Abstract
The P1 primary alkylsulphatase of Pseudomonas C12B was purified 1500-fold to homogeneity by a combination of streptomycin sulphate precipitation of nucleic acids, (NH4)2SO4 fractionation and chromatography on columns of DEAE-cellulose, Sephacryl S-300 and butyl-agarose. The protein was tetrameric with an Mr of 181000-193000, and exhibited maximum activity at pH 6.1. Primary alkyl sulphates of carbon-chain length C1-C5 or above C14 were not substrates, but the intermediate homologues were shown to be substrates, either by direct assay (C6-C9 and C12) or by gel zymography (C10, C11, C13 and C14). Increasing the chain length from C6 to C12 led to diminishing Km. Values of delta G0' for binding substrates to enzyme were dependent linearly on chain length, indicating high dependence on hydrophobic interactions. Vmax./Km values increased with increasing chain length. Inhibition by alk-2-yl sulphates and alkane-sulphonates was competitive and showed a similar dependence on hydrophobic binding. The P1 enzyme was active towards several aryl sulphates, including o-, m- and p-chlorophenyl sulphates, 2,4-dichlorophenyl sulphate, o-, m- and p-methoxyphenyl sulphates, m- and p-hydroxyphenyl sulphates and p-nitrophenyl sulphate, but excluding bis-(p-nitrophenyl) sulphate and the O-sulphate esters of tyrosine, nitrocatechol and phenol. The arylsulphatase activity was weak compared with alkylsulphatase activity, and it was distinguishable from the de-repressible arylsulphatase activity of Pseudomonas C12B reported previously. Comparison of the P1 enzyme with the inducible P2 alkylsulphatase of this organism, and with the Crag herbicide sulphatase of Pseudomonas putida, showed that, although there are certain similarities between any two of the three enzymes, very few properties are common to all three.
Full text
PDF

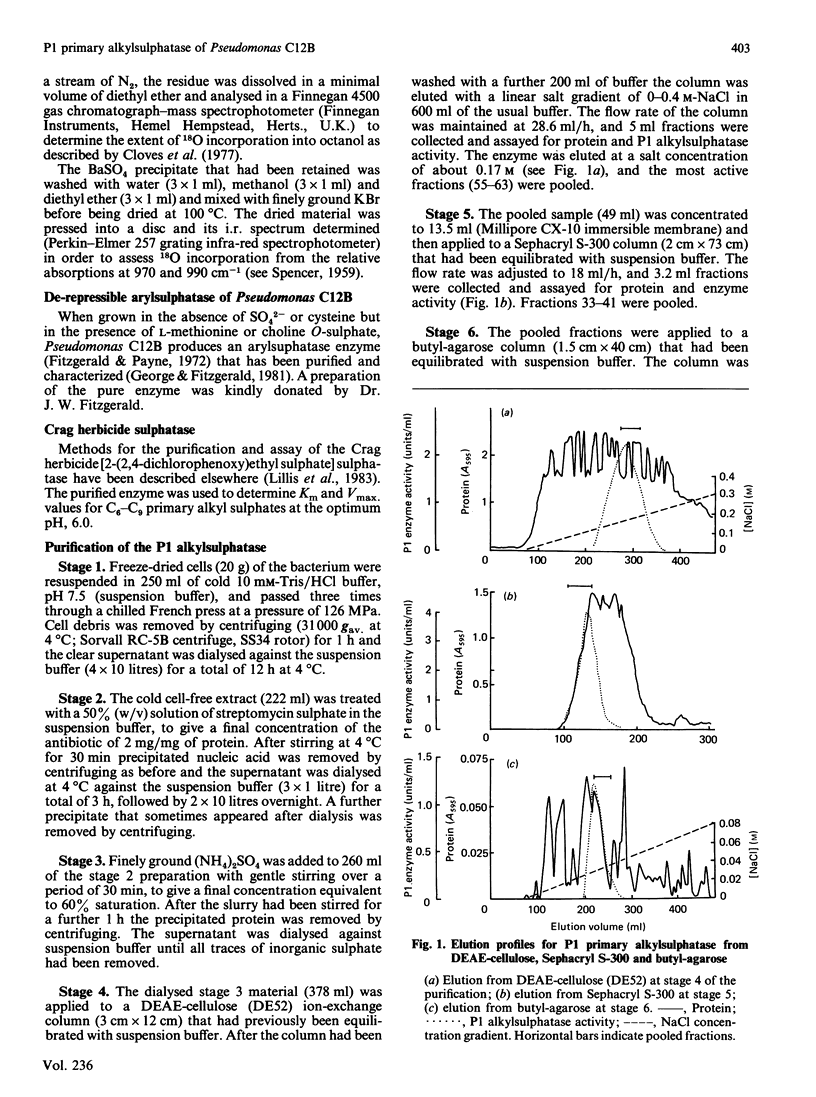




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew B., Dodgson K. S., Gorham S. D. Purification and properties of the S1 secondary alkylsulphohydrolase of the detergent-degrading micro-organism, Pseudomonas C12B. Biochem J. 1978 Mar 1;169(3):659–667. doi: 10.1042/bj1690659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew B., Dodgson K. S., Matcham G. W., Shaw D. J., White G. F. A novel mechanism of enzymic ester hydrolysis. Inversion of configuration and carbon-oxygen bond cleavage by secondary alkylsulphohydrolases from detergent-degrading micro-organisms. Biochem J. 1977 Sep 1;165(3):575–580. doi: 10.1042/bj1650575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
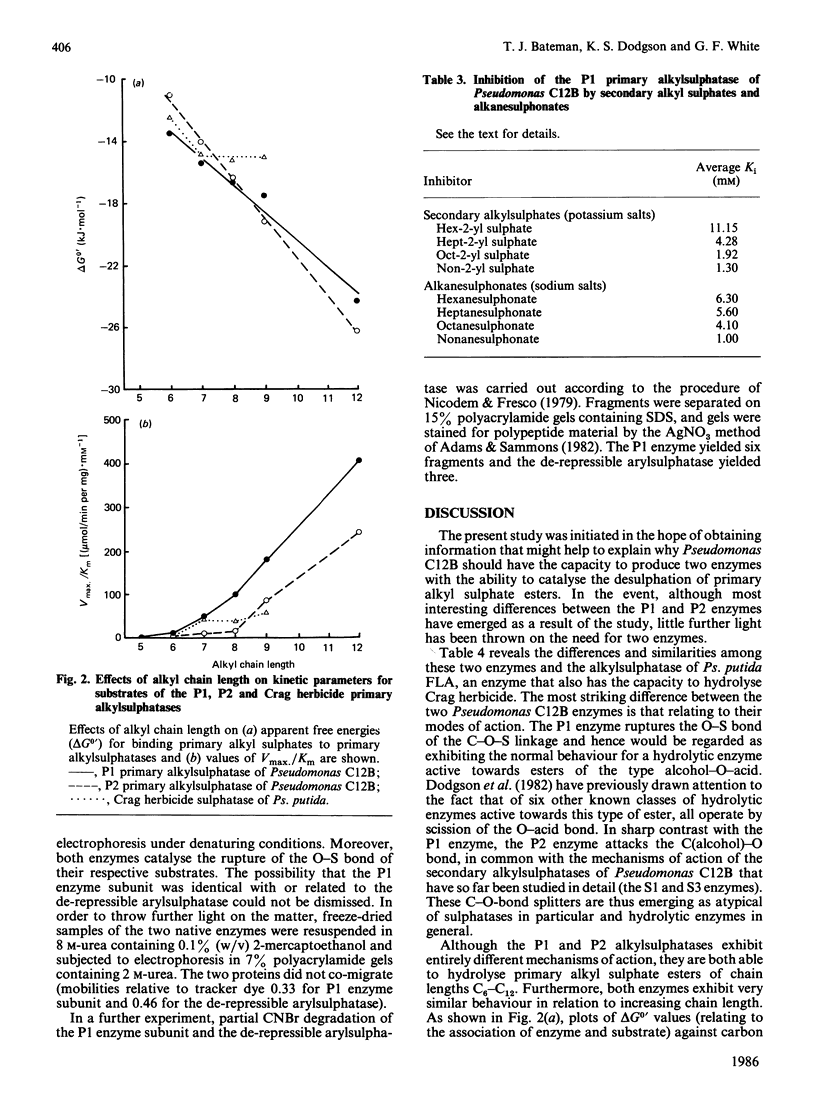
- Bryan J. K. Molecular weights of protein multimers from polyacrylamide gel electrophoresis. Anal Biochem. 1977 Apr;78(2):513–519. doi: 10.1016/0003-2697(77)90111-7. [DOI] [PubMed] [Google Scholar]
- Cloves J. M., Dodgson K. S., Games D. E., Shaw D. J., White G. F. The mechanism of action of primary alkylsulphohydrolase and arylsulphohydrolase from a detergent-degrading micro-organism. Biochem J. 1977 Dec 1;167(3):843–846. doi: 10.1042/bj1670843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloves J. M., Dodgson K. S., White G. F., Fitzgerald J. W. Purification and properties of the P2 primary alkylsulphohydrolase of the detergent-degrading bacterium pseudomonas C12B. Biochem J. 1980 Jan 1;185(1):23–31. doi: 10.1042/bj1850023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloves J. M., Dodgson K. S., White G. F., Fitzgerald J. W. Specificity of P2 primary alkylsulphohydrolase induction in the detergent-degrading bacterium Pseudomonas C12B. Effects of alkanesulphonates, alkyl sulphates and other related compounds. Biochem J. 1980 Jan 1;185(1):13–21. doi: 10.1042/bj1850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B., WILLIAMS K. Studies on sulphatases. 13. The hydrolysis of substituted phenyl sulphates by the arylsulphatase of Alcaligenes metalcaligenes. Biochem J. 1956 Oct;64(2):216–221. doi: 10.1042/bj0640216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson K. S., Fitzgerald J. W., Payne W. J. Chemically defined inducers of alkylsulphatases present in Pseudomonas C12B. Biochem J. 1974 Jan;138(1):53–62. doi: 10.1042/bj1380053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Payne W. J. The regulation of arylsulphatase formation in Pseudomonas C 12 B. Microbios. 1972 Sep-Oct;6(22):147–156. [PubMed] [Google Scholar]
- George J. R., Fitzgerald J. W. Arylsulfatase from Pseudomonas sp. strain C12B: purification to homogeneity, immunological analysis, and physical properties. J Bacteriol. 1981 Mar;145(3):1428–1431. doi: 10.1128/jb.145.3.1428-1431.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD A. G., TUDBALL N., DODGSON K. S. Infrared studies on sulphate esters. III. O-Sulphate esters of alcohols, amino alcohols and hydroxylated amino acids. Biochim Biophys Acta. 1961 Sep 30;52:413–419. doi: 10.1016/0006-3002(61)90397-3. [DOI] [PubMed] [Google Scholar]
- Lillis V., Dodgson K. S., White G. F., Payne W. J. Initiation of Activation of a Preemergent Herbicide by a Novel Alkylsulfatase of Pseudomonas putida FLA. Appl Environ Microbiol. 1983 Nov;46(5):988–994. doi: 10.1128/aem.46.5.988-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcham G. W., Dodgson K. S. Preparation and characterization of substrates suitable for the study of stereospecific secondary alkylsulphohydrolases of detergent-degrading micro-organisms. Biochem J. 1977 Dec 1;167(3):717–722. doi: 10.1042/bj1670717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcham G. W., Dodgson K. S. Purification, properties and cellular localization of the stereospecific CS2 secondary alkylsulphohydrolase of Comamonas terrigena. Biochem J. 1977 Dec 1;167(3):723–729. doi: 10.1042/bj1670723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. S., Lamberts B. L. Use of coomassie brilliant blue R250 for the electrophoresis of microgram quantities of parotid saliva proteins on acrylamide-gel strips. Biochim Biophys Acta. 1965 Aug 24;107(1):144–145. doi: 10.1016/0304-4165(65)90403-4. [DOI] [PubMed] [Google Scholar]
- Nikodem V., Fresco J. R. Protein fingerprinting by SDS-gel electrophoresis after partial fragmentation with CNBr. Anal Biochem. 1979 Sep 1;97(2):382–386. doi: 10.1016/0003-2697(79)90089-7. [DOI] [PubMed] [Google Scholar]
- Payne W. J., Fitzgerald J. W., Dodgson K. S. Methods for visualization of enzymes in polyacrylamide gels. Appl Microbiol. 1974 Jan;27(1):154–158. doi: 10.1128/am.27.1.154-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. J., Dodgson K. S., White G. F. Substrate specificity and other properties of the inducible S3 secondary alkylsulphohydrolase purified from the detergent-degrading bacterium Pseudomonas C12B. Biochem J. 1980 Apr 1;187(1):181–190. doi: 10.1042/bj1870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B. Studies on sulphatases. 25. The determination of BaSO(3)O by infrared spectroscopy. Biochem J. 1959 Nov;73(3):442–447. doi: 10.1042/bj0730442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. H., Tudball N. Studies on the enzymic degradation of L-serine O-sulphate by a rat liver preparation. Biochem J. 1967 Nov;105(2):467–472. doi: 10.1042/bj1050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loos C. M., van Breda A. J., Meijer A. E., Jöbsis A. C. Biochemical investigation to the reliability of the histochemical demonstration of microsomal arylsulphatase activity in cryostat sections. Histochemistry. 1981;73(2):161–164. doi: 10.1007/BF00493015. [DOI] [PubMed] [Google Scholar]


