial. Gastric Cancer. 2023;26(1):123-131. doi: 10.1007/s10120-022-01335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordstrom J, Gorlatov S, Zhang W, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced fcγ receptor binding properties. Breast Cancer Res. 2011;13(6):R123. doi: 10.1186/bcr3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markham AJD. Margetuximab: First approval. Drugs. 2021;81(5):599-604. doi: 10.1007/s40265-021-01485-2 [DOI] [PubMed] [Google Scholar]
- 33.Catenacci D, Kang Y, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): A single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21(8):1066-1076. doi: 10.1016/S1470-2045(20)30326-0 [DOI] [PubMed] [Google Scholar]
- 34.Gambardella V, Fleitas T, Tarazona N, et al. Towards precision oncology for HER2 blockade in gastroesophageal adenocarcinoma. Ann Oncol . 2019;30(8):1254-1264. doi: 10.1093/annonc/mdz143 [DOI] [PubMed] [Google Scholar]
- 35.Caruso C . ZW25 Effective in HER2-positive cancers. Cancer Discovery. 2019;9(1):8. doi: 10.1158/2159-8290.CD-NB2018-162 [DOI] [PubMed] [Google Scholar]
- 36.Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17(1):1-12. doi: 10.1007/s10120-013-0252-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hecht J, Bang Y, Qin S, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–A randomized phase III trial. Journal of Clinical Oncology. 2016;34(5):443-451. doi: 10.1200/JCO.2015.62.6598 [DOI] [PubMed] [Google Scholar]
- 38.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285(21):1182-1186. doi: 10.1056/NEJM197111182852108 [DOI] [PubMed] [Google Scholar]
- 39.Cleary J, Horick N, McCleary N, et al. FOLFOX Plus ziv-aflibercept or placebo in first-line metastatic esophagogastric adenocarcinoma: A double-blind, randomized, multicenter phase 2 trial. Cancer. 2019;125(13):2213-2221. doi: 10.1002/cncr.32029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs C, Tomasek J, Yong C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31-39. doi: 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 41.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235. doi: 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 42.Li H, Huang H, Zhang T, et al. Apatinib: A novel antiangiogenic drug in monotherapy or combination immunotherapy for digestive system malignancies. Front Immunol. 2022;13:937307. doi: 10.3389/fimmu.2022.937307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Zhu X, Wang Y, et al. Prognostic value and association of Lauren classification with VEGF and VEGFR-2 expression in gastric cancer. Oncol Lett. 2019;18(5):4891-4899. doi: 10.3892/ol.2019.10820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson D, Wu Y, Lin SJO. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19(49):5548-5557. doi: 10.1038/sj.onc.1203957 [DOI] [PubMed] [Google Scholar]
- 45.Mongre R, Mishra C, Shukla A, et al. Emerging Importance of Tyrosine Kinase Inhibitors against Cancer: Quo Vadis to Cure?. Int J Mol Sci. 2021;22(21):11659. doi: 10.3390/ijms222111659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du Z, Lovly C. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17(1):58. doi: 10.1186/s12943-018-0782-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang L, Jiang S, Shi YJ. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001-2020). J Hematol Oncol. 2020;13(1):143. doi: 10.1186/s13045-020-00977-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan D, Zhu Z, Zhang X, Liu J. Targeted therapy for gastric cancer: Current status and future directions (review). Oncol Rep. 2016;35(3):1245-1254. doi: 10.3892/or.2015.4528 [DOI] [PubMed] [Google Scholar]
- 49.Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. JNat Rev Clin Oncol. 2011;8(8):492-503. doi: 10.1038/nrclinonc.2011.45 [DOI] [PubMed] [Google Scholar]
- 50.Ha S, Lee J, Jang J, et al. HER2-positive Gastric cancer with concomitant MET and/or EGFR overexpression: A distinct subset of patients for dual inhibition therapy. Int J Cancer. 2015;136(7):1629-1635. doi: 10.1002/ijc.29159 [DOI] [PubMed] [Google Scholar]
- 51.Fukuda K, Saikawa Y, Takahashi M, et al. Antitumor effect of cetuximab in combination with S-1 in EGFR-amplified gastric cancer cells. IInt J Oncol. 2012;40(4):975-982. doi: 10.3892/ijo.2011.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim M, Lee H, Lee H, Jeon Y, Yang H, Kim WJH. EGFR In gastric carcinomas: Prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52(6):738-746. doi: 10.1111/j.1365-2559.2008.03021.x [DOI] [PubMed] [Google Scholar]
- 53.Chan J, Blaszkowsky L, Enzinger P, et al. A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Ann Oncol . 2011;22(6):1367-1373. doi: 10.1093/annonc/mdq604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi K, Fuse N, Komatsu Y, et al. Phase II study of cetuximab plus S-1/cisplatin therapy in Japanese patients with advanced gastric cancer. Jpn J Clin Oncol. 2021;51(6):879-885. doi: 10.1093/jjco/hyaa276 [DOI] [PubMed] [Google Scholar]
- 55.Richards D, Kocs D, Spira A, et al. Results of docetaxel plus oxaliplatin (DOCOX) ± cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: Results of a randomised phase 2 study. Eur J Cancer.. 2013;49(13):2823-2831. doi: 10.1016/j.ejca.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 56.Okines A, Ashley S, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: Dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol. 2010;28(25):3945-3950. doi: 10.1200/JCO.2010.29.2847 [DOI] [PubMed] [Google Scholar]
- 57.G QA, M S, S C, et al. First-line panitumumab plus docetaxel and cisplatin in advanced gastric and gastro-oesophageal junction adenocarcinoma: results of a phase II trial. Clin Transl Oncol. 2019;22(4):495–502. doi: 10.1007/s12094-019-02151-6 [DOI] [PubMed] [Google Scholar]
- 58.Yazici O, Sendur M, Ozdemir N, Aksoy S. Targeted therapies in gastric cancer and future perspectives. World J Gastroenterol. 2016;22(2):471-489. doi: 10.3748/wjg.v22.i2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohtsu A, Shah M, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968-3976. doi: 10.1200/JCO.2011.36.2236 [DOI] [PubMed] [Google Scholar]
- 60.Rini B. Sunitinib. Expert Opin Pharmacother. 2007;8(14):2359-2369. doi: 10.1517/14656566.8.14.2359 [DOI] [PubMed] [Google Scholar]
- 61.Lee K, Park S, Oh D, et al. Phase I study of sunitinib plus capecitabine/cisplatin or capecitabine/oxaliplatin in advanced gastric cancer. Invest New Drugs. 2013;31(6):1547-1558. doi: 10.1007/s10637-013-0032-y [DOI] [PubMed] [Google Scholar]
- 62.Boku N, Muro K, Machida N, et al. Phase I study of sunitinib plus S-1 and cisplatin in Japanese patients with advanced or metastatic gastric cancer. Invest New Drugs.. 2014;32(2):261-270. doi: 10.1007/s10637-013-9948-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marin-Acevedo J, Kimbrough E, Lou YJ. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14(1):45. doi: 10.1186/s13045-021-01056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marin-Acevedo J, Dholaria B, Soyano A, et al. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J Hematol Oncol. 2018;11(1):39. doi: 10.1186/s13045-018-0582-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagchi S, Yuan R, Engleman E. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223-249. doi: 10.1146/annurev-pathol-042020-042741 [DOI] [PubMed] [Google Scholar]
- 66.Leach D, Krummel M, Allison JJS. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734-1736. doi: 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- 67.Wei S, Duffy C, Allison J. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069-1086. doi: 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 68.Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188615. doi: 10.1016/j.bbcan.2021.188615 [DOI] [PubMed] [Google Scholar]
- 69.Shitara K, Ajani J, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942-948. doi: 10.1038/s41586-022-04508-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly R, Lee J, Bang Y, et al. Safety and efficacy of durvalumab and tremelimumab alone or in combination in patients with advanced gastric and gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2020;26(4):846-854. doi: 10.1158/1078-0432.CCR-19-2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coutzac C, Pernot S, Chaput N, Zaanan A. Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol. 2019;133:25-32. doi: 10.1016/j.critrevonc.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 72.Doroshow D, Bhalla S, Beasley M, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18(6):345-362. doi: 10.1038/s41571-021-00473-5 [DOI] [PubMed] [Google Scholar]
- 73.Janjigian Y, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40. doi: 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamath S, Kalyan A, Benson A. Pembrolizumab for the treatment of gastric cancer. Expert Rev Anticancer Ther. 2018;18(12):1177-1187. doi: 10.1080/14737140.2018.1526084 [DOI] [PubMed] [Google Scholar]
- 75.Fuchs C, Doi T, Jang R, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA oncology. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fashoyin-Aje L, Donoghue M, Chen H, et al. FDA Approval summary: Pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24(1):103-109. doi: 10.1634/theoncologist.2018-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Darvin P, Toor S, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1-11. doi: 10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250. doi: 10.3389/fimmu.2019.02250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Q, Li J, Zheng H, et al. Adoptive cellular immunotherapy for solid neoplasms beyond CAR-T. Mol Cancer. 2023;22(1):28. doi: 10.1186/s12943-023-01735-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitra A, Barua A, Huang L, Ganguly S, Feng Q, He B. From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol. 2023;14:1188049. doi: 10.3389/fimmu.2023.1188049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferreros P, Trapero I. Interleukin Inhibitors in Cytokine Release Syndrome and Neurotoxicity Secondary to CAR-T Therapy. Diseases. 2022;10(3):41. doi: 10.3390/diseases10030041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zimmermann K, Kuehle J, Dragon AC, et al. Design and Characterization of an “All-in-One” Lentiviral Vector System Combining Constitutive Anti-GD2 CAR Expression and Inducible Cytokines. Cancers (Basel). 2020;12(2):375. doi: 10.3390/cancers12020375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Y, Li D, Qin DY, et al. Interleukin-armed chimeric antigen receptor-modified T cells for cancer immunotherapy. Gene Ther. 2017;25(3):192-197. doi: 10.1038/gt.2017.81 [DOI] [PubMed] [Google Scholar]
- 84.Maalej KM, Merhi M, Inchakalody VP, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. 2023;22(1):20. doi: 10.1186/s12943-023-01723-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan J, Tang K, Luo Y, et al. Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: A single-arm, phase 2 study. Lancet Oncol. 2023;24(11):1229-1241. doi: 10.1016/S1470-2045(23)00436-9 [DOI] [PubMed] [Google Scholar]
- 86.Perera Molligoda Arachchige AS. Human NK cells: From development to effector functions. Innate Immun. 2021;27(3):212-229. doi: 10.1177/17534259211001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfefferle A, Jacobs B, Haroun-Izquierdo A, Kveberg L, Sohlberg E, Malmberg K-J. Deciphering Natural Killer Cell Homeostasis. Front Immunol. 2020;11:812. doi: 10.3389/fimmu.2020.00812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545-553. doi: 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shafer P, Kelly LM, Hoyos V. Cancer Therapy With TCR-Engineered T Cells: Current Strategies, Challenges, and Prospects. Front Immunol. 2022;13:835762. doi: 10.3389/fimmu.2022.835762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Estelle B, Célia G, Nicolas C, Stéphane DJSA. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci Adv. 2023;9(7):eadf3700. doi: 10.1126/sciadv.adf3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe K, Kuramitsu S, Posey AD, June CH. Expanding the Therapeutic Window for CAR T Cell Therapy in Solid Tumors: The Knowns and Unknowns of CAR T Cell Biology. Front Immunol. 2018;9:2486. doi: 10.3389/fimmu.2018.02486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kominsky S. Claudins: Emerging targets for cancer therapy. Expert Rev Mol Med. 2006;8(18):1-11. doi: 10.1017/S1462399406000056 [DOI] [PubMed] [Google Scholar]
- 93.Gowrikumar S, Singh A, Dhawan P. Role of Claudin Proteins in Regulating Cancer Stem Cells and Chemoresistance-Potential Implication in Disease Prognosis and Therapy. Int J Mol Sci.. 2019;21(1):53. doi: 10.3390/ijms21010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashimoto I, Oshima TJC. Claudins and Gastric Cancer: An Overview. Cancers (Basel). 2022;14(2):290. doi: 10.3390/cancers14020290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jun K, Kim J, Jung J, Choi H, Chin H. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg. 2014;12(2):156-162. doi: 10.1016/j.ijsu.2013.11.022 [DOI] [PubMed] [Google Scholar]
- 96.Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol.. 2006;208(5):633-642. doi: 10.1002/path.1922 [DOI] [PubMed] [Google Scholar]
- 97.Athauda A, Chau I. Claudin 18.2-a FAST-moving target in gastric cancer? Ann Oncol. 2021;32(5):584-586. doi: 10.1016/j.annonc.2021.02.021 [DOI] [PubMed] [Google Scholar]
- 98.Singh P, Toom S, Huang YJ. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10(1):105. doi: 10.1186/s13045-017-0473-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.American Association for Cancer Research. Gastric Cancer CAR T-cell Target Antigen ID'd. Cancer Discovery. 2021;11(12):2954. doi: 10.1158/2159-8290.cd-nb2021-0390 [DOI] [PubMed] [Google Scholar]
- 100.Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14(23):7624-7634. doi: 10.1158/1078-0432.CCR-08-1547 [DOI] [PubMed] [Google Scholar]
- 101.Lordick F, Shitara K, Janjigian YJ. New agents on the horizon in gastric cancer. Ann Oncol. 2017;28(8):1767-1775. doi: 10.1093/annonc/mdx051 [DOI] [PubMed] [Google Scholar]
- 102.Türeci O, Sahin U, Schulze-Bergkamen H, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: The MONO study. Ann Oncol. 2019;30(9):1487-1495. doi: 10.1093/annonc/mdz199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sahin U, Türeci Ö, Manikhas G, et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32(5):609-619. doi: 10.1016/j.annonc.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 104.Jiang H, Shi Z, Wang P, et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. JJ Natl Cancer Inst. 2019;111(4):409-418. doi: 10.1093/jnci/djy134 [DOI] [PubMed] [Google Scholar]
- 105.Qi C, Gong J, Li J, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat Med. 2022;28(6):1189-1198. doi: 10.1038/s41591-022-01800-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cunningham S, Kamangar F, Kim M, et al. Claudin-4, mitogen-activated protein kinase kinase 4, and stratifin are markers of gastric adenocarcinoma precursor lesions. Cancer Epidemiol Biomarkers Prev. 2006;15(2):281-287. doi: 10.1158/1055-9965.EPI-05-0539 [DOI] [PubMed] [Google Scholar]
- 107.Kwon M, Kim S, Jeong H, et al. Claudin-4 overexpression is associated with epigenetic derepression in gastric carcinoma. Lab Invest. 2011;91(11):1652-1667. doi: 10.1038/labinvest.2011.117 [DOI] [PubMed] [Google Scholar]
- 108.Samadani A, Noroollahi S, Mansour-Ghanaei F, et al. Fluctuations of epigenetic regulations in human gastric adenocarcinoma: How does it affect?. Biomed Pharmacother. 2019;109:144-156. doi: 10.1016/j.biopha.2018.10.094 [DOI] [PubMed] [Google Scholar]
- 109.Nakamura J, Tanaka T, Kitajima Y, Noshiro H, Miyazaki K. Methylation-mediated gene silencing as biomarkers of gastric cancer: A review. World J Gastroenterol. 2014;20(34):11991-12006. doi: 10.3748/wjg.v20.i34.11991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eden A, Gaudet F, Waghmare A, Jaenisch RJS. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003;300(5618):455. doi: 10.1126/science.1083557 [DOI] [PubMed] [Google Scholar]
- 111.Gaudet F, Hodgson J, Eden A, et al. Induction of tumors in mice by genomic hypomethylation. Science . 2003;300(5618):489-492. doi: 10.1126/science.1083558 [DOI] [PubMed] [Google Scholar]
- 112.Padmanabhan N, Ushijima T, Tan P. How to stomach an epigenetic insult: The gastric cancer epigenome. Nat Rev Gastroenterol Hepatol. 2017;14(8):467-478. doi: 10.1038/nrgastro.2017.53 [DOI] [PubMed] [Google Scholar]
- 113.Zeng Y, Rong H, Xu J, et al. DNA Methylation: An important biomarker and therapeutic target for gastric cancer. Front Genet. 2022;13:823905. doi: 10.3389/fgene.2022.823905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ebrahimi V, Soleimanian A, Ebrahimi T, et al. Epigenetic modifications in gastric cancer: Focus on DNA methylation. Gene. 2020;742:144577. doi: 10.1016/j.gene.2020.144577 [DOI] [PubMed] [Google Scholar]
- 115.Calcagno D, Gigek C, Chen E, Burbano R, Smith MA. DNA And histone methylation in gastric carcinogenesis. World J Gastroenterol. 2013;19(8):1182-1192. doi: 10.3748/wjg.v19.i8.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeng X, Wang J, Chen S. Methylation modification in gastric cancer and approaches to targeted epigenetic therapy (review). Int J Oncol. 2017;50(6):1921-1933. doi: 10.3892/ijo.2017.3981 [DOI] [PubMed] [Google Scholar]
- 117.Tan H, Zhang S, Zhang J, et al. Long non-coding RNAs in gastric cancer: New emerging biological functions and therapeutic implications. Theranostics. 2020;10(19):8880-8902. doi: 10.7150/thno.47548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fattahi S, Kosari-Monfared M, Golpour M, et al. LncRNAs as potential diagnostic and prognostic biomarkers in gastric cancer: A novel approach to personalized medicine. J Cell Physiol. 2020;235(4):3189-3206. doi: 10.1002/jcp.29260 [DOI] [PubMed] [Google Scholar]
- 119.Yang Z, Guo X, Li G, Shi Y, Li L. Long noncoding RNAs as potential biomarkers in gastric cancer: Opportunities and challenges. Cancer Lett. 2016;371(1):62-70. doi: 10.1016/j.canlet.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 120.Fang X, Pan H, Leng R, Ye D. Long noncoding RNAs: Novel insights into gastric cancer. Cancer Lett. 2015;356:357-366. doi: 10.1016/j.canlet.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 121.Schaalan M, Mohamed W, Fathy S. MiRNA-200c, MiRNA-139 and ln RNA H19; new predictors of treatment response in H-pylori- induced gastric ulcer or progression to gastric cancer. Microb Pathog. 2020;149:104442. doi: 10.1016/j.micpath.2020.104442 [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y, Yan J, Li C, et al. LncRNA H19 induced by helicobacter pylori infection promotes gastric cancer cell growth via enhancing NF-κB-induced inflammation. J Inflamm (Lond). 2019;16:23. doi: 10.1186/s12950-019-0226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ba M, Ba Z, Long H, et al. LncRNA AC093818.1 accelerates gastric cancer metastasis by epigenetically promoting PDK1 expression. Cell Death Dis. 2020;11(1):64. doi: 10.1038/s41419-020-2245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang G, Xu Y, Wang S, et al. LncRNA SNHG17 promotes gastric cancer progression by epigenetically silencing of p15 and p57. J Cell Physiol. 2019;234(4):5163-5174. doi: 10.1002/jcp.27320 [DOI] [PubMed] [Google Scholar]
- 125.Zhu X, Tian X, Yu C, et al. A long non-coding RNA signature to improve prognosis prediction of gastric cancer. Mol Cancer. 2016;15(1):60. doi: 10.1186/s12943-016-0544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shin V, Chu K. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol. 2014;20(30):10432-10439. doi: 10.3748/wjg.v20.i30.10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morgos DT, Stefani C, Miricescu D, et al. Targeting PI3K/AKT/mTOR and MAPK Signaling Pathways in Gastric Cancer. Int J Mol Sci. 2024;25(3):1848. doi: 10.3390/ijms25031848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022;12:819128. doi: 10.3389/fonc.2022.819128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ning G, Zhu Q, Kang W, et al. A novel treatment strategy for lapatinib resistance in a subset of HER2-amplified gastric cancer. BMC Cancer. 2021;21(1):923. doi: 10.1186/s12885-021-08283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24(8):560-575. doi: 10.1038/s41580-023-00585-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rong L, Li Z, Leng X, et al. Salidroside induces apoptosis and protective autophagy in human gastri c cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed Pharmacother. 2020;122:109726. doi: 10.1016/j.biopha.2019.109726 [DOI] [PubMed] [Google Scholar]
- 132.Song Y, Gao N, Yang Z, et al. Comprehensive molecular analyses of notch pathway-related genes to predict prognosis and immunotherapy response in patients with gastric cancer. J Oncol. 2023;2023:1-15. doi: 10.1155/2023/2205083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang M, Yu F, Zhang Y, Li P. Novel insights into Notch signaling in tumor immunity: potential targets for cancer immunotherapy. Front Immunol. 2024;15:1352484. doi: 10.3389/fimmu.2024.1352484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Demitrack ES, Gifford GB, Keeley TM, et al. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34(20):2522-2536. doi: 10.15252/embj.201490583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ullah R, Yin Q, Snell AH, Wan L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer Biol. 2022;85:123-154. doi: 10.1016/j.semcancer.2021.05.010 [DOI] [PubMed] [Google Scholar]
- 136.Helen D, Graham RB, Charles C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 137.Inamdar GS, Madhunapantula SV, Robertson GP. Targeting the MAPK pathway in melanoma: Why some approaches succeed and other fail. Biochem Pharmacol. 2010;80(5):624-637. doi: 10.1016/j.bcp.2010.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.López MJ, Carbajal J, Alfaro AL, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. doi: 10.1016/j.critrevonc.2022.103841 [DOI] [PubMed] [Google Scholar]
- 139.Shi J, Yao D, Liu W, et al. Highly frequent PIK3CA amplification is associated with poor prognosis in gastric cancer. BMC cancer. 2012;12:50. doi: 10.1186/1471-2407-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lei Z, Teng Q, Tian Q, et al. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct Target Ther. 2022;7(1):358. doi: 10.1038/s41392-022-01190-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shimizu T, Marusawa H, Matsumoto Y, et al. Accumulation of somatic mutations in TP53 in gastric epithelium with H elicobacter pylori infection. Gastroenterology. 2014;147(2):407-417.e403. doi: 10.1053/j.gastro.2014.04.036 [DOI] [PubMed] [Google Scholar]
- 142.Bockerstett KA, Lewis SA, Noto CN, et al. Single-Cell transcriptional analyses identify lineage-specific epithel ial responses to inflammation and metaplastic development in the gastr ic corpus. Gastroenterology. 2020;159(6):2116-2129.e2114. doi: 10.1053/j.gastro.2020.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Antonioli L, Fornai M, Blandizzi C, Pacher P, Haskó G. Adenosine signaling and the immune system: When a lot could be too much. Immunol Lett. 2019;205:9-15. doi: 10.1016/j.imlet.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 144.Li Y, Lu L, Wu X, et al. The multifaceted role of long non-coding RNA in gastric cancer: Current Status and future perspectives. Int J Biol Sci. 2021;17(11):2737-2755. doi: 10.7150/ijbs.61410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Q, Wang C, Yang Y, Xu R, Li Z. LncRNA and its role in gastric cancer immunotherapy. Front Cell Dev Biol. 2023;11:1052942. doi: 10.3389/fcell.2023.1052942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Du P, Liu P, Patel R, et al. The value of metabolic LncRNAs in predicting prognosis and immunotherapy efficacy of gastric cancer. Front Oncol. 2023;12:1019909. doi: 10.3389/fonc.2022.1019909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ghafouri-Fard S, Vafaee R, Shoorei H, Taheri M. MicroRNAs in gastric cancer: Biomarkers and therapeutic targets. Gene. 2020;757:144937. doi: 10.1016/j.gene.2020.144937 [DOI] [PubMed] [Google Scholar]
- 148.Hao N-B, He Y-F, Li X-Q, Wang K, Wang R-L. The role of miRNA and lncRNA in gastric cancer. Oncotarget. 2017;8(46):81572-81582. doi: 10.18632/oncotarget.19197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matsuoka T, Yashiro M. Novel biomarkers for early detection of gastric cancer. World J Gastroenterol. 2023;29(17):2515-2533. doi: 10.3748/wjg.v29.i17.2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu X-L, Ren L-F, Wang H-P, et al. Plasma microRNAs as potential new biomarkers for early detection of early gastric cancer. World J Gastroenterol. 2019;25(13):1580-1591. doi: 10.3748/wjg.v25.i13.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zheng J, Jiang X, Jiang K, et al. miR-196a-5p correlates with chronic atrophic gastritis progression to gastric cancer and induces malignant biological behaviors of gastric cancer cells by targeting ACER2. Mol Biotechnol. 2022;65(8):1306-1317. doi: 10.1007/s12033-022-00589-8 [DOI] [PubMed] [Google Scholar]
- 152.Zhang Z, Wu H, Chong W, Shang L, Jing C, Li L. Liquid biopsy in gastric cancer: predictive and prognostic biomarkers. Cell Death Dis. 2022;13(10):903. doi: 10.1038/s41419-022-05350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matsusaka S, Chìn K, Ogura M, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci. 2010;101(4):1067-1071. doi: 10.1111/j.1349-7006.2010.01492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jin Y, Chen D-L, Wang F, et al. The predicting role of circulating tumor DNA landscape in gastric cancer patients treated with immune checkpoint inhibitors. Mol Cancer. 2020;19(1):154. doi: 10.1186/s12943-020-01274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]