Abstract
With the increasing demand for wearable electronics (such as smartwatch equipment, wearable health monitoring systems, and human–robot interface units), flexible energy storage systems with eco‐friendly, low‐cost, multifunctional characteristics, and high electrochemical performances are imperative to be constructed. Nanocellulose with sustainable natural abundance, superb properties, and unique structures has emerged as a promising nanomaterial, which shows significant potential for fabricating functional energy storage systems. This review is intended to provide novel perspectives on the combination of nanocellulose with other electrochemical materials to design and fabricate nanocellulose‐based flexible composites for advanced energy storage devices. First, the unique structural characteristics and properties of nanocellulose are briefly introduced. Second, the structure–property–application relationships of these composites are addressed to optimize their performances from the perspective of processing technologies and micro/nano‐interface structure. Next, the recent specific applications of nanocellulose‐based composites, ranging from flexible lithium‐ion batteries and electrochemical supercapacitors to emerging electrochemical energy storage devices, such as lithium‐sulfur batteries, sodium‐ion batteries, and zinc‐ion batteries, are comprehensively discussed. Finally, the current challenges and future developments in nanocellulose‐based composites for the next generation of flexible energy storage systems are proposed.
Keywords: batteries, composites, energy storage, flexible electronics, nanocellulose, supercapacitors
Recent advances on nanocellulose‐based composites consisting of nanocellulose and other electrochemical materials for emerging flexible energy‐storage devices are comprehensively discussed, with a focus on structure–property–application relationships to optimize their performance. The current challenges and future developments regarding design and fabrication of nanocellulose‐based composites for the next generation of energy‐storage systems are discussed and proposed.
1. Introduction
With the rapid rise of implantable, wearable, and portable electronic devices on the commercial market, wearable electronic devices that appear as gadgets, accessories, and clothing have already been widely used.[ 1 , 2 , 3 ] Especially, with the vigorous development of artificial intelligence and Internet of Things in the era of big data, wearable electronic products have demonstrated broader prospects in the future. Accordingly, the flexible, functional, and reliable electrochemical energy storage (EES) equipment is required to power emerging electronics.[ 4 , 5 ] In particular, the global society is facing a series of challenges, such as global warming, resource scarcity, and severe environmental pollution, so that it is of great interest to fabricate low‐cost and eco‐friendly materials for high‐performance energy storage devices.[ 6 ]
Cellulose, as a highly promising alternative material for petroleum‐based products, is biodegradable, renewable, environmentally friendly, and nontoxic. It has been extensively used in diverse fields, such as fibers and clothes, pulp and paper industry, cosmetics, food industry, and pharmaceutics among many others.[ 7 , 8 , 9 , 10 ] In general, nanocellulose refers to nanostructured cellulose with the diameter less than 100 nm and the length up to several micrometers.[ 11 ] The unique characters of nanocellulose, such as superior mechanical properties (strength 2–3 GPa), low density (1.6 g cm−3), high specific surface area (200–300 m2 g−1), and low thermal expansion coefficient (1 ppm K−1), make them an ideal building block for flexible functional compounds.[ 12 , 13 ] Compared to the conventional energy storage materials (such as carbon‐based materials, conducting polymers, metal oxides, MXene, etc.), nanocellulose is commonly integrated with other electrochemically active materials or pyrolyzed to carbon to develop composites as energy storage materials because of its intrinsic insulation. Nanocellulose‐based composites in the EES systems demonstrate considerable hydrophilic surfaces, abundant hierarchical pore structure (micro‐ and mesopores) for charge storage, and rich absorptive sites to enhance absorption and transport of electrolyte ions.[ 14 , 15 , 16 ] Except for improvement in electrochemical performance, nanocellulose‐based composites with green and abundant raw materials are also beneficial for the reduction of production costs, and construction of environmentally friendly processes.[ 17 , 18 , 19 , 20 ] Thus, nanocellulose‐based composites have been attractive components among numerous candidates for design and fabrication of advanced flexible energy storage devices. In recent years, nanocellulose‐based composites with superior electrochemical performance by combining the advantages of the nanocellulose and electrochemically active materials have been constructed to apply in various kinds of energy storage systems.[ 21 , 22 , 23 , 24 ]
As shown in the Figure 1 , a brief timeline is summarized to demonstrate the evolution and development of nanocellulose‐based composites for advanced energy storage devices. Due to the complexities in the preparation processes and microstructures of different nanocellulose‐based composites, challenges for introducing new features into the composites, such as stretchability, anti‐freezing, and self‐healing properties, have also emerged, which require advanced structural design and operando techniques. Moreover, the influence of nanocellulose component on the EES mechanism of the composite electrodes requires systematic theoretical calculations combined with adequate electrochemical analysis. Although several reviews have been reported with a focus on specific EES application or from viewpoints of structures or preparation strategies on nanocellulose‐based composites,[ 17 , 39 , 40 , 41 ] a comprehensive review of the flexible nanocellulose‐based composites, specifically combining preparation strategies, structural designs, functional applications, as well as emerging features and challenges for EES application is still missing.
Figure 1.
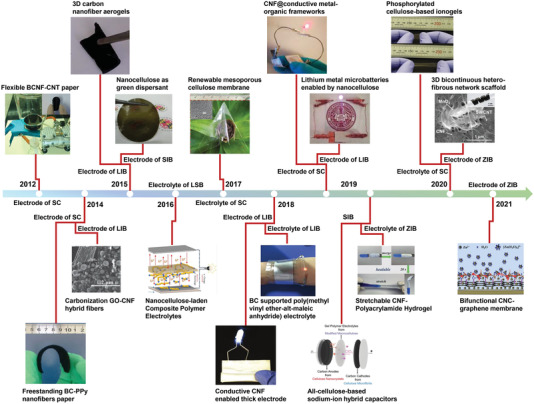
Brief timeline of nanocellulose‐based composites for advanced energy storage devices. Flexible carbon nanotube‐coated bacterial cellulose nanofibrils (BCNF–CNT) paper for supercapacitor (SC) electrode. Reproduced with permission.[ 25 ] Copyright 2012, American Chemical Society. The conductive free‐standing membranes based on bacterial cellulose–polypyrrole nanofibers (BC–PPy) for pseudocapacitor. Reproduced with permission.[ 26 ] Copyright 2014, Elsevier. The negative electrode of carbonization graphene oxide–cellulose nanofibrils (GO–CNF) hybrid fibers for lithium‐ion battery (LIB). Reproduced with permission.[ 18 ] Copyright 2014, Wiley‐VCH. The 3D carbon nanofiber aerogels for the anode of LIB derived from BC with nano‐Fe3O4. Reproduced with permission.[ 27 ] Copyright 2015, The RoyalSociety of Chemistry. Composite films for electrode of sodium‐ion battery (SIB) by using the nanocellulose‐assisted dispersed MoS2 aqueous solutions. Reproduced with permission.[ 28 ] Copyright 2015, Elsevier. The composite nanocellulose‐laden polymer electrolytes for lithium–sulfur battery (LSB). Reproduced with permission.[ 29 ] Copyright 2016, Elsevier. KOH‐saturated flexible, renewable, and transparent cellulose membrane for SC electrolyte. Reproduced with permission.[ 30 ] Copyright 2017, Wiley‐VCH. A conductive nanofiber network with decoupled electron and ion transfer pathways by the conformal electrostatic assembly of neutral carbon black particles on negatively charged CNF for thick electrode of LIB. Reproduced with permission.[ 31 ] Copyright 2018, Wiley‐VCH. BC‐based polymer composite for lithium metal battery as the multifunctional electrolyte. Reproduced with permission.[ 32 ] Copyright 2018, The Royal Society of Chemistry. CNF@conductive metal–organic framework nanopapers for SC electrode. Reproduced with permission.[ 33 ] Copyright 2019, American Chemical Society. The electrode of LIB with 3D printing enabled by nanocellulose. Reproduced with permission.[ 34 ] Copyright 2019, Wiley‐VCH. The sodium‐ion hybrid capacitor with quasi‐solid‐state based on all‐cellulose composites. Reproduced with permission.[ 10 ] Copyright 2019, Wiley‐VCH. The self‐healable and flexible nanocellulose/borax‐crosslinked polyvinyl alcohol hydrogel electrolyte for zinc‐ion hybrid supercapacitor. Reproduced with permission.[ 35 ] Copyright 2019, The Royal Society of Chemistry. The renewable, extremely durable, and highly conducting cellulose‐based ion gel electrolyte for supercapacitor electrolyte. Reproduced with permission.[ 36 ] Copyright 2019, The Royal Society of Chemistry. The self‐supporting CNF/carbon nanotube film with bicontinuous ion/electron transport channels for electrode of aqueous zinc‐ion battery (ZIB). Reproduced with permission.[ 37 ] Copyright 2020, Wiley‐VCH. The cellulose nanowhisker–graphene (CNC–graphene) film as the interface layer for highly reversible and long cycling life Zn metal anode. Reproduced with permission.[ 38 ] Copyright 2019, The Royal Society of Chemistry.
This review specifically aims at offering new perspectives on the combination of nanocellulose with other electrochemical materials to optimize their performances. The synthesis strategies, structure and interface engineering, working mechanisms, and applications in flexible electrodes, electrolytes/separators of flexible nanocellulose‐based composites are highlighted. An outline map of this review is illustrated in Figure 2 . In this review, we start with the preparation methods and properties of nanocellulose, by briefly summarizing the recent research progress on nanocellulose‐based composites from the perspective of processing technologies and micro/nanostructure, with the focus on the relationships of the structure–property–application of these composites for EES. Then, we comprehensively discuss recent specific applications of nanocellulose‐based composites from lithium‐ion batteries (LIBs) and electrochemical supercapacitors (SCs) to emerging EES systems, such as lithium–sulfur batteries (LSBs) and zinc‐ion batteries (ZIBs). Finally, current challenges and potential strategies for further improvement of nanocellulose‐based composites toward the next generation of EES devices are discussed and summarized.
Figure 2.
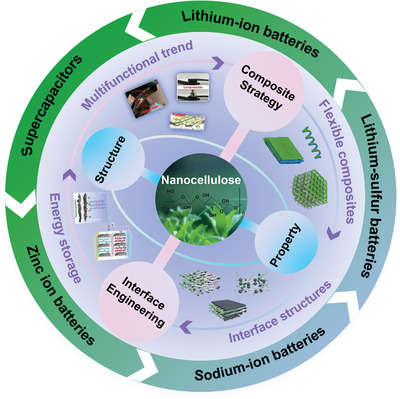
The outline map of this review.
2. Preparation and Properties of Nanocellulose
Cellulose is a polysaccharide which exists as linear chains consisting of repeating anhydro‐d‐glucose units covalently linked by β‐1,4‐glycosidic bonds.[ 42 ] Figure 3a illustrates the hierarchical structure of plants, with focus on the supramolecular structure of cellulose. From the molecular structure of cellulose, abundant hydroxyl groups are present in the cellulose chains, which have high tendency to form strong intrachain and interchain hydrogen bonds.[ 43 ] As a result, multiple cellulose chains form elementary fibrils and further assemble into larger microfibrils and microfibril bundles with the diameters ranging from 5 to 50 nm.[ 44 ] These microfibrils along with hemicellulose and lignin form the cell walls, and the cell walls combined with other components (e.g., proteins and inorganic compounds) eventually form the plant. Depending on the packing arrangements of the cellulose chains, there are two regions, namely ordered (crystalline) and disordered (amorphous) regions, coexisting in the cellulose microfibrils. The cellulose chains are closely packed forming a dense structure in the crystalline domains, while they are randomly entangled in the disordered regions.[ 45 ] Compared with the crystalline regions, disordered regions with a lower density are less stable and easy to be degraded by acids. For example, the disordered regions of cellulose can be hydrolyzed under well controlled conditions (e.g., acid concentration and temperature), and the ordered regions remain as cellulose nanocrystals (CNC).[ 46 ] In addition, cellulose microfibrils within the cell walls of plants can be released by mechanical disintegration, and the obtained nanofibers are generally called cellulose nanofibrils (CNF) that consist of both ordered and disordered regions.[ 47 ] So far, CNC and CNF have been considered as the two main categories of nanocellulose produced from lignocellulosic biomass by a top‐down process.[ 48 ]
Figure 3.
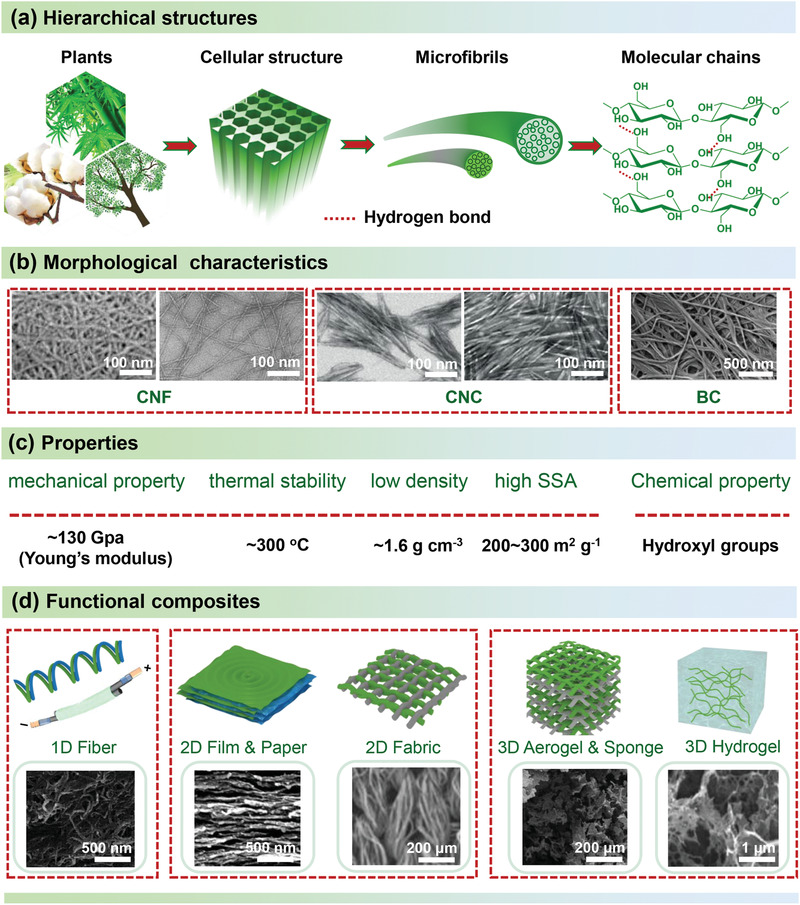
Morphology, properties, and composites of nanocellulose. a) The hierarchical structure of cellulose.[ 2 ] b) The morphology of representative nanocellulose, including cellulose nanofibers (CNF), cellulose nanocrystals (CNC), and bacterial cellulose (BC). Reproduced with permission.[ 49 , 50 , 51 , 52 ] Copyright 2007, American Chemical Society; Copyright 2007, American Chemical Society; Copyright 2014, Wiley‐VCH; Copyright 2020, American Chemical Society. c) Various characteristics in terms of mechanical behavior, thermal stability, density, specific surface area (SSA), and surface chemistry.[ 53 ] d) Schematic illustrations of various structures of nanocellulose composites (including 1D fiber, 2D film, paper, and fabric, and 3D aerogel, sponge, and hydrogel). Reproduced with permission.[ 54 , 55 , 56 , 57 ] Copyright 2011, Wiley‐VCH. Copyright 2018, Elsevier. Copyright 2017, Wiley‐VCH. Copyright 2015, Wiley‐VCH.
As shown in Figure 3b, CNC are rigid rod‐like particles with the diameter in the range of 5–50 nm and length of 100–500 nm, while CNF have larger aspect ratios with a diameter less than 100 nm and a length of several micrometers.[ 58 , 59 ] Typically, CNC are mainly produced by acid hydrolysis or oxidation,[ 60 , 61 , 62 , 63 ] and CNF are obtained by 2,2,6,6‐tetramethylpiperidine‐1‐oxyl radical (TEMPO)‐mediated oxidation[ 50 ] or mechanical fibrillation methods, such as high‐pressure homogenization, high‐intensity ultrasonication, ball milling, and ultrafine grinding.[ 64 , 65 , 66 , 67 ] As another kind of nanocellulose, bacterial cellulose (BC), fabricated through biotechnological assembly, is composed of microfibrils with a diameter of 3 to 4 nm combined into fiber bundles with a thickness below 100 nm and a length up to 100 µm.[ 49 ] Compared with plant cellulose, BC has high purity, high crystallinity (up to 95%), and high degree of polymerization (DP values of 2000–8000) since it is almost pure cellulose containing no lignin and other exotic substances.[ 68 ] In the natural state, BC has an interwoven network structure with a moisture content of more than 98% and good mechanical stability, so that BC can be considered as unique hydrogels. In addition, BC can be further converted into BC nanocrystals (BCNC) and BC nanofibrils (BCNF) by acid hydrolysis and mechanical fibrillation, respectively.[ 69 , 70 ] Except for the above‐mentioned types of nanocellulose, spherical shaped cellulose nanoparticles can also be synthesized by different fabrication strategies from various cellulose precursors.[ 71 , 72 , 73 ] Also, ribbon shaped cellulose nanofibers have been prepared through an electrospinning process from cellulose solutions.[ 74 ] Based on the above discussions on different types of nanocellulose, CNF and BCNF with the web‐like entangled structures and high aspect ratios are ideal building blocks for the fabrication of robust self‐assembled nanostructures, while the self‐assembled nanostructure of CNC is generally brittle due to the lack of an energy‐dissipating amorphous phase and its inability to form entangled networks.[ 75 ] Instead, CNC with high crystallinity is more suitable to be used as reinforcing agent for nanocomposites.[ 76 , 77 , 78 ]
As summarized in Figure 3c, nanocellulose exhibits excellent physiochemical properties, which are attractive to various EES applications.[ 35 , 79 , 80 , 81 , 82 ] There are mainly five categories included of nanocellulose‐based materials: nanocellulose/nanocarbon composites, nanocellulose/conducting polymer composites, nanocellulose/metal compound composites, nanocellulose/organic polymer composites, and ternary or multiple complexes.[ 15 , 83 ] The main roles that nanocellulose‐based composites play in all kinds of supercapacitors and batteries include flexible electrodes, separators, and electrolytes (Figure 4 ). As for flexible electrode materials, they are principally required to demonstrate superior electrical conductivity, high specific surface area, good electrolyte wettability, and chemical stability.[ 81 ] The separator mainly separates the two electrodes of the supercapacitors or batteries to prevent short circuits. In addition, it also displays the function of transporting electrolyte ions. Therefore, the separators need to have the characteristics of insulation, high ionic conductivity, and good thermal stability.[ 84 ] The polymer‐based electrolyte generally needs to exhibit the feature of wide voltage window, temperature range and high ionic conductivity. The gel‐state or solid‐state polymer‐based electrolytes also act as a separator in flexible energy storage devices.[ 85 ]
Figure 4.
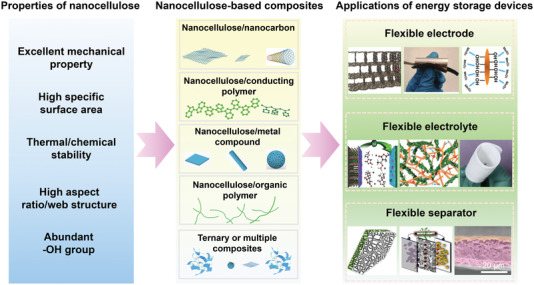
The development of nanocellulose‐based composites for EES of flexible electrode, separator, and electrolyte.
Versatile advantages of nanocellulose make it a highly promising candidate for EES. 1) The nanocellulose with high mechanical strength (Young's modulus of 130 GPa) and good thermal stability makes nanocellulose highly attractive for the fabrication of electrodes and separators with good flexibility and mechanical durability. For instance, Cui et al.[ 86 ] fabricated the enhanced Li+ conducting membrane with flexible nanocellulose. Due to the continuous pathways for Li+ transfer provided by the interconnected network, and abundant electrostatic coordinating sites assisted by negatively charged surface carboxylate groups of CNF, the electrochemical, mechanical, and thermal properties of polyethylene oxide‐based membrane were effectively improved. 2) The low density and high specific surface area facilitate nanocellulose as the building blocks for lightweight and porous electrode materials to enable pore structures of nanocellulose‐based composites within nanometer scale. 3) The abundant hydroxyl groups on the reactive surfaces enable nanocellulose convenient for chemical modification and hybridization with active materials to construct nanocellulose‐based composites. The properties of these composites can be tailored by changing the species of active materials in the composites or changing composite strategies. For example, a bicontinuous ion/electron conduction networks scaffold of intermingled nanofibrous mixture with hydrophilic CNFs and single‐walled carbon nanotubes (SWCNTs) was synthesized to facilitate the redox kinetics (in particular, the intercalation/deintercalation of Zn2+ ions) of MnO2.[ 37 ] The single‐walled carbon nanotubes and CNFs were used to enhance electrolyte accessibility and provide electron conduction channels, respectively. Benefiting from the bicontinuous ion/electron conduction pathways in the electrode, consequently, the rate capability and cyclability of the electrode were significantly improved. 4) The wettability and structure stability of nanocellulose in different electrolytes over a wide potential window position it as a competitive candidate for facilitating ionic transportation. By borax‐assisted crosslinking, Wong and co‐workers[ 35 ] fabricated a zinc‐salt‐containing polyvinyl alcohol (PVA)/CNF hydrogel electrolyte. Due to the CNF reinforcing network, hydrogen bonding, and chain entanglement, the hydrogel electrolytes demonstrated superb viscoelasticity, moderate tensile/compressive strength of 23.1/26.2 kPa, and great adhesive property. 5) The high carbon content, high aspect ratio, and easy processing make nanocellulose a good source material for preparing carbon electrodes with tunable microstructure, high surface area, and doping structure.[ 87 ] Recently, CNC–graphene composite membrane was prepared to mitigate intricately water‐induced corrosion and uncontrollable dendrite growth of zinc metal anode in aqueous ZIB. The work demonstrated that the CNC–graphene interface layer with negative surface charges could simultaneously generate a deanionization shock by spreading cations but screening anions to obtain redirected Zn deposition parallel to the (0002) Zn plane.[ 38 ] To sum up, the development of nanocellulose for EES has received wide attention due to the advantages of its intrinsic properties and structures, as demonstrated in Figure 4.
Among the above‐mentioned merits of nanocellulose, the most important and promising one is that the introduction of nanocellulose into the hybrid materials can make the devices flexible, which can also store charge upon folding, repeated bending, or compressing without significantly reducing the electrochemical performance. To date, a variety of composite approaches, such as spinning, vacuum filtration, and freeze‐drying have been developed for integrating nanocellulose with different active materials,[ 88 , 89 , 90 ] leading to the formation of different structures (Figure 3d), such as 1D fibers, 2D papers/films, and 3D hydrogels/aerogels.[ 54 , 55 , 56 , 91 ]
3. Preparation and Structural Engineering of Nanocellulose‐Based Composites
3.1. Synthesis Strategies
The nanocellulose with good processability and unique properties is highly suitable for the large‐scale production of flexible functional materials. Generally, nanocellulose with rich oxygen‐containing groups can be combined with other materials, which can enhance connections through covalent interactions with other functional groups. A variety of flexible nanocellulose‐based functional materials have been fabricated by designing the surface/interface chemistry, systematically adjusting the structures, and integrating advanced processing technologies with the methods of microfluidic spinning, electrospinning, 3D printing, etc.[ 92 , 93 , 94 ]
1D fiber/wires can be constructed by spinning and twisting (Figure 5a–c), which are attractive for integrating other functional materials and particular properties into macroscopic assemblies with superior electrical and mechanical performances.[ 92 , 95 , 96 ] Moreover, 1D nanocellulose‐based composites can be directly designed into textiles and fabrics, showing significant prospects in flexible electrodes, which are favorable for developing wearable and flexible energy storage devices.[ 97 , 98 ] Furthermore, unconventional architectures can be tailored by 1D nanocellulose‐based composites to suit different energy storage devices for special applications. Typically, based on the dip‐coating and twisting techniques, flexible yarn supercapacitors were fabricated using a carbon nanotube (CNT)@BC membrane as a structural matrix.[ 95 ] Due to the high hydrophilic capillaries and conductivity of CNT@BC network, the polypyrrole (PPy)@CNT@BC electrodes demonstrated improved infiltration of electrolyte and shortened the ion diffusion distance. For twisting method, the preparation process is still not scalable, so that it is still not suitable for large‐scale application, but it can be used to prepare dense and reinforced composite fibers.
Figure 5.
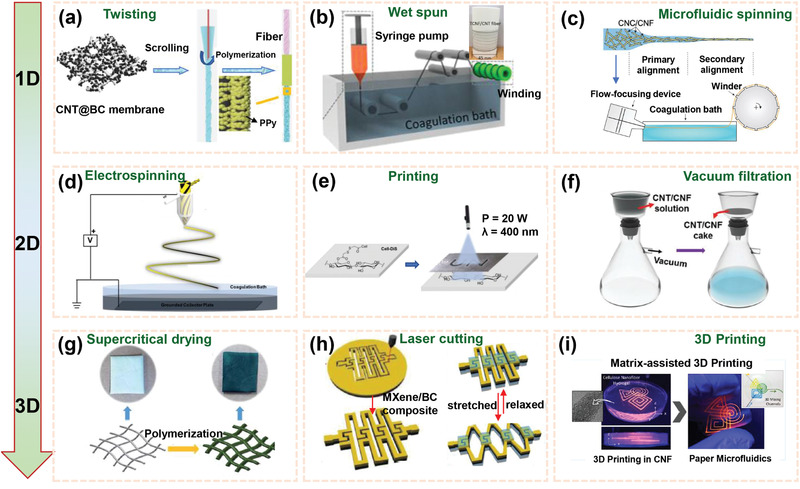
a–i) Typical preparation technologies for fabricating nanocellulose‐based flexible composites. Reproduced with permission.[ 92 , 93 , 94 , 95 , 96 , 100 , 105 , 107 ] Copyright 2018, Wiley‐VCH; Copyright 2019, JohnWiley & Sons, Ltd; Copyright 2017, American Chemical Society; Copyright2020, Springer Nature; Copyright 2019, American Chemical Society; Copyright2017, Wiley‐VCH; Copyright 2018, The Royal Society of Chemistry; Copyright2019, Wiley‐VCH.
2D papers/films can be prepared by mixing nanocellulose with conductive or active materials dispersions followed by electrospinning,[ 93 ] printing,[ 99 ] filtration and drying,[ 100 ] and so forth (Figure 5d–f). The film composites based on nanocellulose are significantly promising in flexible electrodes/separators for energy storage devices. The unique structures and properties of nanocellulose may endow the film composites with good hydrophilic property, mechanical strength, excellent flexibility, as well as optical transparency, depending on the characteristics of the thickness of the as‐obtained films and the nanocellulose‐based‐units.[ 24 , 101 , 102 , 103 ] Vacuum filtration is among the most widely used methods for the preparation of composite films and can be easily achieved in a laboratory with simple equipment. However, the area of obtained composite films is limited by the filter size. Recently, Lee and co‐workers reported the fabrication of solid‐state flexible supercapacitors on paper with an all‐inkjet‐printed process.[ 104 ] To realize high‐precision print image, the CNF‐mediated nanoporous films (as a primer layer) was inkjet‐printed on top of paper. In addition to active carbon and CNTs, Ag nanowires were introduced onto the electrodes to improve electrical conductivity.
3D aerogels/foams can be prepared through supercritical drying/critical point drying/freeze‐drying of mixtures of nanocellulose and other functional materials (Figure 5g).[ 105 ] As‐developed aerogels/foams exhibited high porosity, low density, web‐like entangled structures, which make them good electrodes for flexible EES systems. Aerogels derived from CNF/reduced graphene oxide (RGO)/CNT were developed by freeze‐drying of aqueous dispersions of CNF/GO nanosheets (GONSs)/CNT followed by thermal reduction.[ 106 ] The supercapacitors assembled by the compressed CNF/RGO/CNT aerogels as electrodes exhibited a high specific capacitance of 252 F g−1. The approaches of laser cutting and 3D printing (also called additive manufacturing) are also used for preparing 3D nanocellulose‐based materials.[ 94 , 107 ] Hu and co‐workers fabricated the twistable, bendable, and stretchable microsupercapacitor arrays through laser‐cutting kirigami patterning. Combining facile laser‐cutting kirigami patterning process with high‐performance few‐layered Ti3C2T x flakes (MXene)/BCNF paper offered a promising method for fabricating and designing wearable energy storage devices.[ 107 ]
Over the last few years, significant efforts have been devoted to developing synthesis methods for preparing nanocellulose‐based composites with different morphologies, spatial structures, and properties. The synthesis methods reported to date for nanocellulose‐based composites could be classified into in‐situ and ex‐situ strategies, as illustrated in Figure 6 .
Figure 6.
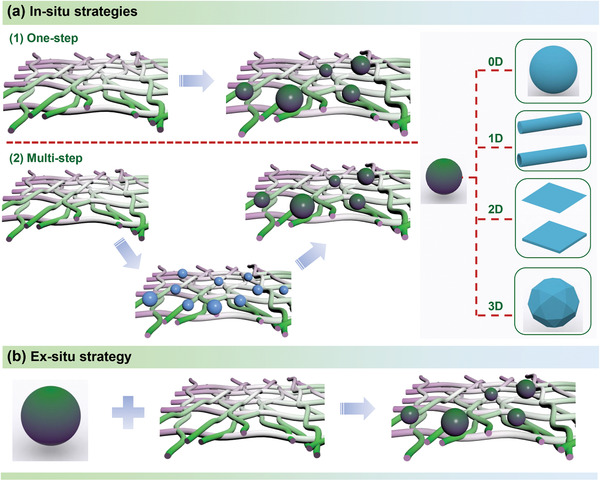
Schematic illustration of a) in‐situ strategies including one‐step in‐situ growth and multistep in‐situ conversion, and b) ex‐situ strategy for preparing nanocellulose‐based composites.
3.2. In‐Situ Strategies for Preparing Nanocellulose‐Based Composites
In‐situ strategies for producing nanocellulose‐based composites have been used extensively for EES specifically, which mainly includes two methods: 1) in‐situ one‐step growth of other materials on nanocellulose; 2) in‐situ multistep generation of other materials on nanocellulose. The in‐situ one‐step method primarily concerns the direct polymerization or growth of compounds (including nanoparticles,[ 108 ] nanowires,[ 109 ] nanoplates,[ 110 ] nanoflowers,[ 111 ] etc.) on an existing nanocellulose substrate and with subsequent development into the desired materials with different properties. In addition to the superior conductivity of in‐situ polymerized conducting polymers, most of the electrochemically active nanoparticles display poor electrical conductivity. Thus, the main difficulty for these approaches is that they need to be further compounded with conductive materials or carbonized when used as electrodes for energy storage devices. For example, conducting polymers such as polyaniline (PANI),[ 112 ] PPy,[ 113 , 114 , 115 , 116 , 117 ] and poly(3,4‐ethylenedioxythiophene) (PEDOT)[ 118 ] are usually polymerized in‐situ and form a homogenous layer on the surface of nanocellulose, resulting in composite films/papers with flexibility and high performances. Wang et al.[ 116 ] produced the polypyrrole‐coated core–shell TEMPO‐oxidized bacterial cellulose (PPy–TOBC) composite networks by in‐situ polymerization, as demonstrated in Figure 7a. The composite of PPy–TOBC film as supercapacitor electrodes demonstrated high specific capacitance (153 F g−1 at 0.2 A g−1) . A flexible nickel–cobalt layered double hydroxide/PANI/BC (NiCo‐LDH/PANI/BC) film with both high mechanical and electrochemical performances was reported by Cheng and co‐workers through in‐situ loading PANI and NiCo‐LDH on BC,[ 105 ] respectively. The film with a 3D conductive network possessed abundant electroactive sites and smooth channels, and as electrodes showed outstanding electrochemical performance. Liu's group[ 119 ] prepared the BC@zeolitic imidazolate framework‐8 (ZIF8) hybrid gel by in‐situ growing ZIF8 on the surface of the BC with electrostatic interaction (N‐containing ligands and Zn ions with —OH groups), as shown in Figure 7b. After freeze‐drying process, the BC@ZIF8 hybrid aerogels were carbonized leading to N‐self‐doped carbon nanofiber (N‐CF) aerogels. The aerogels with 3D interconnected network, highly porous and silk cocoon‐like node morphology demonstrated remarkable capacitance and superior long‐term cycling stability.
Figure 7.
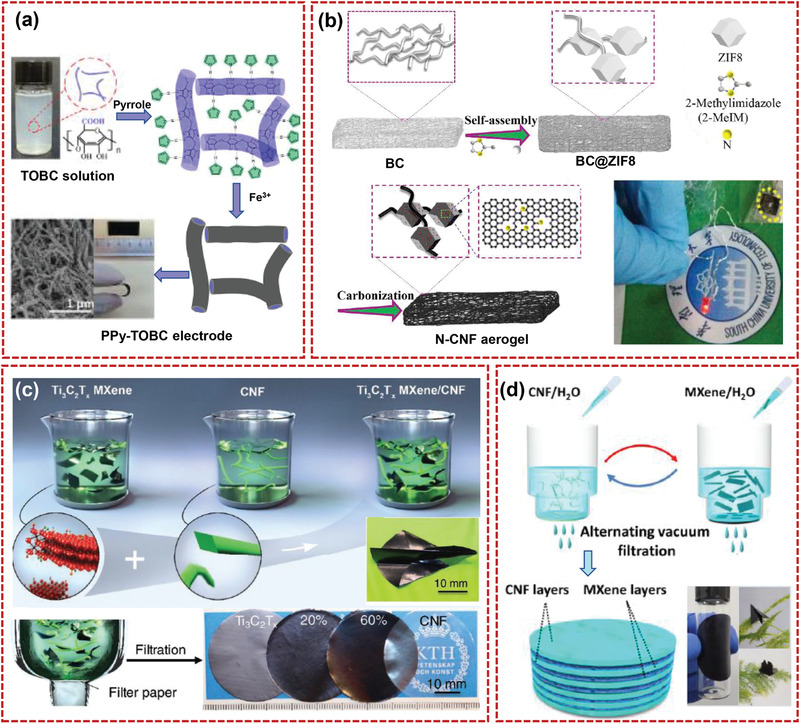
Illustrated procedure for the preparation of a) PPy–TOBC core–shell nanofiber network materials (Reproduced with permission.[ 116 ] Copyright 2016, Elsevier) and b) BC@ZIF8 and N‐CF aerogel by in‐situ strategy (Reproduced with permission.[ 119 ] Copyright 2019, Elsevier). c) The preparation process and flexibility of 2D MXene and 1D CNF multifunctional nanocomposites (Reproduced with permission.[ 120 ] Copyright 2019, Wiley‐VCH) and d) multilayered CNF@MXene films by ex‐situ strategy (Reproduced with permission.[ 129 ] Copyright 2020, American Chemical Society).
3.3. Ex‐Situ Strategies for Preparing Nanocellulose‐Based Composites
Ex‐situ strategies via noncovalent or covalent interactions enable the objective electrochemical materials to be grafted on the nanocellulose surface, which mainly concern the fabrication of active materials in advance with the ideal morphologies and nanostructures (Figure 6b).[ 120 , 121 ] Compared to the in‐situ approaches, ex‐situ strategies overcome the inconsistency between the reaction processes for composites and synthesizing precursors. Distinct interactions including inductive interaction, electrostatic interaction, exchange repulsion, and noncovalent interaction could play important roles for preparing nanocellulose‐based composites.[ 100 , 122 , 123 , 124 ] For example, CNF can be mixed with carbon nanomaterials (CNTs and graphene) to generate uniform dispersions to prepare nanocellulose‐supported composites.[ 125 , 126 ] Shao and co‐workers prepared nonwoven macrofiber mats from the composite solutions of CNF/SWCNTs by wet spinning, which promoted the arrangement of SWCNTs along the axial direction in the macrofiber.[ 97 ] Simultaneously, the aggregation of SWCNTs was effectively prevented by CNF. The macrofiber demonstrated a well‐defined porous structure, which could enhance the diffusion of electrolyte ions. 2D films containing the covalent intercalation of BC fibers and graphene oxide were prepared via filtration of the esterification mixture.[ 127 ] The composite films demonstrated a break elongation of 24% and tensile strength of 18.5 MPa. Flexible, lightweight, and free‐standing Si paper using Cladophora nanocellulose fiber, Si nanoparticles, and CNT as the building blocks were constructed via a vacuum filtration process.[ 128 ] The paper as LIB anodes exhibited improved energy storage performances due to the strong adhesion of uniformly distributed Si nanoparticles to the 3D conductive flexible CNT/Cladophora nanocellulose fiber network. Hamedi and co‐workers[ 120 ] demonstrated multifunctional nanocomposites by blending dispersions of the 2D MXene nanosheets and the 1D CNF, as shown in Figure 7c. The nanocomposites displayed a high mechanical strength of 340 MPa and a good electronic conductivity of 2.95 × 104 S m−1. Otherwise, the lightweight and flexible multilayered CNF@MXene films with excellent mechanical properties were prepared by simple alternating vacuum filtration method (Figure 7d).[ 129 ] The robust film with hierarchical nacre‐like micro–nanostructures demonstrated excellent toughness (2.7 MJ m−3), good tensile strength (112.5 MPa), and flexibility.
3.4. Interface Structural Design of Nanocellulose‐Based Composites
The electrochemical performance of the final energy storage systems can be significantly influenced by the interface exposed to electrolyte and the structure of the electrochemical active material.[ 101 , 130 , 131 ] In this section, from the perspectives of structure and interface engineering, the research progress of nanocellulose‐based composites for application of EES is summarized, which includes the interface construction and micro/nano interface structure between electrochemical materials and nanocellulose components. By promoting the flux of ions across the electrode/electrolyte boundary and increasing the active sites of interfacial reactions, nanocellulose‐based composites with ideal structures have been demonstrated to improve performances of EES systems. Obviously, the nanocellulose plays an important role in transferring electrolyte ions from/to nanocellulose‐based composites during the charge/discharge process. Therefore, constructing a good interfacial contact mode of the electrochemical materials with the nanocellulose component is highly important for improving the kinetics of electrochemical reactions between different components.
Considering the flexibility and radial size of nanocellulose, the nanocellulose‐based composites with diverse dimensions of the electrochemical materials can be mainly classified into three types: nanocellulose‐anchored hybrids, layered structured hybrids, and nanocellulose‐mixed composites, as illustrated in Figure 8 .[ 134 , 135 ] Theoretically, for nanocellulose‐anchored composites, nanocellulose as a support layer could not only effectively prevent the aggregation of the second phase electrochemical materials, but also enhance the mechanical stability by avoiding peeling‐off of the electrochemical materials component from the nanocellulose. Layered structured composites can be constructed with nanocellulose and nanosheets. Among them, nanocellulose acts as a flexible unit, ion transport channel, or nanospacer. For nanocellulose‐mixed composites, utilizing the synergistic effect of nanocellulose and electrochemically active materials gives the composites mechanical robustness, improved electrochemical performance, and good flexibility. Numerous nanocellulose‐based composites, such as nanocellulose anchored conducting polymers, nanocellulose/transition metal compound mixed composites, and layered structured nanocellulose/MXene hybrids have been fabricated for EES.[ 136 , 137 , 138 , 139 , 140 , 141 , 142 ] However, the nanocellulose‐based components may have a certain negative influence on the electron transfer process due to its insulating property. In order to overcome the insulation, Li et al.[ 143 ] deposited a layer multiwalled carbon nanotubes (MWCNTs) onto the surface of BC paper to enhance electron transfer of nanocellulose‐based composites, in which MWCNTs were strongly bonded with BC paper via electrostatic interaction and hydrogen bonding. After the polymerization of PANI, the composites of BC/MWCNT/PANI could provide smoothly electronic transport path, rich active‐sites, and effective electrolyte accessibility. The flexible BC/MWCNT/PANI electrode showed remarkable specific capacitance of 656 F g−1 at 1 A g−1 and appreciable long‐cycling performance (after 1000 cycles over 99.5% capacitance retention). Therefore, designing high‐performance nanocellulose‐based composites for energy storage systems should take the spatial obstruction of the electron transfer kinetics along with the mass (ions) transport process into consideration. In addition, adjusting the orientation of the conductive components in the hybrids is another effective strategy to improve the electron transfer process.
Figure 8.
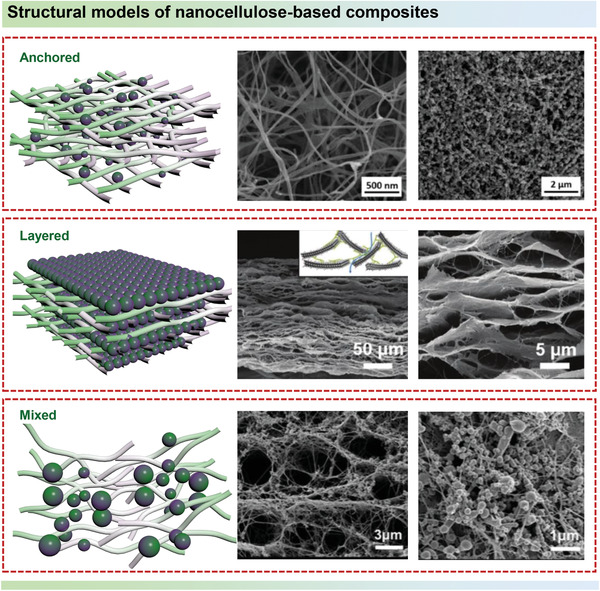
Structural interface models: nanocellulose‐anchored hybrids (Reproduced with permission.[ 132 ] Copyright 2016, Elsevier), layered structured hybrids (Reproduced with permission.[ 89 ] Copyright 2019, Wiley‐VCH), and nanocellulose‐mixed composites (Reproduced with permission.[ 133 ] Copyright 2019, Springer Nature.
4. Supercapacitors
SCs, owing to their rapid charging/discharging rate, superior rate capability, high‐power density, and long cycle life, have shown great potential for fulfilling the ever‐growing power demand.[ 144 , 145 , 146 ] According to the energy storage mechanism, there are typically two types of SCs: electrical double layer capacitors (EDLCs) and pseudocapacitors. For EDLCs, the energy storage process mainly occurs in the accumulation of electrostatic charges on the electrode/electrolyte interface, as shown in Figure 9a. For pseudocapacitors, capacitance is derived from the rapid oxidation–reduction reaction on the surface of electrodes, as illustrated in Figure 10a.[ 147 , 148 ] Although several SCs demonstrate high power density and energy density, there is still big potential for enhancing the electrochemical performances spontaneously. In this section, the latest developments in the flexible nanocellulose‐based composites for application as supercapacitors will be discussed. Electrochemical performances and strategies of various nanocellulose‐based composites for flexible supercapacitor electrodes are summarized and compared in Table 1 .
Figure 9.
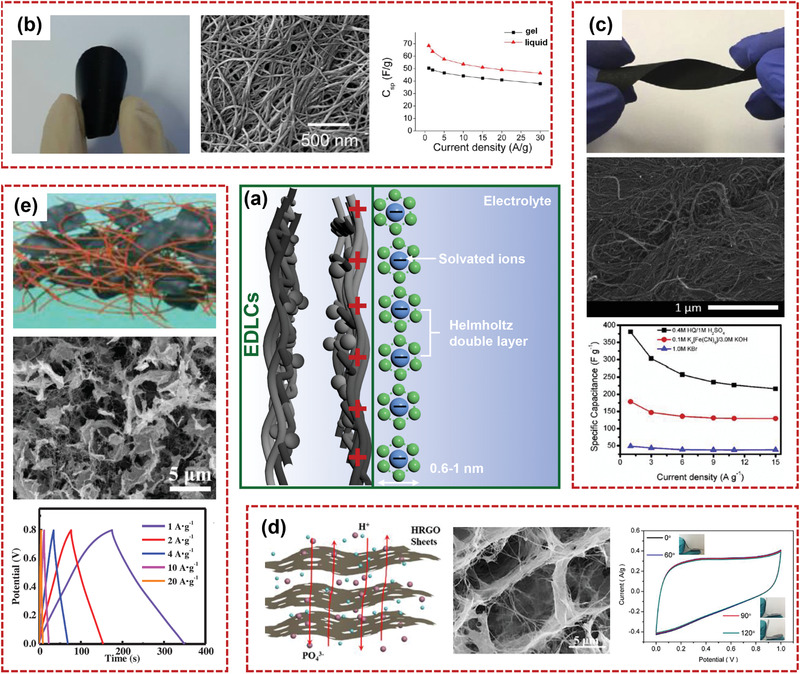
a) Schematic diagram of ion adsorption of EDLCs on the electrode surface. Structures and electrochemical characterizations of b) flexible BC/CNT composite film (Reproduced with permission.[ 25 ] Copyright 2012, American Chemical Society), c) buckypaper/FWCNT@CNF composites (Reproduced with permission.[ 151 ] Copyright 2020, Elsevier), d) holey graphene oxide/bacterial cellulose film (Reproduced with permission.[ 152 ] Copyright 2018, Elsevier), and e) free‐standing carbon nanofiber/graphene nanosheet composites (Reproduced with permission.[ 153 ] Copyright 2018,Wiley‐VCH).
Figure 10.
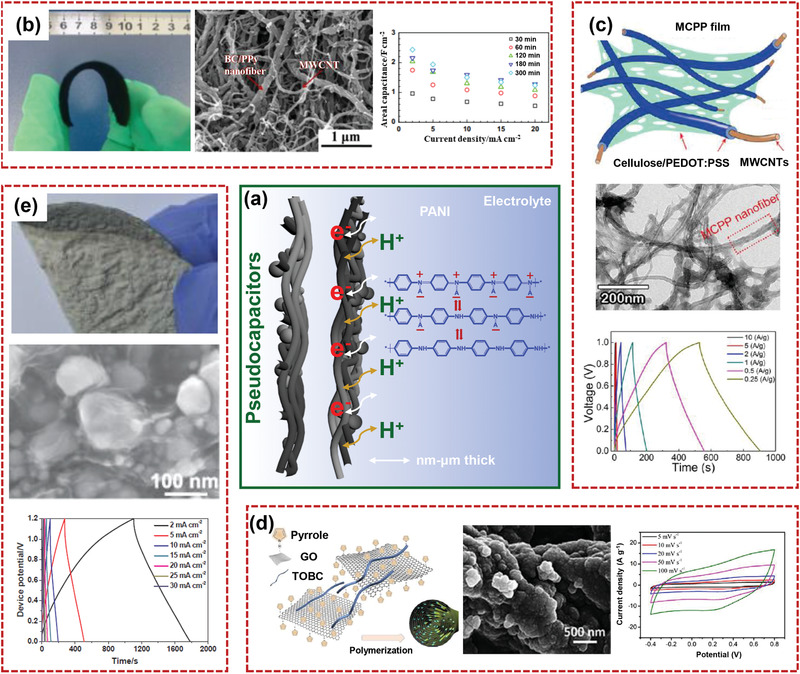
a) Schematic diagram of charge transfer near the electrode surface in pseudocapacitor. PANI/nanocellulose composite has been selected as the active material. Structures and electrochemical characterizations of b) conductive BC/PPy paper electrodes (Reproduced with permission.[ 26 ] Copyright 2014, Elsevier), c) MWCNT‐reinforced cellulose/PEDOT:PSS composite (Reproduced with permission.[ 154 ] Copyright 2017, AmericanChemical Society), d) hierarchical PPy@TEMPO‐oxidized BC/RGO macrofibers (Reproduced with permission.[ 155 ] Copyright 2019, Elsevier), and e) BC/RGO/silver/Fe2O3 film (Reproduced with permission.[ 156 ] Copyright 2017, Elsevier).
Table 1.
Electrochemical performances and strategies of various nanocellulose‐based composites for flexible supercapacitor electrodes
| Electrode | Preparation | Electrolyte | Capacitance (electrode) | Energy density/potential | Cycling stability | Refs. (Year) |
|---|---|---|---|---|---|---|
| EDLC: | ||||||
| BCNF/CNT paper | Vacuum filtering | Ionic‐liquid‐based polymer electrolyte | 50.5 F g−1@1 A g−1 | 15.5 mWh g−1/3 V | 5000 (> 99.5%) | [ 25 ] (2012) |
| Holey RGO/BC film | Culture medium | PVA–H3PO4 | – | 9.2 Wh kg−1/1 V | 5000 (88%) | [ 152 ] (2019) |
| CNT–CNF/PVAB hydrogel | Mixed | CNF/PVAB–KOH | 117.1 F g−1@0.3 A g−1 | –/1 V | 1000 (96.4%) | [ 160 ] (2019) |
| CNC–CNT/PVA–PAA membrane | Electrospun | PVA‐g‐PAA–KCl | 155.5 F g−1@0.5 A g−1 | –/0.8 V | 2000 (92%) | [ 98 ] (2019) |
| CNT@CNF buckypaper | Casted | – | 380.8 F g−1 @ 1 A g−1 | – | 12 000 (120%) | [ 151 ] (2020) |
| Pseudocapacitor: | ||||||
| PANI/Ag/CNF aerogel | Electrodeposition | PVA–H3PO4 | 176 mF cm−2 @ 10 mV s−1 | 10.6 Wh kg−1/0.7 V | – | [ 112 ] (2014) |
| BCNF/PPy paper | Vacuum filtering | LiCl | 2430 mF cm−2 | –/0.8 V | 5000 (94.5%) | [ 26 ] (2014) |
| PPy@TOBC/RGO fiber | Wet spinning | PVA/H2SO4 | 373 F cm−3 @ 0.48 A cm−3 | 8.8 mWh cm−3/1 V | 5000 (79%) | [ 155 ] (2019) |
| CNF/PANI aerogel | Supramolecular self‐assembly | PVA/H2SO4 | 59.26 mF cm−2 | –/1 V | 2000 (75.6%) | [ 156 ] (2019) |
| 3D porous MXene/BC film | Vacuum filtering and freeze casting | PVA/H2SO4 | 2084 mF cm−2 | 252 μWh cm−2/1.4 V | 10 000 (93%) | [ 152 ] (2019) |
| PPy‐coated BC film | Template‐sacrificing polymerization | PVA/H2SO4 | 1710 mF cm−2 @ 0.4 mA cm−2 | 89.8 μWh cm−2/0.8 V | 5000 (68.9%) | [ 158 ] (2019) |
| CNF@c‐MOF film | Interfacial synthesis | PVA/KCl | 125 F g−1 @ 0.33 A g−1 | –/0.7 V | 10 000 (99%) | [ 33 ] (2019) |
4.1. Flexible SCs Based on Nanocellulose‐Based Composites
4.1.1. Flexible Electrodes
At present, carbon materials are considered the most prospective electric double layer materials. 1D CNT, carbon nanofiber (CF), and 2D graphene are the most promising advanced materials for flexible supercapacitors.[ 149 ] CNT and graphene materials can be directly mixed with the highly flexible nanocellulose to act as a free‐standing electrode. Intriguingly, 1D nanocellulose can effectively prevent the accumulation of carbon materials, significantly improve the hydrophilic performance, and enhance the utilization of mesopores of the electrode materials. Kim and co‐workers[ 25 ] developed a BC/CNT paper electrode via vacuum filtering approach for flexible supercapacitor. High flexibility and continuous conductive paths of the paper electrode were achieved due to the entangled structure of 1D CNT and BC substrate. The obtained BC/CNT film was not divided into individual layers over hundreds of repeated bending, showing good mechanical stability. The assembled supercapacitor with BC/CNT film electrode demonstrated high specific capacitance of 50.5 F g−1 (Figure 9b). Jost et al.[ 150 ] fabricated a nanocellulose‐based fibrous flexible supercapacitor by knitting technology. In the welding process, activated carbon (AC) was added into the swelled cellulose yarns. The high carbon mass loadings (0.62 mg cm−1) made the assembled two‐electrode cells deliver a high capacitance of 37 mF cm−1. Lavall and co‐workers[ 151 ] reported a buckypaper (BP) composite (Figure 9c) by employing combination of the double‐ and triple‐walled CNT (FWCNT) with CNF. The generated composite (BP/FWCNT@CNF) was completely flexible and moldable. The BP/FWCNT@CNF film preserved the structure of the FWCNTs, and ensured greater wettability and good electrical conductivity. When hydroquinone (HQ)/H2SO4 was used as a redox electrolyte, the high specific capacitance of 380.8 F g−1 at 1 A g−1 was obtained.
Wan's group reported a holey reduced graphene oxide (HRGO)/BC film with 3D honeycomb structural by biosynthesis approach (Figure 9d).[ 152 ] The HRGO/BC composite films demonstrated a high tensile strength of 204 MPa and could be bended, folded, knotted, and even twisted. Due to the good wettability, holey architecture of HRGO, and continuous 3D honeycomb structure, the solid‐symmetric supercapacitors with HRGO/BC electrodes showed a high specific capacity of 65.9 F g−1. The free‐standing CFs/graphene nanosheet (GN) composites were prepared, as demonstrated in Figure 9e, using BC as a substrate followed by spraying graphene‐dispersed medium and carbonization.[ 153 ] The CFs and GNs were uniformly dispersed in the 3D conductive architecture. Due to the high specific surface area, good structure stability, and fine flexibility of CFs/GNs, the assembled supercapacitor exhibited high capacitance of 215 F g−1 and the extraordinary cycling stability with no capacitance degradation after 5000 cycles.
A promising method for the development of high‐performance flexible supercapacitors is introducing the pseudocapacitive materials with high specific capacitance into the nanocellulose matrix with tough mechanical strength. Conducting polymers aroused great attention for their superior conductivity, cost‐effectiveness, and lightweight. Shen and co‐workers[ 26 ] constructed free‐standing BC/PPy paper electrodes with high conductivity by integrating MWCNTs via environmentally vacuum‐filtering strategy (Figure 10b). With high mass loading of 11.23 mg cm−2, the flexible composite electrodes with porous structure and abundant active sites showed excellent electrochemical performance with the highest capacitance of 2430 mF cm−2. Yu's group[ 154 ] prepared a conductive and flexible cellulose/poly(3,4‐ethylenedioxythiophene)/poly(styrenesulfonate (PEDOT:PSS)) hybrid matrix by supramolecularly assembling 3,4‐ethylenedioxythiophene and cellulose in an ionic liquid. To enhance its conductivity and structural strength, MWCNTs were introduced into the composite matrix. The resulting MWCNT‐reinforced cellulose/PEDOT:PSS (MCPP) composites showed enhanced capacitance (485 F g−1 at 1 A g−1) and cycling durability due to multiple paths for ion and electron transport in electrodes (Figure 10c). The assembled solid‐state supercapacitor based on MCPP showed excellent flexibility and high capacitance of 380 F g−1 at 0.25 A g−1. The micrometer‐scale TOBC/RGO yarns were prepared by wet spinning and hydroiodic acid reduction.[ 155 ] As shown in Figure 10d, PPy was then deposited on the TOBC/RGO yarns by in‐situ polymerization. PPy and RGO interacted strongly with the TOBC nanofibers, which improved electrolyte infiltration. Also, the rich number of mesopores within the composite structure promoted ion and charge transport. Due to the synergistic effects of TOBC, RGO, and PPy, the PPy@TOBC/RGO electrode showed improved specific capacitance to 373 F cm−3 (391 F g−1) at 0.48 A cm−3 (0.5 A g−1). Xiao and co‐workers[ 156 ] prepared a free‐standing BC/RGO/silver/Fe2O3 hybrid film electrode by in‐situ reduction method and vacuum filtration process for high‐performance flexible supercapacitor, as demonstrated in Figure 10e. The flexible BC/RGO/silver/Fe2O3 film delivered two electroactive species (RGO and Fe2O3) and high conductivity with unique morphology. The assembled device showed high areal capacitance of 1132 mF cm−2 and energy density of 226.4 μWh cm−2. In addition, the device also demonstrated stable capacitance performance and good rate capability when it was bent.
Recently, new electrode materials such as metal–organic frameworks (MOFs), and MXenes are also compounded with nanocellulose to obtain higher capacitance performance. Xu and co‐workers[ 33 ] reported a highly flexible, hierarchical porous, conductive nanopapers electrode of CNF@ conductive‐MOF (CNF@c‐MOF) for high performance supercapacitor as shown in Figure 11a,b. The CNF@c‐MOF composite nanofibers demonstrated good mechanical strength, hierarchical porosity, and high conductivity (Figure 11c). The nanopaper electrodes and obtained supercapacitor both showed good electrochemical performances due to the smooth electrolyte transport and charge transfer of CNF@c‐MOF film (Figure 11d,e). By interweaving with BCNF and anchoring redox juglone on CNTs, Tang and co‐workers fabricated a flexible juglone/CNTs/BCNF film electrode.[ 157 ] The flexible chemical electrode of juglone/CNTs/BCNF exhibited good electrochemical performance due to the enhanced charge transfer. The specific capacitance (461.8 F g−1) was more than five times higher than that of CNTs/BC electrode. Furthermore, they used polydopamines (PDAs) as the interfacial modifier for the engagement of ZIF‐67 polyhedrons along the BCNF to prepare PPy‐coated BCNF electrodes via a template‐sacrificing polymerization.[ 158 ] The well‐ordered PPy domains along BCNF afforded electrolyte‐accessible channels. Thus, the assembled flexible supercapacitor demonstrated not only good flexibility, but also a high areal energy density of 89.8 μWh cm−2 at power density of 0.31 mW cm−2. Yuan and co‐workers reported a self‐assembled MXene/BC film electrode with bicontinuous network of interconnected BC and MXene nanosheets (Figure 11f–i).[ 159 ] The MXene/BC film with 3D porous structure provided large ion‐accessible specific surface area, rapid ion transport process, superior mechanical property (43 MPa), and high electrical conductivity. The matched aqueous asymmetric supercapacitor of PANI/BC as cathode and MXene/BC as anode delivered a high capacitance of 925 mF cm−2 at 3 mA cm−2.
Figure 11.
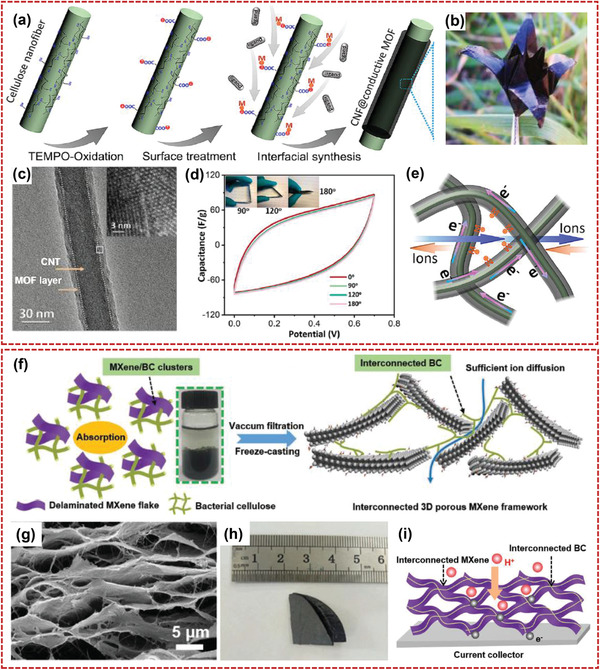
Nanocellulose‐based composites compounded with new electrode materials. a) Preparation procedure of CNF@c‐MOF hybrid nanofibers, b) an origami photograph folded by nanopaper, c) TEM image of a single CNF@c‐MOF hybrid nanofiber, d) cyclic voltammetry (CV) curves under different folding angles of CNF@c‐MOF double‐layer supercapacitor, and e) illustration of the electrolyte ion transport and charge transfer in the CNF@c‐MOF nanopapers. Reproduced with permission.[ 33 ] Copyright 2019, American ChemicalSociety. f) Schematic of synthesis process of MXene/BC composites, g) SEM images of MXene/BC film, h) the folded MXene/BC film, and i) diagrammatic illustration of electrons and ions transport in the MXene/BC electrode. Reproduced with permission.[ 159 ] Copyright 2019, Wiley‐VCH.
With the advancement of technologies such as sensors, wireless communication networks, and microchips, flexible electronic devices are becoming more multifunctional. However, it is still a great challenge to integrate multiple functions into one device. With the necessary electrochemical properties, multifunctional flexible supercapacitors, which are stretchable, self‐healing, health monitoring, and so on, are attractive for portable and wearable electronics. Xu and co‐workers[ 160 ] fabricated a fast self‐healing and pH‐sensitivity supercapacitor with CNT–CNF/polyvinyl alcohol‐borax (CNT–CNF/PVAB) composite hydrogel as electrode and PVAB hydrogel as electrolyte. The CNT–CNF/PVAB composite hydrogel showed fast self‐healing (20 s at room temperature) and pH‐sensitivity due to the reversible dynamic interactions produced by crosslinker of borate ions, CNT–CNF nanohybrids, and PVA chains. The bicomponent CNT–CNF nanohybrids that combined the template function of CNFs and conductivity of CNTs were uniformly dispersed in PVAB matrix. Resulting CNT–CNF/PVAB composite hydrogels with conductive 3D network showed enhanced mechanical property and electrochemical performance (specific capacitance of 117.1 F g−1). Luo and co‐workers[ 161 ] reported a biocompatible and autonomously self‐healing supercapacitor with a hybrid system of tannic acid, CNC, and gelatin methacrylate (GelMA), as shown in Figure 12a–c. With the presence of tannic acid, the conductive agents of PANI and RGO were feasibly coated onto the surface of GelMA–CNC hydrogel via in‐situ polymerization. Obtained SC showed specific capacitance of 1.86 F cm−3 and retained 96% after one cut–healing process. Zhong and co‐workers[ 162 ] constructed a flexible supercapacitor using pressure‐sensitive hydrogels of lignosulfonate/SWCNT (Lig/SWCNT). The assembled biomass‐based SC with cellulose hydrogels as the electrolyte showed good specific capacitance (292 F g−1 at 0.5 A g−1). Remarkably, the flexible supercapacitor presented excellent electrochemical stability even after 1000 bending cycles (Figure 12d–e). Yang's group[ 163 ] reported a foldable supercapacitor electrode through coating the microporous cellulose fibers with SWCNTs, following by infiltration and in‐situ polymerization of aniline monomers. It was found that SWCNTs were wrapped around the interpenetrating cellulose fibers, ultrathin and ultralong PANI nanoribbons were formed in the SWCNT network. The prepared electrode demonstrated a relatively high capacitance (40.5 F cm−3). After repeated folding/unfolding process of 1000 times, the electrode showed almost no loss of capacitance or mechanical failure (Figure 12f,g). With assembling PANI and carboxylated ginger CNFs in a green aqueous medium, Yu and co‐workers[ 164 ] reported a 3D lightweight composite aerogels. The lightweight conductive supramolecular aerogel with hierarchically porous 3D structures showed a good conductivity of 372 S cm−1 and a high areal capacitance of 59.26 mF cm−2. In addition, the supramolecular aerogel could monitor concentration changes of different gases in the external environment and pulse beat signals or human motion signals in different ways.
Figure 12.
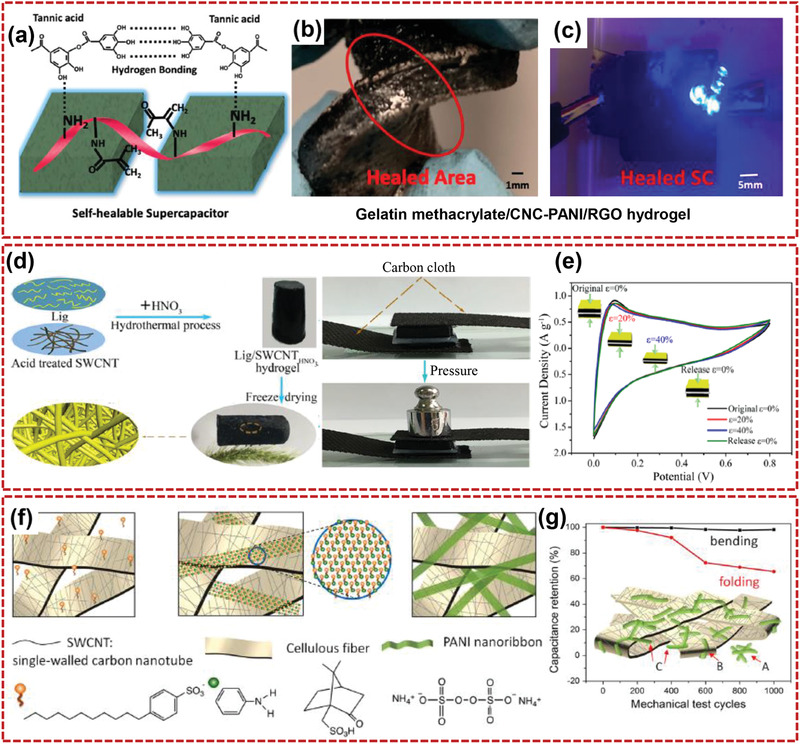
Multifunctional supercapacitors based on nanocellulose‐based composites. a) Schematic diagram of the self‐healing mechanism, b) twisting of self‐healed SC, and c) healed SC lightening the LED. Reproduced with permission.[ 161 ] Copyright 2019, AmericanChemical Society. d) Preparation process of Lig/SWCNT aerogel and a compressing and recovering process of the compressible SC, and e) cyclic voltammetry curves of the compressible SC at various strains. Reproduced with permission.[ 162 ] Copyright 2018, AmericanChemical Society. f) Schematic illustration for the preparation of SWCNT/PANI nanoribbon paper, and g) mechanical properties of SWCNT/PANI nanoribbon paper electrode. Reproduced with permission.[ 163 ] Copyright 2014, Elsevier.
Overall, nanocellulose‐based composite electrode has been a competitive candidate in promoting the development of flexible supercapacitors. For EDLC, nanocellulose can effectively prevent the accumulation of carbon materials, significantly improve the hydrophilic performance, and enhance the utilization of mesopores of the electrode materials. To improve electrochemical performance, great efforts should be aimed at the design of high surface area composite structures. For pseudocapacitor, the electrochemical performance of the nanocellulose‐based composite electrode can be improved by introducing pseudocapacitive materials, and the component of nanocellulose can improve the surface and mechanical properties of the composite electrode. Furthermore, when the active materials form a strong entangled network due to the high aspect ratio of nanocellulose, these could endow the composite electrode with the flexible, compressible, bendable, or even foldable properties. In addition, the interface interaction between the nanocellulose and the active material should be optimized to maximize the synergistic effect, improving the electrochemical performances of nanocellulose‐based composite electrode while maintaining or enhancing its flexibility and mechanical strength. Finally, the multifunctional flexible nanocellulose‐based composite electrode is urgent for further development to meet the increasing demands of wearable, portable, and implantable electronic devices.
4.1.2. Flexible Electrolytes/Separators
Due to the robust mechanical property, hydrophilic performance, refined nanostructure, and high porosity of nanocellulose, it has been served as the separators/electrolytes for supercapacitors.[ 36 , 165 , 166 ] Lee and co‐workers reported a nanoporous cellulose separator with a bilayer nanomat structure and high porosity.[ 167 ] In this work, the top layer was a nanoporous thin mat of terpyridine‐functionalized CNF and the support layer was a thick macroporous mat of electrospun polyvinylpyrrolidone/polyacrylonitrile. The ion transport rate and leakage current were balanced by the unique porous bilayer hierarchical/asymmetric structure. And the cycling performance of assembled devices with this unique nanocellulose‐based separator realized a substantial improvement.
Wu and co‐workers reported a flexible cellulose‐based hydrogel membrane with hierarchical porosity and double‐crosslinked structure (Figure 13a–c).[ 168 ] In the membrane, the cellulose and polyacrylamide (PAM) was crosslinked by PDA. By adjusting the ratio of dopamine/acrylamide, it was found that the key factors affecting the mechanical properties of the hydrogel were the abundant hydrogen bonds distributed in the hydrogel network and the π–π stacking of the catechol groups in the PDA. As the optimized sample of C4‐DM‐40 (cellulose4–PDA–PAM‐40), self‐healing and mechanical properties were better than other hydrogel samples. The hydrogel with Fe3+‐functionalization enhanced the sensitivity and conductivity of the hydrogel (Figure 13d,e). Significantly, activated carbon materials were deposited onto the C4‐DM‐40 hydrogel membrane to construct an integrated microsupercapacitor. The all‐in‐one device showed high volumetric capacitance of 394.1 F cm−3 and areal capacitance of 275.8 mF cm−2 at 10 mV s−1 (Figure 13f). By using a scalable and simple solution‐phase inversion method, Yu's group[ 30 ] demonstrated a renewable, transparent, and flexible mesoporous cellulose membrane (mCel‐membrane). The mCel‐membrane showed high porosity of 71.78% and uniform mesopores of ~24.7 nm. The mCel‐membrane with saturated KOH as electrolyte demonstrated good mechanical robustness and flexibility, high ionic conductivity of 325 mS cm−1, and high electrolyte retention of 451.2 wt%.
Figure 13.
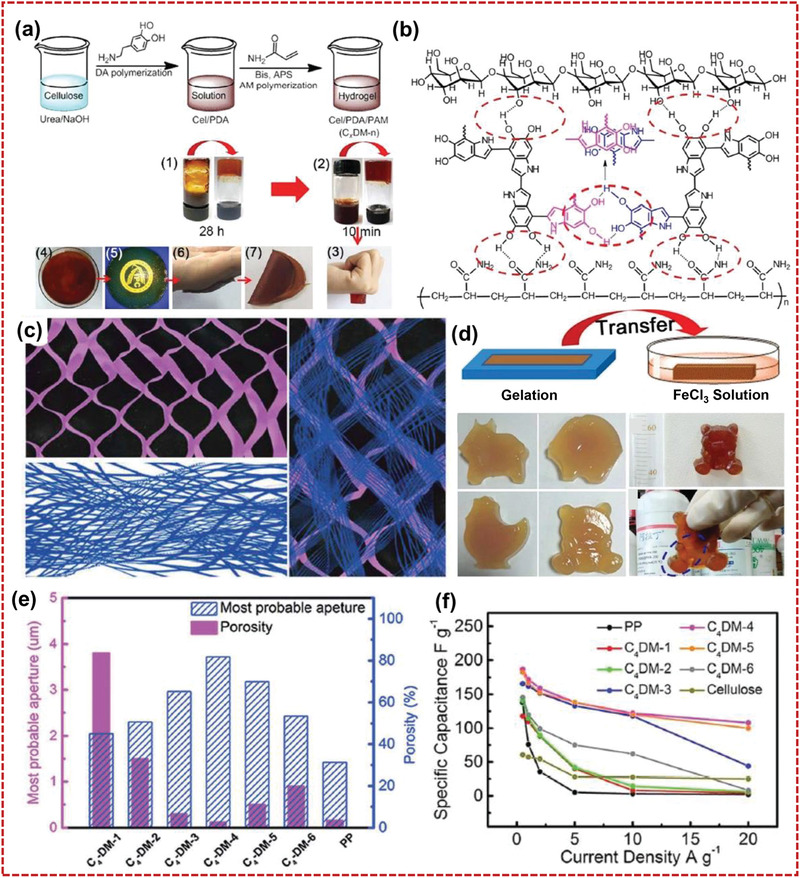
Flexible cellulose–PDA–PAM hydrogel separators. Reproduced with permission.[ 168 ] Copyright2018, The Royal Society of Chemistry. a) Synthetic process of the cellulose–PDA–PAM hydrogel, b) illustration of the molecular structure of cellulose–PDA–PAM hydrogels, c) the preparation process, plasticity, adhesivity, and healable properties of conductive hydrogel, d) 3D structure of cellulose–PAM, cellulose–PDA, and the hierarchical structure of C4‐DM‐40, e) the most probable aperture size and porosity of the C4‐DM‐x, and f) electrochemical performances of supercapacitors with C n ‐DM‐x as separators.
Consequently, the high ion conductivity and stability of gel‐ or solid‐state separator/electrolyte are also essential for improving the energy density and safety of supercapacitors in practical applications. The intrinsic properties of nanocellulose, such as low cost, superior mechanical strength and flexibility, and high thermal and chemical stability, could satisfy the critical requirements of polymer separators or electrolytes. Emerging strategies of adding or modifying redox active materials into nanocellulose‐based electrolytes could enhance the energy density of supercapacitors. Redox additives through the rapid electron transfer and reversible faradaic reaction at the interface of electrode–electrolyte can provide extra pseudocapacitance, thereby significantly enhancing the specific capacitance. Furthermore, the construction of crosslinked networks or ionic bonds in nanocellulose‐based electrolytes can endow supercapacitors with additional functions, such as stretchability and self‐healing property.
Due to various advantages including low cost, strong mechanical strength, and porous network, nanocellulose‐based composite materials have been verified not only to implement in SCs but also to fabricate other kinds of EES systems, especially in rechargeable batteries, such as LIBs, LSBs, sodium‐ion batteries (SIBs), etc.
5. Lithium‐Ion Batteries
LIBs with environmentally benign operation, considerable lifespan, large output voltage, and high energy density, have emerged as the main power source for consumer electronics since 1991 by introduction of Sony Inc.[ 169 ] In principle, by transporting the insertion/deintercalation of Li+ between the anode and cathode, the charging and discharging process of LIB is realized, as shown in Figure 14a. When charging, forced by an external power source, Li+ deintercalates from the cathode and inserts into the anode through the electrolyte. When discharging, Li+ carrying the current from the anode moves back to the cathode. Nanocellulose‐based materials with hierarchical microstructures, robust mechanical performance, and good electrochemical stability have demonstrated great potential to be used in LIBs as electrode components, solid electrolytes, and separator membranes.
Figure 14.
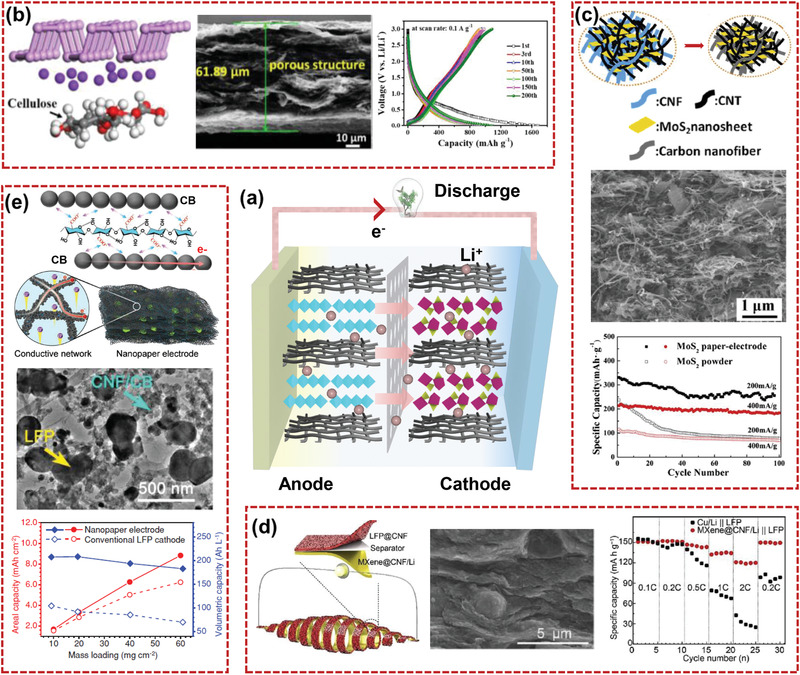
a) Schematic illustration of the working mechanism of LIBs with the nanocellulose‐based composite electrodes. Structures and electrochemical properties of b) lithium‐ion battery anodes of free‐standing black phosphorus/nanocellulose nanowires composite. Reproduced with permission.[ 170 ] Copyright 2020, AmericanChemical Society. c) Flexible MoS2‐based paper‐electrode assembled with carbonized CNF and CNTs. Reproduced with permission.[ 171 ] Copyright 2018, Elsevier. d) Lithium‐ion battery cathodes of flexible MXene@CNF film. Reproduced with permission.[ 174 ] Copyright 2020, Elsevier. e) Li–LFP batteries enabled by conductive cellulose nanofiber. Reproduced with permission.[ 31 ] Copyright 2018, Wiley‐VCH.
5.1. Flexible Electrodes
Electronic equipment continues to develop rapidly in the direction of lightness, miniaturization, eco‐friendly, and integration. There is an urgent need for a matching energy supply system to solve the practical application requirements of intelligent communications, mobile medical, and other fields. Therefore, development and utilization of flexible and multifunctional LIBs are particularly important for wearable and smart electronic devices. Nanocellulose can be combined with other Li‐hosting components as a flexible substrate in anode/cathode composites. Electrochemical performances and strategies of various nanocellulose‐based composites for flexible lithium‐ion battery electrodes are summarized and compared in Table 2 .
Table 2.
Electrochemical performances and strategies of various nanocellulose‐based composites for flexible lithium‐ion battery electrodes
| Electrode | Preparation | Capacitance | Coulombic efficiency | Cycling stability | Refs. (Year) |
|---|---|---|---|---|---|
| Anode: | |||||
| LTO/CNT/CNF film | Extrusion process | 157 mAh g−1 | 100% | 500 (96.4%) | [ 175 ] (2015) |
| Fe3O4‐decorated CF aerogel derived from BC | Hydrothermal and carbonization | 754 mAh g−1 | – | – | [ 27 ] (2015) |
| Cladophora CNF/Si/CNT paper | Paper‐making process | 800 mAh g−1 | >95% | – | [ 128 ] (2016) |
| Carbonized‐CNF/Li | 3D print | 2346 mAh g−1 | – | 3000 (85%) | [ 34 ] (2019) |
| Carbonized MoS2/CNF/CNTs hybrid film | Pressured extrusion | 720 mAh g−1 | 97.8% | – | [ 171 ] (2019) |
| Self‐supporting MXene@CNF/Li composite film | Spin steaming technology‐induced assembly | 1973 mAh g−1 | – | 150 (79.4%) | [ 174 ] (2020) |
| BP@CNF composite film | Vacuum‐assisted filtration | 1020.1 mAh g−1 | – | 400 (84.9%) | [ 170 ] (2020) |
| Cathode: | |||||
| CNF/CB/LFP nanopaper | Freeze‐dryer | 8.8 mAh cm−2 | – | 150 (>90%) | [ 31 ] (2018) |
| Carbonized‐CNF/LFP | 3D print | 167 mAh g−1 | 97.2% | 3000 (85%) | [ 34 ] (2019) |
Li et al.[ 18 ] fabricated a well‐aligned CNF/RGO composite microfibers with wet‐spinning method followed by carbonization. The as‐prepared fibers with high conductivity of 649 ± 60 S cm−1 were demonstrated as anodes of lithium ion battery. The anode showed a stable discharge capacity of 312 mAh g−1. Wang et al.[ 170 ] fabricated a free‐standing black phosphorus/nanocellulose nanowires composite anode by vacuum‐assisted filtration (Figure 14b). The black phosphorus/nanocellulose nanowires composite offered 3D mixed‐conducting network for transportation of Li+ and electrons. Benefiting from the multifunction of nanocellulose, the black phosphorus/nanocellulose nanowires composite showed high capacitance of 1020.1 mAh g−1 at 0.1 A g−1. A flexible MoS2‐based hybrid film with hierarchical structure was fabricated by using TEMPO‐oxidized CNF as fibrous skeletons and biobased binder.[ 171 ] After adding conductive fillers of CNT and carbonization process, the MoS2‐based paper‐electrode nanoarchitectures were finally obtained, as shown in Figure 14c. The flexible paper‐electrode assembled with carbonized CNF, CNT, and ultrathin MoS2 nanosheets demonstrated a high initial discharge specific capacity of 930 mAh g−1. Otherwise, nanocellulose‐derived carbon materials can be employed as versatile conductive porous scaffolds to support active anode materials. Fe3O4 nanoparticles,[ 27 ] SnO2 and Ge nanoparticles,[ 172 ] and MoS2 nanoleaves[ 132 ] have been successfully synthesized on nanocellulose‐derived carbon scaffolds through an in‐situ growth method. A flexible framework of 3D carbonaceous aerogel for supporting Fe2O3 was prepared by using BC.[ 130 ] The amorphous Fe2O3 was tightly coated on the 3D carbonized BC (CBC) with mechanical stability and good electrical conductivity. Benefiting from the hierarchical pores in amorphous Fe2O3@CBC composite and the well‐dispersed Fe2O3 nanoshells, when assembled into a half‐cell, the electrode exhibited high‐rate capability and stable cycling performance.
For LIBs cathodes, the most common active materials are LiMn2O4, LiCoO2, and LiFePO4 in the current commercial market, but the cycling life and rate capability of these electrodes are still limited by the fact of the severe structural degradation during repeated charge/discharge process as well as slow kinetics for electron and Li+ transport.[ 34 , 173 ] Accordingly, nanocellulose could be the flexible matrices for constructing cathode composites with conductive materials to improve the ionic and electronic conductivity of the electrode and alleviate the stress/strain for preserving electrode integrity. By mixing a trace of CNF, Guo and co‐workers constructed a MXene film as Li host with topological structures via spin steaming method.[ 174 ] The MXene@CNF film with enhanced mechanical strength and flexibility demonstrated interlocked topological microstructure, as shown in Figure 14d. The assembled flexible LIB (MXene@CNF–Li film anode matched with flexible LiFePO4/cellulose nanofiber cathodes) showed outstanding stability and high specific capacity. Cao et al.[ 175 ] demonstrated a free‐standing lithium titanate (LTO)/CNT/CNF composite network film by using a pressure‐controlled aqueous extrusion process. The combination of CNF and CNT constructed a strong conductive fibrous network. Moreover, CNT linked with LTO aimed to build electronic conductive paths to help remedy the intrinsically low electronic conductivity of LTO. The flexible anode for LIBs demonstrated good high‐rate cycling performance (142 mAh g−1 even at 10C). A flexible conductive nanofiber network was reported for high‐loading thick electrodes, in which negatively charged CNFs anchored neutral carbon black nanoparticles.[ 31 ] The conductive nanofiber network integrated with lithium iron phosphate (LFP) with robust electrical networks enabled a high‐loaded and compact electrode, which effectively shortened the ion transmission path (Figure 14e). The conductive network with interconnected nanopores not only acted as a nanoscale electrolyte reservoir surrounding the electroactive material, but also acted as ion‐transport channels across the electrode. The assembled flexible Li–LFP batteries exhibited superior volumetric energy density of 538 Wh m−3 due to the fast charge transfer kinetics and compact electrode structure.
Overall, nanocellulose has shown great potential in manufacturing flexible LIBs due to its high mechanical strength, network entanglement structures, and high aspect ratio. However, most of the electrode materials of lithium‐ion batteries reported so far have been simply synthesized by only mixing nanocellulose with active materials, which results in relatively weak interfacial interactions. The interface structure and interactions using the noncovalent or covalent chemical bonds should be considered to improve the interface interaction between nanocellulose and active materials, so as to obtain an electrode with good electrochemical performance, robust mechanical strength, high flexibility, and effective mass loading.
5.2. Flexible Electrolytes/Separators
The separator of LIB is located between the anode and the cathode. It could prevent the anode and the cathode from being short‐circuited due to contact. The electrolyte serves as an electrolyte reservoir to form a channel for transportation of lithium ions during the process of charging/discharging. Due to the excellent mechanical and thermal properties as well as good hydrophilic of nanocellulose‐based paper/film materials, they have been used in LIBs to enhance power density, energy density, safety, and cycle life by influencing the cell kinetics. Recently, Sun et al.[ 29 ] introduced ZIF8 into the CNF system by in‐situ synthesis to improve the pore structure of composite separators. The introduction of ZIF8 led to more homogeneous pore distribution due to the prevented aggregation of CNF. ZIF8–CNF composite separators not only had the advantages of environmentally friendly CNF but also demonstrated superior thermal stability (thermally stable up to 200 °C). These composite separators with fast wetting speed and better surface wettability could decrease the battery interior resistance and electrolyte filling time. The LIBs assembled with ZIF8–CNF composite separator showed better rate capacity, cycling performance, and discharge capacity retention. Huang et al.[ 176 ] prepared TOBC membranes for the separators of LIBs. The TOBC membranes had adequate porosity and desirable affinity with liquid electrolyte and lithium electrode, giving rise to superior electrolyte uptake and small interfacial resistance. To date, the research on nanocellulose‐based separators has mainly concentrated on the development of safe separators with good electrolyte wettability. However, few works focused on chemically functionalized cellulose separators, which may also enhance the performances of the LIBs. Wang et al.[ 177 ] fabricated a flexible redox‐active bilayered nanocellulose‐based separator, which included a redox‐active PPy‐containing support layer and a mesoporous insulating CNF layer (Figure 15a–c). The PPy‐containing layer added extra capacity to the LIBs and provided mechanical support to the CNF layer. The redox‐active separator exhibited high flexibility, and no internal short circuits were observed during the operation of these LIBs. Flexible gel‐ and solid‐state electrolytes can provide greater portability and security to LIBs compared with liquid electrolytes. To achieve this goal, nanocellulose as a candidate has been investigated as the building block for gel‐ or solid‐state electrolytes. Xu et al.[ 178 ] prepared internal crosslinking BC network with high‐strength as the gel polymer electrolyte. The lithium ion channels were created and organic solvents were captured by glycosidic bonds, ether groups, and hydroxyl groups on the BC chains to generate high ionic conductivity. The vertical growth of lithium dendrite was inhibited by BC nanofibers. The assembled batteries with gel polymer electrolyte demonstrated good rate and cycling performances. Du et al.[ 179 ] prepared an environmentally friendly and mechanically robust cellulose gel membrane for electrolyte of LIBs. Cellulose membranes with 5% epichlorohydrin demonstrated not only wide electrochemical stability window, Li+ transference number (0.82), high ionic conductivity (6.34 × 103 mS cm−1), and better interfacial compatibility with electrodes, but also good thermal stability and mechanical strength. Dong et al.[ 32 ] prepared a multifunctional polymer electrolyte of BC supported poly(methyl vinyl ether‐alt‐maleic anhydride) (P(MVE‐MA)) for a 4.45 V class LiCoO2 lithium metal battery (Figure 15d,e). As shown in Figure 15f, the tensile strength of the resulting polymer electrolyte reached 50 MPa, which was attributed to the hydrogen‐bonding interaction between P(MVE‐MA) and BC. Even at 60 °C, LiCoO2 lithium metal battery made with the polymer electrolyte exhibited high capacity retention (after 700 cycles, the capacity retention was 85%). As shown in Figure 15g,h, a commercial LED was lit by as‐fabricated flexible LiCoO2/Li battery with electrolyte of BC supported P(MVE‐MA).
Figure 15.
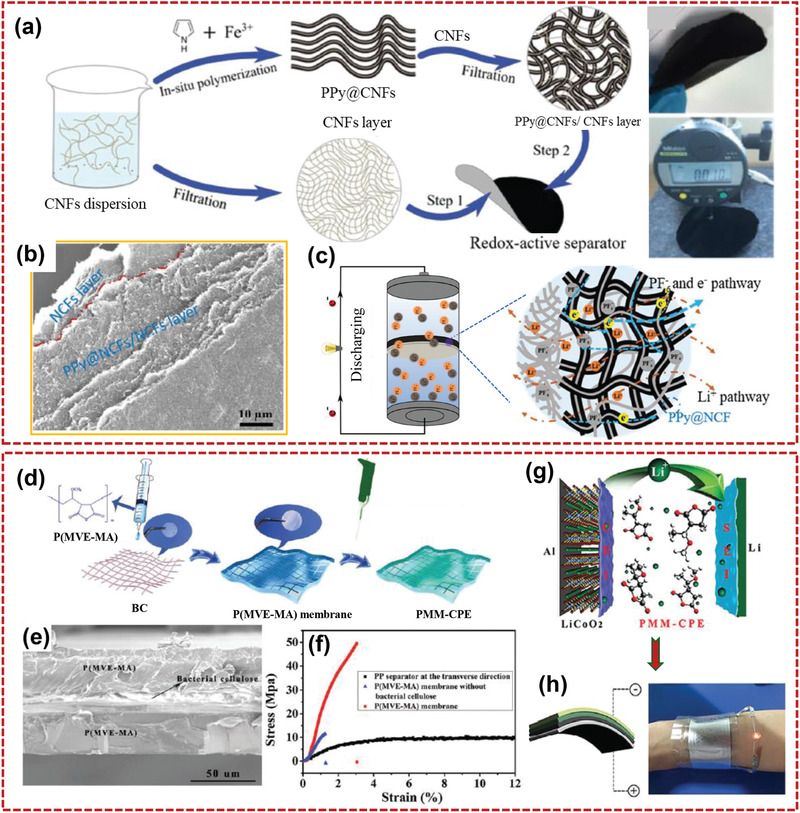
a) Schematic description for the fabrication of the PPy@CNFs/CNFs separator, b) cross‐sectional SEM image of the PPy@CNFs/CNFs separator, and c) working diagram of LFP/Li batteries equipped with the PPy@CNFs/CNFs separator. Reproduced with permission.[ 177 ] Copyright 2017, Wiley‐VCH. d) Schematic representation of the BC supported P(MVE‐MA) fabrication process, e) side‐view SEM image of the BC supported P(MVE‐MA) membrane, f) stress–strain curves of the BC supported P(MVE‐MA) membrane, the P(MVE‐MA) membrane without BC, and polypropylene separator, g) the mechanism of action of PMM‐CPE in the lithium metal battery, and h) photographs of an LED lighting‐up by the flexible LiCoO2/Li battery. Reproduced with permission.[ 32 ] Copyright 2018, The Royal Society of Chemistry.
In short, to enhance the electrochemical performances of LIB, the nanocellulose‐based separator/electrolyte should offer high pore structure to promote the ion migration rate in the electrochemical reaction. Crosslinking network has been introduced to improve the mechanical flexibility of nanocellulose‐based electrolyte. For instance, dual‐crosslinked hydrogel with reversible bonds and strong covalent bonding, such as the dynamic borate ester bonding, ionic bonding, and H‐bonding, could support the nanocellulose–polymer composites with good elasticity and mechanical strength. To realize the multifunctional properties, such as resistance to extreme environments and self‐healing properties, much relevance of external stimuli and molecular interactions among components need to be investigated.
6. Other Flexible Batteries Based on Nanocellulose‐Based Composites
6.1. Lithium–Sulfur Batteries
Lithium–sulfur batteries have captured tremendous attention recently owing to their high theoretical volumetric (2800 Wh L−1) and gravimetric (2500 Wh kg−1) energy density and far beyond higher capacity than that of conventional lithium‐ion batteries. Therefore, the development of high‐performance flexible lithium–sulfur batteries can better meet the needs of flexible wearable electronic devices in the future. Moreover, outstanding properties of elemental sulfur, such as abundant resources and low price, make LSBs a particularly attractive EES.[ 180 ] Nevertheless, there are still remarkable challenges for the practical application of LSBs. First, during electrochemical cycles, the volume changes of sulfur particles would lead to structural changes of active materials and this would result in the reduction of capacity. Second, the insulating sulfur and Li2S make electrochemical kinetics sluggish. Third, polysulfides are easily dissolved in the electrolyte leading to the “shuttle phenomenon.” To address these problems, various attempts have been done for the design and preparation of novel electrodes, electrolytes, and separators.[ 181 ]
6.1.1. Flexible Electrodes
Nanocellulose and active materials can be integrated to fabricate composite electrodes for LSBs. Owing to its rich hydroxyl groups, entangled networks, and high aspect ratio, flexible CNF was developed as a building block to fabricate free‐standing and sandwich‐structured cathode materials with high areal mass loading for long‐life LSBs (Figure 16a–c).[ 182 ] Reasonably designed structure made the electrode with good rate performance and high specific capacity. Interconnected CNF/CNT layers were prepared on both sides of the active layer to supply efficient electron transport and entrap polysulfide species. The synergistic effects of the physical encapsulation by carbonaceous materials (graphene and CNT/CNF fibers) and chemisorption for lithium polysulfides by chemical functionalization (hetero‐N‐doping) resulted in good electronic conductivity and suppression of polysulfide dissolution and migration. The electrode presented a high capacity of ≈8 mAh cm−2 with an areal sulfur loading of 8.1 mg cm−2, and the average Coulombic efficiency was ≈97.3%. Wu et al.[ 183 ] reported a high performance of lithium polysulfide batteries in which a local concentration effect‐derived heterogeneous Li2S2/Li2S deposition took place on dual‐phase MWCNT/CNF/NiCo2S4 self‐standing paper (Figure 16d,e). A high‐performance self‐standing soluble lithium polysulfide (LiPS) host‐MWCNT/CNF/NiCo2S4 (3.5 mg cm−2) could catalyze 2.85 mg cm−2 (based on sulfur) loaded LiPS to demonstrate high specific capacity of 1154 mAh g−1 at 0.1C. The local concentration effect of LiPS was promoted by the insulating phase defect of nano‐CNF, LiPS adsorptive NiCo2S4, and highly electronic conductivity (above 50 S cm−1), which contributed to the stable and fast heterogeneous particle‐shaped deposition of Li2S2/Li2S and high kinetics of the LiPS cathode.
Figure 16.
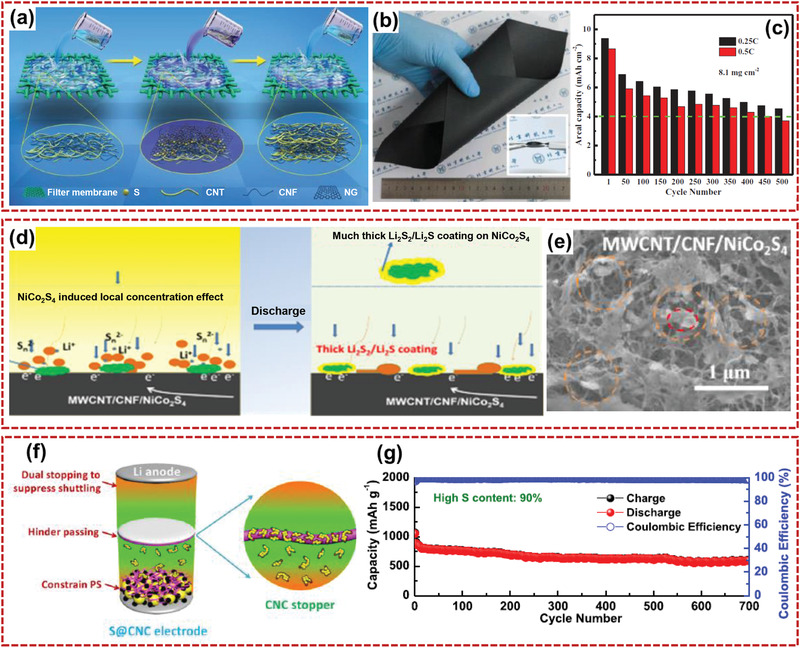
Applications of flexible nanocellulose‐based composites in LSBs: a) Schematic preparation process of the free‐standing electrode, b) photographs of the flexible electrode, and c) areal capacities of the Li–S batteries at different cycles. Reproduced with permission.[ 182 ] Copyright 2017, Wiley‐VCH. d) Schematic illustration of LiPS–Li2S2/Li2S depositing on the paper electrode, and e) SEM image of MWCNT/CNF/NiCo2S4 supported LiPS cells after the first discharge cycle. Reproduced with permission.[ 183 ] Copyright 2020, American Chemical Society. f) Schematic diagram of Li–S battery with a CNC binder, and g) cycling performance of the Li–S battery with a CNC binder and a CNC‐coated separator. Reproduced with permission.[ 185 ] Copyright 2020, American Chemical Society.
6.1.2. Flexible Electrolytes/Separators
The nanoscale microfibrillated cellulose‐laden polymer systems were prepared as electrolyte for Li–S rechargeable battery.[ 184 ] The polymer electrolyte demonstrated outstanding thermal stability, high ionic conductivity, and stable interface toward lithium metal. The battery based on the abundant natural cellulose‐based polymer electrolyte as separator exhibited higher specific capacity, superior Coulombic efficiency, better cycling stability, and rate capability at ambient conditions. Hydrophilic, eco‐friendly, and low‐cost CNC was proposed for high‐performance LSBs as a multifunctional polysulfide stopper.[ 185 ] The functional groups with rich electrons on CNC delivered robust binding energies with polysulfides according to the density functional theory. Furthermore, CNC could also act as a polysulfide stopper on the separator to hinder polysulfide shuttling to the Li anode. Hence, the as‐prepared LSBs demonstrated outstanding cycling performance even under the conditions of high sulfur content of 90 wt% (63 wt% in the cathode), high loading of 8.5 mg cm−2, and high temperature of 60 °C (Figure 16f,g).
Overall, nanocellulose‐based hybrid materials are expected to be used as electrodes, electrolytes, and separators for high performance LSBs. However, there are still challenges, e.g. how to achieve high loading of active materials in the cathode while maintaining high mechanical strength, flexible, and excellent electronic conductivity of the nanocellulose‐based composites. To alleviate the polysulfide shuttle with a stable and persistent effect is still a challenge for the nanocellulose‐based electrodes and separators. Therefore, reasonable design and optimization of hierarchically porous structures and surface/interface characteristics of nanocellulose are essential for the construction of high‐performance LSBs.
6.2. Sodium‐Ion Batteries
Na‐ion batteries recently have attracted widespread attention due to their low cost and abundant reserves of sodium.[ 186 ] SIBs possess similar working principle as LIBs: Na+ migrates in the electrolyte between cathode and anode while the electrons are transported through the external circuit. Nevertheless, the radius of Na+ is ≈55% larger than that of Li+, so that some anode materials which are the high‐quality anode in LIBs exhibit poor storage performances in SIBs.[ 187 ] Generally, the host frameworks with large interstitial spaces in SIBs electrode material were required for Na+ insertion/extraction. In addition to the kinetic issue, the larger Na+ radius also relates to the structural changes of electrode materials that may occur during insertion or extraction. Hence, the development of appropriate electrode materials is essential to improve the performance of SIBs.
6.2.1. Flexible Electrodes
Nanocellulose can be integrated with active materials to fabricate flexible electrodes for SIBs. Hu and co‐workers[ 28 ] showed that using TEMPO‐oxidized CNF as a dispersant can effectively disperse 2D materials (such as MoS2) in aqueous solutions and form hybrids with 2D materials. The CNF/MoS2/CNTs composite films with the optimized ratio can be used as flexible electrodes for SIB anodes. Although with the low content of MoS2, the first cycle discharge capacity was 147 mAh g−1, which was still higher than the capacity of the MoS2/CNTs anode. Nanocellulose could be compounded with active material and converted into functional sodium‐ion electrode material through further carbonization.[ 188 , 189 ] For instance, Yu and co‐workers[ 190 ] demonstrated a binder‐free and free‐standing carbon nanofiber electrode via simple carbonization of BC film. The results showed simutaneous adsorption and co‐intercalation of sodium ions and solvent in ether‐based electrolytes, leading to long cycle life and high Coulombic efficiency at high‐rate capability.
The larger size of Na+ compared to Li+ hinders the kinetics
of the electrochemical reactions. Therefore, nanocellulose‐based composites for reversible electrodes should possess sufficiently interstitial sites and/or large channels. The future challenge is to construct and fabricate novel nanocellulose‐based composites with hierarchical 3D porous structures through advanced synthesis strategies, which contain appropriate storage space for Na+, higher Na+ diffusion coefficients, shorter ions diffusion distance, and fast electron transport.
6.3. Zn‐Ion Batteries
Rechargeable batteries with aqueous electrolytes have received increasing attention due to their low fabrication costs and high safety, as compared to popular LIBs with organic electrolytes. Benefiting from high electrochemical stability in the aqueous electrolyte, metal Zn can be directly applied as anodes in ZIBs. Zn can realize the participation of two electrons in the electrochemical reaction, demonstrating high theoretical specific capacitance (820 mAh g−1 and 5855 mAh cm−3) and low redox potential (−0.763 V vs standard hydrogen electrode). With the advantages of safety, low cost, and high energy density, ZIBs are expected to become a high‐efficiency energy storage devices for next‐generation portable electronic equipment.
6.3.1. Flexible Electrodes
Using Zn‐grown graphite papers as the anode and nanostructured polyaniline–cellulose paper as the cathode, solid‐state flexible aqueous ZIB was prepared.[ 191 ] The flexible gel electrolyte based on CNF demonstrated high ionic conductivity. This ZIB delivered an energy density of 117.5 mWh g−1 when the power density was 0.16 W g−1. It also exhibited a good cyclic stability with high capacitance retention of 84.7% over 1000 cycles. More importantly, after 1000 mechanical bending cycles, the specific capacity retention was still about 91%. As shown in Figure 17a, Yi et al.[ 192 ] adopted the high‐strength, lightweight, low‐cost, and sustainable cellulose yarns as robust and flexible substrates of both cathode (polyaniline) and anode (metal Zn) for aqueous Zn‐ion fibrous battery. The fiber battery delivered an energy density of 153.2 Wh kg−1 at a power density of 0.16 kW kg−1, and the energy density maintained at 61.1 Wh kg−1 at a high‐power density of 6.5 kW kg−1 (Figure 17b). Meanwhile, superior cyclic stability (91.9% over 1000 charge/discharge cycles) and high flexibility (97.5% over 1000 bending cycles) were presented by this fiber battery.
Figure 17.
Applications of nanocellulose‐based composites in flexible Zn‐ion batteries: a) Schematic illustrations of the anode and cathode for the aqueous Zn‐ion fibrous battery, and b) Ragone plot of the Zn‐ion fibrous battery with the inset showing a photo of a powered LED under static loading (100 g). Reproduced with permission.[ 192 ] Copyright 2019, American Chemical Society. c) Synthetic procedure of the PANa–cellulose hydrogel electrolyte, d) cross‐sectional SEM image of the freeze‐dried PANa–cellulose hydrogel, e) schematic diagram of the stretchable flat‐shape zinc–air battery with PANa–cellulose hydrogel electrolyte, and f) photographs of fiber‐shaped zinc–air batteries to light up a smartwatch. Reproduced with permission.[ 193 ] Copyright 2019, American Chemical Society.
6.3.2. Flexible Electrolytes/Separators
Zhao et al.[ 193 ] reported flexible BCNF/PVA composite hydrogel electrolytes (BPCEs) for flexible zinc–air batteries. The mechanical performances of the PVA film were improved by the introduction of BC microfibers. The BPCEs showed superior toughness and mechanical strength due to the novel percolating load‐bearing dual network. By adding 6 wt% of BC, the elongation at break of the 6‐BPCE membrane was doubled and the tensile strength was increased by nine times. Compared to aqueous alkaline electrolytes, the batteries using the 6‐BPCE membranes exhibited higher stability and superior rechargeability. By designing a double‐network alkaline‐tolerant hydrogel electrolyte, Zhi's group[ 194 ] developed the fiber‐shaped (500% stretchable) and flat‐shaped (800% stretchable) zinc–air batteries with superstretchability. In the dual‐network hydrogel electrolyte, sodium polyacrylate (PANa) chains contributed to the formation of soft domains. The carboxyl groups neutralized by hydroxyls and cellulose as potassium hydroxide stabilizer were responsible for vastly enhanced alkaline tolerance (Figure 17c,d). The obtained fiber‐shaped zinc–air battery with superstretchability exhibited a high‐power density. In addition, the power density was slightly increased after stretching to 500% of its original length. Similar phenomena were observed for the 800%‐stretched flat zinc–air batteries. Benefiting from the advantages of the highly soft and alkaline‐tolerant hydrogel electrolyte, the devices after being heavily deformed could also maintain stable power output (Figure 17e,f).
In summary, Zn anode with modifyed nanoporous nanocellulose‐based composites contributed to keeping structural stability and reducing the volume variation. The nanocellulose‐based composite as a buffer layer could significantly suppress hydrogen evolution and Zn corrosion. The nanocellulose‐based composite for Zn cathode materials can serve as a surface coating to protect cathode from structural degradation and dissolution.[ 186 ] Because flexibility/wearability is the development trend of battery, more research on flexible nanocellulose‐based electrolytes/separators for ZIBs is needed. Integrating novel functions to the existing systems requires further exploration and modification from the viewpoint of polymer chemistry, so that the electrolyte is rendered with desired properties catering for practical applications. Further studies of simplified processing technology, new cathode, and mechanism will make the flexible high‐performance ZIBs possible.
7. Conclusion and Perspective
The forthcoming smart electronics era necessitates the development of advanced EES devices with outstanding electrochemical performances, flexibility, lightweight, and environmental friendliness. In this review, the recent progress on nanocellulose‐based composites for flexible EES applications has been summarized, mainly focusing on their rational structural design, interfacial engineering, and mechanisms of energy storage as well as the emerging functions of the constructed EES devices. Many efforts have been devoted to developing advanced nanocellulose‐based composites with reasonable structures and improved electrochemical performances using various fabrication strategies. In all, the superior mechanical strength, low density, and high specific surface area properties of nanocellulose demonstrate significant application in flexible composite electrodes. The high thermal and structure stability, and wettability of nanocellulose could endow the composites with good hydrophilicity and porosity, which show great prospects in flexible electrolytes and separators. Importantly, nanocellulose as building blocks endows the composite materials with great mechanical flexibility, which allows the flexible EES systems for applications as advanced electronics. Although rapid development and substantial achievements of nanocellulose‐based composites for EES have been witnessed in the past few years, many challenges still remain before such EES devices are realized in the widespread fields.
First, the lack of feasible techniques for continuous and large‐scale fabrication of nanocellulose‐based composites is still a limitation to its application in the EESs. For example, nanocellulose is predominantly dispersed in aqueous medium with low concentration (<3 wt%), complicated and time‐consuming processing steps (e.g., drying) are required to fabricate the free‐standing structures, such as films/nanopapers, fibers, and aerogels. To date, only a few strategies (e.g., directly blending, and in‐situ polymerization) have been proposed for compounding nanocellulose with various kinds of active materials, but some of those face the drawbacks of low efficiency, sophisticated process, and so on. Therefore, fabrication of nanocellulose‐based composites with desired electrochemical and physical properties and the development of advanced preparation strategies will continue to gain substantial interests in the future.
Second, although nanocellulose brings free‐standing structure to the composite electrodes, the mechanical strength and flexibility of some of the nanocellulose‐based composite are not sufficient for practical applications. For instance, the self‐assembled structures of low aspect ratio nanocellulose (e.g., CNC) tend to be fragile. Also, the incorporation of active materials will interrupt the hydrogen bonding of nanocellulose, causing a negative effect on the mechanical properties of the composites. Thus, rational selection of material composition and control of their arrangement in the composite, as well as introducing suitable crosslinker should be well investigated in future work for preparation of mechanically robust composites. Furthermore, with the increasing demand for wearable/portable electronics, the development of EES systems have been directed to scalability, integration, functionality, and intelligence. Currently, most nanocellulose‐based composites present a single function and are only applied in specific environmental scenarios. Therefore, it is still a great challenge to integrate multiple functions into a single nanocellulose‐based composite. With the necessary electrochemical properties, the development of multifunctional nanocellulose‐based composites, which are stretchable, self‐healing, low‐temperature resistance, and health monitoring, are attractive for future electronics applications.
Third, although there are several reports about compound modes and structures of nanocellulose‐based composites (e.g., electrochemically active materials anchored on the surface of nanocellulose, sandwiched between nanocellulose layers, or mixed in nanocellulose fibers) with improved electrochemical performance, mainly because of the proposed “synergistic effects” between the nanocellulose component and the other electrochemical active materials, it is still not clear which of these structures could provide the best electrochemical performance in specific EES applications. Therefore, the molecular interaction between active components and nanocellulose in the electrode/electrolyte interface should be fundamentally understood. As well, more effects should be analyzed to elucidate the working mechanisms from both theoretical and experimental aspects. Many theoretical studies have focused on liquid electrolytes so far. However, the charge storage mechanisms in gel or solid flexible electrolytes are due to the trapping of ions in polymer chains and their interaction at diverse scales more complicated and difficult to be analyzed currently, which needs theoretical advances as well as suitable experimental tools. This would permit the production of unique nanocellulose‐based composites with customized properties for practical applications in different EES systems.
Forth, the introduction of insulating nanocellulose component in the composite electrodes will reduce the conductivity and block the transport of electrons in the electrodes, which would cause negative effects on the electrochemical performance of the energy storage devices, especially the rate property. Thus, rational design of the materials constituents, surface/interface engineering, and fabrication techniques need more efforts to compensate for the lack of conductivity of nanocellulose. For instance, constructing interfacial contact modes of the active materials with the nanocellulose to optimize the balance between fast ion diffusion and electron transport is significantly vital for improving the kinetics of electrochemical reactions. In addition, new electrode materials such as MOFs and MXenes are also compounded with nanocellulose to obtain higher electrochemical performance. However, the relationship between the structure and performance, and energy storage mechanism of these composite materials need to be further understood. More potential applications of these nanocellulose‐based composites could be derived by optimizing the multifunctional dimensionalities and morphologies.
Fifth, although various combinations of nanocellulose with different kinds of active materials have been proposed for the preparation of composite electrodes and separators with superior electrochemical properties, which mainly benefit from their high wettability, high mechanical strength, and web‐like entangled porous structures, the design and control of the pore structure of the nanocellulose‐based composites are still limited. It should be pointed out that the pore size and porosity of the composite materials play a pivotal role on the electrochemical performance of the energy storage devices. For the composite electrodes, rational design of pore structure with hierarchical micropore–mesopore structure and large surface area will be of benefit to increasing the contact area of the electrode–electrolyte, decreasing the diffusion resistance of electrolyte ions, and shortening the diffusion length of the electrolyte ions. For the composite separators, suitable porosity and pore size will be conducive to the ionic transport. Therefore, more investigation on the control of pore structure of the nanocellulose‐based composite electrodes will need further efforts in the future work.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
T.X. and H.D. contributed equally to this work. T.X. and C.S. thank the National Natural Science Foundation of China (32071720) and the Key Technology Research and Development Program of Tianjin (19YFZCSN00950) from Tianjin Municipal Science and Technology Bureau for the financial support. K.Z. and P.L. thank the Federal Ministry for Economic Affairs and Energy (BMWi) and the Ministry for Science and Culture of Lower Saxony (MWK) for the financial support of the WIPANO project (FKZ03THW05K14). In addition, H.D. acknowledges the financial support from the China Scholarship Council (No. 201708120052). W.L. and H.L. acknowledge Innovation Project of Excellent Doctoral Dissertation of Tianjin University of Science and Technology (2020005), and Tianjin Research Innovation Project for Postgraduate Students (2020YJSB119, 2020YJSS130).
Open access funding enabled and organized by Projekt DEAL.
Biographies
Ting Xu obtained her Ph.D. degree in chemistry from Beijing University of Chemical Technology in 2020. Now, she is working as a postdoctoral researcher at Tianjin University of Science and Technology. Her research interests include the design and synthesis of nanocellulose‐based flexible supercapacitors, fibrous batteries, and multifunctional flexible polymer electrolyte.

Haishun Du received his master's degree from Tianjin University of Science and Technology in 2017. He is currently a Ph.D. candidate at Auburn University (AU). Prior to joining AU, he worked as a research assistant at Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences. His current research focuses on sustainable preparation of cellulose nanomaterials and their nanocomposites, exploring their potential applications in diverse fields such as flexible electronics, energy storage devices, sensors, and biomedicine. He has authored/coauthored more than 50 peer‐reviewed journal articles and 8 patents.

Chuanling Si is currently a government distinguished professor at Tianjin University of Science and Technology. He obtained his Ph.D. in 2006 in the field of wood chemistry from Kangwon National University, Korea. In the same year, he joined the faculty of Tianjin University of Science and Technology as an associate professor and became a professor in 2011. He is a group leader at Tianjin Key Laboratory of Pulp and Paper. His research focuses on the conversion of biomass to chemicals and materials including cellulose and its derivatives production, nanocellulose‐based functional materials, and biomass‐based energy storage materials.

Peiwen Liu is a project leader in the Department of Wood Technology and Wood‐based Composites, Georg‐August‐University of Göttingen. He was awarded a Ph.D. in wood technology and wood chemistry from Georg‐August‐University of Göttingen in 2020. His research fields are the development of selective and efficient as well as ecologically and economically benign preparation methods and their use in the exploitation of natural polymers.

Kai Zhang is a professor and the head of the department for Wood Technology and Wood‐based Composites, Georg‐August‐University of Göttingen. He studied at Hefei University of Technology (B.Eng.), China, and Dresden University of Technology (M.S. and Ph.D., 2011), Germany. After research stations at Dresden University of Technology, the Pennsylvania State University, and Darmstadt University of Technology, he joined the University of Göttingen in 2015. One of his major research fields is the preparation of functional materials using biobased compounds via diverse chemical and physical approaches.

Xu T., Du H., Liu H., Liu W., Zhang X., Si C., Liu P., Zhang K., Advanced Nanocellulose‐Based Composites for Flexible Functional Energy Storage Devices. Adv. Mater. 2021, 33, 2101368. 10.1002/adma.202101368
Contributor Information
Chuanling Si, Email: sichli@tust.edu.cn.
Peiwen Liu, Email: pliu@uni-goettingen.de.
Kai Zhang, Email: kai.zhang@uni-goettingen.de.
References
- 1. Wang H., Yang Y., Guo L., Adv. Energy Mater. 2017, 7, 1601709. [Google Scholar]
- 2. Liu W., Song M.‐S., Kong B., Cui Y., Adv. Mater. 2017, 29, 1603436. [DOI] [PubMed] [Google Scholar]
- 3. Zhao D., Zhu Y., Cheng W., Chen W., Wu Y., Yu H., Adv. Mater. 2020, 2000619. [DOI] [PubMed] [Google Scholar]
- 4. Gogotsi Y., ACS Nano 2014, 8, 5369. [DOI] [PubMed] [Google Scholar]
- 5. Lu J., Chen Z., Ma Z., Pan F., Curtiss L. A., Amine K., Nat. Nanotechnol. 2016, 11, 1031. [DOI] [PubMed] [Google Scholar]
- 6. Admassie S., Ajjan F. N., Elfwing A., Inganäs O., Mater. Horiz. 2016, 3, 174. [Google Scholar]
- 7. Klemm D., Heublein B., Fink H.‐P., Bohn A., Angew. Chem., Int. Ed. 2005, 44, 3358. [DOI] [PubMed] [Google Scholar]
- 8. Klemm D., Kramer F., Moritz S., Lindstrom T., Ankerfors M., Gray D., Dorris A., Angew. Chem., Int. Ed. 2011, 50, 5438. [DOI] [PubMed] [Google Scholar]
- 9. Lagerwall J. P. F., Schutz C., Salajkova M., Noh J., Park J., Scalia G., Bergstrom L., NPG Asia Mater. 2014, 6, e80. [Google Scholar]
- 10. Xu Z., Xie F., Wang J., Au H., Tebyetekerwa M., Guo Z., Yang S., Hu Y.‐S., Titirici M.‐M., Adv. Funct. Mater. 2019, 29, 1903895. [Google Scholar]
- 11. Du H., Liu W., Zhang M., Si C., Zhang X., Li B., Carbohydr. Polym. 2019, 209, 130. [DOI] [PubMed] [Google Scholar]
- 12. Thiemann S., Sachnov S. J., Pettersson F., Bollström R., Österbacka R., Wasserscheid P., Zaumseil J., Adv. Funct. Mater. 2014, 24, 625. [Google Scholar]
- 13. An B. W., Heo S., Ji S., Bien F., Park J.‐U., Nat. Commun. 2018, 9, 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu H., Du H., Zheng T., Liu K., Ji X., Xu T., Zhang X., Si C., Chem. Eng. J. 2021, 426, 130817. [Google Scholar]
- 15. Chen W., Yu H., Lee S.‐Y., Wei T., Li J., Fan Z., Chem. Soc. Rev. 2018, 47, 2837. [DOI] [PubMed] [Google Scholar]
- 16. Wang Z., Lee Y.‐H., Kim S.‐W., Seo J.‐Y., Lee S.‐Y., Nyholm L., Adv. Mater. 2020, 2000892. [Google Scholar]
- 17. Dutta S., Kim J., Ide Y., Kim J. H., Hossain M. S. A., Bando Y., Yamauchi Y., Wu K. C. W., Mater. Horiz. 2017, 4, 522. [Google Scholar]
- 18. Li Y., Zhu H., Shen F., Wan J., Han X., Dai J., Dai H., Hu L., Adv. Funct. Mater. 2014, 24, 7366. [Google Scholar]
- 19. Kim H., Mattinen U., Guccini V., Liu H., Salazar‐Alvarez G., Lindström R. W., Lindbergh G., Cornell A., ACS Appl. Mater. Interfaces 2020, 12, 41211. [DOI] [PubMed] [Google Scholar]
- 20. Gou J., Liu W., Tang A., J. Mater. Sci. 2020, 55, 10699. [Google Scholar]
- 21. Guo R., Chen J., Yang B., Liu L., Su L., Shen B., Yan X., Adv. Funct. Mater. 2017, 27, 1702394. [Google Scholar]
- 22. Delannoy P.‐E., Riou B., Brousse T., Bideau J. L., Guyomard D., Lestriez B., J. Power Sources 2015, 287, 261. [Google Scholar]
- 23. Huang Y., Zheng M., Lin Z., Zhao B., Zhang S., Yang J., Zhu C., Zhang H., Sun D., Shi Y., J. Mater. Chem. A 2015, 3, 10910. [Google Scholar]
- 24. Li H., Cheng Z., Zhang Q., Natan A., Yang Y., Cao D., Zhu H., Nano Lett. 2018, 18, 7407. [DOI] [PubMed] [Google Scholar]
- 25. Kang Y., Chun S.‐J., Lee S.‐S., Kim B.‐Y., Kim J. H., Chung H., Lee S.‐Y., Kim W., ACS Nano 2012, 6, 6400. [DOI] [PubMed] [Google Scholar]
- 26. Li S., Huang D., Yang J., Zhang B., Zhang X., Yang G., Wang M., Shen Y., Nano Energy 2014, 9, 309. [Google Scholar]
- 27. Wan Y., Yang Z., Xiong G., Luo H., J. Mater. Chem. A 2015, 3, 15386. [DOI] [PubMed] [Google Scholar]
- 28. Li Y., Zhu H., Shen F., Wan J., Lacey S., Fang Z., Dai H., Hu L., Nano Energy 2015, 13, 346. [Google Scholar]
- 29. Sun X., Li M., Ren S., Lei T., Lee S.‐Y., Lee S., Wu Q., J. Power Sources 2020, 454, 227878. [Google Scholar]
- 30. Zhao D., Chen C., Zhang Q., Chen W., Liu S., Wang Q., Liu Y., Li J., Yu H., Adv. Energy Mater. 2017, 7, 1700739. [Google Scholar]
- 31. Kuang Y., Chen C., Pastel G., Li Y., Song J., Mi R., Kong W., Liu B., Jiang Y., Yang K., Hu L., Adv. Energy Mater. 2018, 8, 1802398. [Google Scholar]
- 32. Dong T., Zhang J., Xu G., Chai J., Du H., Wang L., Wen H., Zang X., Du A., Jia Q., Zhou X., Cui G., Energy Environ. Sci. 2018, 11, 1197. [Google Scholar]
- 33. Zhou S., Kong X., Zheng B., Huo F., Strømme M., Xu C., ACS Nano 2019, 13, 9578. [DOI] [PubMed] [Google Scholar]
- 34. Cao D., Xing Y., Tantratian K., Wang X., Ma Y., Mukhopadhyay A., Cheng Z., Zhang Q., Jiao Y., Chen L., Zhu H., Adv. Mater. 2019, 31, 1807313. [DOI] [PubMed] [Google Scholar]
- 35. Chen M., Chen J., Zhou W., Xu J., Wong C.‐P., J. Mater. Chem. A 2019, 7, 26524. [Google Scholar]
- 36. Hong S., Yuan Y., Liu C., Chen W., Chen L., Lian H., Liimatainen H., J. Mater. Chem. C 2020, 8, 550. [Google Scholar]
- 37. Kim S.‐H., Kim J.‐M., Ahn D. B., Lee S.‐Y., Small 2020, 16, 2002837. [Google Scholar]
- 38. Zhang X., Li J., Liu D., Liu M., Zhou T., Qi K., Shi L., Zhu Y., Qian Y., Energy Environ. Sci. 2021, 14, 3120. [Google Scholar]
- 39. Chen C., Hu L., Acc. Chem. Res. 2018, 51, 3154. [DOI] [PubMed] [Google Scholar]
- 40. Wang X., Yao C., Wang F., Li Z., Small 2017, 13, 1702240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim J. H., Lee D., Lee Y.‐H., Chen W., Lee S.‐Y., Adv. Mater. 2018, 31, 1804826. [DOI] [PubMed] [Google Scholar]
- 42. Liu S., Tao D., Bai H., Liu X., J. Appl. Polym. Sci. 2012, 126, E281. [Google Scholar]
- 43. Reza M., Bertinetto C., Kesari K. K., Engelhardt P., Ruokolainen J., Vuorinen T., Sci. Rep. 2019, 9, 3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moon R. J., Martini A., Nairn J., Simonsen J., Youngblood J., Chem. Soc. Rev. 2011, 40, 3941. [DOI] [PubMed] [Google Scholar]
- 45. Lee C. M., Kafle K., Park Y. B., Kim S. H., Phys. Chem. 2014, 16, 10844. [DOI] [PubMed] [Google Scholar]
- 46. Lv D., Du H., Che X., Wu M., Zhang Y., Liu C., Nie S., Zhang X., Li B., ACS Sustainable Chem. Eng. 2019, 7, 9449. [Google Scholar]
- 47. Yang X., Reid M. S., Olsén P., Berglund L. A., ACS Nano 2020, 14, 724. [DOI] [PubMed] [Google Scholar]
- 48. Foster E. J., Moon R. J., Agarwal U. P., Bortner M. J., Bras J., Camarero‐Espinosa S., Chan K. J., Clift M. J. D., Cranston E. D., Eichhorn S. J., Fox D. M., Hamad W. Y., Heux L., Jean B., Korey M., Nieh W., Ong K. J., Reid M. S., Renneckar S., Roberts R., Shatkin J. A., Simonsen J., Stinson‐Bagby K., Wanasekara N., Youngblood J., Chem. Soc. Rev. 2018, 47, 2609. [DOI] [PubMed] [Google Scholar]
- 49. Ifuku S., Nogi M., Abe K., Handa K., Nakatsubo F., Yano H., Biomacromolecules 2007, 8, 1973. [DOI] [PubMed] [Google Scholar]
- 50. Saito T., Kimura S., Nishiyama Y., Isogai A., Biomacromolecules 2007, 8, 2485. [DOI] [PubMed] [Google Scholar]
- 51. Chen W., Li Q., Wang Y., Yi X., Zeng J., Yu H., Liu Y., Li J., ChemSusChem 2014, 7, 154. [DOI] [PubMed] [Google Scholar]
- 52. Liu W., Du H., Zhang M., Liu K., Liu H., Xie H., Zhang X., Si C., ACS Sustainable Chem. Eng. 2020, 8, 7536. [Google Scholar]
- 53. Klemm D., Kramer F., Moritz S., Lindstrm T., Ankerfors M., Gray D., Dorris A., Angew. Chem., Int. Ed. 2011, 50, 5438. [DOI] [PubMed] [Google Scholar]
- 54. Yao J., Ji P., Sheng N., Guan F., Zhang M., Wang B., Chen S., Wang H., Electrochim. Acta 2018, 283, 1578. [Google Scholar]
- 55. Xiong J., Lin M.‐F., Wang J., Gaw S. L., Parida K., Lee P. S., Adv. Energy Mater. 2017, 7, 1701243. [Google Scholar]
- 56. Yang X., Shi K., Zhitomirsky I., Cranston E. D., Adv. Mater. 2015, 27, 6104. [DOI] [PubMed] [Google Scholar]
- 57. Li S., Warzywod J., Wang S., Ren G., Fan Z., Carbon 2016, 124, 212. [Google Scholar]
- 58. Li J., Wei X., Wang Q., Chen J., Chang G., Kong L., Su J., Liu Y., Carbohydr. Polym. 2012, 90, 1609. [DOI] [PubMed] [Google Scholar]
- 59. Kaushik M., Li A. Y., Hudson R., Masnadi M., Li C.‐J., Moores A., Green Chem. 2016, 18, 129. [Google Scholar]
- 60. Wang Q., Zhao X., Zhu J. Y., Ind. Eng. Chem. Res. 2014, 53, 11007. [Google Scholar]
- 61. Ribeiro R. S. A., Pohlmann B. C., Calado V., Bojorge N., Pereira N., Eng. Life Sci. 2019, 19, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu P., Pang B., Dechert S., Zhang X., Zhang K., Angew. Chem. 2020, 132, 3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Montanari S., Roumani M., Heux L., Vignon M., Macromolecules 2005, 38, 1665. [Google Scholar]
- 64. Du H., Parit M., Wu M., Che X., Wang Y., Zhang M., Wang R., Zhang X., Jiang Z., Li B., J. Hazard. Mater. 2020, 400, 123106. [DOI] [PubMed] [Google Scholar]
- 65. Chen W., Yu H., Liu Y., Chen P., Zhang M., Hai Y., Carbohydr. Polym. 2011, 83, 1804. [Google Scholar]
- 66. Kang X., Sun P., Kuga S., Wang C., Zhao Y., Wu M., Huang Y., ACS Sustainable Chem. Eng. 2017, 5, 2529. [Google Scholar]
- 67. Zhang C., Wu M., Yang S., Song X., Xu Y., Cellulose 2020, 27, 7447. [Google Scholar]
- 68. Iguchi M., Yamanaka S., Budhiono A., J. Mater. Sci. 2000, 35, 261. [Google Scholar]
- 69. Singhsa P., Narain R., Manuspiya H., ACS Appl. Nano Mater. 2018, 1, 209. [DOI] [PubMed] [Google Scholar]
- 70. Li Q., Wang Y., Wu Y., He K., Li Y., Luo X., Li B., Wang C., Liu S., Food Hydrocolloids 2019, 88, 180. [Google Scholar]
- 71. Hornig S., Heinze T., Biomacromolecules 2008, 9, 1487. [DOI] [PubMed] [Google Scholar]
- 72. Sharma P. R., Varma A. J., Carbohydr. Polym. 2014, 114, 339. [DOI] [PubMed] [Google Scholar]
- 73. Sharma P. R., Varma A. J., Chem. Commun. 2013, 49, 8818. [DOI] [PubMed] [Google Scholar]
- 74. Nosar M. N., Salehi M., Ghorbani S., Beiranvand S. P., Goodarzi A., Azami M., Cellulose 2016, 23, 3239. [Google Scholar]
- 75. Benítez A. J., Walther A., J. Mater. Chem. A 2017, 5, 16003. [Google Scholar]
- 76. Liu Y., Wang H., Yu G., Yu Q., Li B., Mu X., Carbohydr. Polym. 2014, 110, 415. [DOI] [PubMed] [Google Scholar]
- 77. Carlsson D. O., Lindh J., Nyholm L., Strømmea M., Mihranyan A., RSC Adv. 2014, 4, 52289. [Google Scholar]
- 78. Fiss B. G., Hatherly L., Stein R. S., Friščić T., Moores A., ACS Sustainable Chem. Eng. 2019, 7, 7951. [Google Scholar]
- 79. Zheng Q., Cai Z., Ma Z., Gong S., ACS Appl. Mater. Interfaces 2015, 7, 3263. [DOI] [PubMed] [Google Scholar]
- 80. Long C., Qi D., Wei T., Yan J., Jiang L., Fan Z., Adv. Funct. Mater. 2014, 24, 3953. [Google Scholar]
- 81. Chen L., Huang Z., Liang H., Guan Q., Yu S., Adv. Mater. 2013, 25, 4746. [DOI] [PubMed] [Google Scholar]
- 82. Zhu J. Y., Sabo R., Luo X., Green Chem. 2011, 13, 1339. [Google Scholar]
- 83. Thomas B., Raj M. C., Athira K. B., Rubiyah M. H., Joy J., Moores A., Drisko G. L., Sanchez C., Chem. Rev. 2018, 118, 11575. [DOI] [PubMed] [Google Scholar]
- 84. Song Y., Sheng L., Wang L., Xu H., He X., Electrochem. Commun. 2021, 124, 106948. [Google Scholar]
- 85. Lopez J., Mackanic D. G., Cui Y., Bao Z., Nat. Rev. Mater. 2019, 4, 312. [Google Scholar]
- 86. Zhang X., Li J., Ao H., Liu D., Shi L., Wang C., Zhua Y., Qian Y., Energy Storage Mater. 2020, 30, 337. [Google Scholar]
- 87. Deng S., Zhang Y., Xie D., Yang L., Wang G., Zheng X., Zhu J., Wang X., Yue Y., Pan G., Xia X., Tu J., Nano Energy 2019, 58, 355. [Google Scholar]
- 88. Wan Z., Chen C., Meng T., Mojtaba M., Teng Y., Feng Q., Li D., ACS Appl. Mater. Interfaces 2019, 11, 42808. [DOI] [PubMed] [Google Scholar]
- 89. Tian W., Mohammadi A. V., Reid M. S., Wang Z., Ouyang L., Erlandsson J., Pettersson T., Wågberg L., Beidaghi M., Hamedi M. M., Adv. Mater. 2019, 31, 1902977. [DOI] [PubMed] [Google Scholar]
- 90. Jiang Y., Xie X., Chen Y., Liu Y., Yang R., Sui G., J. Mater. Chem. C 2018, 6, 8679. [Google Scholar]
- 91. Zhan Z., Song Q., Zhou Z., Lu C., J. Mater. Chem. C 2019, 7, 9820. [Google Scholar]
- 92. Nechyporchuk O., Håkansson K. M. O., Gowda K. V, Lundell F., Hagström B., Köhnke T., Adv. Mater. Technol. 2018, 4, 1800557. [Google Scholar]
- 93. Peng Q., Cheng J., Lu S., Li Y., Polym. Adv. Technol. 2020, 31, 15. [Google Scholar]
- 94. Shin S., Hyun J., ACS Appl. Mater. Interfaces 2017, 9, 26438. [DOI] [PubMed] [Google Scholar]
- 95. Wang W., Yang Y., Chen Z., Deng Z., Fan L., Guo W., Xu J., Meng Z., Cellulose 2020, 27, 7649. [Google Scholar]
- 96. Cho S.‐Y., Yu H., Choi J., Kang H., Park S., Jang J.‐S., Hong H.‐J., Kim I.‐D., Lee S.‐K., Jeong H. S., Jung H.‐T., ACS Nano 2019, 13, 9332. [DOI] [PubMed] [Google Scholar]
- 97. Niu Q., Gao K., Shao Z., Nanoscale 2014, 6, 4083. [DOI] [PubMed] [Google Scholar]
- 98. Han J., Wang S., Zhu S., Huang C., Yue Y., Mei C., Xu X., Xia C., ACS Appl. Mater. Interfaces 2019, 11, 44624. [DOI] [PubMed] [Google Scholar]
- 99. Rull‐Barrull J., d'Halluin M., Le Grognec E., Felpin F.‐X., J. Mater. Chem. C 2017, 5, 5154. [Google Scholar]
- 100. Luo W., Hayden J., Jang S.‐H., Wang Y., Zhang Y., Kuang Y., Wang Y., Zhou Y., Rubloff G. W., Lin C.‐F., Hu L., Adv. Energy Mater. 2017, 8, 1702615. [Google Scholar]
- 101. Zhang Y., Shang Z., Shen M., Chowdhury S. P., Ignaszak A., Sun S., Ni Y., ACS Sustainable Chem. Eng. 2019, 7, 11175. [Google Scholar]
- 102. Qu H., Zhang J., Du A., Chen B., Chai J., Xue N., Wang L., Qiao L., Wang C., Zang X., Yang J., Wang X., Cui G., Adv. Sci. 2018, 5, 1700503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wu B., He W., Lu M., Li Z., Qiang H., Polym. Compos. 2020, 41, 1135. [Google Scholar]
- 104. Choi K.‐H., Yoo J., Lee C. K., Lee S.‐Y., Energy Environ. Sci. 2016, 9, 2812. [Google Scholar]
- 105. Wu H., Zhang Y., Yuan W., Zhao Y., Luo S., Yuan X., Zheng L., Cheng L., J. Mater. Chem. A 2018, 6, 16617. [Google Scholar]
- 106. Zheng Q., Cai Z., Ma Z., Gong S., J. Mater. Chem. A 2018, 6, 16617. [Google Scholar]
- 107. Jiao S., Zhou A., Wu M., Hu H., Adv. Sci. 2019, 6, 1900529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Arularasua M. V., Harbb M., Sundaram R., Carbohydr. Polym. 2020, 249, 116868. [DOI] [PubMed] [Google Scholar]
- 109. Chen T., Zhang S., Lin Q., Wang M., Yang Z., Zhang Y., Wang F., Sun L., Nanoscale 2020, 12, 21271. [DOI] [PubMed] [Google Scholar]
- 110. Cui S., Wei P., Li L., Compos. Sci. Technol. 2018, 168, 63. [Google Scholar]
- 111. Su X., Chen W., Han Y., Wang D., Yao J., Appl. Surf. Sci. 2021, 536, 147945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang X., Lin Z., Chen B., Zhang W., Sharma S., Gu W., Deng Y., J. Power Sources 2014, 246, 283. [Google Scholar]
- 113. Wang Z., Tammela P., Zhang P., Huo J., Ericson F., Strømme M., Nyholm L., Nanoscale 2014, 6, 13068. [DOI] [PubMed] [Google Scholar]
- 114. Wang H., Bian L., Zhou P., Tang J., Tang W., J. Mater. Chem. A 2013, 1, 578. [Google Scholar]
- 115. Wang Z., Tammela P., Zhang P., Strømme M., Nyholm L., J. Mater. Chem. A 2014, 2, 16761. [Google Scholar]
- 116. Wang F., Kim H.‐J., Park S., Kee C.‐D., Kim S.‐J., Oh I.‐K., Compos. Sci. Technol. 2016, 128, 33. [Google Scholar]
- 117. Wang Z., Carlsson D. O., Tammela P., Hua K., Zhang P., Nyholm L., Strømme M., ACS Nano 2015, 9, 7563. [DOI] [PubMed] [Google Scholar]
- 118. Wang Z., Tammela P., Huo J., Zhang P., Strømme M., Nyholm L., J. Mater. Chem. A 2016, 4, 1714. [Google Scholar]
- 119. Chen H., Liu T., Mou J., Zhang W., Jiang Z., Liu J., Huang J., Liu M., Nano Energy 2019, 63, 103836. [Google Scholar]
- 120. Tian W., Mohammadi A. V., Reid M. S., Wang Z., Ouyang L., Erlandsson J., Pettersson T., Wågberg L., Beidaghi M., Hamedi M. M., Adv. Mater. 2019, 31, 1902977. [DOI] [PubMed] [Google Scholar]
- 121. Cao W., Ma C., Tan S., Ma M., Wan P., Chen F., Nano‐Micro Lett. 2019, 11, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hou M., Hu Y., Xu M., Li B., Cellulose 2020, 27, 9457. [Google Scholar]
- 123. Wang J., Ran R., Sunarso J., Yin C., Zou H., Feng Y., Li X., Zheng X., Yao J., J. Power Sources 2017, 347, 259. [Google Scholar]
- 124. Etman A. S., Wang Z., Ghazaly A. E., Sun J., Nyholm L., Rosen J., ChemSusChem 2019, 12, 5157. [DOI] [PubMed] [Google Scholar]
- 125. Gao K., Shao Z., Li J., Wang X., Peng X., Wang W., Wang F., J. Mater. Chem. A 2013, 1, 63. [Google Scholar]
- 126. Gao K., Shao Z., Wang X., Zhang Y., Wang W., Wang F., RSC Adv. 2013, 3, 15058. [Google Scholar]
- 127. Liu Y., Zhou J., Zhu E., Tang J., Liu X., Tang W., J. Mater. Chem. C 2015, 3, 1011. [Google Scholar]
- 128. Wang Z., Xu C., Tammela P., Huo J., Strømme M., Edström K., Gustafsson T., Nyholm L., J. Mater. Chem. A 2015, 3, 14109. [Google Scholar]
- 129. Zhou B., Zhang Z., Li Y., Han G., Feng Y., Wang B., Zhang D., Ma J., Liu C., ACS Appl. Mater. Interfaces 2020, 12, 4895. [DOI] [PubMed] [Google Scholar]
- 130. Huang Y., Lin Z., Zheng M., Wang T., Yang J., Yuan F., Lu X., Liu L., Sun D., J. Power Sources 2016, 307, 649. [Google Scholar]
- 131. Zhang X., Cui X., Lu C.‐H., Li H., Zhang Q., He C., Yang Y., Chem. Eng. J. 2020, 401, 126031. [Google Scholar]
- 132. Zhang F., Tang Y., Yang Y., Zhang X., Lee C.‐S., Electrochim. Acta 2016, 211, 404. [Google Scholar]
- 133. Jyothibasu J. P., Kuo D.‐W., Lee R.‐H., Cellulose 2019, 26, 4495. [Google Scholar]
- 134. France K. J. D., Hoare T., Cranston E. D., Chem. Mater. 2017, 29, 4609. [Google Scholar]
- 135. Ma L., Bi Z., Xue Y., Zhang W., Huang Q., Zhang L., Huang Y., J. Mater. Chem. A 2020, 8, 5812. [Google Scholar]
- 136. France K., Zeng Z., Wu T., Nyström G., Adv. Mater. 2020, 2000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang X., Li H., Zhang W., Huang Z., Tsui C. P., Lu C., He C., Yang Y., Electrochim. Acta 2019, 301, 55. [Google Scholar]
- 138. Zhang X., Zhao J., Xia T., Li Q., Ao C., Wang Q., Zhang W., Lu C., Deng Y., Energy Storage Mater. 2020, 31, 135. [Google Scholar]
- 139. Fei G., Wang Y., Wang H., Ma Y., Guo Q., Huang W., Yang D., Shao Y., Ni Y., ACS Sustainable Chem. Eng. 2019, 7, 8215. [Google Scholar]
- 140. Huang J., Qian X., An X., Li X., Guan J., Cellulose 2020, 27, 5829. [Google Scholar]
- 141. Chen L., Yu H., Wang D., Yang T., Yao J., Tam K. C., ACS Sustainable Chem. Eng. 2019, 7, 11823. [Google Scholar]
- 142. Chen Z., Hu Y., Zhuo H., Liu L., Jing S., Zhong L., Peng X., Sun R., Chem. Mater. 2019, 31, 3301. [Google Scholar]
- 143. Li S., Huang D., Zhang B., Xu X., Wang M., Yang G., Shen Y., Adv. Energy Mater. 2014, 4, 1301655. [Google Scholar]
- 144. Wang Y., Song Y., Xia Y., Chem. Soc. Rev. 2016, 45, 5925. [DOI] [PubMed] [Google Scholar]
- 145. Yan J., Wang Q., Wei T., Fan Z., Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar]
- 146. Wang F., Wu X., Yuan X., Liu Z., Zhang Y., Fu L., Zhu Y., Zhou Q., Wu Y., Huang W., Chem. Soc. Rev. 2017, 46, 6816. [DOI] [PubMed] [Google Scholar]
- 147. Simon P., Gogotsi Y., Nat. Mater. 2020, 19, 1151. [DOI] [PubMed] [Google Scholar]
- 148. Yan J., Li S., Lan B., Wu Y., Lee P. S., Adv. Funct. Mater. 2020, 30, 1902564. [Google Scholar]
- 149. Sumboja A., Liu J., Zheng W. G., Zong Y., Zhang H., Liu Z., Chem. Soc. Rev. 2018, 47, 5919. [DOI] [PubMed] [Google Scholar]
- 150. Jost K., Durkin D. P., Haverhals L. M., Brown E. K., Langenstein M., De Long H. C., Trulove P. C., Gogotsi Y., Dion G., Adv. Energy Mater. 2014, 5, 1401286. [Google Scholar]
- 151. Santos M. C. G., da Silva D. R., Pinto P. S., Ferlauto A. S., Lacerda R. G., Jesus W. P., da Cunha T. H. R., Ortega P. F. R., Lavall R. L., Electrochim. Acta 2020, 349, 136241. [Google Scholar]
- 152. Guan F., Chen S., Sheng N., Chen Y., Yao J., Pei Q., Wang H., Chem. Eng. J. 2019, 360, 829. [Google Scholar]
- 153. Luo H., Xiong P., Xie J., Yang Z., Huang Y., Hu J., Wan Y., Xu Y., Adv. Funct. Mater. 2018, 28, 1803075. [Google Scholar]
- 154. Zhao D., Zhang Q., Chen W., Yi X., Liu S., Wang Q., Liu Y., Li J., Li X., Yu H., ACS Appl. Mater. Interfaces 2017, 9, 13213. [DOI] [PubMed] [Google Scholar]
- 155. Sheng N., Chen S., Yao J., Guan F., Zhang M., Wang B., Wu Z., Ji P., Wang H., Chem. Eng. J. 2019, 368, 1022. [Google Scholar]
- 156. Zou Z., Xiao W., Zhang Y., Yu H., Zhou W., Appl. Surf. Sci. 2020, 500, 144244. [Google Scholar]
- 157. Fang D., Zhou J., Sheng L., Tang W., Tang J., Chem. Eng. J. 2020, 396, 125325. [Google Scholar]
- 158. Zhou J., Yuan Y., Tang J., Tang W., Energy Storage Mater. 2019, 23, 594. [Google Scholar]
- 159. Wang Y., Wang X., Li X., Bai Y., Xiao H., Liu Y., Liu R., Yuan G., Adv. Funct. Mater. 2019, 29, 1900326. [Google Scholar]
- 160. Han J., Wang H., Yue Y., Mei C., Chen J., Huang C., Wu Q., Xu X., Carbon 2019, 149, 1. [Google Scholar]
- 161. Hsu H. H., Liu Y., Wang Y., Li B., Luo G., Xing M., Zhong W., ACS Sustainable Chem. Eng. 2020, 8, 6935. [Google Scholar]
- 162. Peng Z., Zou Y., Xu S., Zhong W., Yang W., ACS Appl. Mater. Interfaces 2018, 10, 22190. [DOI] [PubMed] [Google Scholar]
- 163. Ge D., Yang L., Fan L., Zhang C., Xiao X., Gogotsi Y., Yang S., Nano Energy 2015, 11, 568. [Google Scholar]
- 164. Wang D., Yu H., Qi D., Ramasamy M., Yao J., Tang F., Tam K. C., Ni Q., ACS Appl. Mater. Interfaces 2019, 11, 24435. [DOI] [PubMed] [Google Scholar]
- 165. Rana H. H., Park J. H., Gund G. S., Park H. S., Energy Storage Mater. 2020, 25, 70. [Google Scholar]
- 166. Tang X., Lui Y., Merhi A. R., Chen B., Ding S., Zhang B., Hu S., ACS Appl. Mater. Interfaces 2017, 9, 44429. [DOI] [PubMed] [Google Scholar]
- 167. Kim J.‐H., Gu M., Lee D. H., Kim J.‐H., Oh Y.‐S., Min S. H., Kim B.‐S., Lee S.‐Y., Nano Lett. 2016, 16, 5533. [DOI] [PubMed] [Google Scholar]
- 168. Li L., Lu F., Wang C., Zhang F., Liang W., Kuga S., Dong Z., Zhao Y., Huang Y., Wu M., J. Mater. Chem. A 2018, 6, 24468. [Google Scholar]
- 169. Li M., Lu J., Chen Z., Amine K., Adv. Mater. 2018, 30, 1800561. [DOI] [PubMed] [Google Scholar]
- 170. Wang R., Dai X., Qian Z., Zhong S., Chen S., Fan S., Zhang H., Wu F., ACS Appl. Mater. Interfaces 2020, 12, 31628. [DOI] [PubMed] [Google Scholar]
- 171. Cao S., Shi L., Miao M., Fang J., Zhao H., Feng X., Electrochim. Acta 2019, 298, 22. [Google Scholar]
- 172. Wang B., Li X., Luo B., Yang J., Wang X., Song Q., Chen S., Zhi L., Small 2013, 9, 2399. [DOI] [PubMed] [Google Scholar]
- 173. Kim P. J., Kim K., Pol V. G., Energy Storage Mater. 2019, 19, 179. [Google Scholar]
- 174. Wang C.‐Y., Zheng Z.‐J., Feng Y.‐Q., Ye H., Cao F.‐F., Guo Z.‐P., Nano Energy 2020, 74, 104817. [Google Scholar]
- 175. Cao S., Feng X., Song Y., Xue X., Liu H., Miao M., Fang J., Shi L., ACS Appl. Mater. Interfaces 2015, 7, 10695. [DOI] [PubMed] [Google Scholar]
- 176. Huang C., Ji H., Yang Y., Guo B., Luo L., Meng Z., Fan L., Xu J., Carbohydr. Polym. 2020, 230, 115570. [DOI] [PubMed] [Google Scholar]
- 177. Wang Z., Pan R., Ruan C., Edström K., Strømme M., Nyholm L., Adv. Sci. 2018, 5, 1700663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Xu D., Wang B., Wang Q., Gu S., Li W., Jin J., Chen C., Wen Z., ACS Appl. Mater. Interfaces 2018, 10, 17809. [DOI] [PubMed] [Google Scholar]
- 179. Du Z., Su Y., Qu Y., Zhao L., Jia X., Mo Y., Yu F., Du J., Chen Y., Electrochim. Acta 2019, 299, 19. [Google Scholar]
- 180. Seh Z. W., Sun Y., Zhang Q., Cui Yi, Chem. Soc. Rev. 2016, 45, 5605. [DOI] [PubMed] [Google Scholar]
- 181. Tao T., Lu S., Fan Y., Lei W., Huang S., Chen Y., Adv. Mater. 2017, 29, 1700542. [DOI] [PubMed] [Google Scholar]
- 182. Yu M., Ma J., Xie M., Song H., Tian F., Xu S., Zhou Y., Li B., Wu D., Qiu H., Wang R., Adv. Energy Mater. 2017, 7, 1602347. [Google Scholar]
- 183. Wu H., Wang L., Bi J., Li Y., Pang X., Li Z., Meng Q., Liu H., Wang L., ACS Appl. Mater. Interfaces 2020, 12, 15228. [DOI] [PubMed] [Google Scholar]
- 184. Nair J. R., Bella F., Angulakshmi N., Stephan A. M., Gerbaldi C., Energy Storage Mater. 2016, 3, 69. [Google Scholar]
- 185. Liu J., Li Y., Xuan Y., Zhou L., Wang D., Li Z., Lin H., Tretiak S., Wang H., Wang L., Guo Z., Zhang S., ACS Appl. Mater. Interfaces 2020, 12, 17592. [DOI] [PubMed] [Google Scholar]
- 186. Xiang X., Zhang K., Chen J., Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar]
- 187. Chayambuka K., Mulder G., Danilov D. L., Notten P. H. L., Adv. Mater. 2015, 27, 5343.26275211 [Google Scholar]
- 188. Wang M., Yang Y., Yang Z., Gu L., Chen Q., Yu Y., Adv. Sci. 2017, 4, 1600468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kim Y., Yeom S., Lee J.‐E., Kang S., Kang H., Lee G.‐H., Kim M., Lee S., Lee H.‐W., Chae H., J. Power Sources 2020, 468, 228371. [Google Scholar]
- 190. Yang H., Xu R., Yu Y., Energy Storage Mater. 2019, 22, 105. [Google Scholar]
- 191. Ma Y., Xie X., Lv R., Na B., Ouyang J., Liu H., ACS Sustainable Chem. Eng. 2018, 6, 8697. [Google Scholar]
- 192. Yi H., Ma Y., Zhang S., Na B., Zeng R., Zhang Y., Lin C., ACS Sustainable Chem. Eng. 2019, 7, 18894. [Google Scholar]
- 193. Zhao N., Wu F., Xing Y., Qu W., Chen N., Shang Y., Yan M., Li Y., Li L., Chen R., ACS Appl. Mater. Interfaces 2019, 11, 15537. [DOI] [PubMed] [Google Scholar]
- 194. Ma L., Chen S., Wang D., Yang Q., Mo F., Liang G., Li N., Zhang H., Zapien J. A., Zhi C., Adv. Energy Mater. 2019, 9, 1803046. [Google Scholar]


