Abstract
The nonstructural protein NS1 of the autonomous parvovirus minute virus of mice (MVMp) is cytolytic when expressed in transformed cells. Before causing extensive cell lysis, NS1 induces a multistep cell cycle arrest in G1, S, and G2, well reproducing the arrest in S and G2 observed upon MVMp infection. In this work we investigated the molecular mechanisms of growth inhibition mediated by NS1 and MVMp. We show that NS1-mediated cell cycle arrest correlates with the accumulation of the cyclin-dependent kinase (Cdk) inhibitor p21cip1 associated with both the cyclin A/Cdk and cyclin E/Cdk2 complexes but in the absence of accumulation of p53, a potent transcriptional activator of p21cip1. By comparison, MVMp infection induced the accumulation of both p53 and p21cip1. We demonstrate that p53 plays an essential role in the MVMp-induced cell cycle arrest in both S and G2 by using p53 wild-type (+/+) and null (−/−) cells. Furthermore, only the G2 arrest was abrogated in p21cip1 null (−/−) cells. Together these results show that the MVMp-induced cell cycle arrest in S is p53 dependent but p21cip1 independent, whereas the arrest in G2 depends on both p53 and its downstream effector p21cip1. They also suggest that induction of p21cip1 by the viral protein NS1 arrests cells in G2 through inhibition of cyclin A-dependent kinase activity.
Autonomous parvoviruses are nonenveloped linear single-stranded DNA viruses. To accomplish their lytic cycle, they require proliferating cells, since they are strongly dependent on cell factors associated with the S phase of the cell cycle (11, 16, 50, 52). However, they cannot induce cell proliferation as do oncogenic viruses. Cell proliferation is not the only prerequisite; autonomous parvoviruses also depend on cell factors associated with the differentiation state of the cell (51). Consequently, parvoviral infection is often lethal or highly malformative when it occurs in a fetus or neonate and comparatively innocuous when it occurs in adults. Another consequence of these parvovirus requirements is their oncotropism. Parvovirus infection interferes with the development of tumors in vivo and leads to extensive lysis of transformed cells in vitro (reviewed in reference 45). The nonstructural viral protein NS1 plays an essential role in the oncolytic action of parvoviruses. NS1 is an essential multifunctional protein that regulates the viral cycle at several levels. It is required for viral DNA replication (reviewed in reference 12) and viral gene expression. It is a potent transcriptional activator, acting primarily on the viral late promoter p38, although it is also reported to modulate heterologous promoters (6, 29, 30, 34). NS1 can directly interact with the transcription factor SP1 (28, 33) and, in vitro, with the proteins TFIIA(α/β) and TBP (33). In addition, NS1 displays ATPase and helicase activities (27, 38) and a site-specific nicking activity (10, 14). It is found covalently attached to the 5′ end of intracellular replicating viral DNA (10, 14), and in vitro assays have shown that the endonuclease activity of NS1 is activated by sequence-specific binding cell factors (7, 8, 32) and a member of the high-mobility-group proteins 1/2 (9, 13). NS1, furthermore, is cytotoxic when expressed in transformed cell lines in vitro (5, 36) and in vivo (48).
Cell lines that have stably integrated the coding sequence for NS1 under the control of a dexamethasone-inducible promoter have been previously produced. Cell lysis occurs after several days of expression of NS1. However, cell proliferation is already impaired within a few hours, with an accumulation in the G1, S, and G2 phases, as observed by flow cytometry (40, 41). In this study, we examined the molecular mechanisms of NS1-induced cell cycle arrest and we compared how NS1 expression and minute virus of mice (MVMp) infection impinge on cell division regulators. The cell cycle is described as the regulated succession of the S phase, during which DNA replication occurs, and mitosis (M), separated by the two gap periods (G1 and G2). Transitions from one phase to the next and progression through each phase are regulated by different cyclin-dependent kinases (Cdks) through phosphorylation of various substrates. The activity of these kinases is variously controlled by phosphorylation, association with activators (cyclins) or inhibitors (Cdk inhibitors), and localization within the cell (reviewed in references 43 and 49). Different cyclin/Cdk complexes are activated sequentially during the cell cycle. Cyclin D/Cdk4 or Cdk6 initiates proliferation from G0 to G1, cyclin E/Cdk2 promotes the transition from G1 to S, cyclin A/Cdk2 or Cdc2 promotes the S phase and entry into the prophase of mitosis (21), and cyclin B/Cdc2 controls the accomplishment of mitosis (reviewed in reference 39). p53-dependent and -independent induction of the pleiotropic Cdk inhibitor p21cip1 is a common mechanism of growth arrest in different physiological situations (20; reviewed in reference 22). Specifically, p53-mediated up-regulation of p21cip1 has been shown to arrest cells in G1 (20). Overexpression of p53 also causes a G2 arrest through the extinction of cyclin B-associated kinase activity, resulting from both a direct inhibition of the kinase by the p53 effector GADD45 and transcriptional repression of the Cdc2 and cyclin B promoters (42, 53, 56). In this study, we investigated the requirement of p53 and p21cip1 in the cell cycle arrest induced by MVMp infection and the status of S and G2 cyclin/Cdk complexes in NS1-expressing and MVMp-infected cells.
MATERIALS AND METHODS
Cells and viruses.
The FRNS1.25 line and its derivatives transformed with c-Ha-ras (FRNS1.25EJ1) or polyomavirus middle T antigen (FRNS1.25MT4–1) are rat fibroblasts stably transfected with an inducible vector, where the expression of the MVMp protein NS1 is driven by a mouse mammary tumor virus (MMTV) long terminal repeat (LTR) (36). FREJ4 cells are rat fibroblasts transformed with c-Ha-ras (54). NIH 3T3 cells are normal mouse fibroblasts, MEFip53° cells are p53-null (−/−) immortalized mouse embryonic fibroblasts (MEFs) (2), and SAOS-2 is a p53−/− human osteosarcoma cell line (ATCC HTB-85).
Primary MEFs were prepared from 12.5-day embryos from p53 wild-type (+/+) or null embryos (18) as described elsewhere (25). MEF p21cip1−/− are primary MEFs in the 129/C57BL/6 mixed genetic background (17). Primary MEFs were used before passage 5.
All cell lines were cultured in Dulbecco's modified essential medium supplemented with 10% fetal calf serum and 1% sodium pyruvate. MVMp infection was carried out as follows. The cells were rinsed with phosphate-buffered saline (PBS) (1.5 mM KH2PO4, 8.1 mM Na2HPO4, 140 mM NaCl, 2.7 mM KCl [pH 7.2 to 7.4]) supplemented with CaCl2 (0.9 mM) and MgSO4 (0.9 mM) (PBS-Ca-Mg). The cells were then incubated for 1 h in 1 ml of PBS-Ca-Mg buffer with or without the virus (multiplicity of infection, 10 infectious particles per cell). The cells were harvested after another 24-h incubation in culture medium at 37°C.
Western blotting.
Viral NS1 and endogenous cell cycle proteins were visualized by immunoblotting. Cells treated or not treated with 10−5 M dexamethasone or infected with MVMp were rinsed and scraped in cold PBS. For proteins purified on p9 beads, the protocol described for immunoprecipitation was used. For proteins analyzed from crude extracts, cells were lysed in 2% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl (pH 6.8), 10% glycerol, 0.1% bromophenol blue, and 2% β-mercaptoethanol and subjected to three cycles of boiling (5 min) and freezing. The samples were fractionated by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide) and electrophoretically transferred to a nitrocellulose membrane in 20 mM Tris-HCl, 150 mM glycine, and 20% methanol (pH 8). The nitrocellulose membranes were blocked in PBS–0.1% Tween 20–5% nonfat dry milk for NS1, p34cdc2, p21cip1, p27kip1, and cyclins A, B, and E. The blots were incubated with a polyclonal rabbit antiserum raised against a bacterial fusion protein containing an MVMp-NS1-specific carboxy-terminal amino acid sequence (31) or with rabbit polyclonal antibody raised against cyclin A (H-432) (1:1,000 dilution, sc-741; Santa Cruz) or cyclin E (M-20) (1:1,000 dilution, sc-481; Santa Cruz). Mouse monoclonal antibodies were used to detect p34cdc2 (IgG2a 17; 1:1,000 dilution; Santa Cruz sc-54), p33cdk2 (polyclonal serum kindly provided by F. Hall), cyclin B1 (IgG1 GNS1; 1:1,000 dilution; Santa Cruz sc-245), and p27kip1 (IgG1 K25020; 1:1,000 dilution; Transduction Laboratories; 1:1,000 dilution). p21cip1 was detected with pooled mouse monoclonal immunoglobulin G (IgG) derived from clones CP36 (IgG1K, epitope amino acids 1 to 80) and CP74 (IgG2BK, epitope amino acids 1 to 80) (Euromedex, Mundolsheim, France). Immunocomplexes were revealed with an anti-rabbit antibody linked to horseradish peroxidase (ECL detection system from Amersham). The p53 protein was detected by immunoprecipitation of cellular extracts labeled with [35S]methionine/cysteine.
Metabolic labeling.
Cells were infected by MVMp at a multiplicity of infection of 10 for 24 h or treated with 10−5 M dexamethasone to induce the expression of NS1 during the indicated times. Cells were washed twice with PBS followed by a 1-h preincubation with cysteine- and methionine-free Dulbecco's modified essential medium supplemented with 1% glutamine. Cells were labeled for 2 h in the same medium with 150 μCi of Pro-mix 35S (Amersham)/ml in the presence of the proteasome inhibitor N-CBZ-Leu-Leu-Leu-AL (Sigma) at a concentration of 10 μM. The same experiment realized in the absence of the proteasome inhibitor also indicates no variation in the level of p53 in NS1-expressing cells (data not shown). Equal aliquots were lysed in CHRIS buffer (50 mM Tris-HCl [pH 8.0], 10% glycerol, 200 mM NaCl, 0.5% Nonidet P-40, and 0.1 mM EDTA) supplemented with 10-μg/ml concentrations of each of the following protease inhibitors: leupeptin, aprotinin, N-p-tosyl-l-lysine chloromethyl ketone (TLCK), N-tosyl-l-phenylalanine chloromethyl ketone (TPCK), and phenylmethylsulfonyl fluoride. Labeled p53 was recovered by immunoprecipitation with the monoclonal antibody Pab421 (hybridoma supernatant), separated by SDS-polyacrylamide gel electrophoresis, and detected by autoradiography. As a positive control, FREJ4 cells were γ-irradiated (1 gray) and then cultured with the proteasome inhibitor N-CBZ-Leu-Leu-Leu-AL at a concentration of 10 μM during the 8 h before metabolic labeling and harvesting.
Coimmunoprecipitation.
Cells (2 × 106 cells) were lysed in 500 μl of homogenization buffer (25 mM morpholinepropanesulfonic acid [MOPS] [pH 7.2], 15 mM EGTA, 15 mM MgCl2, 2 mM dithiothreitol, 1 mM sodium vanadate, 1 mM NaF, 1 mM disodium phenylphosphate, 60 mM β-glycerophosphate, 15 mM nitrophenylphosphate, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 μg of soybean trypsin inhibitor (SBTI) per ml, 100 μM benzamidine) and sonicated. The homogenates were centrifuged for 10 min at 18,000 × g and 4°C, and then 450 μl of supernatant was added to rabbit polyclonal anti-cyclin A (diluted 1:1,000; J. Pines) or anti-cyclin E (diluted 1:100; K. Keyomarsi) antibodies. After 1 h on ice, 50 μl of a 1:5 dilution of protein A-Sepharose beads in bead buffer (50 mM Tris-HCl [pH 7.4], 5 mM NaF, 250 mM NaCl, 5 mM EDTA, 0.1% NP-40, 5 mM EGTA, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 μg of SBTI per ml, and 100 μM benzamidine) was added. Samples were incubated at 4°C for 1 h on a rocking wheel. The beads were rinsed three times with 1 ml of bead buffer. For immunoblot analysis, the pellet resuspended in 50 μl of loading buffer was boiled for 5 min, loaded onto an SDS-polyacrylamide gel, and electrophoresed. The presence of the Cdk inhibitor p21cip1 in the immunoprecipitated complex was determined by Western blotting as described above. Alternatively, histone H1 kinase assays were performed as described elsewhere (35) by incubating the beads for 10 min at 30°C after dilution in 25 μl of kinase buffer (50 mM Tris-HCl [pH 7.4], 5 mM EGTA, 10 mM MgCl2, 1 mM dithiothreitol) in the presence of 0.2 mCi of [γ-32P]ATP (Dupont NEN)/ml and 1 mg of histone H1 (Boehringer-Mannheim)/ml. The samples were then spotted on phosphocellulose paper (P81; Whatman) that was rinsed in diluted phosphoric acid. The radioactivity incorporated in histone H1 was counted in a Beckman scintillation counter.
Flow cytometry analysis.
Cells were harvested by trypsinization and rinsed with PBS. After centrifugation, the pellet (105 to 106 cells) was suspended in 1 ml of PBS and kept on ice for 5 min. The cell suspension was then fixed by drop-wise addition of 9 ml of precooled (−20°C) 80% ethanol under violent shaking. Fixed samples were kept at 4°C until used (15). For staining, the cells were centrifuged, resuspended in PBS, digested with RNase A (100 μg/ml; Boehringer), and treated with propidium iodide (50 μg/ml; Sigma) for 15 min. Fixed and stained cells were analyzed by flow cytometry with a fluorescence-activated cell sorter (FACScan; Becton Dickinson). The propidium iodide emission signal was monitored at 575 nm by means of an appropriate filter (band-pass width, 26). Ten thousand events per sample were collected, stored, and analyzed by the Lysis II system (Becton Dickinson). The distribution of cells among the various phases of the cell cycle was quantified by means of the Cell Fit program (Becton Dickinson).
RESULTS
p53 accumulates following MVMp infection.
Rat fibroblasts become arrested in the S and G2 phases of the cell cycle upon MVMp infection or NS1 expression (40, 41). During the viral cycle, parvoviral genome amplification leads to the accumulation of strand breaks in the viral genome that may be detected as signals of chromatin damage. As DNA damage commonly leads to cell cycle arrest through the accumulation of p53 (for reviews see references 23 and 44), we determined whether p53 accumulates upon MVMp infection. Wild-type p53 cells (FREJ4), permissive to the viral infection, were infected for 24 h with MVMp and labeled with [35S]methionine/cysteine, and p53 was detected after immunoprecipitation (Fig. 1, left panel). p53 was not detectable in uninfected cells but accumulated readily after infection. In situ indirect immunofluorescence revealed that p53 was present in both the nucleus and cytoplasm of MVMp-infected cells (Fig. 2A). Since the nuclear localization of p53 is related to its activation (47), we concluded that MVMp infection triggers accumulation of active p53.
FIG. 1.
p53 accumulates in rat fibroblasts upon MVMp infection but not in NS1-expressing cells. FREJ4 cells were infected or not infected for 24 h with MVMp or irradiated with γ rays as a positive control (left panel). FRNS1.25EJ1 cells were cultivated in the presence of dexamethasone (Dex) for the indicated amounts of time to induce NS1 expression (right panel). Before harvest, cells were metabolically labeled for 2 h using [35S]methionine; and [35S]cysteine; p53 was then immunoprecipitated and revealed by autoradiography.
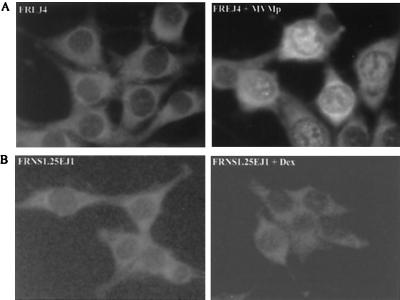
FIG. 2.
Localization of p53 in rat fibroblasts upon MVMp infection or NS1 expression. p53 was visualized by indirect immunofluorescence. (A) MVMp-infected (right panel) and uninfected (left panel) FREJ4 cells. (B) FRNS1.25EJ1 cells induced by dexamethasone (Dex) to express NS1 (right panel) or not induced (left panel).
MVMp-induced cell cycle arrest is p53 dependent.
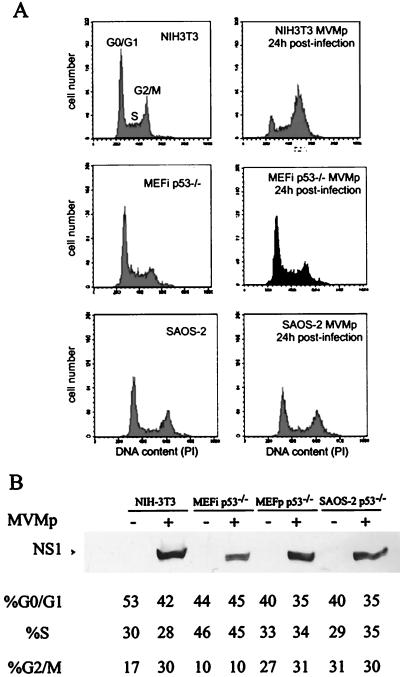
In order to investigate the role of p53 in the cell cycle arrest induced by MVMp, we analyzed two p53−/− cell lines (MEFip53° [immortalized MEFs] and SAOS-2 [a human osteosarcoma cell line]) and one p53+/+ cell line (NIH 3T3 [immortalized mouse fibroblasts]). Cells were infected for 24 h, and their DNA contents were determined by flow cytometry. While MVMp infection led to a massive accumulation of NIH 3T3 cells in the S and G2 phases, no perturbation of the cell cycle distribution was observed in the two p53-null cell lines, MEFip53° and SAOS-2 (Fig. 3A). As the absence of cell cycle perturbation could be explained by nonpermissiveness to the virus, we assessed the efficiency of MVMp infection in the different cell lines by analyzing NS1 expression. Western blot analysis showed that NS1 accumulated at similar levels in the various cell lines (Fig. 3B). In addition, in situ immunodetection showed that 80% of the NIH 3T3 cells, 50% of the MEFip53° cells, and 50% of the SAOS-2 cells expressed NS1 (data not shown). Consistently, plating efficiency experiments indicated that NIH 3T3 cells are very sensitive to MVMp infection while MEFip53° and SAOS-2 cells are resistant to MVMp infection (data not shown). In order to firmly establish the essential role of p53 in MVMp-induced cell cycle arrest, we next compared isogenic p53+/+ and p53−/− cells. Primary mouse fibroblasts were isolated from p53−/− or p53+/+ mouse embryos and infected by MVMp, and their DNA contents were estimated by flow cytometry (Fig. 4). As described above for immortalized cell lines, p53+/+ MEFs accumulated in the S and G2 phases upon MVMp infection, whereas p53−/− MEFs exhibited no changes in the cell cycle distribution. We thus concluded that the cell cycle arrest consequent to MVMp infection is p53 dependent.
FIG. 3.
p53 is involved in the cell cycle arrest induced by MVMp infection. (A) Effects of MVMp infection on the cell cycle distribution profile are compared in p53+/+ cells (NIH 3T3) and in p53−/− cells (immortalized MEFip53−/− and SAOS-2). Exponentially growing cells were infected with MVMp (right panels) or mock treated (left panels) as indicated. Cells were harvested 24 h later and processed for flow cytometric analysis of the DNA contents. Peaks of cells in G0/G1, S, and G2/M are indicated in the upper left panel. (B) Expression levels of the viral protein NS1 were measured by immunoblotting to ascertain the efficacy of infection. MEFp p53−/− are primary MEFs derived from p53-null embryos. The percentage of cells in each phase of the cell cycle is indicated below the gel. +, infected cells; −, mock-treated cells.
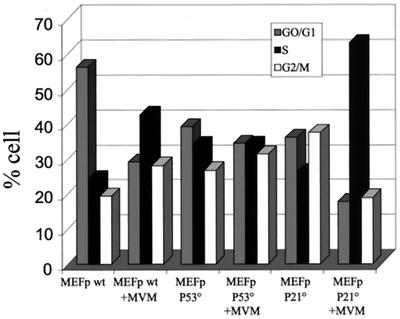
FIG. 4.
p21cip1 is involved in the cell cycle arrest induced by MVMp infection. The requirement of p21cip1 and p53 in the MVMp-induced cell cycle arrest was investigated in primary MEFs (MEFp) derived from either wild-type (wt), p21cip1-null (P21°), or p53-null (P53°) embryos. Cells were infected with MVMp or mock treated, harvested 24 h later, and processed for flow cytometric analysis of DNA contents. Bars represent the percentage of cells in the G0/G1, S, and G2/M phases of the cell cycle.
MVMp-induced G2 arrest depends on p21cip1.
p53 acts as a transcriptional activator and exerts part of its cytostatic effect through the induction of p21cip1, also known as an inhibitor of the cyclin/Cdks that control the S and G2 phases. We therefore investigated the role of p21cip1 in MVMp-induced cell cycle arrest. Primary MEFs derived from wild-type, p53−/−, and p21cip1−/− mouse embryos were infected for 24 h with MVMp, and the distribution of cells among the different phases of the cell cycle was compared to that of uninfected cells (Fig. 4). While p53−/− MEFs exhibited no changes in the cell distribution, p21cip1−/− MEFs massively accumulated in the S phase upon MVMp infection but not in the G2 phase, suggesting that p21cip1 is involved in the G2 phase arrest but not in the S phase arrest. p21cip1 could thus be the downstream effector of p53 in the induction of the G2 block but not in the arrest in the S phase.
p53 does not accumulate upon NS1 expression.
As previously described (40, 41), expression of the viral protein NS1 alone leads to cell cycle arrest with an accumulation of cells in the G1, S, and G2 phases, thus reproducing the effects of the viral infection on the cell cycle. Accumulation of p53 during NS1-induced cell cycle arrest was investigated in a Ha-ras- transformed rat fibroblast cell line into which had been integrated the NS1 coding sequence under the control of the dexamethasone-inducible MMTV LTR promoter (FRNS1.25EJ1). Both immunoprecipitation (Fig. 1, right panel) and in situ immunofluorescence (Fig. 2B) indicated the absence of p53 accumulation in NS1-expressing cells, in contrast with our observations (described above) for MVMp-infected cells. The failure of the inducible cell line to accumulate p53 upon dexamethasone treatment may be explained by the low levels of NS1 expression rather than by the intrinsic inability of NS1 to induce p53. However, low NS1 levels are sufficient to arrest cell proliferation, suggesting that the NS1 cytostatic effect does not require p53 accumulation.
p21cip1 is induced following NS1 protein expression and MVMp infection.
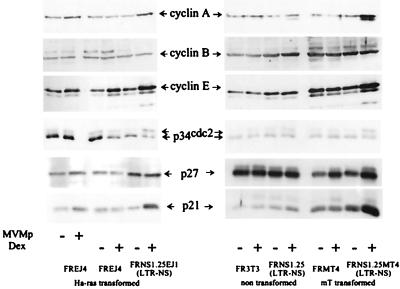
We next investigated how cell cycle regulators are modified upon either MVMp infection or NS1 expression. Thus, we compared infected cells to different cell lines into which had been integrated the NS1 coding sequence under the control of the dexamethasone-inducible MMTV LTR promoter. We used rat fibroblasts transformed with either the Ha-ras oncogene (FRNS1.25EJ1) or the polyomavirus middle T antigen (FRNS1.25MT4.1) that stop dividing and undergo lysis when induced to express NS1 (36). Nontransformed rat fibroblasts (clone FRNS1.25) expressing NS1 at levels similar to those obtained in FRNS1.25MT4.1 cells but resistant to NS1-induced lysis were used as a control (36). Levels of cyclins A, B, and E, the kinase p34cdc2, and the Cdk inhibitors p21cip1 and p27kip1 were measured 24 h after infection or induction (Fig. 5). Control cell lines lacking the LTR-NS1 construct were used to distinguish the effects of dexamethasone treatment.
FIG. 5.
NS1 expression and MVMp infection induce accumulation of cyclin A and the Cdk inhibitor p21cip1 and inhibitory phosphorylation of the p34cdc2 kinase. Cyclins A, B, and E, the kinase p34cdc2, and the Cdk inhibitors p21cip1 and p27kip1 were detected by immunoblotting in rat fibroblasts after infection with MVMp or induction of NS1 expression with dexamethasone. FRNS1.25, FRNS1.25EJ1, and FRNS1.25MT4.1 rat fibroblasts contain the LTR-NS1 construct. The former is not transformed. The two latter are transformed with the Ha-ras oncogene and the polyomavirus middle T antigen, respectively. Control cell lines without the LTR-NS1 construct were used to distinguish either effects of NS1 or dexamethasone treatment. FR3T3 cells are nontransformed rat fibroblasts. FREJ4 and FRMT4 are transformed rat fibroblasts. The former is transformed with the Ha-ras oncogene, and the latter is transformed with the polyomavirus middle T antigen. The cells were harvested 24 h after infection or dexamethasone treatment. Proteins were purified on p9 beads from cellular extracts before detection by Western blotting, except for cyclin B.
In cells transformed with the middle T antigen, NS1 induced the accumulation of p21cip1, cyclin A, and cyclin E. The appearance of a slowly migrating form of p34cdc2, usually considered to be the hyperphosphorylated, inactive form of the kinase, was also detected. In these cells, p27kip1 accumulates upon treatment with dexamethasone, independent of NS1 expression. Similar results were obtained in the Ha-ras-transformed cells, except that cyclin E accumulated after addition of dexamethasone in the absence of NS1 expression, although to a lesser extent than in NS1-expressing cells. By comparison, nontransformed cells only slightly accumulated p21cip1 and the inactive phosphorylated form of p34cdc2 upon NS1 expression. Consistently, these cells exhibit a decreased growth rate when expressing NS1 (36), which is associated with a slight accumulation of cells in G2 (40). Finally, MVMp infection also induced the accumulation of p21cip1 and cyclin A in Ha-ras-transformed fibroblasts, although to a lesser extent than NS1 expression. By contrast, cyclin E did not accumulate in MVMp-infected cells. This is consistent with the fact that the viral cycle is initiated when cells enter the S phase, at a time when cyclin E has been degraded, while dexamethasone-treated cells express NS1 independent of the cell cycle phase, thus allowing a block in G1 when cyclin E is expressed (41).
Altogether these data focused on the potential role of the Cdk inhibitor p21cip1 in the cytostatic effects of both MVMp infection and NS1 expression.
p21cip1 is associated with cyclin/Cdk complexes.
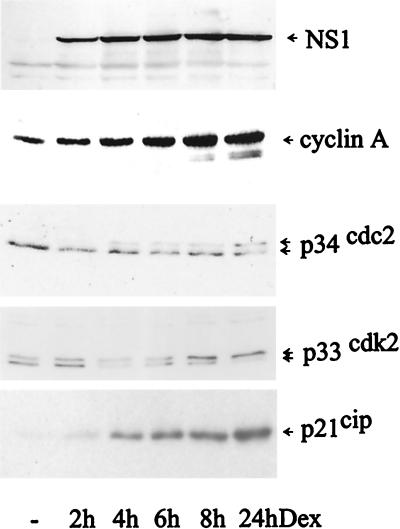
p21cip1 is best known for its ability to bind and inhibit the G1- and S-phase kinase complexes cyclin D/Cdk4, cyclin E/Cdk2, and cyclin A/Cdk2 (24), and it is less inhibitory towards cyclin B/Cdc2, which controls the M phase (reviewed in reference 49). Therefore, the causative role of NS1 in the accumulation of p21cip1 and cyclin A and the shift of p34cdc2 and p33cdk2 was studied in a kinetics experiment. Figure 6 shows that NS1 is already detectable 2 h after dexamethasone treatment, while p21cip1 and cyclin A accumulate within 4 h, concomitant with the appearance of the slowly migrating form of p34cdc2, representing the inactive tyrosine-phosphorylated form of the kinase. Identical results were obtained for p33cdk2 (Fig. 6).
FIG. 6.
Kinetics of NS1, cyclin A, p21cip1, p34cdc2, and p33cdk2 expression in FRNS1.25EJ1. The cells were treated with dexamethasone and harvested at the indicated times. Crude cell extracts (for cyclin A, NS1, and p33cdk2) or extracts purified on p9 beads (for p21cip1 and p34cdc2) were used for detection by Western blotting. Hence, the signal observed here for p21 represents the fraction of the protein which is bound to cyclin/Cdk complexes.
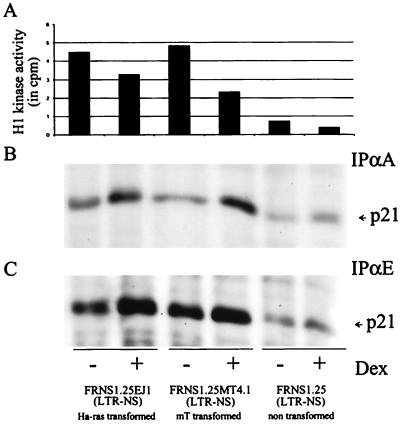
In order to determine whether increasing levels of cyclin A would lead to a parallel increase of the associated kinase activity, we measured the cyclin A-associated histone H1 kinase activity in NS1-expressing cell lines. Figure 7A shows that the kinase activity was significantly reduced upon NS1 expression in both untransformed and transformed cells, in spite of higher levels of cyclin A in transformed cells (Fig. 5). As cyclin A is rate limiting for the kinase activity (1), this result suggested that a fraction of the cyclin A/Cdk complexes was inhibited. We therefore assayed cyclin A immunoprecipitates for the presence of p21cip1, which is also accumulated upon expression of NS1. We found, in NS1-expressing cells, a larger amount of p21cip1 associated with cyclin A immunoprecipitates (Fig. 7B) that paralleled the increase in cyclin A and p21cip1 levels. p21cip1 was also detected in cyclin E immunoprecipitates, at levels proportional to cyclin E levels (Fig. 7C). These results suggest that in NS1-expressing cells, inhibition of cyclin A-dependent kinase activity resulted from both binding to the p21cip1 inhibitor and inhibitory phosphorylation of either of the kinase subunits (Cdc2 and Cdk2).
FIG. 7.
NS1-induced p21cip1 is associated with the cyclin A/Cdk and cyclin E/Cdk complexes and impairs cyclin A kinase activity. Exponentially growing cells were cultured in the presence (+) or absence (−) of dexamethasone for 24 h prior to harvesting. Proteins immunoprecipitated with anti-cyclin A (IPαA) (panels A and B) or anti-cyclin E (IPαE) (panel C) antibodies were analyzed for histone H1 kinase activity (A) and the presence of p21cip1 by immunoblotting (B and C).
DISCUSSION
Our data clearly demonstrate the essential role of p53 in MVMp-induced cell cycle arrest in the S and G2 phases. MVMp infection led to the accumulation of p53, and p53−/− cells were resistant to the cytostatic effects of MVMp. Yet the ability of p53 to induce p21cip1 cannot be wholly responsible for the p53-induced cell cycle arrest, since only the G2 arrest was abrogated in MVMp-infected p21cip1−/− cells. The p21cip1 protein is thus involved in the observed G2 block, but it is not required to arrest cell DNA replication. This is in good agreement with the previous observation that the p21cip1/p53 pathway does not seem to be involved in the S phase arrest (55) and the increasingly described role of p21cip1 in a G2 block (3, 4, 19, 37).
In contrast to MVMp infection, the NS1-induced cell cycle arrest was not associated with p53 accumulation. This observation is surprising since we have previously shown that NS1 expression leads to the appearance of nicks in the cellular chromatin (41), which is a well-known p53 induction signal. Although p53 activation without accumulation (26) upon NS1 expression cannot be excluded, these observations suggest that the cell cycle perturbations caused by MVMp infection involve mechanisms in addition to those mediated by NS1. Accordingly, similar observations were made with the related virus adeno-associated virus (AAV). The expression of the cytostatic protein Rep does not induce p53 accumulation, whereas infection by AAV does (K. Raj, P. Ogston, and P. Beard, VIIIth Parvovirus Workshop, abstr. p94, 2000.). Interestingly, inactivated particles also induce p53 but empty viral capsids do not, revealing that nonreplicating viral DNA by itself, with hairpin structures at both ends, elicits a DNA damage response. Most probably, the p53 accumulation in MVMp-infected cells is also triggered by the presence of the linear single-stranded DNA.
We gained further insights into the molecular mechanisms of MVMp-induced cell cycle arrest through the analysis of the cell cycle regulators. Indeed, both MVMp-infected and NS1-expressing cells accumulated the Cdk inhibitor p21cip1 that was found associated with cyclin A/Cdk and cyclin E/Cdk2 complexes in NS1-expressing cells. In both situations, accumulation of p21cip1 could thus counterbalance the concomitant accumulation of the Cdk activating cyclins A and E. This is sufficient to explain the reduced cyclin A kinase activity and cell cycle arrest observed in NS1-expressing cells. In addition, cyclin E- and cyclin B-dependent kinases were not detectable in NS1-expressing cells (our unpublished results) while the p34cdc2 and p33cdk2 kinases were shifted towards the phosphorylated inactive form. These observations, and the fact that cyclin A is rate limiting for prophase entry, indicate that the NS1 protein operates to arrest cells in G2 through inhibition of the cyclin A-dependent kinase. Our results also show that the role of p21 is essential in this process. Considering viral infection, cell cycle arrest occurs in a precise period of time, after S phase entry and before mitosis promoting factor activation, which is favorable to viral replication. Since cyclin A/Cdk2 is the only limiting factor for cell cycle progression during this entire period (21), its inhibition is also sufficient to explain the S phase arrest of NS1-expressing or MVMp-infected cells.
Noteworthy, p21cip1 was accumulated to much higher levels in NS1-expressing clones than in infected cells in which both NS1 and p53 are produced, both being potent inducers of p21cip1. In contrast, NS1 expression alone did not cause any accumulation of p53. Moreover, the amount of NS1 expressed in infected cells was higher than in conditionally expressing cells. One would thus expect higher p21cip1 levels in MVMp-infected cells than in NS1-expressing cells. A partial explanation may be that 100% of the cells stably express NS1 in dexamethasone-treated cultures, while less than 50% of MVMp-infected cells do so. Another contributing factor could be the trapping of NS1 molecules by many viral targets in infected cells for purposes other than p21cip1 induction. During the viral cycle, NS1 interacts with viral chromatin to activate the viral promoters, to cleave viral DNA multimers, and to exert its helicase activity, allowing parvoviral DNA replication. Hence, although p21cip1 may play a major role in the G2 arrest detected in NS1-expressing cells, this role may be masked during infection because NS1 is trapped by other targets and because other cell cycle-stopping factors such as p53, acting at least partly via a different route, are induced. It is also noteworthy that MVMp infection does not cause the appearance of the slowly migrating inactive form of the p34cdc2 kinase, which is found to have accumulated following NS1 expression. This observation lends further support to the hypothesis that the mechanisms underlying MVMp- and NS1-induced cell cycle arrest are different. Similarly, MVMp infection induces the accumulation of p53 in a way unrelated to the NS1-induced nicks in the cellular chromatin (41).
In conclusion, our data clearly identify a p53-dependent pathway in the cell cycle block induced by MVMp infection since, in the absence of p53, MVMp-infected fibroblasts did not accumulate in S/G2. Protein p53-dependent arrest in G2 may be explained by the ability of p53 to induce p21cip1 since MVMp-infected p21cip1−/− cells did not accumulate in this phase. Yet the downstream effector(s) of p53 in the MVMp-induced arrest in the S phase is still unknown. p53-dependent arrest of cell DNA replication clearly does not involve p21cip1, since p21cip1−/− cells still accumulate in the S phase. The cytostatic effect of the NS1 protein expressed alone most probably involves p21cip1, as p21cip1 accumulates massively in cells blocked when induced to express NS1 and p21cip1 is found associated with cyclin/Cdk complexes. It has been recently reported that the S phase arrest mediated by the related AAV replication protein Rep78 involves the accumulation of the hypophosphorylated form of pRB (46). Rep78 shares several properties with NS1: specific DNA binding, site-specific endonuclease, helicase, and ATPase activities, and a cytostatic effect. However, NS1, unlike Rep78, is cytotoxic (5). In Rep78-expressing cells, accumulation of the hypophosphorylated form of pRB leads to the down-regulation of the E2F target genes, cyclin A and cdc2, as well as cyclin B. On the contrary, NS1 expression leads to the accumulation of cyclin A and does not affect levels of cyclin B and Cdc2 expression, suggesting that Rep78 and NS1 involve different pathways to exert their cytostatic activity. Although we cannot formally exclude the possibility of an additional role of pRB in MVMp-induced S phase arrest, our data clearly identify p53 as an essential mediator of S and G2 phase arrest and p21cip1 as a mediator of G2 arrest in MVMp-infected cells.
ACKNOWLEDGMENTS
This work was supported by the Central Fractionation Department of the Red Cross (Bruxelles-Capitale convention no. 96B183), by the International Brachet Stiftung, by the Fonds National de la Recherche Scientifique (grants to A.O.D.B. and P.C.-F.), by the Association pour la Recherche sur le Cancer and Fondation de France (grants to J.S.-T. and C.B.), and by Deutsche Forschungsgemeinschaft (grant to H.S.).
The monoclonal antibody Pab421 and the p53−/− immortalized MEFs were kindly provided by B. Henglein, Institut Curie, Paris, France. We thank Phil Leder and Alan Wang for the p21cip1−/− MEFs. We are indebted to Laurent Meijer for his involvement and stimulating discussions and to all members of his laboratory in Roscoff, France, for efficiency and help. We thank Jean Dubuisson and Yvan de Launoit for their help. We also thank Suzanne Mousset for the cell lines displaying inducible NS1 expression and Marcel Tuijnder for precious technical assistance.
REFERENCES
- 1.Arooz T, Yam C H, Siu W Y, Lau A, Li K K, Poon R Y. On the concentrations of cyclins and cyclin-dependent kinases in extracts of cultured human cells. Biochemistry. 2000;39:9494–9501. doi: 10.1021/bi0009643. [DOI] [PubMed] [Google Scholar]
- 2.Attardi L D, Lowe S W, Brugarolas J, Jacks T. Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 1996;15:3693–3701. [PMC free article] [PubMed] [Google Scholar]
- 3.Barboule N, Lafon C, Chadebech P, Vidal S, Valette A. Involvement of p21 in the PKC-induced regulation of the G2/M cell cycle transition. FEBS Lett. 1999;444:32–37. doi: 10.1016/s0014-5793(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 4.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 5.Caillet-Fauquet P, Perros M, Brandenburger A, Spegelaere P, Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990;9:2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen J, Cotmore S F, Tattersall P. Minute virus of mice transcriptional activator protein NS1 binds directly to the transactivation region of the viral P38 promoter in a strictly ATP-dependent manner. J Virol. 1995;69:5422–5430. doi: 10.1128/jvi.69.9.5422-5430.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen J, Cotmore S F, Tattersall P. A novel cellular site-specific DNA-binding protein cooperates with the viral NS1 polypeptide to initiate parvovirus DNA replication. J Virol. 1997;71:1405–1416. doi: 10.1128/jvi.71.2.1405-1416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen J, Cotmore S F, Tattersall P. Parvovirus initiation factor PIF: a novel human DNA-binding factor which coordinately recognizes two ACGT motifs. J Virol. 1997;71:5733–5741. doi: 10.1128/jvi.71.8.5733-5741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello E, Saudan P, Winocour E, Pizer L, Beard P. High mobility group chromosomal protein 1 binds to the adeno-associated virus replication protein (Rep) and promotes Rep-mediated site-specific cleavage of DNA, ATPase activity and transcriptional repression. EMBO J. 1997;16:5943–5954. doi: 10.1093/emboj/16.19.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotmore S F, Nuesch J P, Tattersall P. Asymmetric resolution of a parvovirus palindrome in vitro. J Virol. 1993;67:1579–1589. doi: 10.1128/jvi.67.3.1579-1589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotmore S F, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 12.Cotmore S F, Tattersall P. DNA replication in the autonomous parvoviruses. Semin Virol. 1995;6:271–281. [Google Scholar]
- 13.Cotmore S F, Tattersall P. High-mobility group 1/2 proteins are essential for initiating rolling-circle-type DNA replication at a parvovirus hairpin origin. J Virol. 1998;72:8477–8484. doi: 10.1128/jvi.72.11.8477-8484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotmore S F, Tattersall P. In vivo resolution of circular plasmids containing concatemer junction fragments from minute virus of mice DNA and their subsequent replication as linear molecules. J Virol. 1992;66:420–431. doi: 10.1128/jvi.66.1.420-431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darzynkiewicz Z, Gong J, Traganos F. Analysis of DNA content and cyclin protein expression in studies of DNA ploidy, growth fraction, lymphocyte stimulation and the cell cycle. Methods Cell Biol. 1994;41:421–435. doi: 10.1016/s0091-679x(08)61732-x. [DOI] [PubMed] [Google Scholar]
- 16.Deleu L, Fuks F, Spitkovsky D, Hörlein R, Faisst S, Rommelaere J. Opposite transcriptional effects of cyclic AMP-responsive elements in confluent or p27KIP-overexpressing cells versus serum-starved or growing cells. Mol Cell Biol. 1998;18:409–419. doi: 10.1128/mcb.18.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 18.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 19.Dulic V, Stein G H, Far D F, Reed S I. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.el-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 21.Furuno N, den Elzen N, Pines J. Human cyclin A is required for mitosis until mid prophase. J Cell Biol. 1999;147:295–306. doi: 10.1083/jcb.147.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gartel A L, Tyner A L. The growth-regulatory role of p21 (WAF1/CIP1) Prog Mol Subcell Biol. 1998;20:43–71. doi: 10.1007/978-3-642-72149-6_4. [DOI] [PubMed] [Google Scholar]
- 23.Giaccia A J, Kastan M B. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 24.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 25.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 26.Hupp T R, Sparks A, Lane D P. Small peptides activate the latent sequence-specific DNA binding function of p53. Cell. 1995;83:237–245. doi: 10.1016/0092-8674(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 27.Jindal H K, Yong C B, Wilson G M, Tam P, Astell C R. Mutations in the NTP-binding motif of minute virus of mice (MVM) NS-1 protein uncouple ATPase and DNA helicase functions. J Biol Chem. 1994;269:3283–3289. [PubMed] [Google Scholar]
- 28.Krady J K, Ward D C. Transcriptional activation by the parvoviral nonstructural protein NS-1 is mediated via a direct interaction with Sp1. Mol Cell Biol. 1995;15:524–533. doi: 10.1128/mcb.15.1.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legendre D, Rommelaere J. Targeting of promoters for trans activation by a carboxy-terminal domain of the NS-1 protein of the parvovirus minute virus of mice. J Virol. 1994;68:7974–7985. doi: 10.1128/jvi.68.12.7974-7985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legendre D, Rommelaere J. Terminal regions of the NS-1 protein of the parvovirus minute virus of mice are involved in cytotoxicity and promoter trans inhibition. J Virol. 1992;66:5705–5713. doi: 10.1128/jvi.66.10.5705-5713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legrand C, Rommelaere J, Caillet-Fauquet P. MVM(p) NS-2 protein expression is required with NS-1 for maximal cytotoxicity in human transformed cells. Virology. 1993;195:149–155. doi: 10.1006/viro.1993.1355. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q, Astell C R. A murine host cell factor required for nicking of the dimer bridge of MVM recognizes two CG nucleotides displaced by 10 base pairs. Virology. 1996;224:105–113. doi: 10.1006/viro.1996.0511. [DOI] [PubMed] [Google Scholar]
- 33.Lorson C, Pearson J, Burger L, Pintel D J. An Sp1-binding site and TATA element are sufficient to support full transactivation by proximally bound NS1 protein of minute virus of mice. Virology. 1998;240:326–337. doi: 10.1006/viro.1997.8940. [DOI] [PubMed] [Google Scholar]
- 34.Lorson C, Pintel D J. Characterization of the minute virus of mice P38 core promoter elements. J Virol. 1997;71:6568–6575. doi: 10.1128/jvi.71.9.6568-6575.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meijer L, Kim S H. Chemical inhibitors of cyclin-dependent kinases. Methods Enzymol. 1997;283:113–128. doi: 10.1016/s0076-6879(97)83011-x. [DOI] [PubMed] [Google Scholar]
- 36.Mousset S, Ouadrhiri Y, Caillet-Fauquet P, Rommelaere J. The cytotoxicity of the autonomous parvovirus minute virus of mice nonstructural proteins in FR3T3 rat cells depends on oncogene expression. J Virol. 1994;68:6446–6453. doi: 10.1128/jvi.68.10.6446-6453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niculescu A B, III, Chen X, Smeets M, Hengst L, Prives C, Reed S I. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. . (Erratum, 18:1763.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuesch J P, Cotmore S F, Tattersall P. Expression of functional parvoviral NS1 from recombinant vaccinia virus: effects of mutations in the nucleotide-binding motif. Virology. 1992;191:406–416. doi: 10.1016/0042-6822(92)90202-z. [DOI] [PubMed] [Google Scholar]
- 39.Ohi R, Gould K L. Regulating the onset of mitosis. Curr Opin Cell Biol. 1999;11:267–273. doi: 10.1016/s0955-0674(99)80036-2. [DOI] [PubMed] [Google Scholar]
- 40.Op De Beeck A, Anouja F, Mousset S, Rommelaere J, Caillet- Fauquet P. The nonstructural proteins of the autonomous parvovirus minute virus of mice interfere with the cell cycle, inducing accumulation in G2. Cell Growth Differ. 1995;6:781–787. [PubMed] [Google Scholar]
- 41.Op De Beeck A, Caillet-Fauquet P. The NS1 protein of the autonomous parvovirus minute virus of mice blocks cellular DNA replication: a consequence of lesions to the chromatin? J Virol. 1997;71:5323–5329. doi: 10.1128/jvi.71.7.5323-5329.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Passalaris T M, Benanti J A, Gewin L, Kiyono T, Galloway D A. The G2 checkpoint is maintained by redundant pathways. Mol Cell Biol. 1999;19:5872–5881. doi: 10.1128/mcb.19.9.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pines J. Four-dimensional control of the cell cycle. Nat Cell Biol. 1999;1:E73–E79. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- 44.Rich T, Allen R L, Wyllie A H. Defying death after DNA damage. Nature. 2000;407:777–783. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- 45.Rommelaere J, Cornelis J J. Antineoplastic activity of parvoviruses. J Virol Methods. 1991;33:233–251. doi: 10.1016/0166-0934(91)90024-t. [DOI] [PubMed] [Google Scholar]
- 46.Saudan P, Vlach J, Beard P. Inhibition of S-phase progression by adeno-associated virus rep78 protein is mediated by hypophosphorylated pRb. EMBO J. 2000;19:4351–4361. doi: 10.1093/emboj/19.16.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaulsky G, Goldfinger N, Tosky M S, Levine A J, Rotter V. Nuclear localization is essential for the activity of p53 protein. Oncogene. 1991;6:2055–2065. [PubMed] [Google Scholar]
- 48.Shen X Z, Tsung H C, Yang Y W, Yang Q H, Xiao S D. A preliminary analysis of antineoplastic activity of parvovirus MVMp NS-1 proteins. Cell Res. 1997;7:217–227. doi: 10.1038/cr.1997.22. [DOI] [PubMed] [Google Scholar]
- 49.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 50.Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972;10:586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tattersall P. Susceptibility to minute virus of mice as a function of host cell differentiation. In: Ward D C, Tattersall P, editors. Replication of mammalian parvoviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1978. pp. 131–149. [Google Scholar]
- 52.Tattersall P, Gardiner E M. Autonomous parvovirus-host-cell interactions. In: Tijssen P, editor. Handbook of parvoviruses. Vol. 1. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 111–121. [Google Scholar]
- 53.Taylor W R, DePrimo S E, Agarwal A, Agarwal M L, Schonthal A H, Katula K S, Stark G R. Mechanisms of G2 arrest in response to overexpression of p53. Mol Biol Cell. 1999;10:3607–3622. doi: 10.1091/mbc.10.11.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Hille B, Duponchel N, Salome N, Spruyt N, Cotmore S F, Tattersall P, Cornelis J J, Rommelaere J. Limitations to the expression of parvoviral nonstructural proteins may determine the extent of sensitization of EJ-ras-transformed rat cells to minute virus of mice. Virology. 1989;171:89–97. doi: 10.1016/0042-6822(89)90514-x. [DOI] [PubMed] [Google Scholar]
- 55.Wyllie F S, Haughton M F, Bond J A, Rowson J M, Jones C J, Wynford T D. S phase cell-cycle arrest following DNA damage is independent of the p53/p21(WAF1) signalling pathway. Oncogene. 1996;12:1077–1082. [PubMed] [Google Scholar]
- 56.Zhan Q, Antinore M J, Wang X W, Carrier F, Smith M L, Harris C C, Fornace A J., Jr Association with Cdc2 and inhibition of Cdc2/cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18:2892–2900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]