Abstract
Foreign glycoproteins expressed in recombinant vesicular stomatitis virus (VSV) can elicit specific and protective immunity in the mouse model. We have previously demonstrated the expression of respiratory syncytial virus (RSV) G (attachment) and F (fusion) glycoprotein genes in recombinant VSV. In this study, we demonstrate the expression of RSV F and G glycoproteins in attenuated, nonpropagating VSVs which lack the VSV G gene (VSVΔG) and the incorporation of these RSV proteins into recombinant virions. We also show that intranasal vaccination of mice with nondefective VSV recombinants expressing RSV G (VSV-RSV G) or RSV F (VSV-RSV F) elicited RSV-specific antibodies in serum (by enzyme-linked immunosorbent assay [ELISA]) as well as neutralizing antibodies to RSV and afford complete protection against RSV challenge. In contrast, VSVΔG-RSV F induced detectable serum antibodies to RSV by ELISA, but no detectable neutralizing antibodies, yet it still protected from RSV challenge. VSVΔG-RSV G failed to induce any detectable serum (by ELISA) or neutralizing antibodies and failed to protect from RSV challenge. The attenuated, nonpropagating VSVΔG-RSV F is a particularly attractive candidate for a live attenuated recombinant RSV vaccine.
Respiratory syncytial virus (RSV) is the major respiratory pathogen of infants and children and a major cause of morbidity and mortality worldwide (15). The World Health Organization ranks respiratory diseases as the leading cause of death in children, and RSV is responsible for a significant proportion of this mortality (3). Each year in the United States, approximately 100,000 infants and children are hospitalized for RSV-related disease, and the rates have significantly increased over the last 2 decades (47). RSV is a ubiquitous pathogen. Seasonal epidemics occur each year (during the winter months in the northern hemisphere), and nearly every child is infected by the age of 2 years (22). RSV is recognized as a major pathogen of adults, particularly in the immunocompromised and elderly populations (12, 21). Individuals with lung disease, such as cystic fibrosis, are particular prone to RSV disease (1, 53). The risk factors for severe RSV disease are well described. Infants and children with a history of prematurity, lung disease, congenital heart disease, or immunodeficiency are at risk for severe RSV disease (17, 20, 36) though even otherwise normal infants are prone to severe infection.
The prevention of RSV disease remains a significant challenge for the medical and scientific communities. There is no vaccine currently available to protect against RSV infection. A previous formalin-inactivated vaccine candidate in the 1960s induced severe disease upon subsequent natural infection with RSV (29, 32). This experience has hampered the development and assessment of RSV vaccine candidates. During the past several years, various promising live attenuated RSV vaccine candidates have been tested in the human population. Two live, cold-passaged, temperature-sensitive subgroup A viruses were immunogenic and phenotypically stable in seronegative children. However, these two potential vaccine candidates were underattenuated and caused symptomatic disease in children (31). A cold-passaged, attenuated subgroup B virus was over attenuated in human subjects. Interestingly, this mutant contained a deletion of the SH and G genes which did not limit its growth in cell culture (30). A live attenuated RSV vaccine candidate recently reported induced nasal congestion, fussiness, and anorexia in young infants, negating its potential as a vaccine in this age group (57). The immunogenicity of subunit vaccines has been demonstrated in specific human populations; however, the efficacy of this approach has not yet been demonstrated in seronegative infants (5, 13, 18, 37, 39, 51).
The RSV virion envelope contains three viral encoded glycoproteins (8). RSV G (attachment) and F (fusion), the major antigenic glycoproteins, are responsible for viral attachment and penetration. The function of the third glycoprotein, SH, is unknown. RSV G and/or F are target antigens for vaccine development because antibodies directed against either G or F can neutralize RSV infectivity in animal models (49, 56). Passive transfer of high titers of human RSV-neutralizing antibody can protect experimental animals against RSV disease (40). Furthermore, infants that acquire RSV-specific antibody transplacentally are less prone to severe RSV infection (16, 24, 33). Both a high titer of RSV immunoglobulin and a humanized monoclonal antibody specific for RSV F reduces the severity of disease in infants with an underlying risk factor for RSV disease (4, 19). Although passive immunization is not a practical means of protecting large populations against RSV disease, these findings suggest that vaccines based on the RSV G and/or F glycoprotein may elicit protective immunity. Subunit vaccines based on purified RSV F glycoprotein elicit RSV-specific antibodies in seropositive individuals (5, 13, 18, 37, 39, 51).
Recombinant vesicular stomatitis virus (VSV) expressing foreign genes has shown tremendous potential as a vaccine vector. VSV, the prototypic member of the Rhabdoviridae family, is a nonsegmented negative-strand RNA virus encoding five structural proteins. Attenuated VSV recombinants expressing influenza hemagglutinin protect mice from influenza challenge (41, 42) and produce neutralizing titers to influenza virus in monkeys (unpublished data). A VSV recombinant expressing measles virus hemagglutinin induced neutralizing antibodies and afforded protection against subsequent measles virus infection (45). Recombinant VSV expressing human immunodeficiency virus (HIV) envelope protein induced specific antibodies, and this response was increased by subsequent boost immunizations with recombinant VSV exchange vectors which contain serologically distinct VSV G glycoproteins (43).
We have previously demonstrated the expression of RSV envelope glycoproteins in recombinant VSV (28). The two major antigenic glycoproteins, RSV G and F, were expressed in cells infected with recombinant viruses, and the proteins were incorporated into virions. In this study, we demonstrate the biochemical features of attenuated, nonpropagating VSVs expressing RSV glycoproteins G and F. These nonpropagating viruses (ΔG) lack the VSV G gene, which is essential for infectivity, and are propagated on BHK cells that supply the VSV G glycoprotein in trans. Recombinant and nonpropagating viruses were used to inoculate experimental animals. In this report, we also describe the immune response to RSV elicited by preimmunization with recombinant and replication defective VSVs expressing RSV antigens and the protection afforded by these viruses against RSV challenge.
MATERIALS AND METHODS
Cells and viruses.
Baby hamster kidney cells (BHK-21; American Type Tissue Collection) and human larynx carcinoma cells (HEp-2; American Type Tissue Collection) were maintained in Dulbecco's minimal essential media (DMEM) supplemented with 5 or 10% fetal bovine serum (FBS). Growth of BHK cells expressing VSV G (BHK-G) under inducible tetracycline control and propagation of VSVΔG viruses has been described previously (46). Working stocks of VSV and wild-type VSV recombinants has been described previously (28). Viral titers of frozen stocks were determined on BHK cells (VSV and wild-type VSV recombinants) or BHK-G cells (VSVΔG recombinants) by using standard plaque assays. RSV A2 (supplied by Peter Collins, National Institutes of Health) was propagated, and titers on HEp-2 cells were determined. Preparation of purified RSV was performed using a modification of techniques described elsewhere (48). Titers of frozen RSV stocks were determined by standard plaque assays.
Construction of VSVΔG plasmids.
Plasmids used to recover the VSVΔG-RSV G and VSVΔG-RSV F were derived from full-length VSV clones containing the RSV genes (28). The original full-length VSV genomic plasmids contained RSV G between the VSV M and G genes and RSV F between the VSV G and L genes. VSV G was deleted using appropriate restriction enzymes which removed the VSV G along with its transcriptional start and stop signals. In the resulting VSVΔG plasmids, the RSV glycoprotein genes replace the VSV G gene.
Transfection and recovery of VSVΔG viruses expressing RSV glycoproteins.
Recovery of VSVΔG viruses has been described previously (46). Briefly, the recovery system is based upon synthesis of antigenomic RNA along with expression of the VSV N, P, L, and G genes. VSV N, P, L, and G genes (contained on separate plasmids) and the antigenomic sequences are under bacteriophage T7 control. The plasmids were transfected into BHK cells infected with vaccinia virus expressing T7 polymerase. The antigenomic RNA in a complex with the N protein serves as template for the synthesis of negative strand genome by the VSV L-P polymerase complex. Because these genomic plasmids lack VSV G, a plasmid encoding this protein was added to the transfection. Two days following transfection, supernatants were collected and filtered (0.2 μm) to remove the vaccinia virus. Expression of RSV glycoproteins was confirmed by immunofluorescence. Recombinant virus was plaque purified and propagated on BHK G cells.
Immunofluorescence microscopy.
Infected cell monolayers were fixed with a 3% paraformaldehyde solution. Monoclonal antibodies I1 and I14 were used to bind VSV G (35). Human anti-RSV antiserum (Massachusetts Public Health Biological Laboratories, Boston, Mass.) was used to bind RSV G and F glycoproteins. Bound antibodies were detected using either anti-mouse or anti-human antibody conjugated to Texas Red fluorophore (Jackson ImmunoResearch Laboratories, West Grove, Pa.). Fixed cells were visualized and photographed with a Nikon Microphot-FX microscope equipped with a 40× planapochromat objective.
Western blot analysis.
Cell lysates and purified virus were prepared as described elsewhere (28). Proteins were separated by electrophoresis through a 10% polyacrylamide gel. Following electrophoresis, proteins were transferred to a nitrocellulose filter as described elsewhere (44). The filters were dried, blocked in 5% blotto (Carnation nonfat milk in phosphate-buffered saline [PBS]) and incubated with a primary goat anti-RSV antibody (Virostat, Portland, Maine) diluted 1:250 in blotto. Following several washes, the filters were incubated with a horseradish peroxidase-conjugated donkey anti-goat antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.) diluted 1:10,000 in 150 mM NaCl, 50 mM Tris-HCl, pH 7.6. Binding of the secondary antibody was detected using an ECL Western blotting system (Amersham Pharmacia Biotech Inc., Piscataway, N.J.).
Inoculation of mice.
Four-week-old female BALB/c mice (Charles River Laboratories) were housed in filtered isolette cages for the duration of the experiments. Several days after arrival, mice were inoculated with virus. Animals were anesthetized with Metofane (methoxyflurane, Mallinckrodt Veterinary, Mundelein, Ill). Mice (five animals per group) were inoculated intranasally with 104 PFU (wild-type recombinant VSVs, VSVΔG, and VSVΔG-RSV G) or 1.25 × 103 PFU (VSVΔG-RSV F) contained within 25 μl. The VSVΔG-RSV F could not be grown to titers equivalent to those of the other VSVΔG viruses and, therefore, a lower inoculum was used. Boost immunizations were given at 4-week intervals. Mice immunized with VSV and wild-type recombinant viruses received one boost immunization while mice receiving VSVΔG recombinants received two boost immunizations. Four weeks following the last boost immunization, mice were challenged with 1.2 × 105 PFU of RSV (50 μl).
Neutralization assays.
Blood samples were collected from anesthetized animals by retro-orbital bleed and were allowed to clot at room temperature. Following removal of the clots, sample were centrifuged (TOMY MYX-150 centrifuge with a TMA-11 fixed angle rotor), and clarified sera was collected. Pooled sera from each group was heat inactivated at 56°C for 45 to 60 min. Twofold dilutions of sera were prepared in 96-well plates. An equal volume of RSV (∼150 PFU) was added to each well and the plates were incubated at 37°C for 1 h. A serum-free control well was used for each titration. Subsequently, 5,000 HEp-2 cells were added to each well and the plated were incubated at 37°C, 5% CO2, for several days. Neutralization titer was defined as the last dilution which completely inhibited RSV-induced cytopathic effects (CPE).
Enzyme-linked immunosorbent assay (ELISA).
RSV-infected HEp-2 cell lysates were used as antigen and prepared using a modification of techniques described elsewhere (14). Briefly, HEp-2 cell monolayers were infected with RSV with a multiplicity of infection (MOI) of 5. Monolayers were scraped when ∼75% of the cells demonstrated CPE. Cells were centrifuged and resuspended in 0.5% NP-40 and incubated on ice for 10 min. Cell lysate suspensions were centrifuged (Eppendorf microfuge) for 1 min at 4°C to pellet cell nuclei. Supernatants were collected and stored at −20°C.
ELISAs were performed using modification of techniques described by Anderson et al. (2) and Furze et al. (14). Briefly, 96-well plates (Costar EIA/RIA 96-well plates; Corning Incorporated, Corning, N.Y.) were coated with RSV-infected cell lysates and incubated overnight at 37°C. Lysate solutions were removed and plates were blocked with PBS containing 0.5% gelatin and 0.05% Tween 20 for 1 h at room temperature. Wells were washed with PBS containing 0.05% Tween (PT). Mouse sera and control antibody, diluted in PBS containing 0.5% gelatin, 0.05% Tween 20, and 2% FBS (PGTF), were added to the wells and allowed to react for 1 h at 37°C. Following five washes with PT solution, horseradish peroxidase-conjugated goat anti-mouse (Jackson ImmunoResearch Laboratories) diluted in PGTF (1:10,000) was added to each well, and plates were incubated for 1 h at 37°C. After five washes with PT solution, bound secondary antibody was detected with 3,3′,5,5′-tetramethyl benzidine (TMB; Pierce, Rockford, Ill.). Chromogenic reactions were stopped with 5 N H3PO4. Optical densities (OD) were read at 450 nm (DynaTech MR5000).
Recovery of RSV from infected animals.
Four days after RSV challenge, mice were sacrificed (CO2 asphyxiation). Bronchial alveolar lavage was performed as follows. The trachea was dissected and canulated with a 0.58-mm polyethylene tubing (Becton Dickinson) and secured with suture. One milliliter of sterile PBS was infused and aspirated four times. BAL fluid was diluted 1:10 in viral freezing media (DMEM, 5% FBS, 100 mM MgSO4, 50 mM HEPES, pH 7.5) and snap frozen in liquid N2. Viral titrations of thawed specimens were performed on HEp-2 cells. Following lavage, the left heart was perfused with PBS to remove blood from the pulmonary vasculature. Lungs were excised bilaterally, rinsed in PBS, placed in a preweighed Nunc cryotube vial containing viral freezing media, and snap frozen in liquid N2. To recover virus from lung tissue, specimens were thawed, suspended in 9 volumes (wt/vol) of viral freezing media, and homogenized in Dounce homogenizers. RSV titers were determined by a standard serial dilution plaque assay on Vero cells. Plaque assays were performed in duplicate with a 1% methylcellulose, DMEM, 5% FBS overlay. Cell monolayers were stained with 1% crystal violet (in 0.85% NaCl, 40% formaldehyde) to visualize plaques.
Histopathology.
Following tracheal canulation, lungs of sacrificed animals were inflated with 10% neutral buffered formalin. Specimens were fixed overnight and subsequently embedded in paraffin, sectioned and stained with hemoxylin and eosin. Photomicroscopy was performed with an Nikon Optiphot microscope using a Kontron Elektronik Prog/Res/3012 digital camera. Objective magnification was 20×.
RESULTS
We have previously described the cloning and expression of RSV envelope glycoproteins G (attachment) and F (fusion) in recombinant VSV (28). Cells infected with recombinant VSV expressing RSV G (VSV-RSV G) or RSV F (VSV-RSV F) displayed the RSV glycoproteins on their surface. Both RSV glycoproteins were incorporated into progeny virions. The RSV F glycoprotein expressed by recombinant virus was biologically active. The VSV-RSV F recombinant induced syncytium formation and was able to infect through a pH-independent pathway. In the present study, expression of RSV G and RSV F in nonpropagating VSV is described. The capacity of both wild-type and nonpropagating virus to induce immunity to and protect experimental animals from RSV is also described.
Expression of RSV glycoproteins in recombinant VSV lacking VSV G gene.
Our laboratory has previously described the recovery of nonpropagating VSV lacking the VSV G gene (VSVΔG) (46). The VSV G gene was deleted from full-length cDNA VSV genomic plasmids containing the RSV G or RSV F gene such that the RSV genes replaced VSV G in the viral genome (Fig. 1). These plasmids were derived from VSV genomic clones previously described which contained the RSV G gene upstream of the VSV G gene or RSV F downstream of the VSV G gene (28). Recovery of VSVΔG viruses requires transfection of vaccinia virus T7-infected cells with plasmids encoding the VSV L, N, P, and G genes as well as the full-length antigenomic plasmid lacking the VSV G gene. VSVΔG virus were propagated on a modified BHK cell line expressing the VSV G under inducible tetracycline control (BHK-G cells).
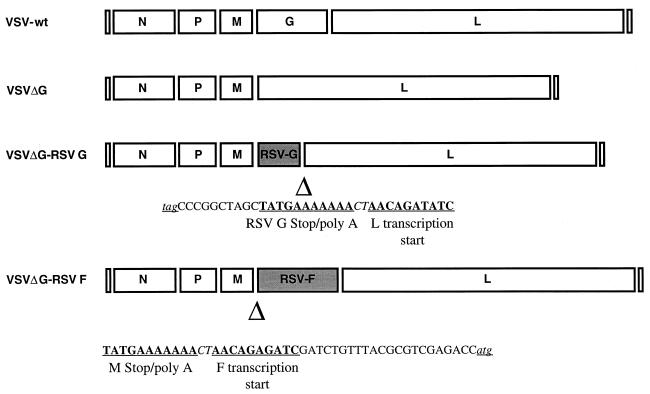
FIG. 1.
Schematic representation of RSV glycoprotein genes in recombinant VSVs lacking the VSV G gene (VSVΔG). Maps displaying gene order are in the antigenomic orientation. Maps of wild-type VSV and VSVΔG recombinants represent viruses used as controls. Maps of VSV-RSV G and VSV-RSV F were published previously (28). VSVΔG-RSV recombinants were derived from wild-type VSV-RSV cDNAs. Deletions of the VSV G gene in VSVΔG-RSV G and VSVΔG-RSV F recombinants are designated (Δ) beneath the respective maps. The newly created RSV G/VSV L and VSV M/RSV F gene junctions are displayed under the linear maps. cDNA sequences corresponding to the positive-sense RNA are shown in the 5′ to 3′ orientation. The transcription stop poly(A) signal and transcription start are labeled. The RSV G termination codon (TAG) and the RSV F initiation codon (ATG) are underlined and are represented in lowercase letters. The intergenic dinucleotide CT is italicized.
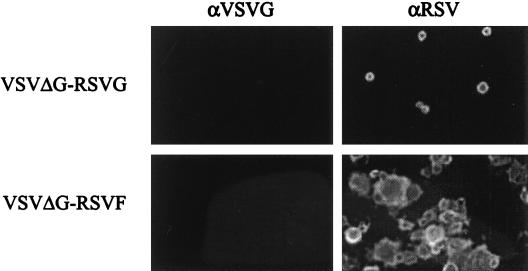
Expression of RSV G and RSV F in VSVΔG-infected cells was confirmed by immunofluorescence (Fig. 2) and Western blot analysis (Fig. 3). Purified virus from BHK-G cells was passaged onto BHK cells at an MOI of 0.1. Eighteen hours after infection, cells were fixed and RSV glycoprotein expression was confirmed by immunofluorescence. VSVΔG-RSV G-infected BHK monolayers displayed evidence of single-cell infection. In contrast, VSVΔG-RSV F-induced cell fusion and syncytium formation. The fusogenic characteristics of the VSVΔG-RSV F were similar to wild-type VSV expressing RSV F (VSV-RSV F) (28). Infection of BHK cells with VSVΔG-RSV F did not result in the production of infectious progeny virus as serial passage of VSVΔG-RSV F infected BHK cell supernatants failed to showed evidence of infectivity (data not shown). VSV G was not detected in VSVΔG-RSV G- or VSVΔG-RSV F-infected cells. Although the RSV glycoproteins were incorporated into VSVΔG virions, RSV specific antibodies failed to inhibit infection of VSVΔG viruses in BHK cells (data not shown). The infectivity of these viruses is therefore based on the presence of VSV G which is supplied in trans by the BHK G cell line.
FIG. 2.
Expression of RSV glycoproteins in VSVΔG recombinant viruses. BHK cell monolayers were infected with either VSVΔG-RSV G or VSVΔG-RSV F at an MOI of 0.1. At 18 h postinfection, monolayers were fixed and screened by immunofluorescence. Primary antibody specificity is shown along the top of the figure and virus is indicated along the left of the figure. Objective magnification, 25×.
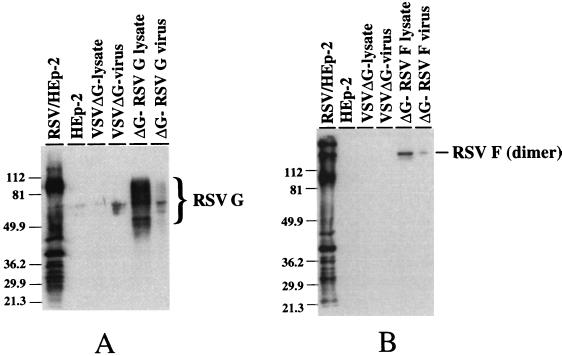
FIG. 3.
Western blot analysis of cell lysates and purified virions. (A) Proteins from uninfected and infected cell lysates were separated in a 12% polyacrylamide (SDS) gel and transferred to nitrocellulose. The blot was probed with a goat anti-RSV antiserum, and antibody binding was detected by ECL following binding with a horseradish conjugated-donkey anti-goat immunoglobulin G. The position of the heavily glycosylated RSV G is noted. Positions of molecular weight markers are noted on the left. (B) Proteins were separated in a 12% polyacrylamide (SDS) gel under nonreducing conditions. Samples were treated with SDS at room temperature prior to electrophoresis. Detection of the proteins bound by the goat anti-RSV antiserum is described above. The position of the RSV F 140-kDa dimer is noted, as are the positions of the molecular mass markers.
Detection of RSV G and F from infected cell lysates and recombinant VSVΔG virions.
Detection of RSV glycoproteins in VSVΔG-RSV G- and VSVΔG-RSV F-infected cells was accomplished by Western blotting. BHK cells were infected with VSVΔG viruses at an MOI of 1.0 and cell lysates were obtained at 18 h postinfection. As shown in Fig. 3A, RSV G was detected in VSVΔG-RSV G-infected cells. RSV G, during wild-type RSV infection, is heavily glycosylated with O- and N-linked glycans, increasing its apparent molecular mass from 32.6 to ∼90 kDa (9, 55). The proteins detected between the unprocessed RSV G and the ∼90-kDA form likely represents RSV G with various degrees of glycosylation. The RSV G synthesized in the VSVΔG-RSV G-infected BHK cells was more heterogeneous than that observed in RSV-infected Hep-2 cells (Fig. 3A). This difference may be due to the inherent properties of the cell lines. The 70-kDa RSV F glycoprotein migrates in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as a 140-kDa dimer under nonreducing conditions (52). As shown in Fig. 3B, a 140-kDa protein was detected in VSVΔG-RSV F-infected cells. and it comigrated with a protein in RSV-infected HEp-2 cell lysates. Similar patterns of RSV glycoproteins were detected in recombinant wild-type VSV-RSV G- and VSV-RSV F-infected cell lysates (28).
Both RSV G and RSV F are incorporated into recombinant wild-type virions (28), and therefore, we predicted the same would be true for the VSVΔG recombinants. To establish this, proteins from purified VSVΔG-RSV G and VSVΔG-RSV F virions were screened by immunoblotting. The majority of the RSV G present in VSVΔG-RSV G virions appears to be of higher molecular weight (Fig. 3A). This suggested that the RSV G that had been fully processed in the Golgi apparatus and subsequently inserted into the cell membrane was incorporated into virions. The dimeric form of RSV F was also present in recombinant VSVΔG-RSV F virions (Fig. 3b). The efficiency of incorporation of RSV glycoproteins into VSVΔG virions could not be determined, as detection of these proteins was possible only when using immunological techniques.
Immunogenicity of wild-type and VSVΔG recombinant viruses expressing RSV glycoproteins.
Four-week-old BALB/c mice were inoculated intranasally with wild-type and VSVΔG recombinant viruses. Each group of five mice was given two (wild-type recombinants) or three (VSVΔG recombinants) boost immunizations at 4-week intervals. Two weeks following the last boost immunization, animals were bled and RSV neutralizing and ELISA antibodies were measured. RSV neutralizing antibody titers were determined with a standard plaque assay with serial dilutions of serum. As displayed in Table 1, wild-type VSV recombinants VSV-RSV G- and VSV-RSV F-induced serum RSV neutralizing antibody titers of 1:16 and 1:32, respectively. VSVΔG-RSV G and VSVΔG-RSV F failed to induce serum RSV neutralizing titers as determined by the plaque reduction assay.
TABLE 1.
Serum RSV neutralizing titers, ELISA titers and protection in mice immunized with recombinant VSVa
| Virusf | Serum neutralizing titerb | RSV ELISA titerc | Protectiond |
|---|---|---|---|
| Pre-immune | <1:8 | NDe | ND |
| VSV rwt | <1:8 | <64 | − |
| VSV-RSV G | 1:16 | 128 | + |
| VSV-RSV F | 1:32 | 4,096 | + |
| VSVΔG | <1:8 | <64 | − |
| VSVΔG-RSV G | <1:8 | <64 | − |
| VSVΔG-RSV F | <1:8 | 1,024 | + |
| Human anti-RSV | 1:1,024 | ND | ND |
| MAbαF | ND | 4,096 | ND |
Serum was collected 2 weeks after boost immunizations.
Neutralizing titers were determined on Vero cells and are reported as the last dilution in which RSV CPE were absent. RSV-specific antiserum (human anti-RSV serum) was used as a positive control.
Dilution of pooled mouse serum which corresponded to an OD450 of 0.5.
Protection against RSV replication in BAL fluid and lung tissue.
ND, not determined.
rwt, recombinant wild type; MAbaF, RSV F-specific mouse monoclonal antibody.
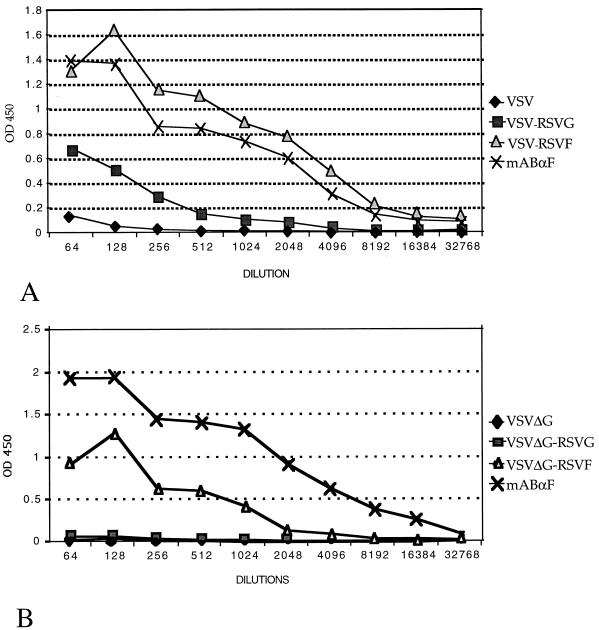
Serum RSV specific antibodies were determined by ELISA. Serial dilutions of serum from immunized mice were incubated in ELISA plate wells coated with a standard amount of RSV viral proteins. Both wild-type VSV recombinants expressing RSV glycoproteins elicited serum RSV specific antibodies (Fig. 4A). VSV-RSV F immunization induced higher titers of RSV-specific antibodies than did VSV-RSV G immunization. VSVΔG-RSV F, but not VSVΔG-RSV G, induced an RSV-specific antibody response (Fig. 4B). These results were in contrast to the neutralizing antibody titer results (Table 1), in which serum from mice immunized with VSVΔG-RSV F recombinants expressing RSV proteins failed to neutralize RSV.
FIG. 4.
ELISA of serum from immunized mice. ELISA plates were coated with RSV-infected HEp-2 cell lysates. Serial dilutions of pooled serum from each group of immunized mice (recombinant wild-type VSV [A] and recombinant VSVΔG [B]) were incubated in coated wells. Bound antibody was detected by a colorimetric reaction following binding of a horseradish peroxidase-conjugated anti-mouse antibody. OD at 450 nm (OD450) are plotted against the inverse of the dilution factor. An RSV F-specific mouse monoclonal antibody (mAbαF) was used as a positive control.
VSV and VSVΔG recombinant viruses protect mice from RSV challenge.
Four weeks following the final boost immunization, mice were challenged with RSV. Each animal was infected intranasally with 1.2 × 105 PFU (50 μl). At 4 days postchallenge, the animals were sacrificed and RSV titers were determined in bronchoalveolar lavage (BAL) fluid and lung tissue. Four days postchallenge was chosen for sacrifice because RSV titers in BAL fluid and lung tissue peaked at this time, as determined by a time course assay (data not shown). RSV replicated to significant titers in mice which were previously immunized with either wild-type VSV or VSVΔG (Fig 5). RSV concentration were ∼104 PFU/ml in BAL fluid and ∼106 PFU/g of lung tissue in those animals which were immunized with either wild-type VSV or VSVΔG. RSV was not detected in either BAL fluid or lung tissue from any mouse immunized with VSV-RSV G, VSV-RSV F, or VSVΔG-RSV F. VSVΔG-RSV G failed to protect from RSV replication. Viral titers from mice immunized with VSVΔG-RSV G were essentially identical to those of mice immunized with VSVΔG. Protection from RSV replication correlated with RSV specific serum antibody (ELISA; Fig. 4) but not with RSV serum neutralizing antibody (Table 1).
FIG. 5.
RSV titers in BAL fluid and lung homogenates from immunized mice. BAL fluid and lung homogenates were prepared 4 days after challenge with RSV. BAL fluid titers (PFU/ml) and lung homogenate titers (PFU/g of tissue) are displayed in a log scale. RSV titers were determined on Vero cells by standard plaque assays. The lower level of detection was 50 PFU/ml of BAL fluid and 50 PFU/g of lung tissue. RSV titers in BAL fluid (∗) and lung homogenates (∗∗) were below the level of detection as indicated.
VSV recombinants expressing RSV antigens induce lymphocytic response in RSV-challenged animals.
To further assess the immune response to RSV in immunized animals, the histopathological features in lung sections were studied. Wild-type VSV and VSVΔG immunized animals displayed histopathological features of RSV infection 4 days after challenge (Fig. 6). Interstitial disease was observed. Hemorrhage and fibrin deposits were noted in the alveolar spaces. Endothelial damage was also noted. Animals immunized with recombinant and replication defective viruses expressing RSV antigens displayed a dense lymphocytic infiltrate. The intense mononuclear cell infiltrate was noted in both the perivascular and peribronchial areas. This reaction was less prominent in animals immunized with either VSVΔG-RSV G and VSVΔG-RSV F. Bronchial epithelium damage was observed in VSV- and VSVΔG-immunized animals, whereas the bronchial epithelium was intact in animals immunized with VSV and VSVΔG viruses expressing RSV antigens. The significance of these observations is addressed in the Discussion.
FIG. 6.
Lung histopathology following RSV challenge. Mice were immunized with the virus noted in each photomicrograph. Lung sections were obtained 4 days following RSV challenge. Bronchial airways (B) are noted. Objective magnification, 20×.
DISCUSSION
We have demonstrated that recombinant VSV and nonpropagating VSV expressing RSV antigens can protect experimental animals against RSV infection. Wild-type recombinant VSV expressing RSV G or F elicited RSV-specific antibody and low levels of serum RSV-neutralizing antibody following intranasal inoculations. Both wild-type recombinant viruses induced complete immunity to RSV replication. Four days following challenge, at the peak of RSV replication in naive, age-matched mice, RSV could not be detected in BAL fluid or in lung tissue of immunized mice. Following initial immunizations, mice tolerated intranasal inoculation with recombinant VSV. Transient weight loss was noted (data not shown), though this phenomenon was similar to previous observations from our laboratory with wild-type VSV and recombinant VSV expressing influenza hemagglutinin (42).
The protection afforded by VSV-RSV and VSVΔG-RSV recombinants correlated with induction of serum RSV-specific antibody. Serum RSV-neutralizing antibodies were not required for protection against RSV replication following challenge. Immunization with VSVΔG-RSV F failed to induce detectable serum neutralizing antibodies, though it protected mice against RSV infection. It is unclear whether the presence of serum RSV-specific antibodies is sufficient for protection. Local mucosal immunity elicited by recombinant VSVs and/or induction of cellular immunity, not examined in this report, may play a role in inhibiting RSV replication. However, nonneutralizing antibodies may be solely responsible for protection. In vitro nonneutralizing monoclonal antibodies reactive with the VSV G glycoprotein protect mice against lethal VSV challenge (34).
VSVΔG recombinants expressing RSV F, but not RSV G, elicited complete sterilizing immunity against RSV replication. The difference in the protection elicited by VSVΔG-RSV F and VSVΔG-RSV G could not be explained by the difference in expression of the RSV glycoproteins. Both RSV G and RSV F glycoproteins were expressed on infected cell surfaces and incorporated into virions. This difference may be explained by the antigenic properties of the RSV glycoproteins and/or the biological features of the VSVΔG viruses. RSV G is heavily glycosylated (9, 55) and therefore may not elicit a robust immune response. However, wild-type VSV-RSV G elicits sterilizing immunity and other recombinant viral systems expressing RSV G induce antibody responses (49). These observations suggest that the antigenic properties of RSV G were not likely to account for the lack of protection afforded by the VSVΔG-RSV G recombinant. However, the level of antigen expression in the immunized host may account for the difference between the immunogenic properties of the VSVΔG recombinant viruses. The fusogenic properties of the VSVΔG-RSV F recombinant virus may lead to cell-to-cell spread of the viral infection in the immunized animal, thus increasing the antigenic signal.
RSV vaccination based on nonpropagating VSV expressing RSV F is promising for several reasons. VSVΔG-RSV F elicits sterilizing immunity in our animal model. Of the two major RSV glycoproteins, RSV F is a more practical antigen for recombinant or subunit vaccines since RSV F is more highly conserved between RSV subgroups A and B compared to RSV G (23, 27). In addition, RSV G contains two regions which display significant variability within each subgroup (7, 50). Furthermore, immunoprophylaxis with a humanized monoclonal antibody directed against the RSV F glycoprotein has been shown to decrease severity of disease in infants and children with underlying lung disease and/or prematurity (4). Vaccination with VSVG-RSV F has distinct advantages over other viral vectors based systems (49, 56) in that the vaccine can be administered intranasally and the vector is replication defective.
Intranasal inoculation likely confers local immunity (not assayed) since we demonstrated that induction of serum neutralizing antibody was not required for protection in VSVΔG-RSV F-immunized mice. Immunization with VSVΔG in experimental animals appears to be safe. Animals tolerated intranasal inoculations well and did not display transient weight loss as that observed with the wild-type recombinants (data not shown). These observations were similar to previous work with VSVΔG expressing influenza hemagglutinin (41). The replication-defective properties of the VSVΔG-RSV F limit viral propagation and pathogenesis, therefore increasing the safety of this potential vaccine candidate. Lack of an immune response to VSV G in animals inoculated with VSVΔG recombinants allows for the increased protective effect of boost immunizations (41).
Following RSV challenge, histopathological findings in animals primed with VSV and VSVΔG recombinants expressing RSV antigens were consistent. Dense peribronchial and perivascular lymphocytic infiltrates were observed. However, histopathological findings did not correlate with protection. For example, mice primed with either VSVΔG-RSV G or VSVΔG-RSV F displayed similar histopathological changes in lung tissue, yet mice primed with VSVΔG-RSV G were not protected against RSV replication. In contrast to animals primed with RSV antigens, animals primed with either control virus, wild-type VSV, or VSVΔG, displayed histopathological features upon challenge consistent with RSV disease, such as interstitial changes and alveolitis. The significance of these observations is unclear. The lymphocytic response is likely a reaction to RSV in mice primed with RSV antigens. Earlier studies have demonstrated that extensive pulmonary histopathology characterized by lymphocytic infiltrates occurs in RSV primed-BALB/c mice upon RSV challenge (11). The lymphocytic response may represent a protective cell mediated immune response to RSV. Alternatively, the cellular infiltration may represent a destructive process. A pathological response to RSV re-exposure would be limited to the animal model. RSV infection in the natural host induces incomplete protective immunity; in infants and young children, the severity of RSV illness decreases with subsequent infections (22).
The immune and inflammatory responses elicited by potential RSV vaccines are significant concerns. During clinical trails in the 1960s, a formalin-inactivated RSV (FI-RSV) vaccine induced enhanced disease upon natural exposure to RSV (29, 32). Animal studies have provided some insight into the mechanisms of this aberrant reaction. In the BALB/c mouse model, primary RSV infection results in bronchiolitis, aveololitis, and a predominant Th1 response. This Th1 response is characterized by relatively high levels of gamma interferon (IFN-γ) and low levels of interleukin-4 (IL-4) (25, 26). In contrast, immunization with FI-RSV leads to a predominantly Th2 response characterized by increased levels of IL-4 and IL-5 and enhanced pulmonary histopathology (10, 54). However, the findings of a recent study suggests that enhanced pathology may not be RSV antigen specific. Boelen et al. found that, in the BALB/c model, severe lung pathology and Th2 cytokine profile were induced by either FI-RSV or mock antigen comprised of formalin-treated HEp-2 cells (6). This phenomenon was also observed in the cotton rat model (38). Therefore, further studies are required to determine the basis of the immune and inflammatory response in lung tissue following RSV challenge in animals immunized with VSV and VSVΔG recombinants. This may require the study of these viruses in many genetic backgrounds of mice and in other species.
In conclusion, recombinant and nonpropagating VSVs expressing RSV antigens provide protective immunity against RSV infection. These results further demonstrate the utility of VSV-based vaccine vectors. Intranasal immunity induced by live attenuated RSVs or recombinant viruses expressing RSV antigens may provide the best protection for young infant and children who are at risk for severe RSV disease.
ACKNOWLEDGMENTS
We are indebted to Robert Homer for his assistance in the review of the histopathology specimens and Irene Visintin for her technical expertise in obtaining the photomicrographs. We thank Edward “Z” Zelazny for his assistance in the mouse work and the members of the Yale Animal Care Facility for their care of the experimental animals. We thank JoAnn Falato for her assistance.
This work was supported by NIH grants AI-01469 (J.S.K.) and AI 24345 (J.K.R.), the Yale Child Health Research Center (NIH grant HD27757), and the Charles H. Hood Foundation, Boston Massachusetts (J.S.K.). Anjeanette Roberts was supported by a Cancer Research Institute Fellowship.
REFERENCES
- 1.Abman S H, Ogle J W, Butler-Simon N, Rumack C M, Accurso F J. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J Pediatr. 1988;113:826–830. doi: 10.1016/s0022-3476(88)80008-8. [DOI] [PubMed] [Google Scholar]
- 2.Anderson L J, Hierholzer J C, Bingham P G, Stone Y O. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J Clin Microbiol. 1985;22:1050–1052. doi: 10.1128/jcm.22.6.1050-1052.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. Acute respiratory infections: the forgotten pandemic. Bull W H O. 1998;76:101–103. , 105–107. [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 5.Belshe R B, Anderson E L, Walsh E E. Immunogenicity of purified F glycoprotein of respiratory syncytial virus: clinical and immune responses to subsequent natural infection in children. J Infect Dis. 1993;168:1024–1029. doi: 10.1093/infdis/168.4.1024. [DOI] [PubMed] [Google Scholar]
- 6.Boelen A, Andeweg A, Kwakkel J, Lokhorst W, Bestebroer T, Dormans J, Kimman T. Both immunisation with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine. 2000;19:982–991. doi: 10.1016/s0264-410x(00)00213-9. [DOI] [PubMed] [Google Scholar]
- 7.Cane P A, Matthews D A, Pringle C R. Identification of variable domains of the attachment (G) protein of subgroup A respiratory syncytial viruses. J Gen Virol. 1991;72:2091–2096. doi: 10.1099/0022-1317-72-9-2091. [DOI] [PubMed] [Google Scholar]
- 8.Collins P L, McIntosh K, Chancock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 9.Collins P L, Mottet G. Oligomerization and post-translational processing of glycoprotein G of human respiratory syncytial virus: altered O-glycosylation in the presence of brefeldin A. J Gen Virol. 1992;73:849–863. doi: 10.1099/0022-1317-73-4-849. [DOI] [PubMed] [Google Scholar]
- 10.Connors M, Giese N A, Kulkarni A B, Firestone C Y, Morse III H C, Murphy B R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors M, Kulkarni A B, Firestone C Y, Holmes K L, Morse III H C, Sotnikov A V, Murphy B R. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couch R B, Englund J A, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;102:2–9. doi: 10.1016/S0002-9343(97)00003-X. , 25–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falsey A R, Walsh E E. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in the institutionalized elderly. Vaccine. 1997;15:1130–1132. doi: 10.1016/s0264-410x(97)00002-9. [DOI] [PubMed] [Google Scholar]
- 14.Furze J, Wertz G, Lerch R, Taylor G. Antigenic heterogeneity of the attachment protein of bovine respiratory syncytial virus. J Gen Virol. 1994;75:363–370. doi: 10.1099/0022-1317-75-2-363. [DOI] [PubMed] [Google Scholar]
- 15.Glezen P, Denny F W. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 16.Glezen W P, Paredes A, Allison J E, Taber L H, Frank A L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 17.Groothuis J R, Gutierrez K M, Lauer B A. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics. 1988;82:199–203. [PubMed] [Google Scholar]
- 18.Groothuis J R, King S J, Hogerman D A, Paradiso P R, Simoes E A. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis. 1998;177:467–469. doi: 10.1086/517377. [DOI] [PubMed] [Google Scholar]
- 19.Groothuis J R, Simoes E A, Levin M J, Hall C B, Long C E, Rodriguez W J, Arrobio J, Meissner H C, Fulton D R, Welliver R C, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 20.Hall C B, Powell K R, MacDonald N E, Gala C L, Menegus M E, Suffin S C, Cohen H J. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 21.Han L L, Alexander J P, Anderson L J. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 22.Henderson F W, Collier A M, Clyde W A, Jr, Denny F W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 23.Hendry R M, Burns J C, Walsh E E, Graham B S, Wright P F, Hemming V G, Rodriguez W J, Kim H W, Prince G A, McIntosh K, et al. Strain-specific serum antibody responses in infants undergoing primary infection with respiratory syncytial virus. J Infect Dis. 1988;157:640–647. doi: 10.1093/infdis/157.4.640. [DOI] [PubMed] [Google Scholar]
- 24.Holberg C J, Wright A L, Martinez F D, Ray C G, Taussig L M, Lebowitz M D. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 25.Hussell T, Openshaw P J. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79:2593–5601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- 26.Hussell T, Spender L C, Georgiou A, O'Garra A, Openshaw P J. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol. 1996;77:2447–2455. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- 27.Johnson P R, Jr, Olmsted R A, Prince G A, Murphy B R, Alling D W, Walsh E E, Collins P L. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987;61:3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn J S, Schnell M J, Buonocore L, Rose J K. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology. 1999;254:81–91. doi: 10.1006/viro.1998.9535. [DOI] [PubMed] [Google Scholar]
- 29.Kapikian A Z, Mitchell R H, Chanock R M, Shvedoff R A, Stewart C E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 30.Karron R A, Buonagurio D A, Georgiu A F, Whitehead S S, Adamus J E, Clements-Mann M L, Harris D O, Randolph V B, Udem S A, Murphy B R, Sidhu M S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karron R A, Wright P F, Crowe J E, Jr, Clements-Mann M L, Thompson J, Makhene M, Casey R, Murphy B R. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J Infect Dis. 1997;176:1428–1436. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 32.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 33.Lamprecht C L, Krause H E, Mufson M A. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J Infect Dis. 1976;134:211–217. doi: 10.1093/infdis/134.3.211. [DOI] [PubMed] [Google Scholar]
- 34.Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984;51:208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefrancois L, Lyles D S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology. 1982;121:168–174. doi: 10.1016/0042-6822(82)90126-x. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald N E, Hall C B, Suffin S C, Alexson C, Harris P J, Manning J A. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med. 1982;307:397–400. doi: 10.1056/NEJM198208123070702. [DOI] [PubMed] [Google Scholar]
- 37.Paradiso P R, Hildreth S W, Hogerman D A, Speelman D J, Lewin E B, Oren J, Smith D H. Safety and immunogenicity of a subunit respiratory syncytial virus vaccine in children 24 to 48 months old. Pediatr Infect Dis J. 1994;13:792–798. doi: 10.1097/00006454-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Piedra P A, Faden H S, Camussi G, Wong D T, Ogra P L. Mechanism of lung injury in cotton rats immunized with formalin-inactivated respiratory syncytial virus. Vaccine. 1989;7:34–38. doi: 10.1016/0264-410x(89)90008-x. [DOI] [PubMed] [Google Scholar]
- 39.Piedra P A, Grace S, Jewell A, Spinelli S, Bunting D, Hogerman D A, Malinoski F, Hiatt P W. Purified fusion protein vaccine protects against lower respiratory tract illness during respiratory syncytial virus season in children with cystic fibrosis. Pediatr Infect Dis J. 1996;15:23–31. doi: 10.1097/00006454-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Prince G A, Hemming V G, Horswood R L, Chanock R M. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985;3:193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 41.Roberts A, Buonocore L, Price R, Forman J, Rose J K. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. 1999;73:3723–3732. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts A, Kretzschmar E, Perkins A S, Forman J, Price R, Buonocore L, Kawaoka Y, Rose J K. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72:4704–4711. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose N F, Roberts A, Buonocore L, Rose J K. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J Virol. 2000;74:10903–10910. doi: 10.1128/jvi.74.23.10903-10910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Schlereth B, Rose J K, Buonocore L, ter Meulen V, Niewiesk S. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J Virol. 2000;74:4652–4657. doi: 10.1128/jvi.74.10.4652-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnell M J, Johnson J E, Buonocore L, Rose J K. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell. 1997;90:849–857. doi: 10.1016/s0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 47.Shay D K, Holman R C, Newman R D, Liu L L, Stout J W, Anderson L J. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 48.Srinivasakumar N, Ogra P L, Flanagan T D. Characteristics of fusion of respiratory syncytial virus with HEp-2 cells as measured by R18 fluorescence dequenching assay. J Virol. 1991;65:4063–4069. doi: 10.1128/jvi.65.8.4063-4069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stott E J, Ball L A, Young K K, Furze J, Wertz G W. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol. 1986;60:607–613. doi: 10.1128/jvi.60.2.607-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullender W M, Mufson M A, Anderson L J, Wertz G W. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J Virol. 1991;65:5425–5434. doi: 10.1128/jvi.65.10.5425-5434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tristram D A, Welliver R C, Mohar C K, Hogerman D A, Hildreth S W, Paradiso P. Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18–36 months old. J Infect Dis. 1993;167:191–195. doi: 10.1093/infdis/167.1.191. [DOI] [PubMed] [Google Scholar]
- 52.Walsh E E, Cote P J, Fernie B F, Schlesinger J J, Brandriss M W. Analysis of the respiratory syncytial virus fusion protein using monoclonal and polyclonal antibodies. J Gen Virol. 1986;67:505–513. doi: 10.1099/0022-1317-67-3-505. [DOI] [PubMed] [Google Scholar]
- 53.Wang E E, Prober C G, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;311:1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 54.Waris M E, Tsou C, Erdman D D, Zaki S R, Anderson L J. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wertz G W, Krieger M, Ball L A. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J Virol. 1989;63:4767–4776. doi: 10.1128/jvi.63.11.4767-4776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wertz G W, Stott E J, Young K K, Anderson K, Ball L A. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987;61:293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright P F, Karron R A, Belshe R B, Thompson J, Crowe J E, Boyce T G, Halburnt L L, Reed G W, Whitehead S S, Anderson E L, Wittek A E, Casey R, Eichelberger M, Thumar B, Randolph V B, Udem S A, Chanock R M, Murphy B R. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]