Abstract
A small animal model of arterial insufficiency is presented which involves unilateral femoral artery ligation and section. Invoked alterations in metabolism and perfusion of the affected muscle mass have been investigated 12 h, 4, 7 and 14 days post-ligation by 31P-n.m.r. and microsphere infusion, both at rest and during isometric muscle contraction at 1 Hz. At rest, the concentration of phosphocreatine was similar to the mean control value (36.0 +/- 1.0 mM) from 4 days post-ligation, but was significantly lower at 12 h (28.5 +/- 3.6 mM). Inorganic phosphate concentrations were significantly elevated for 7 days post-ligation. No significant differences were noted in intramuscular pH. Upon stimulation of the affected muscle mass, a time-dependent improvement in phosphocreatine utilization was observed such that 14 days post-ligation phosphocreatine utilization was not significantly different from mean control values. A similar amelioration was noted for the contraction-induced fall in intramuscular pH. At rest, no significant differences in bloodflow to the muscles of the ligated limb compared with the unaffected contralateral limb were observed. However, isometric contraction of the affected muscle mass resulted in a markedly reduced hyperaemic response 12 h post-ligation. Thereafter, a time-dependent improvement in tissue perfusion during stimulation was observed which paralleled the improvements in phosphocreatine utilization and intramuscular pH changes. The results presented are discussed with respect to the interrelationship between oxygen delivery, high energy phosphate utilization and force maintenance.
Full text
PDF
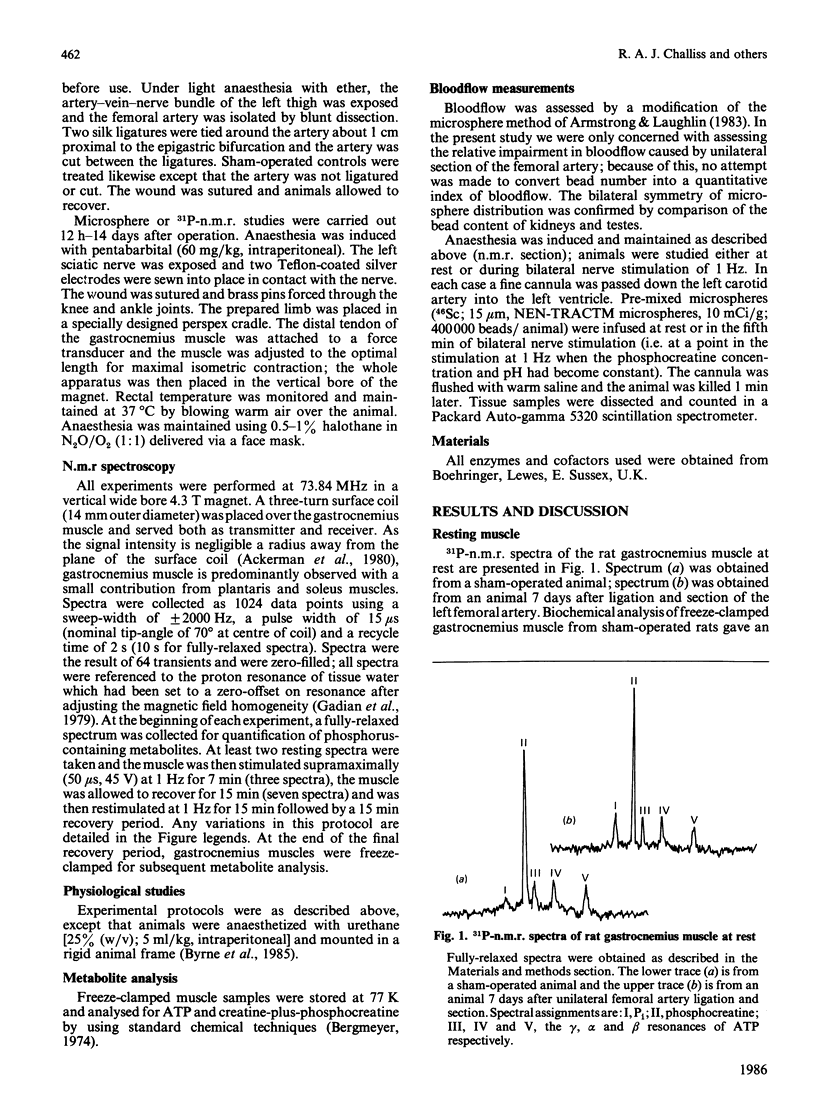

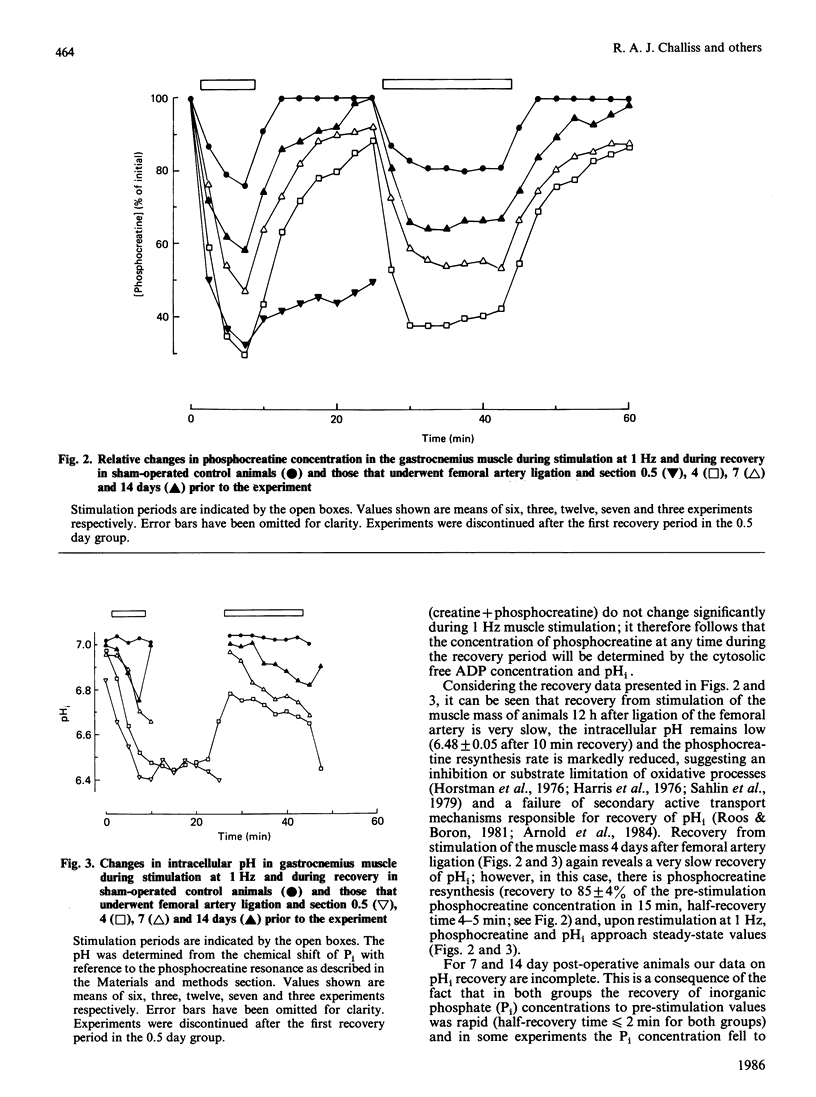
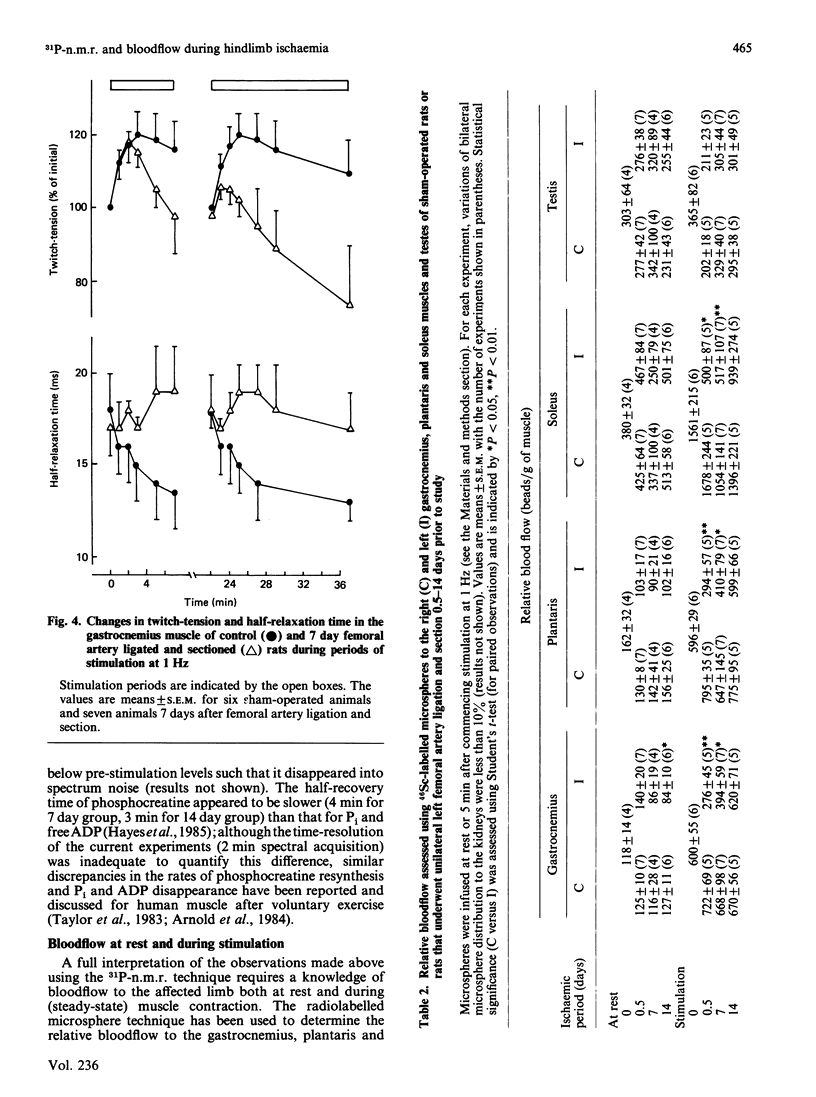
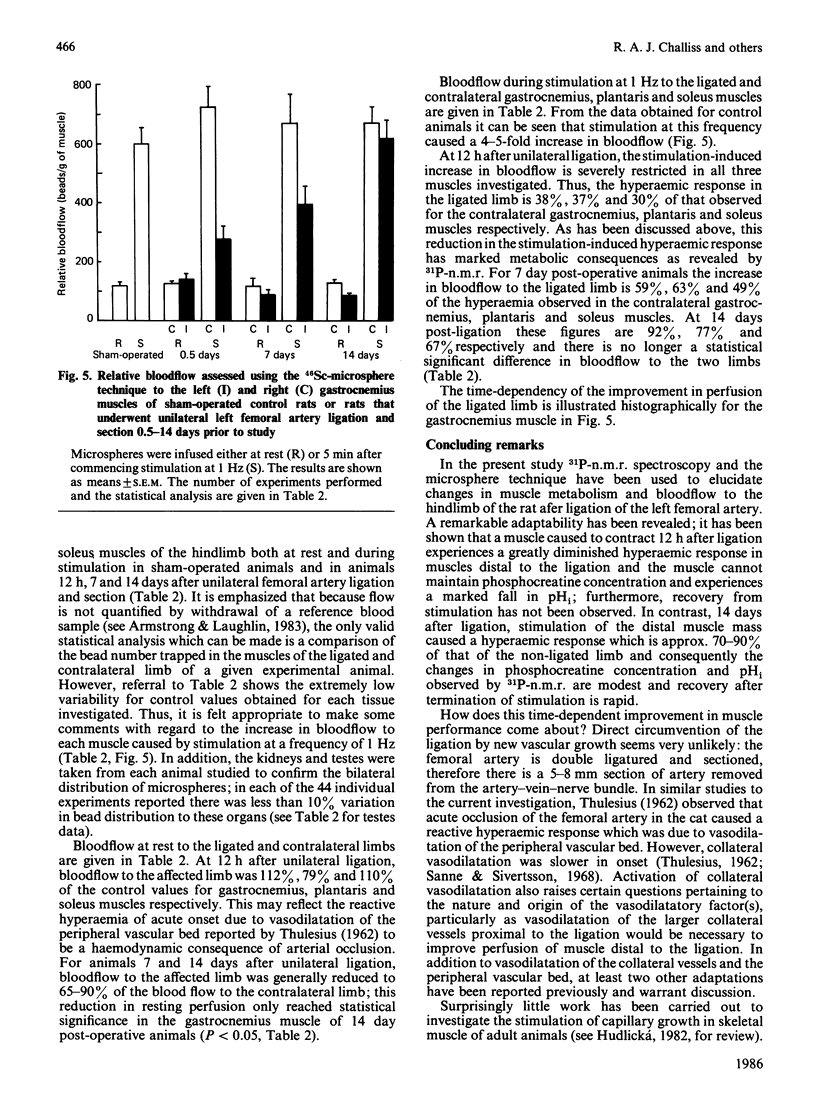

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. B., Laughlin M. H. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol. 1983 Nov;344:189–208. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D. L., Matthews P. M., Radda G. K. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. 1984 Sep;1(3):307–315. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Klinkerfuss G. H., Terjung R. L., Molé P. A., Holloszy J. O. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972 Feb;222(2):373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Beis I., Newsholme E. A. The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J. 1975 Oct;152(1):23–32. doi: 10.1042/bj1520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund-Fellenius A. C., Walker P. M., Elander A., Holm S., Holm J., Scherstén T. Energy metabolism in relation to oxygen partial pressure in human skeletal muscle during exercise. Biochem J. 1981 Nov 15;200(2):247–255. doi: 10.1042/bj2000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund A. C., Hammarsten J., Holm J., Scherstén T. Enzyme activities in skeletal muscles from patients with peripheral arterial insufficiency. Eur J Clin Invest. 1976 Nov 30;6(6):425–429. doi: 10.1111/j.1365-2362.1976.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Byrne E., Hayes D. J., Shoubridge E. A., Morgan-Hughes J. A., Clark J. B. Experimentally induced defects of mitochondrial metabolism in rat skeletal muscle. Biological effects of the mitochondrial uncoupling agent 2,4-dinitrophenol. Biochem J. 1985 Jul 1;229(1):101–108. doi: 10.1042/bj2290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassin S., Gilbert R. D., Bunnell C. E., Johnson E. M. Capillary development during exposure to chronic hypoxia. Am J Physiol. 1971 Feb;220(2):448–451. doi: 10.1152/ajplegacy.1971.220.2.448. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Heat production and chemical changes during isometric contractions of the human quadriceps muscle. J Physiol. 1975 Oct;251(2):303–315. doi: 10.1113/jphysiol.1975.sp011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975 Oct;251(2):287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. R., Salmons S. The reorganization of subcellular structure in muscle undergoing fast-to-slow type transformation. A stereological study. Cell Tissue Res. 1981;220(3):449–471. doi: 10.1007/BF00216750. [DOI] [PubMed] [Google Scholar]
- Gadian D. G., Radda G. K., Brown T. R., Chance E. M., Dawson M. J., Wilkie D. R. The activity of creatine kinase in frog skeletal muscle studied by saturation-transfer nuclear magnetic resonance. Biochem J. 1981 Jan 15;194(1):215–228. doi: 10.1042/bj1940215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. J., Klug G. A., Reichmann H., Seedorf U., Wiehrer W., Pette D. Exercise-induced fibre type transitions with regard to myosin, parvalbumin, and sarcoplasmic reticulum in muscles of the rat. Pflugers Arch. 1984 Apr;400(4):432–438. doi: 10.1007/BF00587545. [DOI] [PubMed] [Google Scholar]
- Green H. J., Reichmann H., Pette D. Fibre type specific transformations in the enzyme activity pattern of rat vastus lateralis muscle by prolonged endurance training. Pflugers Arch. 1983 Nov;399(3):216–222. doi: 10.1007/BF00656718. [DOI] [PubMed] [Google Scholar]
- Hammarsten J., Bylund-Fellenius A. C., Holm J., Scherstén T., Krotkiewski M. Capillary supply and muscle fibre types in patients with intermittent claudication: relationships between morphology and metabolism. Eur J Clin Invest. 1980 Aug;10(4):301–305. doi: 10.1111/j.1365-2362.1980.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Edwards R. H., Hultman E., Nordesjö L. O., Nylind B., Sahlin K. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. 1976 Dec 28;367(2):137–142. doi: 10.1007/BF00585149. [DOI] [PubMed] [Google Scholar]
- Hayes D. J., Challiss R. A., Radda G. K. An investigation of arterial insufficiency in rat hindlimb. An enzymic, mitochondrial and histological study. Biochem J. 1986 Jun 1;236(2):469–473. doi: 10.1042/bj2360469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Honig C. R. Hypoxia in skeletal muscle at rest and during the transition to steady work. Microvasc Res. 1977 May;13(3):377–398. doi: 10.1016/0026-2862(77)90105-4. [DOI] [PubMed] [Google Scholar]
- Horstman D. H., Gleser M., Delehunt J. Effects of altering O2 delivery on VO2 of isolated, working muscle. Am J Physiol. 1976 Feb;230(2):327–334. doi: 10.1152/ajplegacy.1976.230.2.327. [DOI] [PubMed] [Google Scholar]
- Hudlická O. Growth of capillaries in skeletal and cardiac muscle. Circ Res. 1982 Apr;50(4):451–461. doi: 10.1161/01.res.50.4.451. [DOI] [PubMed] [Google Scholar]
- Hudlická O., Tyler K. R., Srihari T., Heilig A., Pette D. The effect of different patterns of long-term stimulation on contractile properties and myosin light chains in rabbit fast muscles. Pflugers Arch. 1982 Apr;393(2):164–170. doi: 10.1007/BF00582940. [DOI] [PubMed] [Google Scholar]
- Idström J. P., Subramanian V. H., Chance B., Schersten T., Bylund-Fellenius A. C. Oxygen dependence of energy metabolism in contracting and recovering rat skeletal muscle. Am J Physiol. 1985 Jan;248(1 Pt 2):H40–H48. doi: 10.1152/ajpheart.1985.248.1.H40. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., Stainsby W. N. Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir Physiol. 1968 May;4(3):292–300. doi: 10.1016/0034-5687(68)90035-2. [DOI] [PubMed] [Google Scholar]
- Ljungqvist A., Unge G. Capillary proliferative activity in myocardium and skeletal muscle of exercised rats. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):306–307. doi: 10.1152/jappl.1977.43.2.306. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Kuchmerick M. J., Brown T. R. Application of 31P-NMR spectroscopy to the study of striated muscle metabolism. Am J Physiol. 1982 Jan;242(1):C1–11. doi: 10.1152/ajpcell.1982.242.1.C1. [DOI] [PubMed] [Google Scholar]
- Pette D., Ramirez B. U., Müller W., Simon R., Exner G. U., Hildebrand R. Influence of intermittent long-term stimulation on contractile, histochemical and metabolic properties of fibre populations in fast and slow rabbit muscles. Pflugers Arch. 1975 Dec 19;361(1):1–7. doi: 10.1007/BF00587333. [DOI] [PubMed] [Google Scholar]
- Pette D., Smith M. E., Staudte H. W., Vrbová G. Effects of long-term electrical stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflugers Arch. 1973 Feb 6;338(3):257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Harris R. C., Hultman E. Resynthesis of creatine phosphate in human muscle after exercise in relation to intramuscular pH and availability of oxygen. Scand J Clin Lab Invest. 1979 Oct;39(6):551–558. doi: 10.3109/00365517909108833. [DOI] [PubMed] [Google Scholar]
- Sahlin K. Intracellular pH and energy metabolism in skeletal muscle of man. With special reference to exercise. Acta Physiol Scand Suppl. 1978;455:1–56. [PubMed] [Google Scholar]
- Sanne H., Sivertsson R. The effect of exercise on the development of collateral circulation after experimental occlusion of the femoral artery in the cat. Acta Physiol Scand. 1968 Jul;73(3):257–263. doi: 10.1111/j.1748-1716.1968.tb04104.x. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Bland J. L., Radda G. K. Regulation of creatine kinase during steady-state isometric twitch contraction in rat skeletal muscle. Biochim Biophys Acta. 1984 Sep 14;805(1):72–78. doi: 10.1016/0167-4889(84)90038-7. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol. 1982 Sep;243(3):R271–R280. doi: 10.1152/ajpregu.1982.243.3.R271. [DOI] [PubMed] [Google Scholar]
- THULESIUS O. Haemodynamic studies on experimental obstruction of the femoral artery in the cat with special reference to the peripheral action of vasoactive substances. Acta Physiol Scand Suppl. 1962;57(199):1–95. doi: 10.1111/j.1748-1716.1962.tb02621.x. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Bore P. J., Styles P., Gadian D. G., Radda G. K. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983 Jul;1(1):77–94. [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Walter U., Müller S. Evidence for a Na-K-ATPase-inhibitor in erythrocytes of patients with essential hypertension. Eur J Clin Invest. 1985 Aug;15(4):209–214. doi: 10.1111/j.1365-2362.1985.tb00170.x. [DOI] [PubMed] [Google Scholar]


