Abstract
Objective
Multidisciplinary rehabilitation facilitates post-stroke functional recovery, but is associated with resource and accessibility barriers. This study evaluated the combination of a wearable device-assisted system (WEAR) and conventional therapy for post-stroke rehabilitation.
Methods
This randomized, controlled, parallel group, clinical trial was conducted at two rehabilitation centers. A WEAR system was developed featuring sensors and application program-embedded smartphones. Stroke patients within 12 weeks of onset and modified Rankin Scale (mRS) scores of 2 to 4 were randomized into a wearable group (WG, WEAR + conventional rehabilitation) or control group (CG, conventional rehabilitation) for 90 days. The primary outcome was mRS score changes within 90 days.
Results
Among 127 stroke patients enrolled (76 men [59.8%]; mean age: 57.5 years), 63 and 64 patients were randomized to WG and CG, respectively. Both groups showed significant improvements in mRS scores. Between-group repeated measures analysis adjusted for sex, age and number of rehabilitation sessions showed greater improvement in mRS scores within 90 days in the WG than in the CG (estimate: 0.73).
Conclusions
This combined WEAR and conventional rehabilitation approach may improve post-stroke functional recovery compared with conventional rehabilitation alone. The WEAR system permits remote monitoring and recording of rehabilitation in various settings.
This clinical trial was retrospectively registered at www.clinicaltrials.gov with the Unique Identifier NCT04997408.
Keywords: Wearable rehabilitation system, randomized controlled trial, stroke, rehabilitation, customized application, device-assisted therapy, functional recovery
Background
Stroke remains the leading cause of chronic physical disability worldwide and the second leading cause of acute mortality. 1 The global burden of stroke is increasing1,2 despite substantial progress in prevention and acute treatment of ischemic and hemorrhagic stroke over the last few decades.3–6 Adherence to multidisciplinary rehabilitation guidelines after acute stroke is associated with improved outcomes.7–9 Current stroke guidelines from academic organizations recommend early and continuous rehabilitation to facilitate functional recovery and improve prognosis.7–9 However, there are several barriers to adherence to rehabilitation recommendations for stroke patients, including the patient’s condition, the engagement of caregivers, availability of facilities and other environmental factors. The efficacy of rehabilitation could be improved by increasing rehabilitation time using mobile communication devices.7,10 According to the US Food and Drug Administration, digital health has a broad scope of application that includes mobile health, telemedicine, telehealth, wearable devices and personalized medicine. 11 Many types of digital devices and technologies have been used to deliver health interventions, including wearable medical devices. 12
Wearable devices have been proposed as an advanced technology to facilitate post-stroke rehabilitation. 13 To supplement subjective evaluation by medical staff, wearable sensors can provide quantifiable data of specific performed activities. They can also record the activity of different parts of the body simultaneously during individual or multiple tasks.14,15 The long-term evaluation of rehabilitation programs using such data could help medical staff to better understand patients’ abilities and progress, complementing clinical assessment for individualized adjustments. The use of wearable devices has been evaluated for use in diagnostics, recovery/adaptation evaluation, extended training outside rehabilitation institutes and training of patients and caregivers for post-stroke rehabilitation. 13 Devices embedded with inertial measurement units (IMU) provide readings from accelerometers and gyroscopes to determine the positions of the attached body parts for evaluation. 16
We proposed a system that included (1) wearable devices with IMU for detecting the positions of body parts, (2) smartphones for recording and transmitting patient data, providing real-time feedback, and receiving instructions from staff, (3) servers for recording and analyzing the data and (4) a computer to allow staff to review the performance data of each patient. 17
We conducted a trial to test this proposed system, which we named the WEarable device-Assisted Rehabilitation (WEAR) system, for patients with stroke. The aim of the trial was to investigate the efficacy of implementing a 30-minute smartphone- or smartwatch-assisted session compared with conventional in-person rehabilitation for stroke patients. A survey to investigate patients’ perceptions and intentions to use the system was conducted using a questionnaire designed according to the Unified Theory of Acceptance and Use of Technology (UTAUT) model. 18
Methods
Overview
This randomized, controlled, parallel group clinical trial was conducted at two rehabilitation centers: Landseed International Hospital, Taiwan, and Antai Tian-Sheng Memory Hospital, Taiwan, from April 2016 to April 2018. The study was approved by the institutional review boards of both study sites (LS-14-001-B1 and TSMH 16-074-2.1). All participants and/or their legal representatives were informed about the study purpose and procedures, including academic publication. Recruitment was conducted by trained nurses following strict guidelines to ensure that participation was voluntary. Written informed consent was obtained prior to participant inclusion. Participants’ identities were protected by anonymizing their data by linking patients to a unique study number devoid of personal identifiers. No participants contacted us after providing their data. The reporting of this study conforms to the CONSORT guidelines. This clinical trial was retrospectively registered at www.clinicaltrials.gov with the Unique Identifier: NCT04997408 on 31 July 2021.
The WEAR system (Supplementary Figure 1) was developed by the Wireless Network and Multimedia Laboratory at National Central University, Taiwan, through multidisciplinary collaboration between stroke neurologists, physiatrists, nurses and therapists from the two hospitals. The development and validation of the WEAR system are described in detail in the Supplementary Material figures and table.
Participants
Patients were eligible for enrollment if they were aged 20 to 75 years, had received a diagnosis of stroke within 4 to 12 weeks of onset, had a designated caregiver willing to participate and had a baseline modified Rankin Scale (mRS) score of 2 to 4. We excluded patients with serious disabling comorbidities that might have affected participation and those who planned to participate in post-acute stroke rehabilitation at other hospitals.
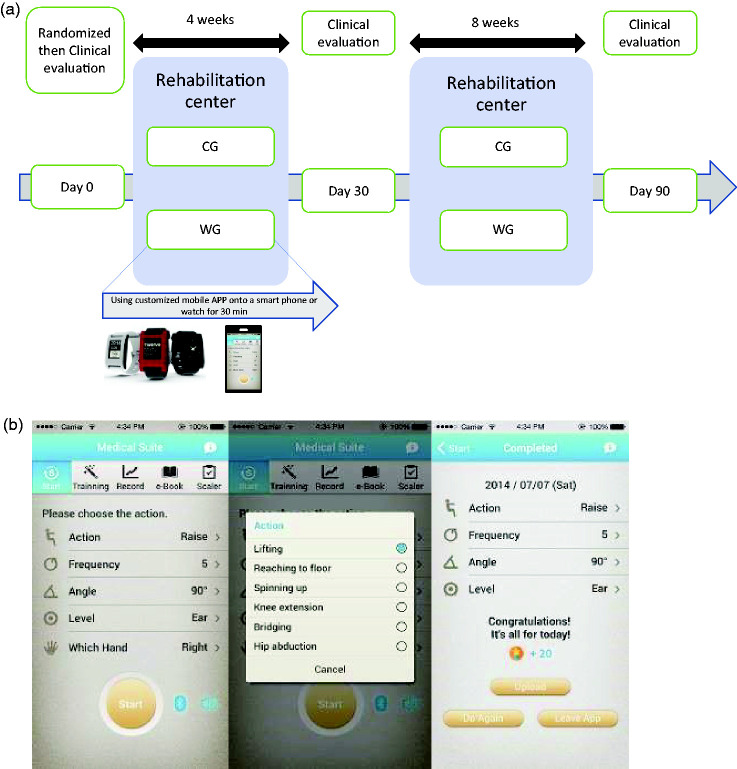
Eligible patients were randomly assigned in a 1:1 ratio to a group that received the WEAR intervention plus conventional rehabilitation (WG) or a control group (CG) who received only conventional rehabilitation. The randomization sequence was generated using a computer and the results were sealed in opaque envelopes. The randomization list was prepared by a statistician who had no clinical role in the study. The study flow of randomization, intervention and evaluation days is summarized in Figure 1(a).
Figure 1.
The WEAR system (a) and the English version of its user interface (b). The Chinese version can be downloaded from Google Play. CG, control group; WG, wearable group; WEAR, WEarable device-Assisted Rehabilitation.
Study interventions
All patients were scheduled to receive regular conventional rehabilitation therapy by therapists at rehabilitation centers at least three times per week. There was a consensus regarding the implementation of the application (app) rehabilitation practice in addition to conventional rehabilitation; a senior therapist was in charge at each hospital during the study. The protocol and recommended intensity of the app rehabilitation treatment was the same in the two hospitals. Physical therapists selected and adapted the exercises for individual patients based on their disability and functional assessment results.
Patients in the WG received approximately 60 minutes of traditional physical and occupational therapy and another 30 minutes of WEAR using a customized mobile app loaded onto a smartphone or smartwatch. Patients in the CG received approximately 90 minutes of conventional physical and traditional therapy. At the hospitals, WG patients and their caregivers were provided with a smartphone loaded with the rehabilitation app, which included six standardized exercises aimed at improving trunk mobility and upper and lower limb mobility [see Additional file 1]. Patients and their (formal or informal) caregivers were asked to perform a selected set of exercises for 3 months, and attended a monthly evaluation session with therapists and neurologists. If discharge occurred before the end date of the study period, then the program continued with outpatient rehabilitation performed using the customized exercise app.
Rehabilitation programs
One of the most popular therapies for stroke patients is proprioceptive neuromuscular facilitation (PNF), 19 which was developed in the 1940s. PNF is a dynamic approach to facilitate post-stroke motor recovery and improves motor performance using maximal resistance to facilitate movement. PNF focuses on spiral and diagonal movement patterns to promote a larger neuromuscular response in proprioceptors. The receptors involved in proprioception transmit joint signals to the spinal cord and then to the brain, facilitating bi- or multi-articular muscle activities. The PNF method includes the following maneuvers: (1) resistance; (2) reinforcement; (3) manual contact; (4) body position and body mechanics; (5) verbal stimulation/commands; (6) vision; (7) traction and approximation and (8) stretch. 20
We used six assisted rehabilitation programs for the trunk and upper and lower limbs in accordance with rehabilitation practice. The programs were based on the following core activities: lifting, spinning up, reaching the floor, knee extension, hip abduction, and bridging (Supplementary Figure 2 a–f). These daily exercises were designed based on the PNF model and incorporate three-dimensional movements that include diagonal and spiral movement patterns and their combinations in the sagittal, coronal and transverse planes, corresponding to pitch (movement through the horizontal axis), roll (movement through the frontal axis) and yaw (movement through the vertical axis). These exercises can be modified for patients at different functional stages, and for the same patients as they progress through different Brunnstrom stages of stroke recovery. 21
Details of the app rehabilitation treatment process are described in Additional file 1. The therapists demonstrated these movements on the smartphones and smartwatches using the app, and recorded accurate sensor values as the thresholds for the algorithms used by the app.
The whole system was externally validated by an independent therapist who was not involved in the development stage. This therapist performed standard correct and incorrect movements that were used to determine the sensitivity and specificity of the app (Supplementary Table 1). The final version of the WEAR system included public education of stroke prevention and treatment, Framingham stroke risk scores, app-assisted stroke rehabilitation, a calendar to log daily performance and prompt the uploading of records, and server and web-based management systems (Figure 1(b)).
After correct installation of the app on the smartphones or smartwatches, the devices were attached to the appropriate limbs or trunk following evaluation by the therapists, as in daily practice. The therapist could choose one of the designed app programs for patients at the desired intensity (angles and levels) to begin the interactive program while performing the in-person rehabilitation programs. The devices detect the movements, transmit them to the server and provide real-time feedback. The therapists instructed patients and their caregivers on how the systems worked. Demonstrative pictures and videos were used to guide patients in performing each movement. During the patients’ regular visits to the rehabilitation center, the therapists evaluated patients’ functional status and provided further instructions based on the stored records. Patients could choose to run the rehabilitation programs on either their smartphones or their smartwatches. Feedback based on the records was available on the system’s web interface. Once the app was activated, the system automatically recorded and uploaded users’ activities, including performed programs, frequency, duration and success rates. Physicians, therapists and nurses could use the system to monitor patients’ app use in real time.
After the WG patients and their caregivers had received sufficient instruction from the therapists, we encouraged patients to perform the assigned rehabilitation programs at home after discharge. The study coordinator and authorized personnel monitored the website every working day to examine the transmitted data on the rehabilitation activities of all participants, either in the rehabilitation centers or at home. The information was shared with the therapists and the physicians in charge. The study coordinator contacted any patients whose transmitted data indicated that they might need further assistance and/or instruction.
Acceptance of the WEAR system
To explore patients’ acceptance and perceptions of the WEAR system, we developed a questionnaire based on the UTAUT model. 18 Cronbach's alpha indicated that the internal consistency reliability of the questionnaire was high (alpha: 0.963). The content validity index of content appropriateness was 0.79, and the content validity index of clarity of recommendations was 0.86, confirming the suitability and comprehensibility of the questionnaire.
The survey was conducted with patients at one center (Landseed International Hospital), using a cross-sectional assessment at baseline for both groups and a follow-up assessment at 30 days (D30) and 90 days (D90) for the WG.
The UTAUT model comprises four determining factors of technology use: performance expectancy, effort expectancy, social influence and facilitating conditions; these factors influence behavioral intention and thus technology use. 22
Outcome measures
All patients underwent a comprehensive clinical evaluation at the start of the study that recorded sex, age, type of stroke, date of stroke onset and affected side. Detailed data on patients’ daily functional scores, which were assessed by the therapist assisting with the rehabilitation, were also collected to evaluate primary and secondary outcomes.
The primary outcome was the change in mRS score from baseline (D0) to D30 and D90. The mRS is a widely used measure of functional outcomes and activity limitations in stroke therapies.23,24 This activity-level measure has been widely used in a range of stroke studies and its application and interpretation is well accepted. However, the mRS does not assess motor function, physical function, movement execution or level of independence. Therefore, we used secondary outcomes to assess other aspects of functional recovery, balance and upper and lower motor ability D0, D30, and D90. We used the following measures, which are routinely used to assess acute stroke patients during admission: the National Institute of Health Stroke scale (NIHSS); the Barthel index (BI) 25 to assess functional recovery of activities of daily living; the Brunnstrom stages of recovery; the Functional Independence Measure (FIM) to assess motor domains, such as self-care, continence, transfers, and locomotion, and cognitive domains; 26 the Berg Balance Scale (BBS) to assess static and dynamic balance ability; 27 and the Wolf Motor Function Test-Functional Ability Scale (WMFT-FAS) to assess upper extremity motor ability. 28
We also conducted focus group interviews with recruited patients and their caregivers, rehabilitation therapists, researchers in post-acute stroke care, the managers of nursing homes and the study investigators. The aim of the interviews was to investigate respondents’ willingness to use the WEAR, and the costs, benefits and barriers involved in applying this system outside of hospital and rehabilitation center settings. During the interview, we shared information about the results of the study and comprehensively discussed aspects that may affect the potential implementation of the system in external settings such as patients’ homes and nursing homes.
Statistical analyses
We calculated the sample size using a two-sided, two-sample t-test assuming 80% power and a type I error of 0.05.29,30 Considering a dropout rate of 20%, the final estimation of the required sample size was 108 participants. A power analysis was performed using G*Power V3.1.9.4. 31
All outcome measures were analyzed based on the intention-to-treat principle, and missing data were addressed according to the last observation carried forward method.32,33 We compared the two groups (WG and CG) on demographics, stroke types, clinical characteristics, rehabilitation engagement items, and scores on the five rehabilitation scales. To assess differences in clinical and demographic characteristics of the patients in the two groups, we used the independent samples t-test for continuous variables, the Pearson chi-square test or Fisher’s exact test for dichotomous variables or the Mann–Whitney U test for ordinal variables (univariate analysis). 34 For the primary outcome, the changes in mRS scores from baseline over the course of the treatment period (from D0 to D30, from D0 to D90) were further compared using the independent samples t-test or the Mann–Whitney U test. The secondary outcomes were determined using the paired t-test or Mann–Whitney U test.
To analyze the outcome at three timepoints, we compared the two groups at multiple time points using a series of individual group comparisons (repeated measures procedures). The within-subject factor was set as time and the between-subject factor was set as group, with an analysis of covariance (ANCOVA) model adjusted using the baseline parameters as covariates. 35 To control the effect of covariate variables, we examined not only the changes in variance of the dependent variable but also the relationship between the dependent variable and the covariates across different levels of a qualitative variable. ANCOVA differentiates between the variance change in the dependent variable explained by changes in the covariate variable and the variance change explained by changes in the levels of the qualitative variables. Therefore, it reduces the error variance of the dependent variable and makes it easier to determine the significance of the variables; it also increases the analytical power. Significant effects identified by the ANCOVA were further examined with a post hoc between-group comparison (pairwise multiple comparisons) of mRS scores using the Mann–Whitney U test.33,36,37 Before conducting the post hoc test (on between-subject factors), the homogeneity of the variances between the groups was first tested. A value of P ≥ 0.05 indicates that the variances are homogeneous, and that a multiple comparison procedure can be applied, such as the least significant difference, the Bonferroni method or the Tukey test. 38
A two-sided value of P < 0.05 was considered significant. SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA) was used to perform all statistical analyses.
Results
A total of 140 patients were evaluated from April 2016 to April 2018, and 127 met the eligibility criteria. After randomization, 63 participants were assigned to the WG and 64 to the CG. The WG included 41 participants with smartphones (ASUS® Zenfone 5) and 22 participants with smartwatches (Pebble® Time).
The details of baseline clinical characteristics are shown in Table 1. Of the 127 patients (mean age, 57.45 ± 9.29 years), 59.8% were men and 74.4% had ischemic stroke. The mean time from stroke to screening was 20.42 ± 25.70 days (interquartile range [IQR] 5–23 days). The median baseline NIHSS score was 5 (IQR 2.0–8.0) in each group, with no difference between groups. There was no between-group difference in the median Brunnstrom stage score, which was 4 in both groups (P = 0.348 for upper limbs, and P = 0.391 for lower limbs). However, there was a significant between-group difference in the distribution of mRS scores 2, 3 and 4 (more patients in the WG group scored 2 whereas more patients in the CG group scored 4; P = 0.039) (Table 1). Excluding two WG patients and four CG patients who did not participate in any treatment sessions after randomization, patients participated in an average of 23 sessions (63.9%) in the WG group and 17 sessions (47.2%) in the CG group out of the 36 assigned rehabilitation sessions. Thirty-seven patients (18 in the WG and 19 in the CG groups) were unavailable to complete the D30 and D90 study evaluations (Figure 2). WG patients who were discharged from the hospital without completing the 36 sessions were encouraged to continue rehabilitation at the same hospital facility or at home. All patients who dropped out of the study completed at least four sessions before discharge. No serious adverse events or discomfort were observed following the rehabilitation.
Table 1.
Demographic and baseline characteristics of the enrolled participants according to randomization groups.
| Characteristic | Wearable group (n = 63) | Control group (n = 64) | P value |
|---|---|---|---|
| Age, years, mean ± SD | 58.73 ± 8.91 | 56.19 ± 9.55 | 0.123 |
| Sex, n (%) | 0.538 | ||
| Male | 36 (57.14) | 40 (62.50) | |
| Stroke type, n (%) a | 0.475 | ||
| Ischemic | 48 (77.42) | 46 (71.88) | |
| Hemorrhagic | 14 (22.58) | 18 (28.12) | |
| From stroke onset to screening, days, mean ± SD | 23.57 ± 25.43 | 17.31 ± 25.78 | 0.171 |
| Affected side, n (%) b | 0.532 | ||
| Left | 33 (52.38) | 30 (47.62) | |
| Right | 30 (47.62) | 33 (52.38) | |
| Rehabilitation sessions, median (IQR) | 23.0 (12.0–34.0) | 17.0 (4.0–34.0) | <0.001 |
| Baseline clinical outcome score | |||
| NIHSS, median (IQR) | 5.0 (2.0–8.0) | 5.0 (2.0–8.0) | 0.759 |
| Brunnstrom stage, median (IQR) c | |||
| Upper extremity | 4.0 (4.0–5.0) | 4.0 (3.0–5.0) | 0.348 |
| Lower extremity | 4.0 (4.0–5.0) | 4.0 (3.0–5.0) | 0.391 |
| mRS, n (%) | 0.039 | ||
| 2 | 27 (42.86) | 15 (23.43) | |
| 3 | 17 (26.98) | 19 (29.69) | |
| 4 | 18 (29.03) | 30 (46.88) | |
NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; IQR, interquartile range; SD, standard deviation.
One individual in the wearable group had a subarachnoid hemorrhage and was excluded from the calculations.
One individual in the control group had an affected brainstem and was excluded from the calculations.
Seven individuals in the wearable group and three individuals in the control group had missing Brunnstrom assessments at baseline and were excluded from the calculations.
Figure 2.
CONSORT participant flow chart.
During the study period, we encouraged patients and their caregivers to use the WEAR at home after appropriate instruction. The system records showed that three patients performed the instructed programs at home for 5, 5, and 22 days, respectively. The patient with the longest engagement time engaged in 516 minutes of WEAR at home, equivalent to almost six rehabilitation sessions as defined in the study.
Primary outcome
The analysis of the mRS score distribution showed that both groups experienced significant improvements at D30 and D90 compared with baseline (WG: median 2, [IQR 1–3], P < 0.0001; CG: median 3; [IQR 2–4], P < 0.0001 at D90, respectively) (Table 2).
Table 2.
Primary outcome (modified Rankin Scale score) by timepoint and changes from baseline at 30 and 90 days
| Day of evaluation | Assessment Median (IQR) | Changes from D0 Median (IQR) | Within-group P value a | Between group P value b | Estimate | Post hoc P value c | |
|---|---|---|---|---|---|---|---|
| WG (n = 63) | Baseline (D0) | 3.0 (2.0–4.0) | Reference | Reference | 0.019 | −0.73 | 0.023 |
| D30 | 2.0 (2.0–4.0) | 0 (−1.0 to 0) | 0.002 | ||||
| D90 | 2.0 (1.0–3.0) | 0 (−1.0 to 0) | <0.0001 | ||||
| CG (n = 64) | Baseline (D0) | 3.0 (3.0–4.0) | Reference | Reference | Reference | ||
| D30 | 3.0 (2.0–4.0) | 0 (−1.0 to 0) | <0.0001 | ||||
| D90 | 3.0 (2.0–4.0) | 0 (−1.0 to 0) | <0.0001 |
WG, wearable group; CG, control group; IQR, interquartile range; D0, baseline; D30, 30 days; D90, 90 days.
Significant within-group difference.
Comparison of between-group change from baseline to D90 using ANCOVA test, adjusting for sex, age and number of rehabilitation sessions.
Post hoc comparison between groups using Mann–Whitney U test.
Further repeated measures ANCOVA analysis was performed to compare the interaction between time and group. The results showed a significant between-group effect for mRS score (P = 0.023), with WG patients showing more improvement than CG patients (estimate −0.73 in the post hoc comparison between groups), after adjusting for age, sex and number of rehabilitation sessions (Table 2).
Secondary outcomes
The mean scores on the BI, FIM and BBS and the median scores on the WMFT-FAS at different time points are shown in Table 3. Both groups showed significant improvements in BI, FIM and BBS scores at D30 and D90, respectively (all P < 0.0001). Both groups also showed significant improvements in WMFT-FAS scores at D30 and D90. In the between-group comparison, WG patients showed significantly more improvement than CG patients in scores on the BI at D0 (P = 0.014) and D90 (P = 0.039), on the FIM at D0 (P = 0.001) and D90 (P = 0.012), on the BBS at D0 (P = 0.025) and D90 (P = 0.002), and on the WMFT-FAS at D90 (P = 0.038). However, there were no significant between-group differences in the mean change in BI, BBS, FIM and WMFT-FAS scores from baseline to D90 (Table 3).
Table 3.
Secondary outcomes by timepoint and change from baseline at 90 days.
| Wearable group (n = 63) |
Control group (n = 64) |
Between group P value b | Difference in the mean change at D90 (95% CI) c | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Within-group P value a | Mean ± SD | Within-group P value a | |||
| BI | ||||||
| Baseline (D0) | 70.71 ± 25.54 | Reference | 58.57 ± 29.22 | Reference | 0.014 | −2.93 (−9.43 to 3.56) |
| D30 | 78.49 ± 25.30 | <0.0001 | 73.13 ± 27.57 | <0.0001 | 0.285 | |
| D90 | 83.02 ± 23.06 | <0.0001 | 73.67 ± 28.09 | <0.0001 | 0.039 | |
| FIM | ||||||
| Baseline (D0) | 76.57 ± 20.87 | Reference | 61.63 ± 27.16 | Reference | 0.001 | −4.16 (−15.33 to 7.00) |
| D30 | 85.79 ± 37.31 | <0.0001 | 77.10 ± 40.70 | <0.0001 | 0.306 | |
| D90 | 84.98 ± 34.83 | <0.0001 | 74.05 ± 37.95 | <0.0001 | 0.012 | |
| BBS | ||||||
| Baseline (D0) | 33.66 ± 16.15 | Reference | 26.43 ± 17.92 | Reference | 0.025 | −0.14 (−4.26 to 3.97) |
| D30 | 41.43 ± 15.54 | <0.0001 | 36.23 ± 17.22 | <0.0001 | 0.115 | |
| D90 | 44.71 ± 13.21 | <0.0001 | 37.07 ± 16.95 | <0.0001 | 0.002 | |
| WMFT-FAS, median (IQR) | ||||||
| Baseline (D0) | 3.0 (0.0–5.0) | Reference | 3.0 (0.0–5.0) | Reference | 0.142 | −0.009 (−0.33 to 0.32) |
| D30 | 3.0 (0.0–5.0) | 0.001 | 4.0 (0.0–5.0) | 0.007 | 0.099 | |
| D90 | 4.0 (0.0–5.0) | <0.0001 | 3.0 (1.0–5.0) | 0.0007 | 0.038 | |
CI, confidence interval; BI, Barthel index; FIM, Functional Independence Measure; BBS, Berg Balance Scale; WMFT-FAS, Wolf Motor Function Test-Functional Ability Scale; IQR, interquartile range; SD, standard deviation; WG, wearable group; CG, control group; D0, baseline; D30, 30 days; D90, 90 days.
Comparison within groups using the paired t-test.
Comparison between groups using the ANCOVA test and adjusting for sex, age and number of rehabilitation sessions.
Adjusted for sex, age and number of rehabilitation sessions; and no significant between-group difference.
Acceptance of the WEAR system
We conducted expert validation tests prior to administering the UTAUT questionnaire.
There were no between-group differences in performance expectancy, effort expectancy and facilitating conditions at baseline, but WG patients had significantly higher scores on social influence (P = 0.02) and behavioral intention (P = 0.002). The follow-up survey of WG patients demonstrated significantly increased scores from baseline to D30 and D90 on performance expectancy (D30: P = 0.002; D90: P = 0.007), effort expectancy (D30: P = 0.0001; D90: P < 0.001) and facilitating conditions (D30: P = 0.003; D90: P < 0.001) (Table 4). Non-significant increases in social influence and behavioral intention scores were also shown at D30 and D90 follow-up.
Table 4.
Acceptance of the WEAR system at baseline in the WG and CG and changes from baseline in the WG.
| Baseline assessment |
30 days assessment |
90 days assessment |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | WG (n = 56) | CG (n = 60) | P value | WG (n = 56) | Change from Baseline | P value a | WG (n = 56) | Change from Baseline | P value a |
| UTAUT score, mean +−SD | |||||||||
| PE | 3.58 ± 0.63 | 3.41 ± 0.56 | 0.133 | 3.88 ± 0.68 | 0.30 ± 0.69 | 0.002 | 3.90 ± 0.80 | 0.32 ± 0.85 | 0.007 |
| EE | 3.33 ± 0.79 | 3.33 ± 0.56 | 0.993 | 3.80 ± 0.72 | 0.47 ± 0.86 | 0.0001 | 3.91 ± 0.63 | 0.58 ± 0.86 | <0.001 |
| SI | 3.67 ± 0.62 | 3.41 ± 0.58 | 0.021 | 3.84 ± 0.65 | 0.17 ± 0.69 | 0.08 | 3.87 ± 0.74 | 0.20 ± 0.77 | 0.06 |
| FC | 3.50 ± 0.70 | 3.33 ± 0.63 | 0.168 | 3.83 ± 0.68 | 0.33 ± 0.78 | 0.003 | 3.94 ± 0.65 | 0.43 ± 0.74 | <0.001 |
| BI | 3.72 ± 0.64 | 3.36 ± 0.59 | 0.002 | 3.88 ± 0.70 | 0.15 ± 0.61 | 0.06 | 3.89 ± 0.75 | 0.17 ± 0.77 | 0.099 |
WG, wearable group; CG, control group; UTAUT, Unified Theory of Acceptance and Use of Technology; SD, standard deviation; PE, performance expectancy; EE, effort expectancy; FC, facilitating conditions; SC, social influence; BI, behavioral intention; WEAR, WEarable device-Assisted Rehabilitation.
Comparison within groups using paired t-test.
Discussion
Our study showed that stroke patients in both groups experienced significant improvement over 90 days, and WG patients showed significantly, although slightly, better functional recovery than CG patients as assessed by the primary outcome (mRS scores) during the study period. The results suggest the benefits of incorporating wearable devices into post-stroke rehabilitation.
The lack of significant differences in initial NIHSS scores and Brunnstrom stages suggest that patients in the two groups had similar severity and functional impairment. However, the groups had different distributions of initial mRS scores. Compared with the WG, the CG group had worse BI, FIM and BBS baseline scores. This difference may reflect the fact that the study assessed different aspects of disability, functional impairment and health. 39 Our primary outcome was mRS score; randomized controlled rehabilitation and pharmaceutical studies have demonstrated that the mRS is a validated global measure of post-stroke activity levels. 40 Of the several statistical methods used to analyze changes in mRS scores over time, the comparison of initial and final dichotomized mRS score changes between the two groups may not reflect clinically meaningful changes in function. 41 Therefore, we focused on comparing individual changes in mRS scores between the WG and CG to evaluate the progress of each patient. We analyzed patients’ mRS scores using repeated measurements, using an ANCOVA model adjusted for sex, age and number of rehabilitation sessions to identify significant changes across different stroke severities within and between the two groups. 42
Both groups experienced significant improvements in the primary outcome and all secondary outcomes at D30 and D90. There were also non-significant improvements in BI, FIM and BBS scores at D90 in the CG but not in the WG, which might reflect the significantly lower baseline scores on the three measures of the CG compared with the WG. Scores on the mRS, BI and FIM are highly correlated, although the FIM contains more cognitive domain response categories than the BI. The non-significant differences found in the between-group analysis of BI, FIM, BBS and WMFT-FAS scores within 90 days may indicate similar rehabilitation efficacy in both groups. 43 We suspect that the greater improvement in mRS scores in the WG was a result only of more rehabilitation sessions; 42 therefore, we adjusted for the number of rehabilitation sessions in the ANCOVA model. Furthermore, the higher number of rehabilitation sessions did not necessarily indicate more overall rehabilitation time in the WG. In the early WG implementation stages, the therapists had to explain to participants how the WEAR worked and help them set up the app and devices in 30 minutes, which may have reduced the in-person rehabilitation time compared with the CG. This setup may have increased the efficacy of the WG in the later stages. The present findings indicate that therapists could integrate app rehabilitation using the WEAR system with in-hospital rehabilitation to facilitate functional improvement. The consecutive records of rehabilitation activity in the system could also help multidisciplinary team members monitor and analyze individuals’ performances. 44 In this study, three patients used the WEAR at home, and one performed almost six equivalent sessions. This finding suggests that the WEAR system could increase in-home self-practice rehabilitation, thus overcoming time and location constraints, limitations in medical resources and safety concerns44,45 and enhancing rehabilitation intensity and functional recovery.46,47
Using the technology-assisted system, the quantity and intensity of rehabilitation sessions could be increased beyond in-person therapy to facilitate recovery.10,47 The sensor-driven system, which features real-time and continuous recording, also helps to overcome the difficulty of monitoring performance accuracy and compliance with recommended activity outside the centers and specialized institutes.48,49 Our system could help to bridge the gap between the advised post-stroke rehabilitation intensity and the insufficient rehabilitation therapy resources provided by medical systems. 49 For example, it could permit more inpatient rehabilitation in the wards, assisted by trained nursing staff, and more outpatient activities, with the help of formal and informal caregivers under supervision using remote monitoring and recording. Although the system is not very expensive, further research is needed to perform cost-benefit analyses.
The UTAUT questionnaire was used to explore patients’ perceptions and intention to use the WEAR system. The WG showed significantly higher scores on social influence and behavioral intention. As the questionnaire was administered after randomization, this finding probably reflects the expectation of WG patients that they would use the WEAR system, and the knowledge of CG patients that they would have no opportunity to use it. Using focus group interviews, we investigated the willingness to use the WEAR system among patients, caregivers and rehabilitation center therapists. We found that compared with therapists, patients and caregivers were more willing to accept the assistance of the WEAR system for rehabilitation. The follow-up survey of WG patients showed significantly increased scores on performance expectancy, effort expectancy and facilitating conditions at D30 and D90, which suggests that these are the main factors that affect use intention. The non-significant increase in social influence and behavioral intention scores at D90 may have been a result of higher baseline scores, but it suggests that the willingness to use the system remained constant in the WG. The influence of other factors, such as age, experience and caregiver support should be further investigated.50,51
All stroke care pathways were modified to avoid delayed reperfusion therapy for acute ischemic stroke participants 52 and to ensure the safety of participants, caregivers and multidisciplinary care teams during the COVID-19 pandemic period. 53 Inpatient rehabilitation should be continued as much as possible for appropriate patients. 54 However, outpatient and community rehabilitation services may be limited,45,55 increasing the need for rehabilitation both inside and outside the institute. 53 Although a systematic review did not support the superiority of telerehabilitation, it has been demonstrated to be noninferior to outpatient rehabilitation. 47 Our results suggest that integrating a wearable device-assisted app with conventional rehabilitation could help stroke survivors to continue their advised rehabilitation programs with similar efficacy.
There were several study limitations. The difference in baseline scores between the two groups may have affected the final results and their interpretation. The inclusion of the study patients was based on mRS scores. The mRS is a well-accepted scale with few grades and modest interrater reliability, which may partly explain the lack of difference between the two groups, consistent with NIHSS scores. However, the difference in initial disability and function between the two groups could have been better assessed using more graded and specific measures for the secondary outcomes. We recommend further studies to recruit stroke patients using more finely graded functional scales to allow for more detailed assessment of these differences. The number of app rehabilitation and conventional rehabilitation sessions during our study were in line with local rehabilitation protocols; however, they may not be optimal. 56 The primary outcome results may have been less significant if the CG had received a similar number of rehabilitation sessions. Furthermore, only a few patients used the system and transmitted data to the server outside the hospital. Implementation of this system requires more information technology literacy and additional efforts from physicians, therapists, patients and their caregivers. Fortunately, these factors will likely improve over time with increased penetration of smartphones, smartwatches and more immersive internet environments. Further investigation to expand the application of technology in stroke rehabilitation in the community is warranted.
Conclusion
This study demonstrated that the WEAR system is feasible, safe and may have beneficial effects over conventional therapy on post-stroke rehabilitation. Patients who received both the WEAR and conventional rehabilitation by therapists showed facilitated functional recovery and persistent improvement of stroke. Rehabilitation using combined modalities could increase the duration and frequency of therapy activities, enhance caregiver engagement, and provide timely feedback to patients through longitudinal monitoring by multidisciplinary teams. Further investigation is warranted to explore more applications of the these types of systems and their efficacy in both inpatient and outpatient rehabilitation.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605241281425 for Improving patient outcomes in acute and subacute stroke using a wearable device-assisted rehabilitation system: a randomized controlled trial by Hsin-Ju Ho, Li-Ching Wu, Eric Hsiao-Kung Wu, Shu-Fang Lee, Te-Hsiu Lee, Sheng-Hua Chiang, Chun-Hsiung Chen, Hui-Yu Chen, Shiuan-Jia Pan, Yu-Wei Chen and on behalf of the WEAR-Stroke Study Group in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605241281425 for Improving patient outcomes in acute and subacute stroke using a wearable device-assisted rehabilitation system: a randomized controlled trial by Hsin-Ju Ho, Li-Ching Wu, Eric Hsiao-Kung Wu, Shu-Fang Lee, Te-Hsiu Lee, Sheng-Hua Chiang, Chun-Hsiung Chen, Hui-Yu Chen, Shiuan-Jia Pan, Yu-Wei Chen and on behalf of the WEAR-Stroke Study Group in Journal of International Medical Research
Acknowledgements
The authors would like to express their gratitude to all participants who were committed to this study during this important period. Additionally, the authors are grateful to Mei-Chiun Tseng, Saio-Shan Yen, Ching-Chung Tseng, Ya-Yen Yang, Pei-Yun Cai, Tzung-Ming Tsai, Meng-Ju Wu, Yu-Jui Chang, Shan-Yu Chen, Chih-Chun Chung, and Yu-Chen Hsieh for their generous assistance.
Author contributions: H.J.H. conducted the statistical analyses and collaborated in writing and editing the manuscript. H.K.W. designed the Wearable-Assisted Rehabilitation (WEAR) system. S.F.L. tested the WEAR system. T.H.L., S.H.C., C.H.C., H.Y.C., and S.J.P collaborated in data collection. L.C.W supervised the statistical analyses. Y.W.C. supervised the project, including the study design, data collection, and analysis and interpretation, and edited the manuscript. All authors interpreted the participant data, and reviewed and approved the final manuscript.
The authors declare that there is no conflict of interests.
Funding: This work was partly supported by the “Cross-platform smartphone-enabled stroke prevention and rehabilitation health system” project, by the Ministry of Science and Technology, Taiwan (NSC-101-2811-E-008-013), by collaborative projects of the National Central University with Landseed International Hospital, Taiwan (NCU-LSH-101-A-011, NCU-LSH-102-A-004), and by the Ministry of Health and Welfare, Taiwan (MOHW104-TDU-M-212-112010, MOHW105-TDU-M-212-122008).
ORCID iD: Hsin-Ju Ho https://orcid.org/0000-0001-9755-1746
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental material for this article is available online.
References
- 1.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021; 20: 795–820. 20210903. DOI: 10.1016/s1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao L, Tan E, Kim J, et al. Telemedicine for stroke: quantifying the long-term national costs and health benefits. Front Neurol 2021; 12: 804355. 20220620. DOI: 10.3389/fneur.2021.804355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. 2019/10/31. DOI: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 4.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021; 52: e364–e467. 20210524. DOI: 10.1161/str.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke 2022; 53: e282–e361. 20220517. DOI: 10.1161/str.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 6.Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021; 6: I–LXII. 20210219. DOI: 10.1177/2396987321989865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: e98–e169. 2016/05/06. DOI: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 8.Teasell R, Salbach NM, Foley N, et al. Canadian Stroke best practice recommendations: rehabilitation, recovery, and community participation following stroke. Part one: rehabilitation and recovery following stroke; 6th edition update 2019. Int J Stroke 2020; 15: 763–788. 20200127. DOI: 10.1177/1747493019897843. [DOI] [PubMed] [Google Scholar]
- 9.Royal College of Physicians, Intercollegiate Stroke Working Party. The fifth edition of the National Clinical Guideline for Stroke. 2016. https://www.hse.ie/eng/about/who/cspd/ncps/stroke/resources/2016-national-clinical-guideline-for-stroke-5th-edition.pdf
- 10.Burridge JH, Lee ACW, Turk R, et al. Telehealth, wearable sensors, and the internet: will they improve stroke outcomes through increased intensity of therapy, motivation, and adherence to rehabilitation programs? J Neurol Phys Ther 2017; 41: S32–S38. DOI: 10.1097/NPT.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 11.Administration UFaD. Digital Health Center of Excellence, https://www.fda.gov/medical-devices/digital-health-center-excellence (2024, accessed July 15 2024).
- 12.Jiang X, Ming WK, You JH. The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res 2019; 21: e13166. 20190617. DOI: 10.2196/13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maceira-Elvira P, Popa T, Schmid AC, et al. Wearable technology in stroke rehabilitation: towards improved diagnosis and treatment of upper-limb motor impairment. J Neuroeng Rehabil 2019; 16: 142. 20191119. DOI: 10.1186/s12984-019-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klassen TD, Semrau JA, Dukelow SP, et al. Consumer-based physical activity monitor as a practical way to measure walking intensity during inpatient stroke rehabilitation. Stroke 2017; 48: 2614–2617. 20170807. DOI: 10.1161/strokeaha.117.018175. [DOI] [PubMed] [Google Scholar]
- 15.Gawronska A, Pajor A, Zamyslowska-Szmytke E, et al. Usefulness of mobile devices in the diagnosis and rehabilitation of patients with dizziness and balance disorders: a state of the art review. Clin Interv Aging 2020; 15: 2397–2406. 2020/12/31. DOI: 10.2147/CIA.S289861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang CC, Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors (Basel) 2010; 10: 7772–7788. 20100820. DOI: 10.3390/s100807772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie DR, Bird ML, Menon C, et al. Perspectives on the prospective development of stroke-specific lower extremity wearable monitoring technology: a qualitative focus group study with physical therapists and individuals with stroke. J Neuroeng Rehabil 2020; 17: 31. 20200225. DOI: 10.1186/s12984-020-00666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouédraogo F, Auger LP, Moreau E, et al. Acceptability of telerehabilitation: experiences and perceptions by individuals with stroke and caregivers in an early supported discharge program. Healthcare (Basel) 2024; 12: 365. 20240131. DOI: 10.3390/healthcare12030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollock A, Baer G, Campbell P, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev 2014; 4: CD001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck SSADBM. PNF in Practice-An Illustrated Guide. Third ed.: Springer-Verlag Berlin Heidelberg, 2008. [Google Scholar]
- 21.Ozen S, Senlikci HB, Guzel S, et al. Computer game assisted task specific exercises in the treatment of motor and cognitive function and quality of life in stroke: a randomized control study. J Stroke Cerebrovasc Dis 2021; 30: 105991. 20210719. DOI: 10.1016/j.jstrokecerebrovasdis.2021.105991. [DOI] [PubMed] [Google Scholar]
- 22.Liu CH, Chen YT, Kittikowit S, et al. Using unified theory of acceptance and use of technology to evaluate the impact of a mobile payment app on the shopping intention and usage behavior of middle-aged customers. Front Psychol 2022; 13: 830842. 20220303. DOI: 10.3389/fpsyg.2022.830842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer SC, Le V, Saver JL, et al. Intense arm rehabilitation therapy improves the modified Rankin Scale score: association between gains in impairment and function. Neurology 2021; 96: e1812–e1822. 2021/02/17. DOI: 10.1212/WNL.0000000000011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F, Zhang S, Zhou F, et al. Early physical rehabilitation therapy between 24 and 48 h following acute ischemic stroke onset: a randomized controlled trial. Disabil Rehabil 2022; 44: 3967–3972. DOI: 10.1080/09638288.2021.1897168. [DOI] [PubMed] [Google Scholar]
- 25.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006; 5: 603–612. DOI: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 26.Chumney D, Nollinger K, Shesko K, et al. Ability of Functional Independence Measure to accurately predict functional outcome of stroke-specific population: systematic review. J Rehabil Res Dev 2010; 47: 17–29. DOI: 10.1682/jrrd.2009.08.0140. [DOI] [PubMed] [Google Scholar]
- 27.Park SH, Lee YS. The diagnostic accuracy of the Berg Balance Scale in predicting falls. West J Nurs Res 2017; 39: 1502–1525. 2016/10/28. DOI: 10.1177/0193945916670894. [DOI] [PubMed] [Google Scholar]
- 28.Duff SV, He J, Nelsen MA, et al. Interrater reliability of the Wolf Motor Function Test-Functional Ability Scale: why it matters. Neurorehabil Neural Repair 2015; 29: 436–443. 20141016. DOI: 10.1177/1545968314553030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Berg M, Crotty MP, Liu E, et al. Early supported discharge by caregiver-mediated exercises and e-health support after stroke: a proof-of-concept trial. Stroke 2016; 47: 1885–1892. 2016/06/16. DOI: 10.1161/STROKEAHA.116.013431. [DOI] [PubMed] [Google Scholar]
- 30.Aprile I, Germanotta M, Cruciani A, et al. Upper limb robotic rehabilitation after stroke: a multicenter, randomized clinical trial. J Neurol Phys Ther 2020; 44: 3–14. 2019/12/14. DOI: 10.1097/NPT.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41: 1149–1160. DOI: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 32.Lim JY, Koh JH, Paik NJ. Intramuscular botulinum toxin-A reduces hemiplegic shoulder pain: a randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke 2008; 39: 126–131. 2007/12/01. DOI: 10.1161/STROKEAHA.107.484048. [DOI] [PubMed] [Google Scholar]
- 33.Valiengo LC, Goulart AC, De Oliveira JF, et al. Transcranial direct current stimulation for the treatment of post-stroke depression: results from a randomised, sham-controlled, double-blinded trial. J Neurol Neurosurg Psychiatry 2017; 88: 170–175. 2016/11/07. DOI: 10.1136/jnnp-2016-314075. [DOI] [PubMed] [Google Scholar]
- 34.Correia FD, Nogueira A, Magalhaes I, et al. Home-based rehabilitation with a novel digital biofeedback system versus conventional in-person rehabilitation after total knee replacement: a feasibility study. Sci Rep 2018; 8: 11299. 20180726. DOI: 10.1038/s41598-018-29668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.England TJ, Hedstrom A, O'Sullivan SE, et al. Remote ischemic conditioning after stroke trial 2: a phase IIb randomized controlled trial in hyperacute stroke. J Am Heart Assoc 2019; 8: e013572. 20191121. DOI: 10.1161/JAHA.119.013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeung LF, Lau CCY, Lai CWK, et al. Effects of wearable ankle robotics for stair and over-ground training on sub-acute stroke: a randomized controlled trial. J Neuroeng Rehabil 2021; 18: 19. 2021/01/31. DOI: 10.1186/s12984-021-00814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeung LF, Ockenfeld C, Pang MK, et al. Randomized controlled trial of robot-assisted gait training with dorsiflexion assistance on chronic stroke patients wearing ankle-foot-orthosis. J Neuroeng Rehabil 2018; 15: 51. 2018/06/20. DOI: 10.1186/s12984-018-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra P, Singh U, Pandey CM, et al. Application of student's t-test, analysis of variance, and covariance. Ann Card Anaesth 2019; 22: 407–411. DOI: 10.4103/aca.ACA_94_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Organization WH. International Classification of Functioning, Disability and Health (ICF). World Health Organization, 2001.
- 40.Quinn TJ, Dawson J, Walters MR, et al. Functional outcome measures in contemporary stroke trials. Int J Stroke 2009; 4: 200–205. DOI: 10.1111/j.1747-4949.2009.00271.x. [DOI] [PubMed] [Google Scholar]
- 41.Erler KS, Wu R, DiCarlo JA, et al. Association of modified Rankin Scale with recovery phenotypes in patients with upper extremity weakness after stroke. Neurology 2022; 98: e1877–e1885. 20220311. DOI: 10.1212/wnl.0000000000200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore JL, Nordvik JE, Erichsen A, et al. Implementation of high-intensity stepping training during inpatient stroke rehabilitation improves functional outcomes. Stroke 2020; 51: 563–570. 20191230. DOI: 10.1161/strokeaha.119.027450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pohl J, Held JPO, Verheyden G, et al. Consensus-based core set of outcome measures for clinical motor rehabilitation after stroke-a Delphi study. Front Neurol 2020; 11: 875. 20200902. DOI: 10.3389/fneur.2020.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jekauc D, Rayling S, Klopp S, et al. Effects of a web-based rehabilitation aftercare on subjective health, work ability and motivation: a partially randomized controlled trial. BMC Musculoskelet Disord 2021; 22: 366. 20210419. DOI: 10.1186/s12891-021-04239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venketasubramanian N, Anderson C, Ay H, et al. Stroke care during the COVID-19 pandemic: international expert panel review. Cerebrovasc Dis 2021; 50: 245–261. 2021/03/24. DOI: 10.1159/000514155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klassen TD, Dukelow SP, Bayley MT, et al. Higher doses improve walking recovery during stroke inpatient rehabilitation. Stroke 2020; 51: 2639–2648. 20200819. DOI: 10.1161/strokeaha.120.029245. [DOI] [PubMed] [Google Scholar]
- 47.Cramer SC, Dodakian L, Le V, et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: a randomized clinical trial. JAMA Neurol 2019; 76: 1079–1087. DOI: 10.1001/jamaneurol.2019.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gache K, Leleu H, Nitenberg G, et al. Main barriers to effective implementation of stroke care pathways in France: a qualitative study. BMC Health Serv Res 2014; 14: 95. 2014/03/01. DOI: 10.1186/1472-6963-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keane S, Lincoln M, T S. Retention of allied health professionals in rural New South Wales: a thematic analysis of focus group discussions. BMC Health Serv Res 2012; 12: 175–186. DOI: 10.1186/1472-6963-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan YK, Tang YM, Teng L. A comparative analysis of digital health usage intentions towards the adoption of virtual reality in telerehabilitation. Int J Med Inform 2023; 174: 105042. 20230318. DOI: 10.1016/j.ijmedinf.2023.105042. [DOI] [PubMed] [Google Scholar]
- 51.Wang MY, Chen H, Gong C, et al. Understanding the use intention and influencing factors of telerehabilitation in people with rehabilitation needs: a cross-sectional survey. Front Public Health 2023; 11: 1274080. 20231031. DOI: 10.3389/fpubh.2023.1274080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegler JE, Zha AM, Czap AL, et al. Influence of the COVID-19 Pandemic on Treatment Times for Acute Ischemic Stroke: The Society of Vascular and Interventional Neurology Multicenter Collaboration. Stroke 2021; 52: 40–47. 20201130. DOI: 10.1161/strokeaha.120.032789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leira EC, Russman AN, Biller J, et al. Preserving stroke care during the COVID-19 pandemic: Potential issues and solutions. Neurology 2020; 95: 124–133. 20200508. DOI: 10.1212/wnl.0000000000009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovasc Dis 2020; 29: 104881. 20200417. DOI: 10.1016/j.jstrokecerebrovasdis.2020.104881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prvu Bettger J, Thoumi A, Marquevich V, et al. COVID-19: maintaining essential rehabilitation services across the care continuum. BMJ Glob Health 2020; 5: e002670. DOI: 10.1136/bmjgh-2020-002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernhardt J, Hayward KS. What Is next after this well-conducted, but neutral, multisite trial testing self-rehabilitation approaches? Stroke 2021; 52: 1948–1950. 20210429. DOI: 10.1161/strokeaha.121.034533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605241281425 for Improving patient outcomes in acute and subacute stroke using a wearable device-assisted rehabilitation system: a randomized controlled trial by Hsin-Ju Ho, Li-Ching Wu, Eric Hsiao-Kung Wu, Shu-Fang Lee, Te-Hsiu Lee, Sheng-Hua Chiang, Chun-Hsiung Chen, Hui-Yu Chen, Shiuan-Jia Pan, Yu-Wei Chen and on behalf of the WEAR-Stroke Study Group in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605241281425 for Improving patient outcomes in acute and subacute stroke using a wearable device-assisted rehabilitation system: a randomized controlled trial by Hsin-Ju Ho, Li-Ching Wu, Eric Hsiao-Kung Wu, Shu-Fang Lee, Te-Hsiu Lee, Sheng-Hua Chiang, Chun-Hsiung Chen, Hui-Yu Chen, Shiuan-Jia Pan, Yu-Wei Chen and on behalf of the WEAR-Stroke Study Group in Journal of International Medical Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.