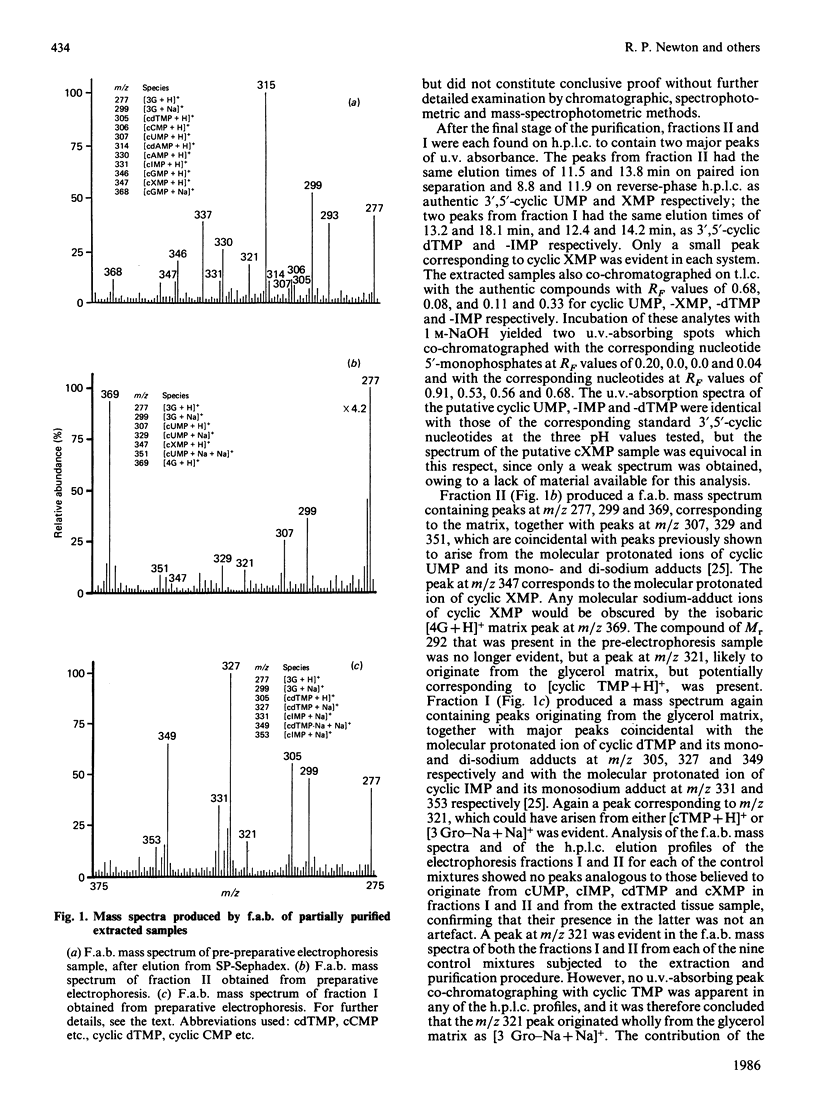
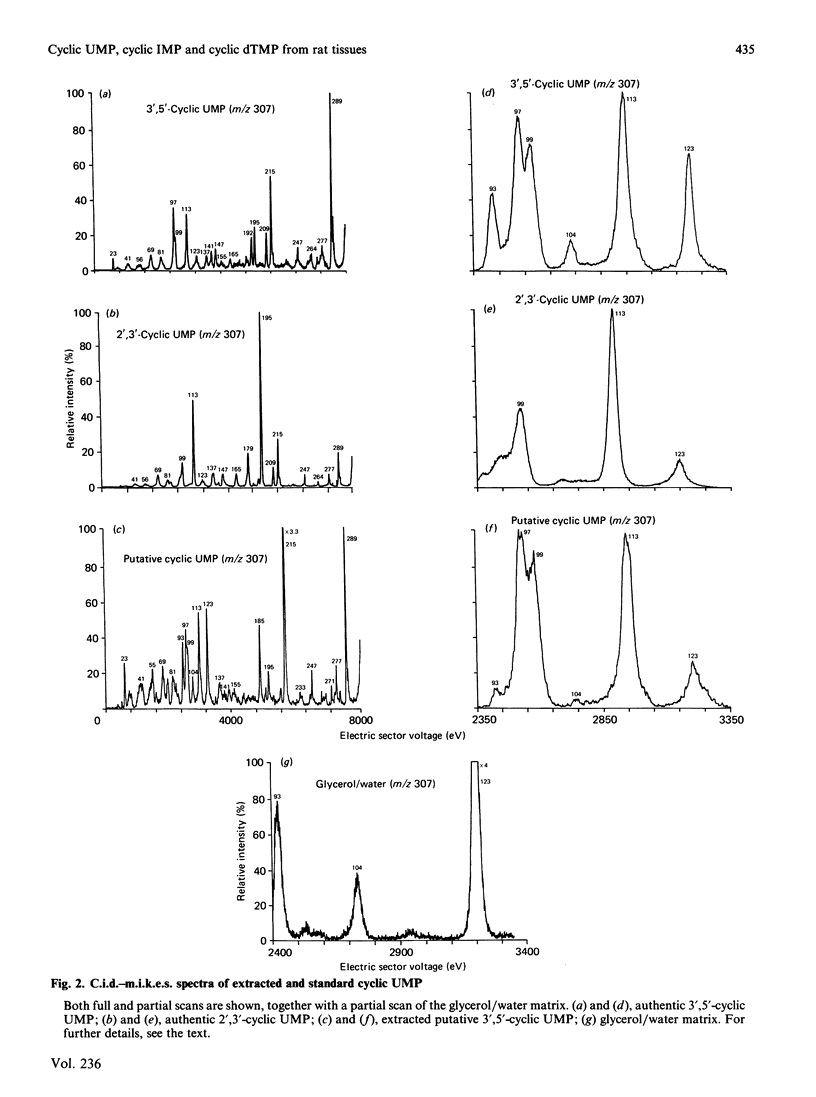
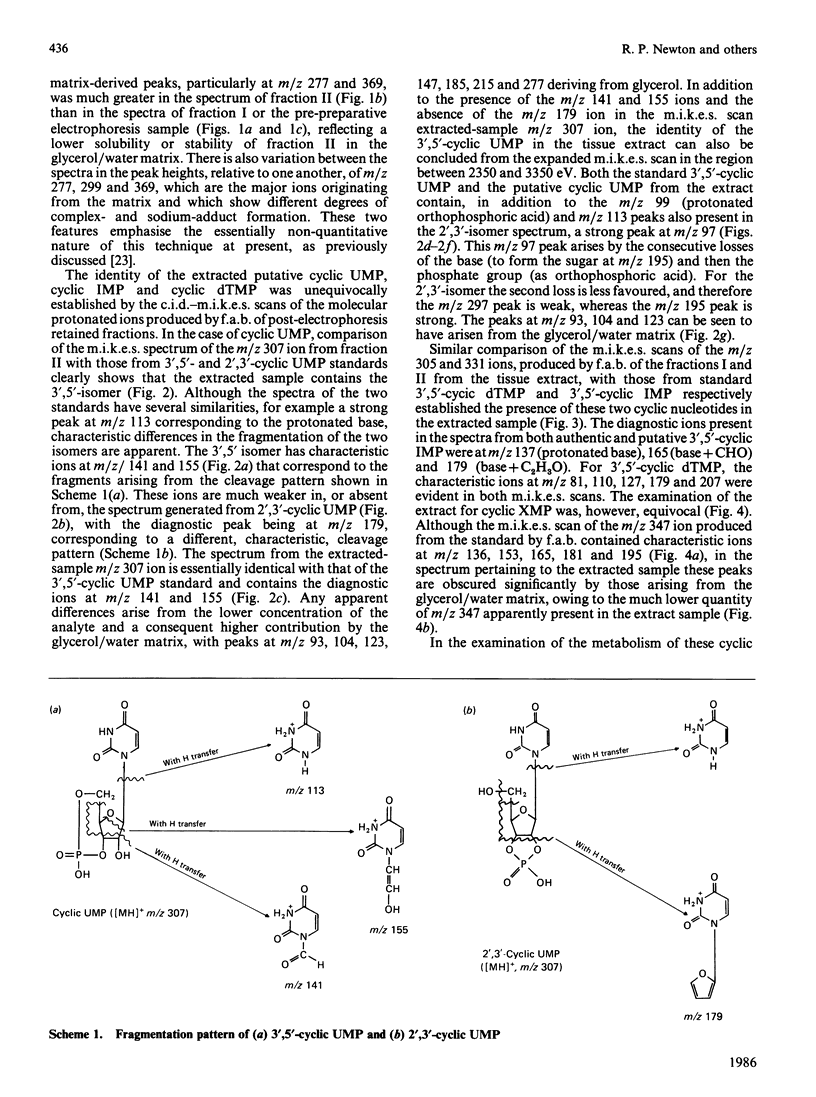
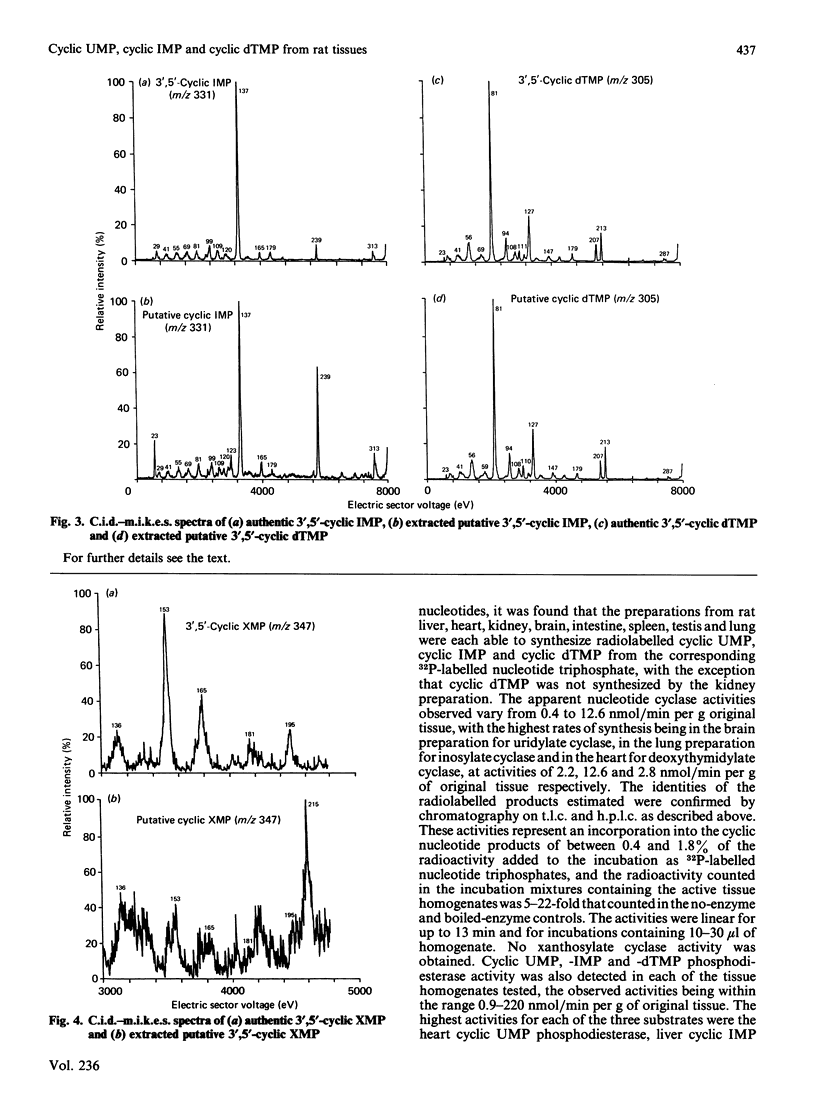
Abstract
The large-scale extraction and partial purification of endogenous 3',5'-cyclic UMP, 3',5'-cyclic IMP and 3',5'-cyclic dTMP are described. Rat liver, kidney, heart, spleen and lung tissues were subjected to a sequential purification procedure involving freeze-clamping, perchlorate extraction, alumina and Sephadex ion-exchange chromatography and preparative electrophoresis. The samples thus obtained co-chromatographed with authentic cyclic UMP, cyclic IMP and cyclic dTMP on t.l.c. and h.p.l.c. and the u.v. spectra of the extracted samples were identical with those of the standards. Fast atom bombardment of the three cyclic nucleotide standards yielded mass spectra containing a molecular protonated ion in each case; mass-analysed ion kinetic-energy spectrometry ('m.i.k.e.s') of these ions produced a spectrum unique to the parent cyclic nucleotide. The extracted putative cyclic UMP, cyclic IMP and cyclic dTMP each produced a m.i.k.e.s. identical with that obtained with the corresponding cyclic nucleotide standard. Rat liver, heart, kidney, brain, intestine, spleen, testis and lung protein preparations were each found capable of the synthesis of cyclic UMP, cyclic IMP and cyclic dTMP from the corresponding nucleoside triphosphate, of the hydrolysis of these cyclic nucleotides and of their binding, with the exception that cyclic dTMP was not synthesized by the kidney preparation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. R. Cyclic cytidine 3',5'-monophosphate (cCMP) in cell regulation. Mol Cell Endocrinol. 1982 Nov-Dec;28(3):373–385. doi: 10.1016/0303-7207(82)90134-4. [DOI] [PubMed] [Google Scholar]
- Bloch A. Uridine 3',5'-monophosphate (cyclic UMP). I. Isolation from rat liver extracts. Biochem Biophys Res Commun. 1975 May 5;64(1):210–218. doi: 10.1016/0006-291x(75)90240-5. [DOI] [PubMed] [Google Scholar]
- Brown E. G., Newton R. P., Shaw N. M. Analysis of the free nucloetide pools of mammalian tissue by high-pressure liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):378–388. doi: 10.1016/0003-2697(82)90461-4. [DOI] [PubMed] [Google Scholar]
- Cech S. Y., Ignarro L. J. Cytidine 3',5'-monophosphate (cyclic CMP) formation by homogenates of mouse liver. Biochem Biophys Res Commun. 1978 Jan 13;80(1):119–125. doi: 10.1016/0006-291x(78)91112-9. [DOI] [PubMed] [Google Scholar]
- Cech S. Y., Ignarro L. J. Cytidine 3',5'-monophosphate (cyclic CMP) formation in mammalian tissues. Science. 1977 Dec 9;198(4321):1063–1065. doi: 10.1126/science.22127. [DOI] [PubMed] [Google Scholar]
- Ferguson D. R., Price R. H. Studies on the metabolism of cyclic nucleotides by the toad bladder: the metabolic products of cAMP. FEBS Lett. 1973 Aug 15;34(2):204–206. doi: 10.1016/0014-5793(73)80794-x. [DOI] [PubMed] [Google Scholar]
- Gaion R. M., Krishna G. Cytidylate cyclase: the product isolated by the method of Cech and Ignarro is not cytidine 3',5'-monophosphate. Biochem Biophys Res Commun. 1979 Jan 15;86(1):105–111. doi: 10.1016/0006-291x(79)90387-5. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Suddath J. L., Hardman J. G. Enzymatic formation of inosine 3',5'-monophosphate and of 2'-deoxyguanosine 3',5'-monophosphate. Inosinate and deoxyguanylate cyclase activity. Biochim Biophys Acta. 1975 Jan 23;377(1):174–185. doi: 10.1016/0005-2744(75)90298-3. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Katoh N., Kuo J. F. Purification and properties of cyclic CMP phosphodiesterase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:403–416. [PubMed] [Google Scholar]
- Ishiyama J. Isolation of cyclic 3',5'-pyrimidine mononucleotides from bacterial culture fluids. Biochem Biophys Res Commun. 1975 Jul 8;65(1):286–292. doi: 10.1016/s0006-291x(75)80091-x. [DOI] [PubMed] [Google Scholar]
- Kingston E. E., Beynon J. H., Newton R. P., Liehr J. G. The differentiation of isomeric biological compounds using collision-induced dissociation of ions generated by fast atom bombardment. Biomed Mass Spectrom. 1985 Sep;12(9):525–534. doi: 10.1002/bms.1200120915. [DOI] [PubMed] [Google Scholar]
- Kingston E. E., Beynon J. H., Newton R. P. The identification of cyclic nucleotides from living systems using collision-induced dissociation of ions generated by fast atom bombardment mass spectrometry. Biomed Mass Spectrom. 1984 Jul;11(7):367–374. doi: 10.1002/bms.1200110709. [DOI] [PubMed] [Google Scholar]
- Lawson A. M., Stillwell R. N., Tacker M. M., Tsuboyama K., McCloskey J. A. Mass spectrometry of nucleic acid components. Trimethylsilyl derivatives of nucleotides. J Am Chem Soc. 1971 Feb 24;93(4):1014–1023. doi: 10.1021/ja00733a039. [DOI] [PubMed] [Google Scholar]
- Newton R. P., Salih S. G., Salvage B. J., Kingston E. E. Extraction, purification and identification of cytidine 3',5'-cyclic monophosphate from rat tissues. Biochem J. 1984 Aug 1;221(3):665–673. doi: 10.1042/bj2210665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W. Adenyl cyclase. II. The enzymatically catalyzed formation of adenosine 3',5'-phosphate and inorganic pyrophosphate from adenosine triphosphate. J Biol Chem. 1962 Apr;237:1228–1232. [PubMed] [Google Scholar]
- Soltero-Rigau E., Kruger T. L., Cooks R. G. Identification of barbiturates by chemical ionization and mass-analyzed ion kinetic energy spectrometry. Anal Chem. 1977 Mar;49(3):435–442. doi: 10.1021/ac50011a027. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Wehmann R. E., Parker C. W., Kipnis D. M. Radioimmunoassay for the measurement of cyclic nucleotides. Adv Cyclic Nucleotide Res. 1972;2:51–61. [PubMed] [Google Scholar]
- Walter U., Uno I., Liu A. Y., Greengard P. Identification, characterization, and quantitative measurement of cyclic AMP receptor proteins in cytosol of various tissues using a photoaffinity ligand. J Biol Chem. 1977 Sep 25;252(18):6494–6500. [PubMed] [Google Scholar]
- Wikberg J. E., Wingren G. B., Anderson R. G. Cytidine 3',5' Monophosphate (cCMP) is not an Endogenous nucleotide in normal or regenerating rat livers. Acta Pharmacol Toxicol (Copenh) 1981 Nov;49(5):452–454. doi: 10.1111/j.1600-0773.1981.tb00931.x. [DOI] [PubMed] [Google Scholar]
- Wikberg J. E., Wingren G. B. Investigations on the occurrence of cytidine 3',5' monophosphate (cCMP) in tissues. Acta Pharmacol Toxicol (Copenh) 1981 Jul;49(1):52–58. doi: 10.1111/j.1600-0773.1981.tb00869.x. [DOI] [PubMed] [Google Scholar]
- de Jong E. G., Heerma W., Dijkstra G. The use of collisional activation spectra in the discrimination of stereoisomeric permethylated disaccharides. Biomed Mass Spectrom. 1980 Mar;7(3):127–131. doi: 10.1002/bms.1200070308. [DOI] [PubMed] [Google Scholar]



