Abstract
Rational patterning and tailoring of graphene relies on the disclosure of suitable reagents for structuring the target functionalities on the 2D‐carbon network. Here, a series of hypervalent iodine compounds, namely, 1‐chloro‐1,2‐benziodoxol‐3(1H)‐one, 1,3‐dihydro‐1‐hydroxy‐3,3‐dimethyl‐1,2‐benziodoxole, and 3,3‐dimethyl‐1‐(trifluoromethyl)‐1,2‐benziodoxole is reported to be extremely efficient for a diversified graphene patterning. The decomposition of these compounds generates highly reactive Cl, OH, and CF3 radicals exclusively in the irradiated areas, which subsequently attach onto the graphene leading to locally controlled chlorination, hydroxylation, and trifluoromethylation, respectively. This is the first realization of a patterned hydroxylation of graphene, and the degrees of functionalization of the patterned chlorination and trifluoromethylation are both unprecedented. The usage of these mild reagents here is reasonably facile compared to the reported methods using hazardous Cl2 or ICl and allows for sophisticated pattern designs with nanoscale precision, promising for arbitrary nanomanipulation of graphene's properties like hydrophilicity and conductivity by the three distinct functionalities (Cl, OH, and CF3). Moreover, the attachment of functional entities to these highly functionalized graphene nanoarchitectures is fully reversible upon thermal annealing, enabling a full writing/storing/reading/erasing control over the chemical information stored within graphene. This work provides an exciting clue for target 2D functionalization and modulation of graphene by using suitable hypervalent iodine compounds.
Keywords: chlorination, graphene patterning, hydroxylation, hypervalent iodine compounds, trifluoromethylation
Diversified nanoscale patterning and tailoring of graphene are demonstrated by using hypervalent iodine compounds in combination with laser writing, realizing the first patterned hydroxylation as well as unprecedented degrees of patterned chlorination and trifluoromethylation of graphene. The graphene patterning is completely reversible upon thermal annealing, enabling full writing/storing/reading/erasing control over 2D chemical information on graphene.

1. Introduction
Covalent 2D patterning of distinct functionalities on the graphene framework is crucial for engineering the multifaceted surface properties of graphene for specific demands in such fields as molecular electronics, energy storage/conversion, and catalysis.[ 1 , 2 , 3 , 4 , 5 , 6 , 7 ] Key to this challenging task is the establishment of efficient functionalization reagents in combination with suitable patterning techniques to direct target addends in a well‐defined order on the 2D carbon lattice. In preceding reports, four main approaches for graphene patterning have been demonstrated, namely, laser/plasma writing,[ 8 , 9 , 10 , 11 ] poly(methyl methacrylate) (PMMA)‐assisted lithography,[ 12 , 13 , 14 , 15 ] force‐accelerated patterning,[ 16 ] and space‐controlling by self‐assembly.[ 17 , 18 , 19 ] Recently, our group has considerably improved the efficiency of graphene patterning by importing a pre‐activation treatment using a K/Na alloy[ 11 , 15 , 20 ] or by the implementation of AgF for the bottom‐side fluorination via our newly developed 2D substrate patterning protocol.[ 21 ] Among these patterning approaches the simple and straightforward laser writing method stands out as it bears a number of clear advantages: I) arbitrary pattern design in large scale, II) easy‐to‐access due to the wide range of available laser technology, III) facile patterning procedure without complicated lithography processes including the required removal of the mask, and IV) capability of the in situ investigation of the functionalization process by using the lasers directly integrated in the commercial Raman setup. However, a fatal flaw of this approach is the lack of suitable photosensitive reagents. To date, only three preceding examples of laser‐induced graphene patterning have been reported, namely, benzoyl peroxides (hazardous),[ 10 , 11 , 22 ] a specific fluoropolymer (CYTOP),[ 8 ] and silver trifluoroacetate.[ 23 ] At the same time, using benzoyl peroxides for the laser writing allow only for the establishment of relatively low degrees of functionalization—located in the low functionalization regime of the Cançado curve[ 24 ]—requiring also rather extended long irradiation periods.[ 10 , 11 , 22 ] Despite the relatively high degree of functionalization provided by the CYTOP polymer and silver trifluoroacetate, their post‐patterning removal remains a severe challenge, which is difficult to overcome when targeting the high standards for real applications of graphene nanoarchitectures. Specifically, the removal of polymer CYTOP requires not only a special stripper but also a very long time,[ 8 ] and the usage of silver trifluoroacetate unavoidably generates in situ conductive silver nanoparticles at the patterned areas, which restricts the applications, for example, in electronics.[ 23 ] Another even more important aspect of graphene patterning is the capability of the grafted addends to modulate the properties of the modified graphene since this is directly correlated with the final applications of these graphene nanostructures. Studies in this direction are still very scarce, and the only existing examples are targeted on either electronic structures alteration[ 8 , 19 , 25 ] or surface potential modulation.[ 12 , 14 ] As such, developing new functionalization reagents that can provide not only a very high degree of functionalization but also versatile property tuning abilities (e.g., hydrophilicity and conductivity) is highly desired.
Herein, we report on a series of hypervalent iodine compounds,[ 26 , 27 ] namely, 1‐chloro‐1,2‐benziodoxol‐3(1H)‐one (ClBO), 1,3‐dihydro‐1‐hydroxy‐3,3‐dimethyl‐1,2‐benziodoxole (HOBO), and 3,3‐dimethyl‐1‐(trifluoromethyl)‐1,2‐benziodoxole (MFBO), for an extremely efficient and reversible 2D patterning of graphene. The I—Cl, I—OH, and I—CF3 bonds in these three precursor compounds are rather weak and therefore their homolysis easily takes place under laser irradiation. The resulting Cl, OH, and CF3 radicals are highly reactive and can subsequently undergo addition reactions onto the inert graphene surface exclusively at the irradiated regions. This leads to the first example of patterned hydroxylation of graphene. In situ studies on the functionalization processes reveal that very high degrees of functionalization for all the three functionalization (up to the high functionalization regime of the Cançado curve[ 24 ] for the patterned chlorination and trifluoromethylation) can be easily achieved through tuning the irradiation time. Three unprecedented graphene nanostructures featuring distinct Cl, OH, and CF3 addends have been fabricated by programing the laser pathway. While the attached Cl, OH, and CF3 units have provided plenty of room for fine‐tuning the properties of graphene (e.g., hydrophilicity and conductivity), the added Cl and OH moieties also allow for a subsequent secondary derivatization to pattern otherwise difficult addends on graphene.[ 21 , 28 ] Besides, the resolution of our patterned functionalization technique can reach the nanometer regime and the 2D functionalizations are fully reversible, enabling a further precise tailoring of the properties of graphene and a full writing/storing/reading/erasing control over chemical information on graphene at the nanometer level.
2. Results and Discussion
The graphene patterning sequence together with the corresponding microscopy images of the sample at each step are shown in Figure 1 . First, an organic layer of the respective hypervalent iodine compound was coated onto a monolayer graphene. Note that the usage of reactive SiO2/Si wafers as the substrate is crucial as it allows for the antaratopic addition mode through the bottom‐side quenching by the supernatant SiO2 layer (Figure 1a) to release the strain energy and achieve a high degree of functionalization.[ 21 , 29 , 30 , 31 ] Subsequently, a green laser (see the Experimental Section for the details) was directed onto the graphene sample to trigger the decomposition of the reagents, which generates highly reactive Cl, OH, and CF3 radicals for ClBO, HOBO, and MFBO, respectively, exclusively at the irradiated regions. It should be noted that although the three compounds have seemingly no absorption at 532 nm (Figure S3, Supporting Information), the graphene itself could serve as the photomediator for the reactions as it has absorption in the visible range.[ 32 ] Addition of these radicals onto the irradiated graphene areas leads to a selective chlorination, hydroxylation, and trifluoromethylation of graphene, respectively. After the writing, the organic layer of these residual reagents on graphene was removed by washing, affording three graphene nanostructures denoted as fG‐Cl, fG‐OH, and fG‐CF3 , respectively.
Figure 1.

a) The antaratopic binding topology of the functionalized graphene sample. b) The hypervalent iodine compounds used for graphene patterning in this work. c) Schematic illustration of the graphene 2D‐patterning process (left column) and the corresponding microscopy images of the graphene sample at each step (right columns). I) A layer of the respective reagent was coated onto a monolayer graphene supported by a SiO2/Si wafer. II) Laser writing with a green laser (λ = 532 nm, see the Experimental Section for the detailed parameters) on predefined regions generated highly reactive radical intermediates (Cl, OH, and CF3 radicals for ClBO, HOBO, and MFBO, respectively) which subsequently added onto the graphene surface. III) The residual reagent layer was removed yielding the corresponding patterned graphene samples denoted as fG‐Cl, fG‐OH, and fG‐CF3 , respectively. Scale bars = 5 µm.
The functionalization processes were monitored with in situ Raman spectroscopy as we also use the laser directly equipped in the Raman instrument for the writing. The Raman spectra along with the corresponding I D/I G profile located on the Cançado curve[ 24 ] as a function of the laser irradiation time are depicted in Figure 2 . Clearly, all three graphene samples coated with the respective hypervalent iodine reagents can undergo a laser‐triggered functionalization and the degree of functionalization depends on the laser irradiation time. For the sample coated with HOBO, a pronounced D‐band has been detected after irradiation for 45 s (Figure 2c,d). The generation of a D‐band is indicative for the bonding transformation from sp2 to sp3 hybridization as a result of the covalent functionalization of graphene by OH radicals. Further increasing the laser irradiation time to 270 s leads to a very high degree of hydroxylation with an I D/I G ratio of 2.8 compared to <0.1 for the pristine graphene. Remarkably, even higher degrees of functionalization are observed in the cases of the chlorination and the trifluoromethylation reaction—both can reach the high functionalization regime of the Cançado curve.[ 24 ] For the sample coated with ClBO, irradiation for 240 s has generated a Raman spectrum with a broad D‐ and G‐band (Figure 2a), corresponding to a very high degree of functionalization located in the high functionalization regime of the Cançado curve.[ 24 ] An increasing degree of functionalization in correlation with the laser irradiation time is also demonstrated by the G‐band upshift due to an increasing implementation of electron‐withdrawing Cl atoms on the graphene lattice. For example, after laser irradiation of 240 s, the G‐band is located at 1598 cm−1, resulting in an upshift of 13 cm−1 compared to that of the pristine graphene (1585 cm−1). To our knowledge, such a high degree of chlorination of graphene realized here (Figure 2a) is unprecedented although the used chlorination reagent ClBO is rather mild, which is much higher than the reported values using hazardous Cl2 or ICl.[ 20 , 28 , 33 ] More significantly, the functionalization of graphene using MFBO is much faster and the degree of functionalization is even higher. For example, a high degree of functionalization located in the high functionalization regime of the Cançado curve[ 24 ] can be realized by irradiation for only 2 s. Increasing the irradiation time to 60 s generates a Raman spectrum with an I D/I G ratio of ≈0.5 and a broad fluorescence signal. This indicates an extraordinary high degree of functionalization of graphene and similar broad fluorescence signals have also been observed for extremely high fluorinated graphene.[ 34 ] Such a high degree of trifluoromethylation of graphene is also unprecedented, which even surpasses our recent case using silver trifluoroacetate.[ 23 ] The trifluoromethylation nature is also verified by the continuously increased upshift of the G‐band correlating with the laser time (Figure 2e) due to the electron‐withdrawing property of the CF3 group. To obtain an overall impression of the extraordinary efficiency of these hypervalent iodine compounds for the diversified graphene patterning, we quantified and compared the degree of functionalization realized in this work and preceding reports. As can be seen from Table S1, the degree of chlorination obtained here is ≈25 times of the values in the two reported cases using ICl or Cl2 [ 33 , 35 ] and the degree of trifluoromethylation by MFBO represents the highest degree of functionalization that has been realized for graphene patterning. Hence, using the three hypervalent iodine compounds here as the reactants for graphene patterning leads not only to the first patterned hydroxylation of graphene but also to unprecedentedly high degrees of functionalization for the patterned chlorination and trifluoromethylation, highlighting the superiority of the developed hypervalent iodine compounds for the diversified graphene patterning.
Figure 2.

a,c,e) Raman spectrum of the monolayer graphene coated with ClBO (a), HOBO (c), and MFBO (e). b,d,f) The corresponding I D/I G profile located on the Cançado curve[ 24 ] for the monolayer graphene coated with ClBO (b), HOBO (d), and MFBO (f). L D: the mean distance between defects. The pink and blue dashed lines indicate the positions of the G‐band (1585 cm−1) and 2D‐band (2676 cm−1) of the starting monolayer graphene, respectively. λexc = 532 nm.
A precise control over the degree of functionalization and reaction areas leads to the patterned functionalization of each sample. Specifically, triangle, quadrangle, and pentagon designs (Figure S1, Supporting Information, for the detailed irradiation profiles) are written on fG‐Cl, fG‐OH, and fG‐CF3 using ClBO, HOBO, and MFBO as the reactant, respectively (Figure 1). The graphene samples at each step can be visualized from the corresponding microscopy images (Figure 1c). For fG‐Cl and fG‐OH, the patterned design can be directly distinguished from the microscopy images after the writing step. Besides, the patterned structures can also be deduced from the image after washing, where the irradiated regions show a higher transparency compared to the unfunctionalized areas. A similar increase in the transparency of graphene, based on the implementation of a high covalent addend loading, has also been reported for extensively fluorinated graphene,[ 36 ] and this is a clear indication that in our case an extraordinary high amount of chlorine and hydroxyl functions have been attached to the graphene sheet in the case of fG‐Cl and fG‐OH, respectively. The case of fG‐CF3 is a little bit different where the pentagon pattern cannot be directly visualized from the respective microscopy images. This could be due to the very high rate of functionalization in the case of fG‐CF3 , even at very low laser powers (see Table S2 for details) which consumes only a trace amount of the reagent so that no obvious morphological changes in the covering film of MFBO could be detected optically (Figure S2, Supporting Information). Besides, we cannot observe similar transparency increase of graphene like in the cases of fG‐Cl and fG‐OH. In this regard, our results indicate visually no transparency change of graphene upon trifluoromethylation despite a very high degree of functionalization realized here. In Figure 3 , the respective Raman mean spectra and the corresponding statistical Raman I D/I G histograms are presented. Clearly, the non‐irradiated area shows—for all three samples—a Raman spectrum resembling that of the pristine graphene with an I D/I G ratio of <0.1, indicative for its unfunctionalized nature. In comparison, in the irradiated areas of fG‐Cl, fG‐OH, and fG‐CF3 all Raman spectra exhibit a very intense D‐band, as indicated by the very high I D/I G ratios of about 2.8, and this can directly be taken as a fact for an extensive covalent attachment of the intermediately formed radicals. This is also corroborated by a remarkable upshift of the G‐band in the cases of fG‐Cl (1596 cm−1) and fG‐CF3 (1592 cm−1) in comparison to pristine graphene (1585 cm−1). Such a p‐doping effect on graphene induced by the covalent attachment of Cl, OH, and CF3 units has been further corroborated by the upshift of 2D‐band (Figures 2 and 3 and Figure S6, Supporting Information).[ 31 , 37 ] Besides, the functionalization at these patterned areas is very homogenous as evidenced by a uniform distribution of the respective I D/I G ratios (Figure 3b).
Figure 3.

a) Mean Raman spectra and b) statistical Raman I D/I G histogram of the respective graphene sample before and after laser irradiation. The pink and blue dashed lines indicate the positions of the G‐band (1585 cm−1) and 2D‐band (2676 cm−1) of the starting monolayer graphene, respectively. λexc = 532 nm.
To deduce the diversified graphene patterning imprinted by these hypervalent iodine compounds, large‐scale Raman mappings have been performed for all three graphene samples. As depicted in Figure 4 , the spatially resolved chemical pattern could be clearly visualized in the corresponding I D/I G map, which is in perfect agreement with our input pattern designs (triangle, quadrangle, and pentagon for fG‐Cl, fG‐OH, and fG‐CF3 , respectively, Figure 1c and Figure S1, Supporting Information). In addition to the complicated pattern design demonstrated here, our patterned functionalization using these hypervalent iodine compounds via laser writing provides a very high resolution down to the nanometer level. As shown in Figure 4d,e, after the writing of parallel lines (perpendicular distances: 1 µm) on graphene using HOBO and the removal of the residual HOBO, the acquired Raman D‐band mapping demonstrates the very high precision of ≈200 nm. As the degree of functionalization and pattern design can be facilely controlled at the nanometer level by tuning the laser time and pathway, any desired graphene nanostructures with spatially defined Cl, OH, or CF3 groups can be easily fabricated with nanoscale precision. Considering the property‐tuning ability of Cl, OH, and CF3 (e.g., hydrophilicity and conductivity) as well as the potential derivatization of Cl and OH groups,[ 21 , 28 ] the possibilities are numerous and very exciting.
Figure 4.
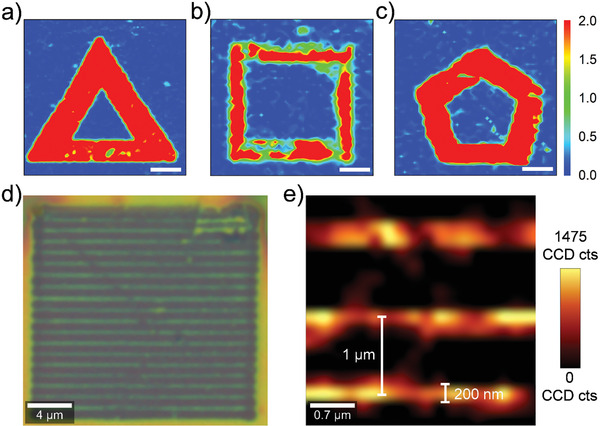
a–c) Raman I D/I G mapping of fG‐Cl (a), fG‐OH (b), and fG‐CF3 (c). Scale bars = 5 µm. d) Optical image showing parallel lines written on graphene using HOBO and e) Raman D‐band map of the sample after the removal of HOBO. λexc = 532 nm.
The highly efficient patterned functionalization, together with the precise modulation of the surface properties of graphene, provided by these hypervalent iodine compounds, were further corroborated by Kelvin probe force microscopy (KPFM) and scanning electron microscopy coupled with energy‐dispersive X‐ray spectroscopy (SEM‐EDS). In principle, successful patterned chlorination, hydroxylation, and trifluoromethylation should lead not only to the covalent attachment of the respective addends onto the patterned regions but also to a change of surface properties of the corresponding graphene areas, owing to the strong electron‐withdrawing abilities of Cl, OH, and CF3 addends. As expected, the elemental distribution of Cl in fG‐Cl (Figure 5e) matches very well with the pattern designs (Figure 1 and Figure S1, Supporting Information) and the Raman I D/I G mappings (Figure 4a). In addition, the surface electrostatic potentials of the patterned areas in fG‐Cl, fG‐OH, and fG‐CF3 are higher than the undisturbed areas by significant differences of 30, 80, and 110 mV, respectively (Figure 5f), representing even more powerful capabilities for the surface potential modulation of graphene in comparison to the two reported cases using diazonium (≈74 mV)[ 12 ] and Diels−Alder (≈100 mV)[ 14 ] reactions. The distinct differences of surface potentials give rise to clear images of these chemical patterns (Figure 5a–c for fG‐Cl, fG‐OH, and fG‐CF3 , respectively), which is in perfect agreement with our input pattern designs (Figure 1 and Figure S1, Supporting Information) as well as the respective Raman I D/I G mappings (Figure 4). In parallel with the accurate pattern design, the nanoscale precision of ≈200 nm realized in this work was further demonstrated by the KPFM characterization by showing a sharp pattern edge in the surface potential line profiles (Figure 5f). Therefore, these results unambiguously demonstrate the diversified and extremely efficient capabilities provided by these hypervalent iodine compounds for not only patterned functionalization but also surface property modulation of graphene.
Figure 5.

a–c) KPFM images of fG‐Cl (a), fG‐OH (b), and fG‐CF3 (c). d) SEM image and e) elemental distribution of Cl of fG‐Cl. f) The corresponding surface potential profiles of fG‐Cl, fG‐OH, and fG‐CF3 . Scale bars = 5 µm.
To verify the reversibility and thermal stability of the graphene nanoarchitectures fG‐Cl, fG‐OH, and fG‐CF3 , temperature‐dependent Raman studies were carried out. As illustrated in Figure 6 , the increase of the temperature leads to the decline of the intensity of the D‐band for all three samples. This is directly correlated with a reversible sp3–sp2 rehybridization of lattice carbon atoms accompanied by the detachment of the covalently linked addends. The defuctionalization processes occur mainly at 200–350 °C and finish upon increasing the temperature to 450 °C. This complete reversibility of our graphene nanoarchitectures provides the opportunity to fine‐tune the attached entities and the degrees of functionalization by thermal annealing. Considering the direct relationship of the attached functionalities with the properties of graphene (e.g., hydrophilic and electronic properties), this simple thermal treatment could serve as another important approach for the precise property manipulation of graphene nanosystems, in addition to the control over the laser time/pathway as demonstrated above.
Figure 6.

Temperature‐dependent Raman spectra of a) fG‐Cl, b) fG‐OH, and c) fG‐CF3 . d) Mean Raman I D/I G ratios extracted from the respective temperature‐dependent Raman spectra. λexc = 532 nm.
To demonstrate the controllability of the overall chemical pattern by thermal annealing, the three graphene samples were annealed at two specific temperatures: 250 and 450 °C. As depicted in Figure 7a–c, chemical patterns can still be recognized after annealing at 250 °C (Figure 4) and the overall degrees of functionalization were adjusted to lower values with I D/I G ratios of ≈0.7–1.0, in comparison to the values prior to the thermal treatment (I D/I G ratios of ≈2.8). A subsequent rise of the temperature to 450 °C results in the complete defunctionalization and removal of the chemical patterns for all three graphene samples, as can be observed in Figure 7d–f. Therefore, with our laser writing, Raman mapping, and thermal treatment sequences, we can enable a full writing/storing/reading/erasing control over the chemical information on graphene.
Figure 7.

a–f) Raman I D/I G mapping of fG‐Cl (a), fG‐OH (b), and fG‐CF3 (c) after thermal annealing at 250 °C, and of fG‐Cl (d), fG‐OH (e), and fG‐CF3 (f) after thermal annealing at 450 °C. λexc = 532 nm. Scale bars = 5 µm.
3. Conclusion
A series of hypervalent iodine compounds, namely, 1‐chloro‐1,2‐benziodoxol‐3(1H)‐one, 1,3‐dihydro‐1‐hydroxy‐3,3‐dimethyl‐1,2‐benziodoxole, and 3,3‐dimethyl‐1‐(trifluoromethyl)‐1,2‐benziodoxole, has been demonstrated to be extremely efficient for the covalent 2D patterning of graphene. This uses the rather weak I—Cl, I—OH, and I—CF3 bonds in the three precursor hypervalent iodine compounds and their consequent homolysis under light irradiation. The afforded Cl, OH, and CF3 radicals are highly reactive, which subsequently undergo addition reaction onto graphene exclusively at the irradiated regions. The degree of functionalization and the design of the patterns with nanoscale precision can be facilely manipulated by tuning the laser irradiation time and pathway, leading to the first patterned hydroxylation of graphene and unprecedentedly high degrees of patterned chlorination and trifluoromethylation of graphene. The success of the laser‐based attachment of functional entities on graphene and the generation of the respective nanoarchitectures was unequivocally demonstrated by Raman, KPFM, and SEM‐EDS measurements. Moreover, the patterned binding of functional moieties is completely reversible upon thermal treatment, enabling a full writing/storing/reading/erasing control over chemical information on graphene. In terms of the strong hydrophilicity of OH unit and hydrophobicity of Cl and CF3 groups, manipulation of the hydrophilicity of the graphene architectures at the nanometer level would be simple and straightforward by our methods reported here, of which many exciting applications like in biology (e.g., cell micropatterning[ 38 , 39 ]) could be envisaged. Moreover, considering the wide variety of hypervalent iodine compounds, and the subsequent secondary derivatization of the attached Cl and OH moieties, our results provide numerous opportunities for arbitrary patterning and property tailoring (e.g., hydrophilicity and conductivity) of graphene at the nanometer level.
4. Experimental Section
Patterned Chlorination of Graphene
ClBO was dip‐coated onto a graphene monolayer supported on a SiO2/Si wafer by immersing the graphene sample in a 0.035 mmol mL−1 acetone/toluene (v/v = 7/5) solution of ClBO for 1 min and subsequent lifting. Immediately after the lifting, a blow‐dry treatment with argon was applied for assisting the film formation. Afterward, the graphene sample with a layer of ClBO was subjected to the laser writing experiments. fG‐Cl with a triangle pattern was prepared by laser writing using a green laser (532 nm, 1.5 mW, 100× objective, 200 s, 0.5 µm step size) and subsequent washing with acetone as well as drying with argon.
Patterned Hydroxylation of Graphene
HOBO was spin‐coated onto a graphene monolayer supported on a SiO2/Si wafer (0.20 mmol mL− 1 acetone solution of HOBO, 4000 rpm, 1 min). Afterward, the graphene sample with a layer of HOBO was subjected to the laser writing experiments. fG‐OH with a quadrangle pattern was prepared by laser writing using a green laser (532 nm, 8 mW, 100× objective, 270 s, 0.5 µm step size) and subsequent washing with acetone as well as drying with argon.
Patterned Trifluoromethylation of Graphene
MFBO was dip‐coated onto a graphene monolayer supported on a SiO2/Si wafer by immersing the graphene sample in a 0.27 mmol mL−1 dichloromethane solution of MFBO for 1 s and subsequent lifting. Immediately after the lifting, a blow‐dry treatment with argon was applied for assisting the film formation. Afterward, the graphene sample with a layer of MFBO was subjected to the laser writing experiments. fG‐CF3 with a pentagon pattern was prepared by laser writing using a green laser (532 nm, 0.25 mW, 100× objective, 2 s, 0.5 µm step size) and subsequent washing with dichloromethane as well as drying with argon.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (Project ID: 182849149 – SFB 953). B.Z. is grateful for the financial support from the China Scholarship Council (CSC) (201706060215).
Open access funding enabled and organized by Projekt DEAL.
Bao L., Zhao B., Yang B., Halik M., Hauke F., Hirsch A., Hypervalent Iodine Compounds as Versatile Reagents for Extremely Efficient and Reversible Patterning of Graphene with Nanoscale Precision. Adv. Mater. 2021, 33, 2101653. 10.1002/adma.202101653
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Bottari G., Herranz M. Á., Wibmer L., Volland M., Rodríguez‐Pérez L., Guldi D. M., Hirsch A., Martín N., D'Souza F., Torres T., Chem. Soc. Rev. 2017, 46, 4464. [DOI] [PubMed] [Google Scholar]
- 2. Zhu Y., Murali S., Cai W., Li X., Suk J. W., Potts J. R., Ruoff R. S., Adv. Mater. 2010, 22, 3906. [DOI] [PubMed] [Google Scholar]
- 3. Chua C. K., Pumera M., Chem. Soc. Rev. 2013, 42, 3222. [DOI] [PubMed] [Google Scholar]
- 4. Georgakilas V., Otyepka M., Bourlinos A. B., Chandra V., Kim N., Kemp K. C., Hobza P., Zboril R., Kim K. S., Chem. Rev. 2012, 112, 6156. [DOI] [PubMed] [Google Scholar]
- 5. Yang Y., Han C., Jiang B., Iocozzia J., He C., Shi D., Jiang T., Lin Z., Mater. Sci. Eng., R 2016, 102, 1. [Google Scholar]
- 6. He M., Jung J., Qiu F., Lin Z., J. Mater. Chem. 2012, 22, 24254. [Google Scholar]
- 7. Kang S. H., Hwang W. S., Lin Z., Kwon S. H., Hong S. W., Nano Lett. 2015, 15, 7913. [DOI] [PubMed] [Google Scholar]
- 8. Lee W. H., Suk J. W., Chou H., Lee J., Hao Y., Wu Y., Piner R., Akinwande D., Kim K. S., Ruoff R. S., Nano Lett. 2012, 12, 2374. [DOI] [PubMed] [Google Scholar]
- 9. Ye D., Wu S.‐Q., Yu Y., Liu L., Lu X.‐P., Wu Y., Appl. Phys. Lett. 2014, 104, 103105. [Google Scholar]
- 10. Edelthalhammer K. F., Dasler D., Jurkiewicz L., Nagel T., Al‐Fogra S., Hauke F., Hirsch A., Angew. Chem., Int. Ed. 2020, 59, 23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bao L., Kohring M., Weber H. B., Hauke F., Hirsch A., J. Am. Chem. Soc. 2020, 142, 16016. [DOI] [PubMed] [Google Scholar]
- 12. Koehler F. M., Luechinger N. A., Ziegler D., Athanassiou E. K., Grass R. N., Rossi A., Hierold C., Stemmer A., Stark W. J., Angew. Chem., Int. Ed. 2009, 48, 224. [DOI] [PubMed] [Google Scholar]
- 13. Sun Z., Pint C. L., Marcano D. C., Zhang C., Yao J., Ruan G., Yan Z., Zhu Y., Hauge R. H., Tour J. M., Nat. Commun. 2011, 2, 559. [DOI] [PubMed] [Google Scholar]
- 14. Li J., Li M., Zhou L.‐L., Lang S.‐Y., Lu H.‐Y., Wang D., Chen C.‐F., Wan L.‐J., J. Am. Chem. Soc. 2016, 138, 7448. [DOI] [PubMed] [Google Scholar]
- 15. Wei T., Kohring M., Chen M., Yang S., Weber H. B., Hauke F., Hirsch A., Angew. Chem., Int. Ed. 2020, 59, 5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bian S., Scott A. M., Cao Y., Liang Y., Osuna S., Houk K. N., Braunschweig A. B., J. Am. Chem. Soc. 2013, 135, 9240. [DOI] [PubMed] [Google Scholar]
- 17. Xia Z., Leonardi F., Gobbi M., Liu Y., Bellani V., Liscio A., Kovtun A., Li R., Feng X., Orgiu E., Samorì P., Treossi E., Palermo V., ACS Nano 2016, 10, 7125. [DOI] [PubMed] [Google Scholar]
- 18. Tahara K., Ishikawa T., Hirsch B. E., Kubo Y., Brown A., Eyley S., Daukiya L., Thielemans W., Li Z., Walke P., Hirose S., Hashimoto S., De Feyter S., Tobe Y., ACS Nano 2018, 12, 11520. [DOI] [PubMed] [Google Scholar]
- 19. Yu M., Chen C., Liu Q., Mattioli C., Sang H., Shi G., Huang W., Shen K., Li Z., Ding P., Guan P., Wang S., Sun Y., Hu J., Gourdon A., Kantorovich L., Besenbacher F., Chen M., Song F., Rosei F., Nat. Chem. 2020, 12, 1035. [DOI] [PubMed] [Google Scholar]
- 20. Wei T., Kohring M., Weber H. B., Hauke F., Hirsch A., Nat. Commun. 2021, 12, 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bao L., Zhao B., Lloret V., Halik M., Hauke F., Hirsch A., Angew. Chem., Int. Ed. 2020, 59, 6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu H., Ryu S., Chen Z., Steigerwald M. L., Nuckolls C., Brus L. E., J. Am. Chem. Soc. 2009, 131, 17099. [DOI] [PubMed] [Google Scholar]
- 23. Wei T., Al‐Fogra S., Hauke F., Hirsch A., J. Am. Chem. Soc. 2020, 142, 21926. [DOI] [PubMed] [Google Scholar]
- 24. Cançado L. G., Jorio A., Ferreira E. H. M., Stavale F., Achete C. A., Capaz R. B., Moutinho M. V. O., Lombardo A., Kulmala T. S., Ferrari A. C., Nano Lett. 2011, 11, 3190. [DOI] [PubMed] [Google Scholar]
- 25. Sessi P., Guest J. R., Bode M., Guisinger N. P., Nano Lett. 2009, 9, 4343. [DOI] [PubMed] [Google Scholar]
- 26. Matoušek V., Pietrasiak E., Schwenk R., Togni A., J. Org. Chem. 2013, 78, 6763. [DOI] [PubMed] [Google Scholar]
- 27. Zhou L., Zhou L., Wang X., Yu J., Yang M., Wang J., Peng H., Liu Z., APL Mater. 2014, 2, 092505. [Google Scholar]
- 28. Sun X., Li B., Lu M., J. Solid State Chem. 2017, 251, 194. [Google Scholar]
- 29. Amsharov K., Sharapa D. I., Vasilyev O. A., Oliver M., Hauke F., Goerling A., Soni H., Hirsch A., Carbon 2020, 158, 435. [Google Scholar]
- 30. Knirsch K. C., Schäfer R. A., Hauke F., Hirsch A., Angew. Chem., Int. Ed. 2016, 55, 5861. [DOI] [PubMed] [Google Scholar]
- 31. Wang Q. H., Jin Z., Kim K. K., Hilmer A. J., Paulus G. L. C., Shih C.‐J., Ham M.‐H., Sanchez‐Yamagishi J. D., Watanabe K., Taniguchi T., Kong J., Jarillo‐Herrero P., Strano M. S., Nat. Chem. 2012, 4, 724. [DOI] [PubMed] [Google Scholar]
- 32. Sun Z., Yan Z., Yao J., Beitler E., Zhu Y., Tour J. M., Nature 2010, 468, 549. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L., Yu J., Yang M., Xie Q., Peng H., Liu Z., Nat. Commun. 2013, 4, 1443. [DOI] [PubMed] [Google Scholar]
- 34. Mazánek V., Jankovský O., Luxa J., Sedmidubský D., Janoušek Z., Šembera F., Mikulics M., Sofer Z., Nanoscale 2015, 7, 13646. [DOI] [PubMed] [Google Scholar]
- 35. Balch A. L., Catalano V. J., Lee J. W., Olmstead M. M., J. Am. Chem. Soc. 1992, 114, 5455. [Google Scholar]
- 36. Feng W., Long P., Feng Y., Li Y., Adv. Sci. 2016, 3, 1500413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Das A., Pisana S., Chakraborty B., Piscanec S., Saha S. K., Waghmare U. V., Novoselov K. S., Krishnamurthy H. R., Geim A. K., Ferrari A. C., Sood A. K., Nat. Nanotechnol. 2008, 3, 210. [DOI] [PubMed] [Google Scholar]
- 38. Oh H. H., Ko Y.‐G., Lu H., Kawazoe N., Chen G., Adv. Mater. 2012, 24, 4311. [DOI] [PubMed] [Google Scholar]
- 39. Wang X., Hu X., Kawazoe N., Yang Y., Chen G., Adv. Funct. Mater. 2016, 26, 7634. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


