Abstract
Bioprinting specific tissues with robust viability is a great challenge, requiring a delicate balance between a densely cellular distribution and hydrogel network crosslinking density. Microtissues composed of tissue‐specific mesenchymal stem cells and extra cellular matrix (ECM) particles provide an alternative scheme for realizing biomimetic cell density and microenvironment. Nevertheless, due to their instability during manufacturing, scarce efforts have been made to date to assemble them using rapid prototyping methods. Here, a novel microtissue bioink with good printability and cellular viability maintenance for digital light processing (DLP) bioprinting is introduced. Generally, the microtissue bioink is prepared by crosslinking acellular matrix microparticles and GelMA hydrogel with a specific proportion. The microtissue bioink exhibits the desired mechanical properties, swelling ratio, and has almost no influences on printability. For instance, a DLP bioprinted ear with a precise auricle structure using microtia chondrocytes microtissue boink is created. Additionally, the chondrocytes in the printed ears show obvious advantages in cell proliferation in vitro and auricular cartilage regeneration in vivo. The microtissue composite bioink for DLP printing not only enables accurate assembly of organ building blocks but also provides a 3D shelter to ensure printed cells' viability.
Keywords: bioinks, digital light porcessing bioprinting, microtissues
A novel microtissue bioink with good printability and cellular viability maintenance for digital light processing (DLP) bioprinting is introduced. The microtissue bioink exhibits desired mechanical properties, swelling ratio, and has almost no influences on printability. For instance, a DLP bioprinted ear with a precise auricle structure using microtia chondrocytes microtissue boink is created, which shows obvious advantages in auricular cartilage regeneration in vivo.
1. Introduction
The combination of 3D bioprinting technology and tissue engineering has recently developed rapidly[ 1 , 2 , 3 ] and is now widely employed in regenerating bone,[ 4 ] cartilage,[ 5 ] heart,[ 6 ] and skin.[ 7 ] Bioprinting specific tissues with robust viability require a delicate balance between a densely cellular distribution and hydrogel network crosslinking density.[ 8 ] The commonly used method for preparing bioinks is to mix cells directly with hydrogel solution.[ 9 , 10 ] However, this method maintains cells in a single‐cell form in the gel, which is not conducive to intercellular interactions, intercellular signaling, and extracellular matrix secretion. Therefore, how to optimize the existing bioink is an important problem.
Effective cartilage regeneration depends on the natural production and deposition of extracellular matrix.[ 11 ] Pati et al.[ 12 ] first developed a method for bioprinting cartilage tissue analogues with novel cartilage acellular matrix (CAM) bioink using a nozzle‐based bioprinter. Zhou et al.[ 5 ] prepared gelatin methacrylate (GelMA)/meniscal extracellular matrix (MECM) bioink and then constructed a biomimetic meniscal scaffold using a customized 3D bioprinting system with dual nozzle and multitemperature printing based on a multilayer biomimetic strategy. Fisch et al.[ 13 ] proposed a bioink calcium‐triggered enzyme crosslinking (CTEC) mechanism based on factor XIII cascade activation and applied it to cartilaginous construct biofabrication. However, these studies generally mixed cells directly with bioink to produce a single‐cell suspension that prevented effective connections between cells, resulting in insufficient extracellular matrix secretion and impairing cartilage maturation. Using cell microspheres, de Melo et al.[ 14 ] employed suspension 3D printing technology to prepare 3D cartilage‐like tissues. Compared with traditional culture methods, this strategy dramatically increased the differentiation of stem cells into chondrocytes and the extracellular matrix deposition in 3D cartilage‐like tissues. However, because this method places a high premium on the printing environment and the mechanical properties of constructs prepared are insufficient, achieving cartilage regeneration in vivo is difficult.
Microtissues are characterized by single‐celled aggregates or microfabricated cell‐laden biomaterials that can simulate a 3D cell growth environment.[ 15 ] By assembling microtissues into ideal structures and implanting them, cell connections and effective substance exchange can be established, which is beneficial for cell proliferation, differentiation, and maintenance of functional phenotypes.[ 16 , 17 ] Simultaneously, tissue microstructure can be precisely controlled, facilitating the extracellular matrix deposition.[ 14 ] Based on the above characteristics, microtissues offer great application potential in constructing functional structures.[ 18 ] Nevertheless, due to their instability during manufacturing, scarce efforts have been made to date to assemble them using rapid prototyping methods.
Here, we present an ECM compound bioink derived from cartilage microtissues and its use in cartilage regeneration, specifically the auricle (Figure 1 ). We first determined the chondrocyte and stem cell characteristics of microtia chondrocytes extracted from abandoned microtia‐tissue samples. CAM was decellularized from porcine ear cartilage tissue, then lyophilized, quickly frozen, and ground into microparticles. The microtia chondrocytes were seeded on CAM microcarriers to construct cartilage microtissues. After 5 d in vitro culture, the cells gradually proliferated and secreted extracellular matrix. Compared with extrusion bioprinting, digital light processing (DLP) bioprinting is highly accurate and causes less mechanical damage to cells, making it more conducive to manufacturing complex structures. As a result, we prepared bioink for DLP bioprinting by mixing the microtissues with GelMA solution, then cultured the constructs in vitro and in vivo, respectively. This approach may provide a new construction strategy for bioprinting and cartilage tissue engineering.
Figure 1.
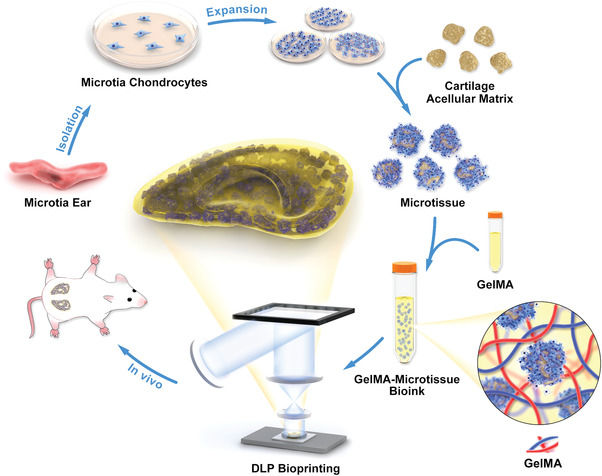
Schematic representation of preparation of microtissue bioink and its application to DLP bioprinting. Microtia chondrocytes were isolated and expanded from microtia ear and then inoculated on the prepared cartilage acellular matrix (CAM) particles to construct microtissues. The GelMA‐microtissue bioink was prepared by mixing the microtissue with GelMA solution, printed to the auricular shape by a DLP‐based 3D bioprinter, then implanted subcutaneously in nude mice for in vivo chondrogenesis experiments.
2. Results
2.1. Extraction and Identification of Microtia Chondrocytes
First, microtia chondrocytes were isolated from microtia tissue by enzyme digestion (Figure 2A). Microtia chondrocytes were previously discovered to have both chondrocyte and stem cell‐like characteristics,[ 19 ] here immunofluorescence staining and q‐RT PCR detection were performed for the characterization of them. Immunofluorescence staining results showed the positive expression of chondrogenesis‐related markers COL2 and Aggrecan (Figure 2B), and the positive expression of stem‐cell‐related markers CD90, CD105 and CD73 (Figure 2C).
Figure 2.
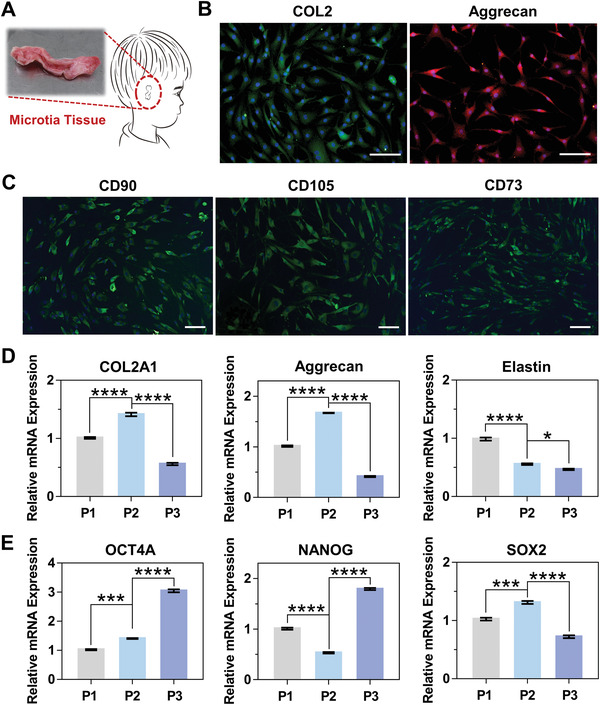
Extraction and identification of microtia chondrocytes. Microtia chondrocytes were previously discovered to have both chondrocyte and stem cell‐like characteristics,[ 19 ] here immunofluorescence staining and q‐RT PCR detection were performed for the characterization of them. A) A diagram of a patient with microtia and the optical image of microtia tissue. B) Immunofluorescence staining of chondrogenesis‐related markers COL2 and Aggrecan of microtia chondrocytes (scale bar: 100 µm). C) Immunofluorescence staining of stem cell‐related markers including CD90, CD105, and CD73 of microtia chondrocytes (scale bar: 100 µm). D,E) mRNA expression analysis of chondrogenesis‐related genes including COL2A1, Aggrecan and Elastin, and stem cell‐related genes including OCT4A, NANOG and SOX2 in P1, P2 and P3 microtia chondrocytes (*p < 0.05, ***p < 0.001, **** p < 0.0001).
As the gene level, the chondrogenesis‐related genes COL2A1, Aggrecan, and Elastin, and stem‐cell‐related genes OCT4A, NANOG and SOX2 had various levels of mRNA expression in P1, P2 and P3 microtia chondrocytes (Figure 2D,E), of these, P3 chondrocytes had the highest expression level of OCT4A and NANOG. Alizarin red S staining demonstrated that after 14 d of osteogenic induction, positive red nodules of various sizes were observed (Figure S1A, Supporting Information), and the osteogenesis‐related genes RUNX2, ALP, OSX, BMP2 and OPN in P1, P2 and P3 microtia chondrocytes also had various level of mRNA expression (Figure S1B, Supporting Information), which showed that microtia chondrocytes had osteogenic differentiation potential. Of these, P3 chondrocytes had the highest expression level of RUNX2, ALP, OSX, and BMP2. After 21 d of adipogenic induction, light red lipid droplets stained by oil red O were observed at magnification (Figure S1C, Supporting Information). Similarly, the adipogenesis‐related genes PPARγ, CEBPα, RXRα, LPL and Adipsin in P1, P2 and P3 microtia chondrocytes had various level of mRNA expression (Figure S1D, Supporting Information), there was almost no statistical difference in gene expression levels between three generations of chondrocytes, which showed that microtia chondrocytes had adipogenic differentiation potential. The above results suggested that the microtia chondrocytes we harvested had both chondrocyte and stem‐cell characteristics. Moreover, P3 microtia chondrocytes seemed to have stronger stemness property, indicating greater differentiation potential. Therefore, P3 microtia chondrocytes were used for subsequent experiments.
2.2. Preparation of Cartilage Acellular Matrix (CAM) and Construction of CAM Microtissues
Porcine ear cartilage was successfully decellularized with a combination of the physical, chemical, and enzymatic process using slightly modified methods described elsewhere, then lyophilized, quick‐frozen in liquid nitrogen, and ground into microparticles (Figure 3A,B). Furthermore, 0.1% peracetic acid solution in combination with 4% ethanol was used for disinfection. Hematoxylin and eosin (H&E) and Masson's trichrome staining confirmed the absence of cells and cell debris in the matrix after decellularization, but obvious cartilage lacuna and extracellular matrix still existed (Figure 3B). Quantitative measurements for dsDNA content were used to evaluate the efficiency of decellularization method, which was found to be optimal for removing the cellular contents with ≈ 98% reduction and only 6.25 ± 1.19 ng of DNA per mg of tissue remaining in CAM (Figure 3C). Dimethylmethylene blue assay was also carried out to estimate the glycosaminoglycan (GAG) content before and after decellularization (Figure 3D). Although there was an observed significant reduction in GAG content for CAM compared to the cartilage tissue controls, this occurrence can be attributed to the detergent‐based decellularization treatments.
Figure 3.
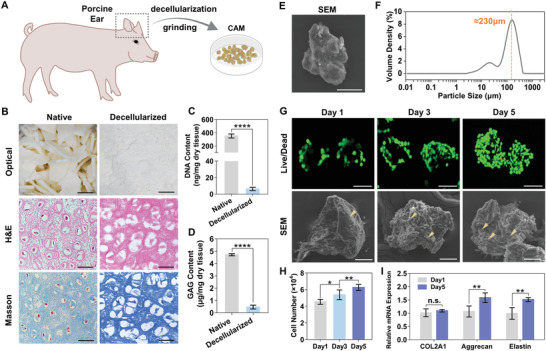
Preparation and biochemical analysis of cartilage acellular matrix (CAM), and construction of CAM microtissues with microtia chondrocytes. A) Schematic of preparation of CAM microparticles. B) Optical images and histological images of hematoxylin and eosin (H&E) and Masson's trichrome stained sections of native (cartilage) and decellularized (CAM) tissue (scale bar: Optical:10 mm, H&E and Masson: 50 µm). C,D) DNA (6.25 ± 1.19 ng of DNA per mg of tissue remaining in dry CAM) and GAG contents of native and decellularized tissue (****p < 0.0001). E) SEM image of CAM microparticle, showed the irregular shape and bumpy surface of CAM microparticles (scale bar: 50 µm). F) Representative image of particle size analysis of CAM microparticles, the particle size distribution is about 230 µm. G) Fluorescence microscopic and 3D reconstructed confocal images of microtissues loaded with microtia chondrocytes stained by live/dead (scale bar: 100 µm) at day 1, day 3, and day 5, and SEM images of them (scale bar: 50 µm). H) Quantification of microtia chondrocytes loading and proliferation on CAM microparticles at day 1, day 3, and day 5 (*p < 0.05, **p < 0.01). I) mRNA expression analysis of chondrogenesis‐related genes in microtissues at day 1 and day 5 (**p < 0.01).
CAM were sifted through the 50 mesh sieves to get CAM microparticles, then scanning electron microscopy (SEM) observation and particle size analysis were performed. The surface morphology was measured by SEM, which showed an irregular shape and bumpy surface of CAM microparticle (Figure 3E). The particle sizes of CAM microparticles were analyzed and the particle size distribution curve was drawn. The volume mean diameter (D v) was analyzed, and the maximum particle diameters below 10%, 50%, and 90% of the total sample volume were expressed as D v(10), D v(50) and D v(90), respectively. Results showed that D v(10) = 22.29 ± 4.88 µm, D v(50) = 111.26 ± 13.28 µm and D v(90) = 230.06 ± 14.66 µm, the particle size distribution was concentrated, which indicates that the sizes of CAM microparticles prepared in this study were relatively uniform (Figure 3F).
Next, microtia chondrocytes were inoculated on CAM microparticles to construct microtissues and cultured for 5 d in vitro. Uniform cell distribution, good cell attachment, and proliferation capacity were achieved (Figure 3G). The number of chondrocytes loaded on 1 mg CAM microparticles was about 4.54 × 104 cells, and further increased to 6.26 × 104 cells after 5 d (Figure 3H). In quantitative analysis, chondrocytes cultured for 5 d expressed a significantly higher amount of Aggrecan and Elastin compared with those cultured for 1 d, however, gene expression of COL2A1 at day 5 was not significantly different with day 1, which might be caused by the insufficient time to cause significant changes in COL2A1 gene level (Figure 3I).
2.3. Preparation and Characterization of CAM Bioink
Different combinations of bioinks were developed by combining various concentrations (0, 1, 5, and 10% w/v) and sizes (0–150, 150–300 and >300 µm CAM microparticles) of CAM with 10% w/v concentration of GelMA. The optical images of the bioink before and after crosslinking showed that the viscosity increased and the transmittance decreased with higher concentration of CAM (Figure 4A, up). SEM showed that inner morphology of the hydrogels were rougher after mixed with CAM microparticles (Figure 4A, down). The rheological properties of the bioinks were studied with Anton Paar MCT 302 rheometer at 37 °C. All preprinted bioinks with different CAM concentrations showed shear thinning behavior, and the higher CAM concentration, the higher viscosity was observed (Figure 4B, left). The viscosity of the bioink before printing was evaluated by subjecting the bioink to a low shear of 1 s−1, after held for 600 s, no significant changes of the viscosity were observed (Figure 4B, right). The storage modulus (G′) of all the bioinks were higher than loss modulus (G″) in the time range, and increased with higher CAM concentration (Figure 4C), which confirmed that all the bioinks can maintain the hydrogel form under dynamic conditions. No significant changes of G’ and G’’ value for the hydrogel were observed by time sweeps (1% strain and 5 rad s−1) after testing for 300 s (Figure 4C). The compressive modulus for the crosslinked bioink increased from 49.21 ± 7.93 kPa to 325.90±12.55 kPa after 10% w/v CAM added (≈6.5 times) (Figure 4D). Rheology and mechanical compression measurements indicated that CAM could increase the viscosity of the bioink before crosslinking and the elasticity after crosslinking. The higher the concentration of CAM, the higher the viscosity, and the stronger the elasticity. The swelling ratios of the crosslinked bioink were found inversely related to the concentration of CAM, which is important for the long‐term stability of the 3D printed hydrogel scaffolds (Figure 4E). Based on the above results, 10% w/v CAM was chosen for the next experiments.
Figure 4.
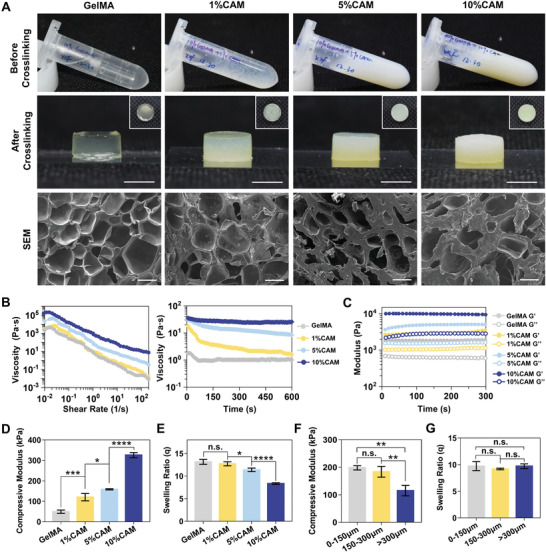
Characterization of the bioink with different CAM concentrations and particle sizes. A) Gross appearances of bioinks before and after crosslinking, and SEM images of the crosslinked GelMA bioinks containing 0%, 1%, 5% and 10% CAM (scale bar: 200 µm). B) Viscosities of preprinted bioinks and C) storage (G′) and loss (G″) modulus of the bioinks measured by rheological testing. D) Compressive modulus and E) swelling ratio of bioinks containing different CAM concentrations (*p < 0.05, ***p < 0.001, ****p < 0.0001). F) Compressive modulus and G) swelling ratio of bioinks containing 10% w/v CAM of different particle sizes (**p < 0.01).
As for the mechanical properties of the bioink containing various sizes (0–150 µm, 150–300 µm and >300 µm) of CAM microparticles, both 0–150 µm and 150–300 µm groups had a compressive modulus significantly higher than that of the >300 µm group, but no statistically significant difference between the 0–150 µm and 150–300 µm groups (Figure 4F). The swelling ratios showed that the difference between the three groups was not statistically significant (Figure 4G).
Since the DLP bioprinting was achieved by layer‐by‐layer light curing, we then tested the sedimentation performance of the bioink (Figure 5 ). After mixing 10%w/v CAM microparticles with 10% w/v GelMA (dissolved with DMEM) and resting at 37 °C, the delamination onset was observed after 1 h in 150–300 µm (Figure 5C) and >300 µm groups (Figure 5D), while the 0–150 µm group (Figure 5B) remained homogeneous. 3 hours later, the delamination of 150–300 µm and >300 µm groups were even more pronounced, while almost no stratification was observed in 0–150 µm group (Figure 5A). The sedimentation distances (d) of the three groups with different particle sizes were then measured for statistical analysis. The results showed that the settlement became more obvious with the increase of the CAM particle size (Figure 5E). From the above results, we can conclude that under the condition of the same mass concentration of CAM, the smaller the particle size, the more the microparticle number, the more uniform the distribution in the system, and the slower the sedimentation rate. Based on the above data, 0–150 µm CAM microparticles were chosen for the subsequent preparation of microtissue bioink to ensure the homogeneity in the DLP bioprinting process.
Figure 5.
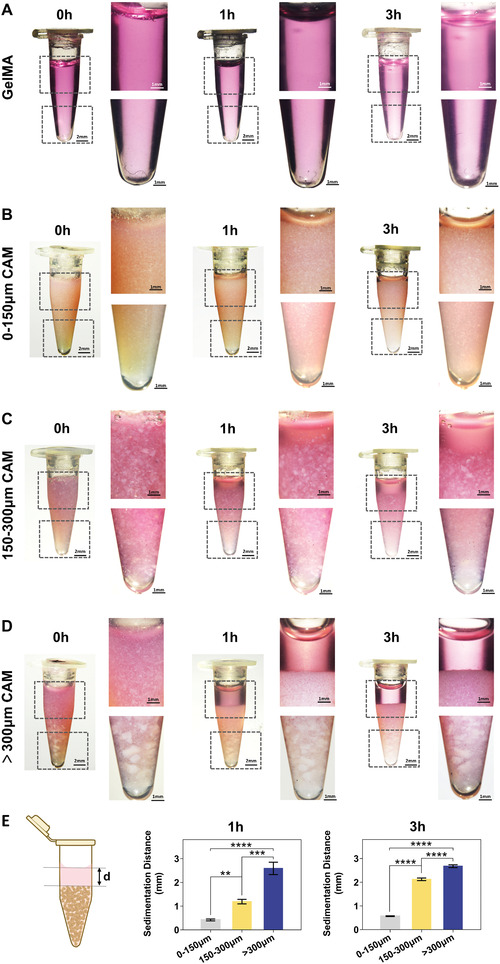
Sedimentation characteristics of bioinks containing 10% w/v CAM of different particle sizes at 37 °C observed under stereomicroscope. A) GelMA group as control, B) 0–150 µm group remained almost homogeneous after 1 and 3 h, and the layer phenomenon was observed in C) 150–300 µm and D) >300 µm groups after 1 and 3 h. E) The sedimentation distances of the three groups with different particle sizes. The results showed that the settlement became more obvious with the increasing of the CAM particle size (**p < 0.01, ***p < 0.001, ****p < 0.0001).
0–150 µm CAM was then mixed with GelMA solution to prepare bioink with the concentration of 10% w/v for DLP printing (Figure 6A). First, the printing parameters of two kinds of bioinks were determined: the light intensity was set as 14 mW cm−2, and the influences of different exposure time and penetration depth on printability were tested respectively (Figure 6B). The results showed that GelMA bioink can be successfully printed with the exposure time of 20 s & depth of 10 µm and the exposure time of 30 s & depth of 30 µm. For GelMA+CAM bioink, the exposure time of 30 s & depth of 20 µm and the exposure time of 35 s & depth of 30 µm can be successfully printed. In order to achieve the shortest exposure time of the entire sample to ensure the survival rate of cells, we determined the following printing parameters by integrating the penetration depth and exposure time: For GelMA bioink, the printing parameters were set as: light intensity 14 mW cm−2, exposure time 30 s and penetration depth 30 µm. As for GelMA+CAM bioink, the printing parameters were set as: light intensity 14 mW cm−2, exposure time 35 s and penetration depth 30 µm.
Figure 6.
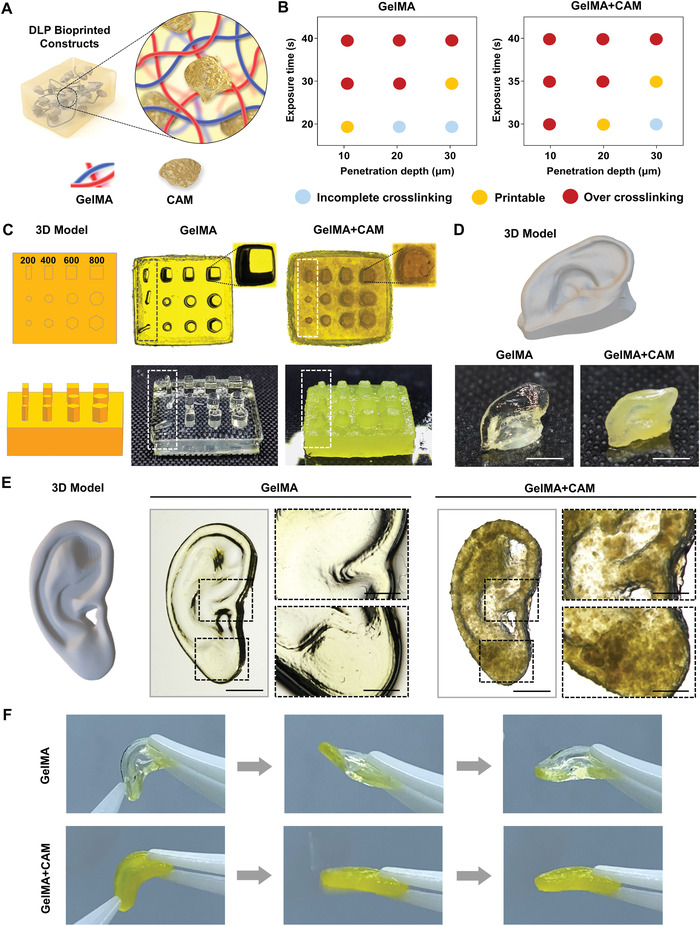
DLP bioprinting using CAM microparticle bioink. A) Schematic of DLP bioprinted constructs using CAM microparticle bioink. B) Preliminary tests for the printing parameter optimization for GelMA and GelMA+CAM bioinks. C) The accuracy testing of GelMA and GelMA+CAM bioinks. Both groups showed good printability, and GelMA+CAM group showed better support performance. D) DLP bioprinting of angular solid auricular structure with GelMA and GelMA+CAM bioinks (scale bar: 5 mm). E) DLP bioprinting of flat solid auricular structure with GelMA and GelMA+CAM bioinks, and the enlarge details of them (scale bar full sample: 5 mm, scale bar close up: 1 mm). F) Flexible properties of printed auricular structures with GelMA and GelMA+CAM bioinks.
Next, we designed different shape models with diameters of 200, 400, 600, and 800 µm for printing accuracy testing. In the top view, in order to show the details of the printed model, the model was photographed with a stereomicroscope; In the front view, in order to highlight the overall morphology and supporting characteristics, the model was photographed with a camera. The results showed that the printing accuracy of both GelMA and GelMA+CAM bioinks could reach 200 µm. However, due to the insufficient mechanical strength of GelMA, the structure collapsed at 200 µm. On the contrary, the GelMA+CAM group remained upright (Figure 6C). In the enlarged detail picture of the printing structure, it can be seen that the model shape of GelMA+CAM group is recognizable, but the edge is rougher compared with that of GelMA group, which may be affected by CAM particle size. However, too small particle size of CAM is not conducive to cell adhesion growth. Therefore, how to strike a balance between printability and good cell growth need to be further explored in future experiments.
The two bioinks were then printed into angular (Figure 6D) and flat auricular structures (Figure 6E) respectively. Macroscopically, the auricular constructs changed from transparent appearance to opaque appearance, accompanied by mechanical hardening. The details of the constructs were observed under the stereomicroscope, both GelMA and GelMA+CAM bioinks could be printed into clear anatomical structures (Figure 6E). When the auricular constructs were pressed down, the GelMA+CAM group had a stronger texture and a faster rebound than GelMA group (Figure 6F and Movie S1, Supporting Information).
2.4. DLP Bioprinting and Long‐Term Culturation In Vitro
Microtia chondrocytes were first inoculated on CAM microparticles and cultured for 5 d to prepare microtissue bioink, followed by DLP bioprinting the constructs (Figure 7A), and an equal number of chondrocytes of the same generation were mixed directly with GelMA as a control group.
Figure 7.
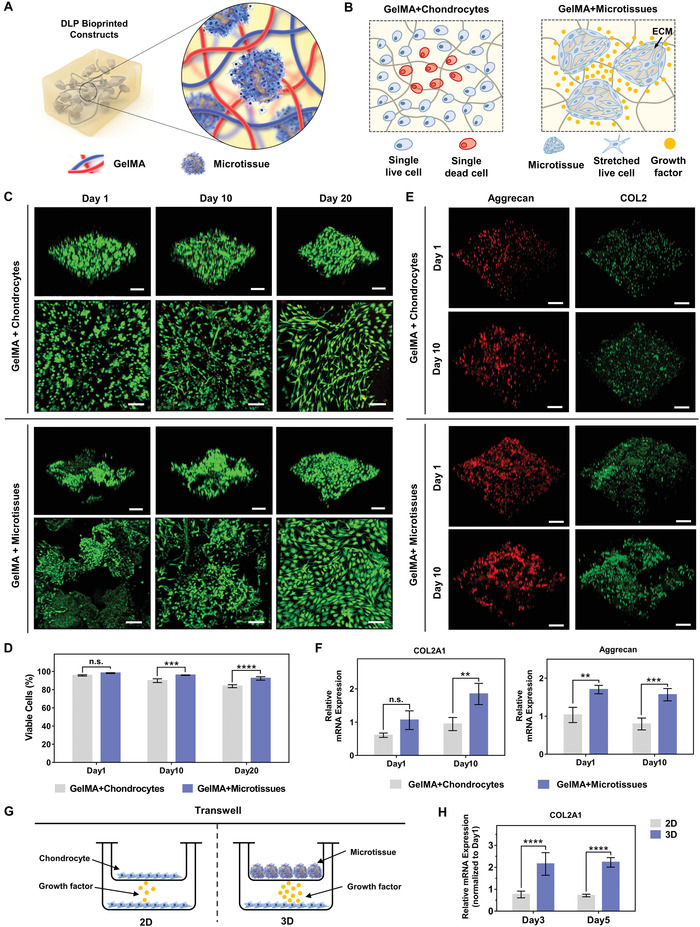
GelMA‐microtissue bioink based DLP bioprinted constructs and long‐term culturation in vitro. A) Schematic of DLP bioprinted constructs using microtissue bioink. B) Schematic diagrams of two bioinks for in vitro culture after printing. In GelMA+chondrocytes group, due to the difficulty of medium infiltration into GelMA, the single cells in internal gel were prone to die due to lack of nutrition. In GelMA+microtissues group, there were more extracellular matrix (ECM) deposits on the microtissues, which facilitated the connection between cells and the secretion of more bioactive substances. C) Fluorescence microscopic and 3D reconstructed confocal images of DLP bioprinted constructs stained by live/dead at day 1, day 10, and day 20 (scale bar: 100 µm). D) Viability of chondrocytes cultured in DLP bioprinted constructs for 1, 10, and 20 d (***p < 0.001, ****p < 0.0001). E) Immunofluorescence staining images of Aggrecan and COL2 in DLP bioprinted constructs cultured in vitro at day 1 and day 10. Aggrecan and COL2 markers were positively expressed in the two groups of chondrocytes after 1 and 10 d in vitro culture (scale bar: 100 µm). F) mRNA expression analysis of chondrocytes in DLP bioprinted constructs at day 1 and day 10 (**p < 0.01, ***p < 0.001). G) Schematic of transwell experiment. 2D stands for co‐culture of chondrocytes with chondrocytes, 3D stands for coculture of chondrocytes with microtissues. H) mRNA expression analysis of chondrocytes in 2D and 3D coculture environment (****p < 0.0001).
The GelMA+chondrocytes group is prone to internal cell death due to lack of nutrition, while the cells in the GelMA+microtissues group could perform intercellular connections and secret more bioactive substances (Figure 7B), which was confirmed by the results of Live/Dead staining. The viability of chondrocytes cultured in auricular constructs was evaluated using Live/Dead staining at time points of day 1, day 10, and day 20 (Figure 7C). Cell viability of bioprinted constructs were 95.68±0.71% and 98.25±0.43% for the GelMA+chondrocytes (control group) and GelMA+microtissues (microtissue group) bioink 1 d postprinting, reflecting the little effect by DLP bioprinting on the survival cell number (Figure 7D, Day 1). After 10 and 20 d culturing in vitro, better cellular viability and more extracellular matrix secretion were observed in the microtissue group, compared to the control group (Figure 7C,D), and the cell viability was above 90% for the entire duration of the experiment in microtissue group (Day 10 = 95.96 ± 0.28%, Day 20 = 92.36 ± 1.91%).
Next, we performed immunofluorescence staining on the in vitro cultured constructs to characterize the differentiation of the chondrocytes during culture. Both groups showed normal expression of Aggrecan and COL2 on Day 1 and Day 10, and more extracellular matrix was observed in GelMA+microtissues group (Figure 7E), which indicated that cells in the constructs cultured in vitro still showed chondrocyte characteristics. In quantitative analysis, as the culture time increased, the mRNA expression level of COL2A1 and Aggrercan in microtissue group gradually increased, and was significantly higher than those in the control group (Figure 7F).
PEGDA, as a bioinert material, is one of the most frequently used synthetic material in tissue engineering. It has been extensively used to increase the stiffness of soft hydrogels without altering the bioactivity of them.[ 20 , 21 ] Based on the above results in GelMA, we mixed the microtissues into 10% w/v PEGDA and photo‐crosslinked them into gel for in vitro culture to preliminary explore whether the microtissues in the bioinert material with dense network also have the ability to promote cell growth and secretion. The results are illustrated in Figure S3 (Supporting Information), after 10 d culturing in vitro, better cellular viability and more extracellular matrix secretion were observed in the microtissue group, compared to the control group. From the above results, we tentatively concluded that the preparation method of the microtissue bioink in this experiment could promote cell growth and extracellular matrix secretion in both soft/bioactive hydrogel and stiff/bioinert hydrogel.
To further explore why cells growed better in microtissue group, transwell experiment was performed, which was delineated as in the schematic (Figure 7G). Briefly, transwell chambers containing chondrocytes and microtissues were respectively inserted into the six‐well plates with equal amounts of chondrocytes in each chamber plated with equal chondrocytes, and then cocultured for 3 and 5 d. Gene expression analysis revealed that compared with monolayer cells, chondrocytes co‐cultured with microtissues showed significantly higher COL2A1 gene expression level (Figure 7H), which may due to more of the active ingredient secretion in the microtissues.
2.5. In Vivo Neocartilage Formation
To meet the practical clinic applications, bioprinted structures not only need to remain stable in vitro culture, but must continue to mature in vivo for a long time. Therefore, we tested the bioprinted auricular constructs in a subcutaneous nude mice model for 6 and 12 weeks, then matured their tissue stability, maturation, and gene expression level (Figure 8 ). Animals were divided into 4 groups described as follows: GelMA (blank group), GelMA+chondrocytes (MC group), GelMA+CAM (CAM group) and GelMA+microtissues (microtissue group). The grafts were harvested 6 and 12 weeks after the implantation (Figure 8A, bottom‐right) and were further evaluated by gross observation, histological staining and mRNA expression analysis. The gross morphology of blank, MC and microtissue groups were relatively intact, however, in CAM group, the texture of the grafts became softer and the structure was completely collapsed (Figure 8B and Movie S2, Supporting Information).
Figure 8.
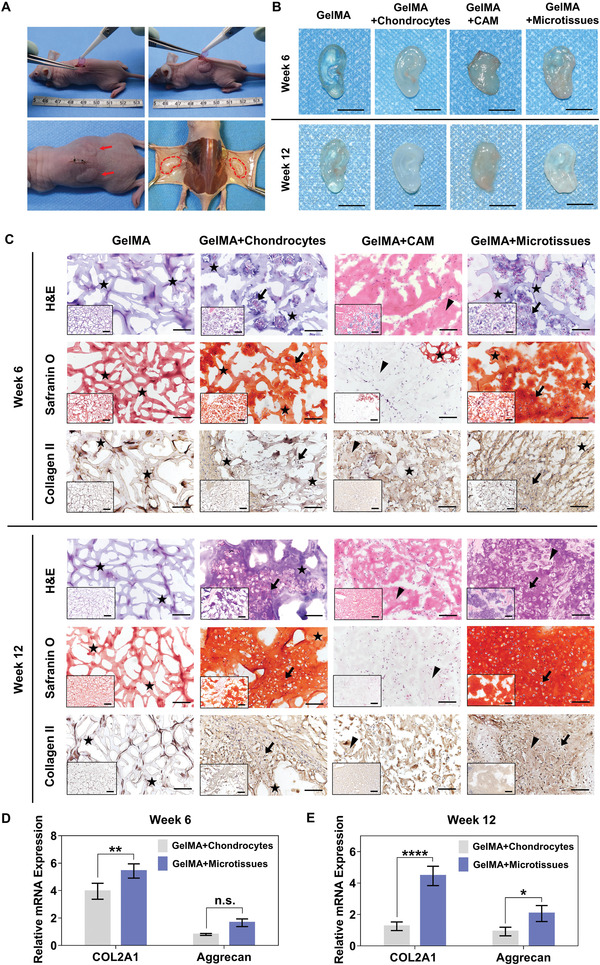
In vivo neocartilage formation. A) Top: the procedure of subcutaneous implantation with auricular constructs in nude mice. Bottom‐left: picture taken right after the subcutaneous implantation of auricular constructs. Bottom‐right: picture taken during explantation of constructs after 12 weeks in vivo. B) Pictures of in vivo constructs at week6 and week12 (scale bar: 1 mm). C) In vivo histological analysis for development of cartilage tissue formation at 6 and 12 weeks after transplantation. Histological and immunohistological stainings for H&E, Safranin O and Collagen II at low magnification (bottom‐left of each picture, scale bar: 500 µm) and at high magnification (scale bar: 100 µm). Asterisk indicates the hydrogel component remaining in the grafts. Triangular arrowhead indicates residual CAM microparticles in the graft. Bold arrow indicates chondrocytes sit in lacunae and are surrounded by the matrix they have secreted. Compared with other three groups, more chondrocytes, red‐stained aggrecan and brown‐stained collagen fibers were observed in GelMA+microtissues group, especially at week12. D,E) mRNA expression analysis of chondrocytes in auricular constructs at week 6 and week 12 (*p < 0.05, **p < 0.01, ****p < 0.0001).
In vivo histological analysis for the development of cartilage tissue formation on 6 and 12 weeks after transplantation was then carried out (Figure 8C). Frozen sections were analyzed by H&E, Safranin O and Collagen II stainings. In the blank group, the histological staining results at week 6 and week 12 showed only residual hydrogel networks (shown in asterisk). Due to its water absorption properties, the dye inside the hydrogel was difficult to be completely removed, so the entire gel network appeared light color, but no cell components were observed inside.
In the MC group, chondrocytes were sparsely arranged in clusters in the hydrogel network at week 6 (bold arrow indicates chondrocytes sit in lacuna and are surrounded by the matrix they have secreted, asterisk indicates residual hydrogel network). At week 12, cell increased in number, cartilage lacunas were clearly visible, and brown‐stained COL2 collagen fibers were observed in immunohistochemical staining, indicating the formation of new cartilage tissue.
In the CAM group, consistent with the gross morphological imaging, at week 6, HE staining showed the pink‐stained acellular matrix and newborn tissue ingrowth, while most of the visual field was unpigmented in Safranin O staining, while the residual CAM microparticles were faintly visible (shown in triangular arrow), and the remaining hydrogels appeared reddish (asterisk). COL2 immunohistochemical staining showed brown‐stained CAM microparticles (triangular arrow) and lightly stained hydrogel component (asterisk), probably due to the cross‐reactivity across genera between human and porcine. At week 12, the original implanted scaffold was further degraded, and the hydrogel composition proportion was further reduced or even disappeared, with only a few residual CAM microparticles (triangular arrow).
In the microtissue group, at week 6, similar to MC group, clusters of chondrocytes were observed, and the presence of cartilage lacunae was faintly observed (bold arrow). At week 12, different from MC group, the hydrogel network basically disappeared and was replaced by new cartilage tissue. At both low magnification and high magnification, large violet‐ and red‐stained regions were shown in HE staining and Safranin O staining respectively, which represented the extracellular matrix of chondrocytes, and a large number of cartilage nuclei were evenly distributed in the cartilage lacunae (bold arrow). COL2 immunohistochemical staining showed a large number of brown‐stained collagen fibers. From the above results, we can conclude that cartilage regeneration was better in the microtissue group.
Gene expression analysis revealed that the mRNA level of chondrogenesis‐related genes COL2A1 in microtissue group was significantly higher compared with the MC group at week 6 (Figure 8D) and week 12 (Figure 8E), and the same for Aggrecan at week 12 (Figure 8E). These results indicated that using microtissues bioink to construct auricular grafts may be more conducive for cartilage regeneration.
Immunofluorescence staining images of anti‐human LaminA/C in subcutaneous auricular constructs at week 6 and week 12 were shown in Figure 9 . A large number of positive cells were found in GelMA+chondrocytes and GelMA+microtissues groups, indicating that most of the proliferating chondrocytes were derived from human sources, while only blue‐stained nuclei were found in the GelMA+CAM group, indicating that the proliferating cells were derived from mice. In the GelMA group, only blue‐stained hydrogel components were visible, with almost no nuclear components.
Figure 9.
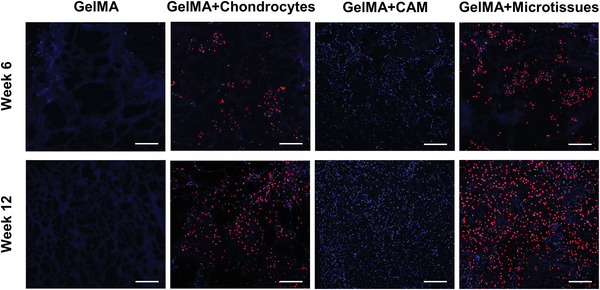
Immunofluorescence staining images of anti‐human LaminA/C in subcutaneous auricular constructs at week6 and week12. A large number of positive cells were found in GelMA+chondrocytes and GelMA+microtissues groups, indicating that most of the proliferating chondrocytes were derived from human sources, while only blue‐stained nuclei were found in the GelMA+CAM group, indicating that the proliferating cells were derived from mice. In the GelMA group, only blue‐stained hydrogel components were visible, with almost no nuclear components (scale bar: 200 µm).
3. Discussion
Although tissue‐engineered auricle is a viable alternative to currently available ear reconstructive options, its clinical translation is a barrier to overcome.[ 22 ] One major reason is the lack of clinically relevant cell sources.[ 23 ] It is an ideal seed cell source for constructing tissue‐engineered ears by extracting microtia chondrocytes from abandoned ear tissue without damaging normal physiological structure and avoiding immune rejection.[ 24 ] Microtia chondrocytes were previously discovered to have a slow dedifferentiation rate, chondrocyte and stem cell‐like characteristics, and the ability to form chondrocytes in vitro.[ 19 ] In 2018, Zhou et al. successfully constructed individually specific shaped cartilage in vitro using microtia chondrocytes and composite biodegradable scaffolds and conducted long‐term follow‐up.[ 25 ] The results indicated that the cartilage was mature, and satisfactory aesthetic effects could be obtained. In this study, microtia chondrocytes were extracted from the residual ear tissue. Their chondrocyte and stem cell characteristics and osteogenic and adipogenic differentiation potential were determined, confirming their viability as a practical cell source for engineering auricular cartilage for clinical application.
As for combining cells with materials, we first chose to construct microtissues in vitro and then conduct 3D bioprinting. Compared with the traditional method of directly inoculating cells onto a scaffold, using a bottom‐up strategy with microtissues could promote cell survival, proliferation, and differentiation by assembling microtissues (similar microarchitectural features to native tissues) as repeating functional units.[ 11 , 26 ] Compared with directly mixing the cells in the hydrogel, preparing microtissues in advance and mixing them with hydrogel could result in a richer extracellular matrix and enhanced intercellular interactions.[ 27 ]
Microtissues are characterized by single‐celled aggregates or microfabricated cell‐laden biomaterials.[ 28 ] However, the mechanical properties of fabricated constructs using single‐celled aggregates or cell‐laden hydrogels are insufficient.[ 14 ] Decellularized extracellular matrix (dECM) is the matrix part left after native tissue decellularization, which retains the structure and functional proteins of natural extracellular matrix and can provide a cell‐specific microenvironment.[ 29 , 30 ] Additionally, it has been demonstrated that cells cultured on dECM derived from their origin tissue improved expansion and differentiation potential.[ 31 ] Simultaneously, dECM can be formed into microparticles while retaining biomimetic mechanical properties, making it an ideal material for microtissue construction.[ 32 ] Besides, cartilage acellular matrix (CAM) microparticles prepared in this experiment exhibited uniform particle sizes and rough surfaces, which may be more conducive to cell adhesion and growth. However, GAG content had a substantial loss in the process of decellularization. Therefore, we thought about the optimization of decellularization methods,[ 33 ] and planned to make the following improvements in subsequent experiments: 1) After digestion with trypsin, a repeated freeze‐thaw process was added to optimize the chemical treatment time. 2) Method for disinfection: The disinfectant contained ethanol/peracetic acid, which could lead to the loss of GAG content. Supercritical CO2 can be tried instead. We next try to make better quality ECM particles by balancing the effect of decellularization and the biological activity of the decellularized tissue through the above methods.
Due to high mechanical properties of CAM microparticles, they were mixed with GelMA and crosslinked to alter the internal microstructure and decrease the hydrogel content, thus improving the bioink's mechanical properties.[ 34 ] Besides, by dissipating energy, CAM microparticles could improve the uniformity of the locally concentrated stress distribution in the hydrogel network. When the force is applied to the composite hydrogels, the GelMA chains are deformed, dissipating energy to the microparticles. The more microparticles, the stronger the dissipation effect, the larger the energy storage modulus.[ 35 ] Therefore, the higher the concentration of CAM microparticles, the stronger the mechanical properties of the bioink. However, as CAM particle sizes increased, the number of particles per unit volume of the bioink decreased, and the hydrogel system's uniformity decreased, resulting in decreased mechanical properties.
The rheological results indicated that CAM bioink possessed shear‐thinning properties, implying that it was injectable, hence expanding its application spectrum.[ 36 ] Additionally, the higher the concentration of CAM microparticles, the higher the viscosity before crosslinking and the stronger the elasticity after crosslinking, which was consistent with compression test results. In addition, regardless of frequency or time variations, the storage modulus of the crosslinked bioink was always greater than the loss modulus, indicating that the constructs could maintain their hydrogel structure under dynamic settings.
All hydrogels exhibit swelling properties in water due to water absorption and the stability of gel's crosslinking networks.[ 37 ] The swelling of CAM microparticles is negligible compared with that of GelMA. As the CAM concentration was increased, the swelling rate gradually decreased because the added CAM microparticles contributed only to bioink's dried weight.[ 35 ] However, the bioink's swelling ratio with 10% w/v CAM microparticles remained above 8, implying that the bioinks added to CAM retained their ability to transport nutrients and metabolites.
Although DLP bioprinting is more accurate and causes less mechanical damage to cells because it is performed by layer‐by‐layer light curing, the bioink is likely to settle during the long printing process when CAM microparticles are added.[ 38 ] Therefore, the sedimentation performances of CAM microparticles with varying particle sizes were evaluated while maintaining CAM concentration.[ 39 ] As demonstrated in the results, the smaller the particle sizes, the smaller the individual mass, the more CAM microparticles per unit volume of bioink, the more uniform the distribution in the system, and thus the more difficult to settle.
Bioink printability is critical for high‐precision DLP printing.[ 40 ] The results disclosed that when 10% w/v CAM microparticles were added to the bioink, the basic printing accuracy remained above 200 µm, and each anatomical structure of the ear could be clearly printed. In addition, since the auricle is a superficial organ, its supportive performance is critical.[ 41 ] The inclusion of CAM microparticles strengthened the structure's elasticity, indicating that the bioink is a suitable material for auricle fabrication.
Since the CAM‐laden bioink is not transparent and opaque, which will significantly decrease the light penetration, it may later affect the crosslinking density of the printed gels and the thickness of the printed gels. We therefore conducted the maximum penetration depth test and maximum printing thickness test. The maximum penetration depth of GelMA and GelMA+10%CAM bioinks were tested by solidifying a 3 mm height cubic model with the light intensity of 14 mW cm‐2. A tentative conclusion could be drawn after triplicate tests that the maximum penetration depth of GelMA was about 250 µm, and GelMA+10%CAM was about 170 µm. Although there may exist certain errors caused by the printer, the measurement method and the material itself over the course of testing, the overall trend showed that the addition of CAM reduces the maximum penetration depth of the bioink. We compensated for this limitation by reducing the penetration depth of each layer while maintaining the same light intensity, and ultimately achieving the printing of the desired shape. As for the maximum printing thickness, when using 0.25% LAP as photoinitiator and 0.01% tartrazine as photoabsorber, the maximum printable thickness of single layer was about 250um, and the printing model would be unstable if the thickness was higher than this.
In previous bioprinting studies, cells were mixed directly with hydrogel for printing, but the growth environment was not ideal due to the lack of effective cell connection and extracellular matrix secretion.[ 33 , 42 ] In this study, chondrocytes were inoculated on CAM microparticles in vitro to construct microtissues engineered to provide a 3D growth environment for cells and promote cell proliferation and extracellular matrix secretion. The microtissues were then mixed with GelMA solution to prepare microtissues bioink for printing. Microtissue bioinks provided efficient intercellular connections that facilitate signal transmission between cells, thereby facilitating cell growth in the hydrogel and extracellular matrix secretion. Additionally, in vivo results indicated that auricular constructs in the microtissues group had a better chondrogenic effect.
However, compared with the GelMA group, the morphology of auricular constructs changed after CAM addition, and no cells have infiltrated the GelMA constructs while the GelMA+CAM constructs appear to have a quite uniform distribution of cells, which is probably because compared with GelMA group, the addition of CAM destroyed the complete gel network inside GelMA and accelerated the degradation of GelMA. Meanwhile, due to better cell affinity, CAM was more conducive to tissue growth.
4. Conclusion
In recent years, 3D bioprinting technology has developed rapidly and found widespread use in tissue engineering. With the continuous development of printing technology, optimization of bioink is becoming more and more important. However, most bioinks are printed by mixing cells directly with hydrogel solution. This method leaves cells in a unicellular state in the hydrogel, which prevents effective cell–cell junctions from being established in time and affects the secretion of their extracellular matrix. In the present study, bioinks based on microtissues are introduced to overcome these limitations. Microtia chondrocytes are combined with cartilage acellular matrix (CAM) microparticles to construct cartilage microtissues, which can simulate the 3D growth microenvironment of cells and establish cell–cell junctions in time. DLP bioprinting of cartilage microtissues mixed with GelMA can produce auricular constructs with high elasticity, high printing accuracy, and low swelling ratio. A large amount of extracellular matrix deposition can be achieved after in vitro culture, and mature cartilage regeneration can be observed after subcutaneous implantation in nude mice for 12 weeks. Cartilage microtissue bioinks have good biocompatibility and mechanical properties, which sheds new light on the optimization of bioink and brings novel hope for the treatment of microtia patients.
5. Experimental Section
Cell Isolation and Expansion
The microtia tissue was obtained from patients (aged 5–17 years) who underwent ear reconstructive surgery at the Department of Plastic Surgery, Wuhan Union Hospital. Informed consent was signed with the donor's family before surgery, and the protocol was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology.he approval number is [2019] IEC (S069).
The abandoned microtia tissue was immediately immersed in sterile normal saline containing 100 U mL−1 penicillin and 100 mg mL−1 streptomycin. The specimen was carefully dissected to strip the fibrous tissue and perichondrium, and then fragmented into 1 mm3. Chondrocytes were isolated with 0.25% trypsin (including 0.02%EDTA, Beyotime, China) for 0.5 h and 0.15% type II collagenase (Sigma‐Aldrich, USA) for 8 h at 37 °C. The obtained digestive liquid was filtered by a single‐cell filter (100 mesh) and collected in a 50 mL centrifuge tube. Centrifugation (1500 rpm, 5 min) was performed to discard the supernatant 3 times. Cell precipitates were suspended in complete medium [High glucose DMEM (H‐DMEM, Hyclone, USA), 10% fetal bovine serum (FBS, Hyclone, USA), 100 U mL−1 penicillin (Sigma‐Aldrich, USA), 0.1 mg mL−1 streptomycin (Sigma‐Aldrich, USA) and 20 ng mL−1 Fibroblast Growth Factor‐basic (bFGF, Peprotech, USA)]. Chondrocytes were then inoculated in the 10 cm cell culture dish (Corning, USA) for culture and expansion.
Immunofluorescence and Induced Differentiation of Microtia Chondrocytes
For immunofluorescence, the expression of CD90 and CD105 was detected using mouse anti‐human CD90 and CD105 monoclonal antibodies (1:100, Abcam, UK), respectively, followed by rabbit anti‐mouse immunoglobulin G (Abcam, UK). The expression of COL2, Aggrecan, and CD73 was detected using rabbit anti‐human COL2, aggrecan and CD73 monoclonal antibodies (1:100, Abcam, UK), respectively, followed by goat anti‐rabbit immunoglobulin G (Abcam, UK). 4′,6‐Diamidino‐2‐phenylindole (DAPI, Sigma‐Aldrich, USA) was used for nuclear staining.
The osteogenic and adipogenic ability of microtia chondrocytes was detected by adipogenic and osteogenic induction solution. Osteogenic differentiation was induced with osteogenic differentiation medium [low‐glucose DMEM (L‐DMEM, Hyclone, USA), 10% fetal bovine serum (FBS), 100 U mL−1 penicillin, 0.1 mg mL−1 streptomycin,10 × 10‐3 m β‐glycerol phosphate, 50 × 10‐3 m vitamin C (Sigma‐Aldrich, USA) and 0.1 × 10‐3 m dexamethasone (Sigma‐Aldrich, USA)] for 14 d. Microtia chondrocytes were fixed with 4% paraformaldehyde for 30–60 min, and 0.1% (pH = 8.0) Alizarin Red S staining (Solarbio, China) solution was added to incubate for 60 min. Adipogenic differentiation was induced with adipogenic differentiation medium [H‐DMEM,10%FBS, 100 U mL−1 penicillin, 0.1 mg mL−1 streptomycin, 5 mg mL−1 insulin (Sigma‐Aldrich, USA), 0.5 × 10‐3 m 3‐isobutyl‐1‐methylxanthine (Sigma‐Aldrich, USA), 200 × 10‐3 m indomethacin (Sigma‐Aldrich, USA) and 1 × 10‐3 m dexamethasone] for 21 d. Microtia chondrocytes were fixed with 4% paraformaldehyde for 30–60 min, and the Oil Red O staining solution kit (Solarbio, China) was used for staining.
Preparation of Cartilage Acellular Matrix (CAM)
Porcine ears were collected from the slaughter house and used with approval. The ears were carefully dissected to strip the fibrous tissue and perichondrium and then fragmented into small pieces. The cartilage pieces were first freeze‐dried for 12 h, then placed in liquid nitrogen for 5 min and frozen ground with a grinder (60 Hz, 60 s) to obtain cartilage microparticles. The microparticles were treated with 0.25% trypsin (containing 0.02%EDTA) in a constant temperature shaker (37 °C, 150 r min−1) for 24 h, in which the new trypsin was changed every 4 h. Trypsinized cartilage tissues were washed with hypertonic buffer solution (1.5 mol L−1 NaCl dissolved in 50 × 10‐3 m Tris‐HCl, pH = 7.6, Sinopharm, China) for 5–8 times until the cartilage serous fluid turned white, and treated with nuclease solution [50 U mL−1 DNase (Sigma‐Aldrich, USA), 1 U mL−1 RNaseA (Sigma‐Aldrich, USA), dissolved in 10 × 10‐3 m Tris‐HCl (Beyotime, China), pH = 7.5] in the constant temperature shaker (37 °C, 80 r min−1) for 20 h. To remove the enzymes, the tissues were washed with the 10 × 10‐3 m Tris‐HCl [containing 10 U mL−1 aprotinin (Sigma‐Aldrich, USA), pH = 8.0] for 20 h and 1% TritonX‐100/PBS (Beyotime, China) solution for 24 h. The decellularized cartilage tissues were washed with PBS for 72 h with changing the solution every 12 h. Then the CAM tissues were frozen ground again (60 Hz,60 s) and sifted through the 50 mesh sieves to get CAM microparticles. To get different particle sizes, CAM microparticles were sifted through the 50100150 mesh sieves and stored at ‐20 °C.
Biochemical Characterization of CAM
To verify the extent of decellularization, the residual DNA and glycosaminoglycan (GAG) were measured and histological sections were analyzed with H&E staining and Masson staining. For DNA and GAG quantification, 20 mg CAM and 20 mg native cartilage tissue were weighed respectively. DNA was extracted according to instructions of Blood/Cell/Tissue Genomic DNA Extraction Kit (TianGen DP304, China) and measured using the Quant‐iTTM PicoGreen dsDNA (ThermoFisher, USA). GAG content was measured with Blyscan Glycosaminoglycan Kit (Biocolor, UK). For histological evaluation, both native and decellularized tissues were fixed in 4% paraformaldehyde (Sinopharm, China). After dehydration, samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) and Masson's trichrome (Solarbio, China). Three independent experiments were performed in triplicate for each group.
Particle Size Analysis
The test was carried out with the laser‐assisted particle size analyzer (Malvern Mastersizer 2000, UK), and the dispersing solvent was distilled water. CAM microtissues to be measured were slowly dispersed into distilled water in the test container, and the test was started when the shading background intensity reached 12%. The refractive coefficients of CAM microtissues and distilled water were 1.50 and 1.33, respectively.
Fabrication of Microtissues
CAM microparticles were treated with 0.1% peracetic acid (Sinopharm, China) solution in combination with 4% ethanol (Sinopharm, China) for 4 h for disinfection, and then washed 3 times with sterile PBS. 500 mg CAM microparticles were added to 50 mL PBS to get 10 mg mL−1 CAM suspension. 2% agarose solution was added to the 12‐well plate in advance to cool to gel. The CAM suspension was blown and mixed, and then added to the 12‐well plate. After CAM microparticles settled into the lower layer, the upper PBS was sucked with the 1 mL syringe. Meanwhile, P3 microtia chondrocytes were digested and suspended into 1 × 106 cell mL−1 cell suspension, dropped on CAM with the inoculation density of 5 × 104 chondrocytes per 1 mg CAM and incubated at 37 °C for 2 h to allow for cell attachment. After that, the complete culture medium [H‐DMEM, 10% FBS, 100 U mL−1 penicillin, 0.1 mg mL−1 streptomycin and 20 ng mL−1 bFGF] was added for long‐time culture.
Assessment of Chondrocytes Viability in Microtissues
For live/dead assay, calcein (Beyotime, China) and PI (Sigma‐Aldrich, USA) were used to stain living cells and dead cells, respectively. Before staining, calcein mother solution was diluted 1000 times with PBS, and 10 mg PI was dissolved in 1 mL acetone and diluted to 1 µg mL‐1 with PBS. The samples were rinsed with PBS for three times in advance. The samples were incubated at 37 °C with calcein working solution for 30 min and then replaced with culture medium for another 30 min. After that, the medium was sucked out, and PI working solution was added and incubated for 5 min. Finally, the samples were rinsed with PBS for three times and observed under the confocal laser microscope (Nikon, Japan).
Cell proliferation within microtissues was detected by Cell Counting Kit‐8 (Beyotime, China). Briefly, standard curves were measured using P3 chondrocytes. After that, microtissues were detected by Cell Counting Kit‐8 at day 1, day 3, and day 5.
Preparation of Bioink
10% w/v GelMA (EFL‐GM‐90, EFL, China) solution was prepared and sterilized in advance, in which the LAP (EFL‐LAP, EFL, China) concentration was 0.25% w/v as photoinitiator, and 0.1% w/v tartrazine (Sinopharm, China) as photoabsorber for DLP bioprinting. In order to observe the effects of different CAM concentrations and particle sizes on the performance of bioink, 1%, 5%, and 10% w/v concentrations and three sizes of CAM microparticles with 0–150 µm, 150–300 µm, and >300 µm were selected for testing, and the suitable CAM concentration and particle size were chosen for the preparation of microtissue bioink.
For the preparation of microtissue bioink, the construction method of microtissues was mentioned above. After 5 d of culture in vitro, the amount of living cells on microtissues was measured, then the microtissues were gently mixed with sterilized 10% w/v GelMA solution. For the bioink as control, the same amount of chondrocytes (about 6.26 × 106 cells in 1 mL GelMA solution) of the same generation were mixed with sterilized 10% w/v GelMA solution directly. All the above operations were carried out at room temperature as soon as possible, and protected from light. The above experiment was repeated for 5 times, and stable microtissue bioink was obtained each time. Replacing GelMA with PEGDA was then tried (M.V. 1000, Aladdin, China) and the above steps were repeated to observe the growth of microtissues in PEGDA.
Scanning Electron Microscopy (SEM)
The surface morphology was measured by Environmental Scanning Electron Microscope (Quanta 200, FEI, USA). All the samples were fixed with 2.5% v/v glutaraldehyde for 4 h, gradient dehydrated by different levels of ethanol (30%, 50%, 70%, 90%, 100%), treated with n‐butanol and then lyophilized. The dried samples were sputtered with gold under vacuum (20 mA,150 s) and observed.
Compression Testing
Disc shaped test specimen of 8 mm diameter and 3 mm height were prepared using bioink with different concentrations and particle sizes of CAM. A 20 g load cell was used for testing. Samples were compressed to a final strain of 30% at a rate of 0.01 mm s−1. The compression modulus was calculated from the slope of the linear 2%‐10% of the stress–strain curve. Three independent experiments were performed in triplicate for each group.
Rheological Examinations
Rheological measurements were conducted on an Anton Paar MCT 302 rheometer (Anton Paar, Austria) at 37 °C. To evaluate the shear thinning behavior, the bioink was placed between the parallel plate subjected to an increasing shear of 0.01–200 s−1. The viscosity of the bioink before printing was evaluated by subjecting the bioink to a low shear of 1 s−1 and held for 600 s. Then the bioink was solidified into a gel sheet with 10 mm diameter and a 1 mm height, the storage and loss modulus were evaluated by time sweeps under fixed strain and frequency conditions (1% strain and 5 rad s−1) for 300 s.
Swelling Ratio
The swelling ratio values were determined following the previous study procedure. Disc‐shaped test specimen of 8 mm diameter and 3 mm height were prepared using bioink with different concentrations and particle sizes of CAM. After photocrosslinking with 405 nm UV, three samples from each condition were soaked in PBS at 37 °C for 12 h and weighed as M1, then lyophilized for 12 h and weighed as M2. The swelling ratio (q) was calculated using Equation q = M1/M2. Three independent experiments were performed in triplicate for each group.
Sedimentation Characteristics of Microtissue in GelMA
The sedimentation characteristics were monitored according to the previous study procedure. Briefly, three particle sizes of 0–150 µm, 150–300 µm and >300 µm CAM microparticles were added into DMEM to the concentration of 10% w/v, left to rest at 37 °C and monitored the sedimentation behavior with time. As for the bright field images at high magnification, the samples standing for 0 h, 1 h and 3 h were solidified and formed at 4 °C respectively, and then placed horizontally under a stereomicroscope for photographing, so as to ensure that the distribution of particles inside the samples remained unchanged. Three samples were tested for each group, and the sedimentation distance was then measured and statistical analysis was done with one‐way ANOVA test.
3D Bioprinting
The 3D auricular model was designed with UG 10.0 software and 3D bioprinting experiments were conducted using Digital Light Projection (DLP) printer (EFL‐BP‐8600, China). The bioink was prepared in advance and mixed by gently blowing, then added on the platform under sterile conditions. For GelMA or GelMA+chondrocytes bioinks, the printing parameters were set as: light intensity 14 mW cm−2, exposure time 30 s, and penetration depth 30 µm. As for GelMA+CAM or GelMA+microtissues bioinks, the printing parameters were set as: light intensity 14 mW cm−2, exposure time 35 s, and penetration depth 30 µm. After conducting the printing process, the constructs were soaked in H‐DMEM for further culturing.
In Vitro Culture
The printed auricular constructs were placed in the complete culture medium [H‐DMEM, 10% FBS, 100 U mL‐1 penicillin, 0.1 mg mL‐1 streptomycin and 20 ng mL‐1 bFGF] for culture, and the culture medium was changed every 3 d for continuous culture for 20 d. The cell growth was observed under a confocal laser microscope at day 1, day 10, and day 20.
To observe the growth of microtissues in different gel networks and to explore whether the construction method of microtissue bioink is universal, GelMA was switched to another gel. PEGDA is a common hydrogel. Different from GelMA, it has denser gel network and higher mechanical strength, but the biocompatibility needs to be improved. Here, 10% w/v PEGDA (M.V. 1000, Aladdin, China) was mixed with microtissues (10% w/v) for in vivo culture, and the cell growth was observed under a confocal laser microscope at day 1 and day 10.
Live/Dead Cell Staining and Immunofluorescence Staining of In Vitro Culture Constructs
For live/dead assay, calcein (Beyotime, China) and PI (Sigma‐Aldrich, USA) were used to stain living cells and dead cells, respectively. Before staining, calcein mother solution was diluted 1000 times with PBS, and 10 mg PI was dissolved in 1 mL acetone and diluted to 1 µg mL‐1 with PBS. The samples were rinsed with PBS for 3 times in advance. The samples were incubated at 37 °C with calcein working solution for 30 min and then replaced with culture medium for another 30 min. After that, the medium was sucked out, and PI working solution was added and incubated for 5 min. Finally, the samples were rinsed with PBS 3 times for 5 min each and observed under the confocal laser microscope (Nikon, Japan). Z‐axis scan length was 250 µm and the experiment was performed in triplicate (n = 3), with the viability of each sample averaged over 3 pictures of randomly chosen positions inside the hydrogel.
For immunofluorescence staining, the expression of COL2 and Aggrecan was detected using rabbit anti‐human COL2 and aggrecan monoclonal antibodies (1:200, Abcam, UK), respectively, followed by goat anti‐rabbit immunoglobulin G (Abcam, UK).
Transwell
For cocultivation experiments, six‐well transwell coculture plates (0.4 µm pore size membrane, Corning, USA) were chosen. 100 µL of chondrocytes were seeded in each lower well plate, at the density of 1 million cells mL−1. Subsequently, transwell chambers containing chondrocytes and microtissues were respectively inserted into the six‐well plates with equal amounts of 1 × 105 chondrocytes in each chamber, and then cocultured for 3 and 5 d. The experiment was repeated three times.
Gene Expression Analysis
For the microtia chondrocytes cultured in petri dish and microtissues, cells were collected by trypsin digestion. For the chondrocytes encapsulated in GelMA (EFL‐GM‐90, EFL, China), GelMA Lysis Buffer (EFL‐GM‐LS‐001, EFL, China) was used to dissolve the gel and release the cells in advance. The total RNA was extracted using TRIzol (ThermoFisher, USA). RNA was reversed and transcribed into cDNA and the real‐time fluorescence quantitative PCR (qRT‐PCR) detection was performed following the manufacturer's recommendation. Briefly, qRT‐PCR was performed using the StepOnePlus Real‐Time PCR System (Applied Biosystems) with the following cycling scheme: 1) Predenaturation (95 °C, 10 min), 2) Denaturation (95 °C) + Annealing extension (60 °C) was used as a cycle, repeated 40 times, 3) Determination of dissolution curve: (95 °C, 15 s) – (60 °C, 60 s) – (95 °C, 15 s). For the detection of mRNA expression, all primers were synthesized by Wuhan Biofavor Biotech. Primers used for qRT‐PCR are presented in Table S1 (Supporting Information). Three independent experiments were performed in triplicate for each group.
Subcutaneous Nude Mice Implantation
All experiments involving animals were performed in accordance with the guidelines of the Ethics Committee of Huazhong University of Science and Technology (approval number SYXK 2016‐0057). Male BALB/c‐nu nude mice (7 weeks old, weighing 17 to 20 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (China). To investigate the effects of CAM microtissues on cartilage regeneration in vivo, 40 mice were divided randomly into 4 groups (n = 10 mice per group). GelMA (CAM‐, chondrocytes‐), GelMA+chondrocytes (CAM‐, chondrocytes+), GelMA+CAM (CAM+, chondrocytes‐) and GelMA+microtissues (CAM+, chondrocytes+) 3D printed auricular constructs were prepared under sterile conditions in advance and soaked in complete medium. Recipient mice were anesthetized by means of inhaled 3% isoflurane. A 10 mm skin incision was made along the midline of the dorsal, two subcutaneous pockets are then bluntly stripped outwards. Auricular constructs were placed under the skin, and 5‐0 cosmetic sutures were used to suture the skin incision intermittently, so as to keep the stent in its original position and avoid damaging the scaffold structure. After 6 and 12 weeks, five randomly selected animals in each treatment group were euthanized, and the grafts were harvested for the following analyses.
An incision along the dorsal midline was created and two subcutaneous pockets were created lateral to the dorsal midline by blunt dissection.
Histological Evaluation
The specimens were placed in the ‐80 °C refrigerator immediately after harvested. 7 µm frozen sections were prepared for all stainings of auricular constructs with cryostat microtome (ThermoFisher, USA) and sections of 9‐11 layer were uniformly selected for staining. Samples were stained with hematoxylin and eosin (H&E) and Safranin O (Solarbio, China). For immunohistochemical evaluation, rabbit anti‐human COL2 monoclonal antibodies (1:100, Abcam, UK) was used, followed by goat anti‐rabbit immunoglobulin G (Abcam, UK).
For immunofluorescence staining, the expression of Lamin A/C was detected using rabbit anti‐human LaminA+LaminC monoclonal antibodies (1:400, Abcam, UK), respectively, followed by goat anti‐rabbit immunoglobulin G (Abcam, UK).
Statistical Analysis
Quantitative results were analyzed with GraphPad Prism (v.7.0.0). One‐way analysis of variance (ANOVA) and student's t‐test were applied to mean comparisons. All data in this study are expressed as the mean values ± standard deviation. Statistically significant differences were considered when p‐values below 0.05 (p < 0.05).
Conflict of Interest
The authors declare no conflict of interest.
Authors Contribution
J.S. and Z.W. designed and supervised the project. X.X. contributed to design experiments and discuss results. X.X., S.W.,and S.M. performed experiments. J.S., Z.W., and N.G. provided financial support. X.X. prepared the manuscript with inputs from all authors. All authors commented on the manuscript.
Supporting information
Supporting Information
Supplemental Movie 1
Supplemental Movie 2
Acknowledgements
The authors appreciate the Analytical & Testing Center of Huazhong University of Science and Technology for SEM measurements. This work was supported by the National Key R&D Program of China (2019YFA0110500), the National Natural Science Foundation of China (No. 82020108020, 82072198, 81873941 and 81701922), and the Natural Science Foundation of Hubei Province (2017CFB263).
Xie X., Wu S., Mou S., Guo N., Wang Z., Sun J., Microtissue‐Based Bioink as a Chondrocyte Microshelter for DLP Bioprinting. Adv. Healthcare Mater. 2022, 11, 2201877. 10.1002/adhm.202201877
Contributor Information
Zhenxing Wang, Email: wangzhenxing@hust.edu.cn.
Jiaming Sun, Email: sunjiaming@hust.edu.cn.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Mandrycky C., Wang Z., Kim K., Kim D. H., Biotechnol. Adv. 2016, 34, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C. T., Biomaterials 2020, 226, 119536. [DOI] [PubMed] [Google Scholar]
- 3. Lee M., Rizzo R., Surman F., Zenobi‐Wong M., Chem. Rev. 2020, 120, 10950. [DOI] [PubMed] [Google Scholar]
- 4. Sun X., Ma Z., Zhao X., Jin W., Zhang C., Ma J., Qiang L., Wang W., Deng Q., Yang H., Zhao J., Liang Q., Zhou X., Li T., Wang J., Bioact. Mater. 2021, 6, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jian Z., Zhuang T., Qinyu T., Liqing P., Kun L., Xujiang L., Diaodiao W., Zhen Y., Shuangpeng J., Xiang S., Jingxiang H., Shuyun L., Libo H., Peifu T., Qi Y., Quanyi G., Bioact. Mater. 2021, 6, 1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee A., Hudson A. R., Shiwarski D. J., Tashman J. W., Hinton T. J., Yerneni S., Bliley J. M., Campbell P. G., Feinberg A. W., Science 2019, 365, 482. [DOI] [PubMed] [Google Scholar]
- 7. Kim B. S., Gao G., Kim J. Y., Cho D. W., Adv. Healthcare Mater. 2019, 8, 1801019. [Google Scholar]
- 8. Murphy S. V., De Coppi P., Atala A., Nat. Biomed. Eng. 2020, 4, 370. [DOI] [PubMed] [Google Scholar]
- 9. Gu Y., Forget A., Shastri V. P., Adv. Sci. 2021, 9, e2103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chimene D., Kaunas R., Gaharwar A. K., Adv. Mater. 2020, 32, 1902026. [DOI] [PubMed] [Google Scholar]
- 11. Kiyotake E. A., Beck E. C., Detamore M. S., Ann. N. Y. Acad. Sci. 2016, 1383, 139. [DOI] [PubMed] [Google Scholar]
- 12. Pati F., Jang J., Ha D. H., Won Kim S., Rhie J. W., Shim J. H., Kim D. H., Cho D. W., Nat. Commun. 2014, 5, 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fisch P., Broguiere N., Finkielsztein S., Linder T., Zenobi‐Wong M., Adv. Funct. Mater. 2021, 31, 2008261. [Google Scholar]
- 14. de Melo B. A. G., Jodat Y. A., Mehrotra S., Calabrese M. A., Kamperman T., Mandal B. B., Santana M. H. A., Alsberg E., Leijten J., Shin S. R., Adv. Funct. Mater. 2019, 29, 1906330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo C., Fang H., Zhou M., Li J., Zhang X., Liu S., Zhou C., Hou J., He H., Sun J., Wang Z., Theranostics 2019, 9, 4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bunpetch V., Zhang Z. Y., Zhang X., Han S., Zongyou P., Wu H., Hong‐Wei O., Biomaterials 2019, 196, 67. [DOI] [PubMed] [Google Scholar]
- 17. Li Y., Liu W., Liu F., Zeng Y., Zuo S., Feng S., Qi C., Wang B., Yan X., Khademhosseini A., Bai J., Du Y., Proc. Natl. Acad. Sci. USA 2014, 111, 13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J., Xu W., Li C., Meng F., Guan Y., Liu X., Zhao J., Peng J., Wang Y., Tissue Eng., Part B 2022, 28. [DOI] [PubMed] [Google Scholar]
- 19. Gu Y., Kang N., Dong P., Liu X., Wang Q., Fu X., Yan L., Jiang H., Cao Y., Xiao R., J. Tissue Eng. Regener. Med. 2018, 12, e1737. [DOI] [PubMed] [Google Scholar]
- 20. Dabiri S. M. H., Samiei E., Shojaei S., Karperien L., Khun Jush B., Walsh T., Jahanshahi M., Hassanpour S., Hamdi D., Seyfoori A., Ahadian S., Khademhosseini A., Akbari M., Small 2021, 17, 2103192. [DOI] [PubMed] [Google Scholar]
- 21. Mamaghani K. R., Naghib S. M., Zahedi A., Rahmanian M., Mozafari M., Mater. Today: Proc. 2018, 5, 15790. [Google Scholar]
- 22. Wiggenhauser P. S., Schantz J. T., Rotter N., Regener. Med. 2017, 12, 303. [DOI] [PubMed] [Google Scholar]
- 23. Zhang L., He A., Yin Z., Yu Z., Luo X., Liu W., Zhang W., Cao Y., Liu Y., Zhou G., Biomaterials 2014, 35, 4878. [DOI] [PubMed] [Google Scholar]
- 24. Kamil S. H., Vacanti M. P., Vacanti C. A., Eavey R. D., Laryngoscope 2004, 114, 2187. [DOI] [PubMed] [Google Scholar]
- 25. Zhou G., Jiang H., Yin Z., Liu Y., Zhang Q., Zhang C., Pan B., Zhou J., Zhou X., Sun H., Li D., He A., Zhang Z., Zhang W., Liu W., Cao Y., EBioMedicine 2018, 28, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wei D. X., Dao J. W., Chen G. Q., Adv. Mater. 2018, 30, 1802273. [DOI] [PubMed] [Google Scholar]
- 27. Amaral A. J. R., Pasparakis G., Acta Biomater. 2019, 90, 21. [DOI] [PubMed] [Google Scholar]
- 28. Luo C., Fang H., Li J., Hou J., Yang J., Yuan Q., Guo L., Zhong A., Wang J., Sun J., Wang Z., J. Biomed. Mater. Res., Part A 2019, 107, 678. [DOI] [PubMed] [Google Scholar]
- 29. De Santis M. M., Alsafadi H. N., Tas S., Bolukbas D. A., Prithiviraj S., Da Silva I. A. N., Mittendorfer M., Ota C., Stegmayr J., Daoud F., Konigshoff M., Sward K., Wood J. A., Tassieri M., Bourgine P. E., Lindstedt S., Mohlin S., Wagner D. E., Adv. Mater. 2021, 33, 2005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abaci A., Guvendiren M., Adv. Healthcare Mater. 2020, 9, 2000734. [DOI] [PubMed] [Google Scholar]
- 31. Yu C., Ma X., Zhu W., Wang P., Miller K. L., Stupin J., Koroleva‐Maharajh A., Hairabedian A., Chen S., Biomaterials 2019, 194, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ratheesh G., Vaquette C., Xiao Y., Adv. Healthcare Mater. 2020, 9, 2001323. [DOI] [PubMed] [Google Scholar]
- 33. Kim B. S., Das S., Jang J., Cho D. W., Chem. Rev. 2020, 120, 10608. [DOI] [PubMed] [Google Scholar]
- 34. Kim M. K., Jeong W., Lee S. M., Kim J. B., Jin S., Kang H. W., Biofabrication 2020, 12, 025003. [DOI] [PubMed] [Google Scholar]
- 35. Hu X., Zhou J., Zhang N., Tan H., Gao C., J. Mech. Behav. Biomed. Mater. 2008, 1, 352. [DOI] [PubMed] [Google Scholar]
- 36. Kessel B., Lee M., Bonato A., Tinguely Y., Tosoratti E., Zenobi‐Wong M., Adv. Sci. 2020, 7, 2001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang Q., Zou Y., Arno M. C., Chen S., Wang T., Gao J., Dove A. P., Du J., Chem. Soc. Rev. 2017, 46, 6255. [DOI] [PubMed] [Google Scholar]
- 38. Gu Z., Fu J., Lin H., He Y., Asian J. Pharm. Sci. 2020, 15, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. You F., Chen X., Cooper D. M. L., Chang T., Eames B. F., Biofabrication 2018, 11, 015015. [DOI] [PubMed] [Google Scholar]
- 40. Huh J., Moon Y. W., Park J., Atala A., Yoo J. J., Lee S. J., Biofabrication 2021, 13, 034103. [DOI] [PubMed] [Google Scholar]
- 41. Van Belleghem S., Torres L., Santoro M. Jr, Mahadik B., Wolfand A., Kofinas P., Fisher J. P., Adv. Funct. Mater. 2020, 30, 1907145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choudhury D., Tun H. W., Wang T., Naing M. W., Trends Biotechnol. 2018, 36, 787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supplemental Movie 1
Supplemental Movie 2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


