Abstract
A small animal model of arterial insufficiency has been used to investigate enzymic alterations in the gastrocnemius, plantaris and soleus muscles of the hypoperfused limb. At 7 days after induction of arterial insufficiency by unilateral femoral artery ligation, there were significant increases in the maximal activities of hexokinase, phosphorylase and 6-phosphofructokinase, whereas the activities of citrate synthase and 2-oxoglutarate dehydrogenase remained unchanged. Similar increases in hexokinase, phosphorylase and 6-phosphofructokinase were still apparent 8-10 weeks after unilateral artery ligation, although only hexokinase remained significantly higher than contralateral control values. No enhancement of oxidative enzyme activities was observed. The results are discussed in relation to the conflicting findings reported by other groups investigating enzymic adaptations in patients with arterial insufficiency.
Full text
PDF
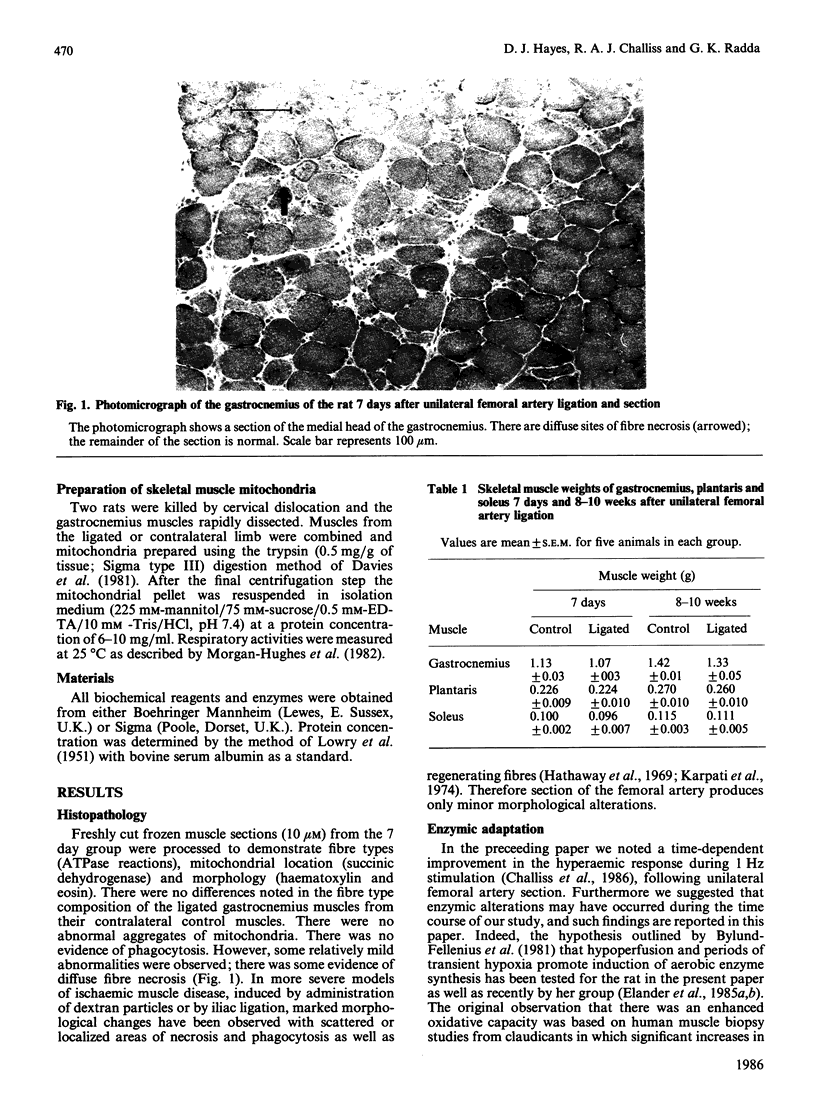
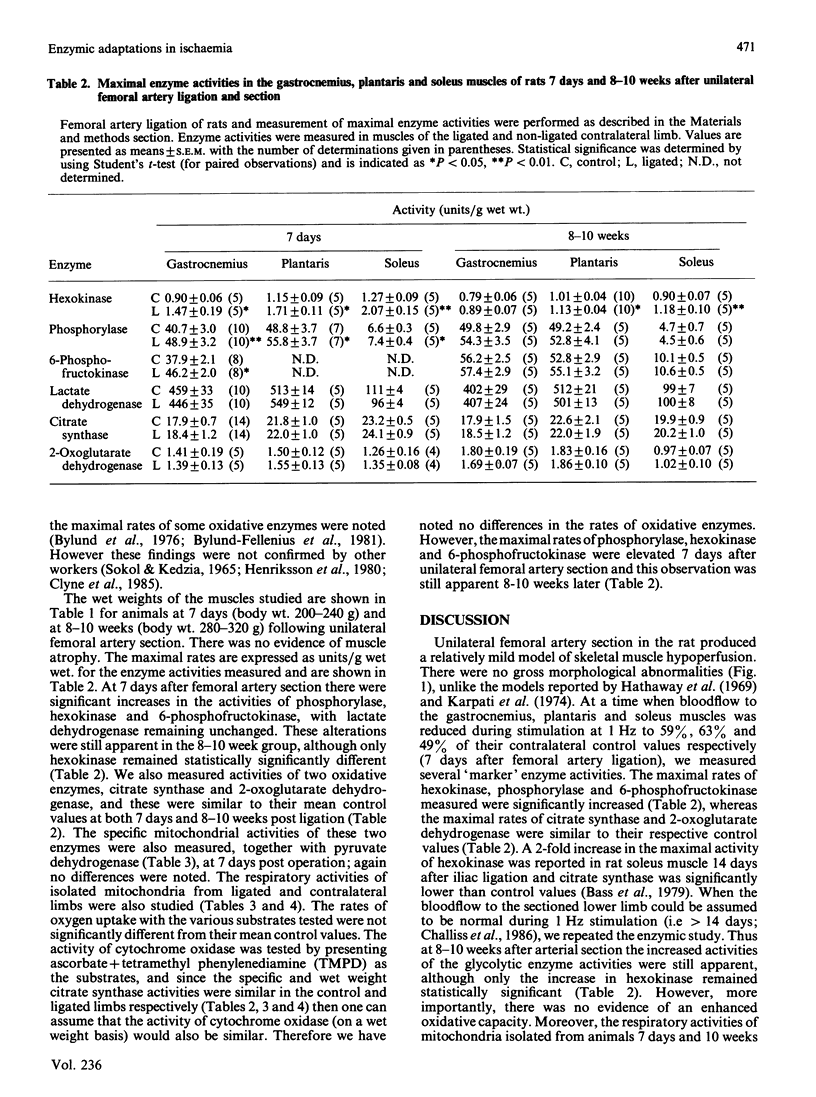


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alp P. R., Newsholme E. A., Zammit V. A. Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem J. 1976 Mar 15;154(3):689–700. doi: 10.1042/bj1540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass A., Gutmann E., Hanzlíková V., Teisinger J. Effects of ischaemia on enzyme-activities in the soleus muscle of the rat. Pflugers Arch. 1979 Mar 16;379(2):203–208. doi: 10.1007/BF00586949. [DOI] [PubMed] [Google Scholar]
- Bylund-Fellenius A. C., Walker P. M., Elander A., Holm S., Holm J., Scherstén T. Energy metabolism in relation to oxygen partial pressure in human skeletal muscle during exercise. Biochem J. 1981 Nov 15;200(2):247–255. doi: 10.1042/bj2000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund A. C., Hammarsten J., Holm J., Scherstén T. Enzyme activities in skeletal muscles from patients with peripheral arterial insufficiency. Eur J Clin Invest. 1976 Nov 30;6(6):425–429. doi: 10.1111/j.1365-2362.1976.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Hayes D. J., Petty R. F., Radda G. K. An investigation of arterial insufficiency in rat hindlimb. A combined 31P-n.m.r. and bloodflow study. Biochem J. 1986 Jun 1;236(2):461–467. doi: 10.1042/bj2360461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne C. A., Mears H., Weller R. O., O'Donnell T. F. Calf muscle adaptation to peripheral vascular disease. Cardiovasc Res. 1985 Aug;19(8):507–512. doi: 10.1093/cvr/19.8.507. [DOI] [PubMed] [Google Scholar]
- Cooney G. J., Taegtmeyer H., Newsholme E. A. Tricarboxylic acid cycle flux and enzyme activities in the isolated working rat heart. Biochem J. 1981 Dec 15;200(3):701–703. doi: 10.1042/bj2000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J., Packer L., Brooks G. A. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys. 1981 Jul;209(2):539–554. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- Elander A., Idström J. P., Holm S., Scherstén T., Bylund-Fellenius A. C. Metabolic adaptation to reduced muscle blood flow. II. Mechanisms and beneficial effects. Am J Physiol. 1985 Jul;249(1 Pt 1):E70–E76. doi: 10.1152/ajpendo.1985.249.1.E70. [DOI] [PubMed] [Google Scholar]
- Elander A., Idström J. P., Scherstén T., Bylund-Fellenius A. C. Metabolic adaptation to reduced muscle blood flow. I. Enzyme and metabolite alterations. Am J Physiol. 1985 Jul;249(1 Pt 1):E63–E69. doi: 10.1152/ajpendo.1985.249.1.E63. [DOI] [PubMed] [Google Scholar]
- Hathaway P. W., Engel W. K., Zellweger H. Experimental myopathy after microarterial embolization; comparison with childhood x-linked pseudohypertrophic muscular dystrophy. Arch Neurol. 1970 Apr;22(4):365–378. doi: 10.1001/archneur.1970.00480220079011. [DOI] [PubMed] [Google Scholar]
- Henriksson J., Nygaard E., Andersson J., Eklöf B. Enzyme activities, fibre types and capillarization in calf muscles of patients with intermittent claudication. Scand J Clin Lab Invest. 1980 Jun;40(4):361–369. doi: 10.3109/00365518009092656. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Janda J., Urbanová D., Mrhová O., Linhart J. The effect of muscular work on the activities of certain enzymes in skeletal muscle in chronic muscular ischaemia. Cor Vasa. 1972;14(4):312–320. [PubMed] [Google Scholar]
- Karpati G., Carpenter S., Melmed C., Eisen A. A. Experimental ischemic myopathy. J Neurol Sci. 1974 Sep;23(1):129–161. doi: 10.1016/0022-510x(74)90148-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Darveniza P., Kahn S. N., Landon D. N., Sherratt R. M., Land J. M., Clark J. B. A mitochondrial myopathy characterized by a deficiency in reducible cytochrome b. Brain. 1977 Dec;100(4):617–640. doi: 10.1093/brain/100.4.617. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Hayes D. J., Clark J. B., Landon D. N., Swash M., Stark R. J., Rudge P. Mitochondrial encephalomyopathies: biochemical studies in two cases revealing defects in the respiratory chain. Brain. 1982 Sep;105(Pt 3):553–582. doi: 10.1093/brain/105.3.553. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Newsholme E. A. The activities of fructose 1,6-diphosphatase, phosphofructokinase and phosphoenolpyruvate carboxykinase in white muscle and red muscle. Biochem J. 1967 May;103(2):391–399. doi: 10.1042/bj1030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge E. A., Challiss R. A., Hayes D. J., Radda G. K. Biochemical adaptation in the skeletal muscle of rats depleted of creatine with the substrate analogue beta-guanidinopropionic acid. Biochem J. 1985 Nov 15;232(1):125–131. doi: 10.1042/bj2320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge E. A., Radda G. K. A 31P-nuclear magnetic resonance study of skeletal muscle metabolism in rats depleted of creatine with the analogue beta-guanidinopropionic acid. Biochim Biophys Acta. 1984 Sep 14;805(1):79–88. doi: 10.1016/0167-4889(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Sokol S., Kedzia H. Etude histo-chimique des muscles des membres inférieurs chez les malades en ischémie chronique. Lyon Chir. 1965 May;61(3):335–343. [PubMed] [Google Scholar]
- Staging lung cancer. Br Med J. 1980 Jul 12;281(6233):94–95. [PMC free article] [PubMed] [Google Scholar]
- Urbanová D., Janda J., Mrhová O., Linhart J. Enzyme changes in the ischaemia of skeletal muscle and the effect of physical conditioning. A histochemical study. Histochem J. 1974 Mar;6(2):147–155. doi: 10.1007/BF01011803. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Newsholme E. A. The maximum activities of hexokinase, phosphorylase, phosphofructokinase, glycerol phosphate dehydrogenases, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxykinase, nucleoside diphosphatekinase, glutamate-oxaloacetate transaminase and arginine kinase in relation to carbohydrate utilization in muscles from marine invertebrates. Biochem J. 1976 Dec 15;160(3):447–462. doi: 10.1042/bj1600447. [DOI] [PMC free article] [PubMed] [Google Scholar]



