Abstract
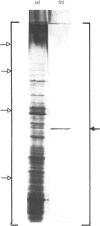
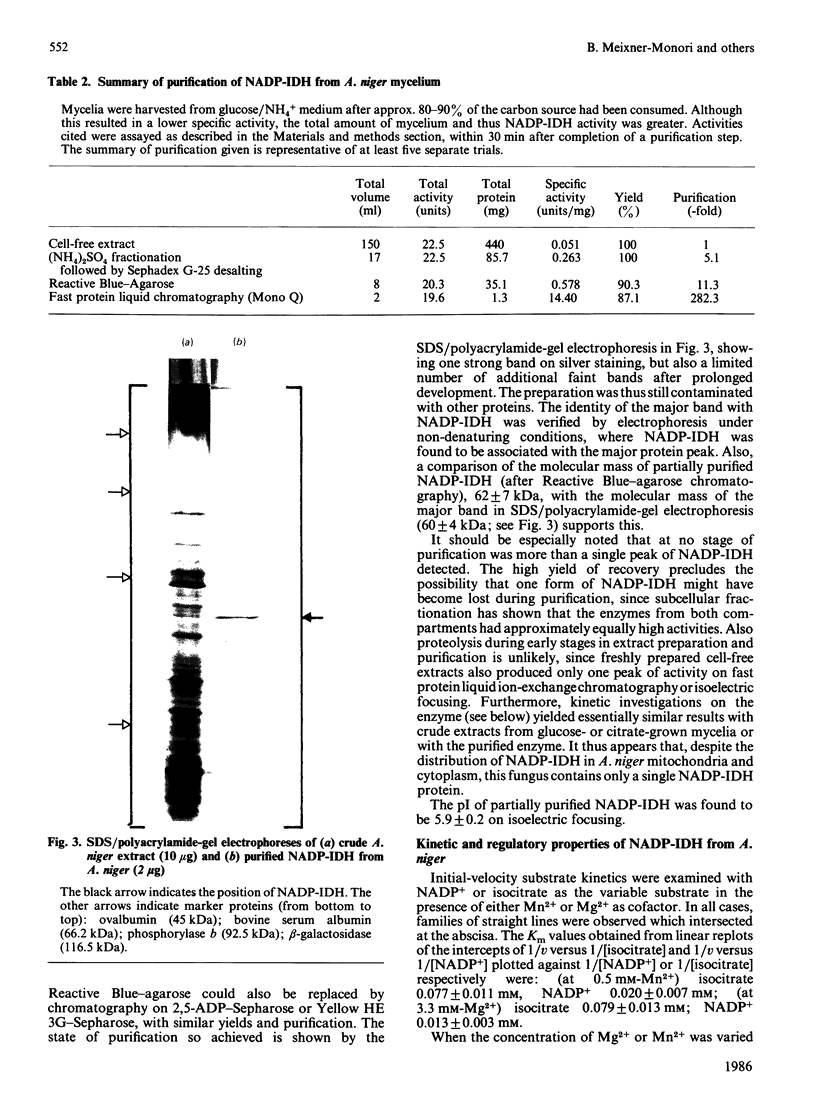
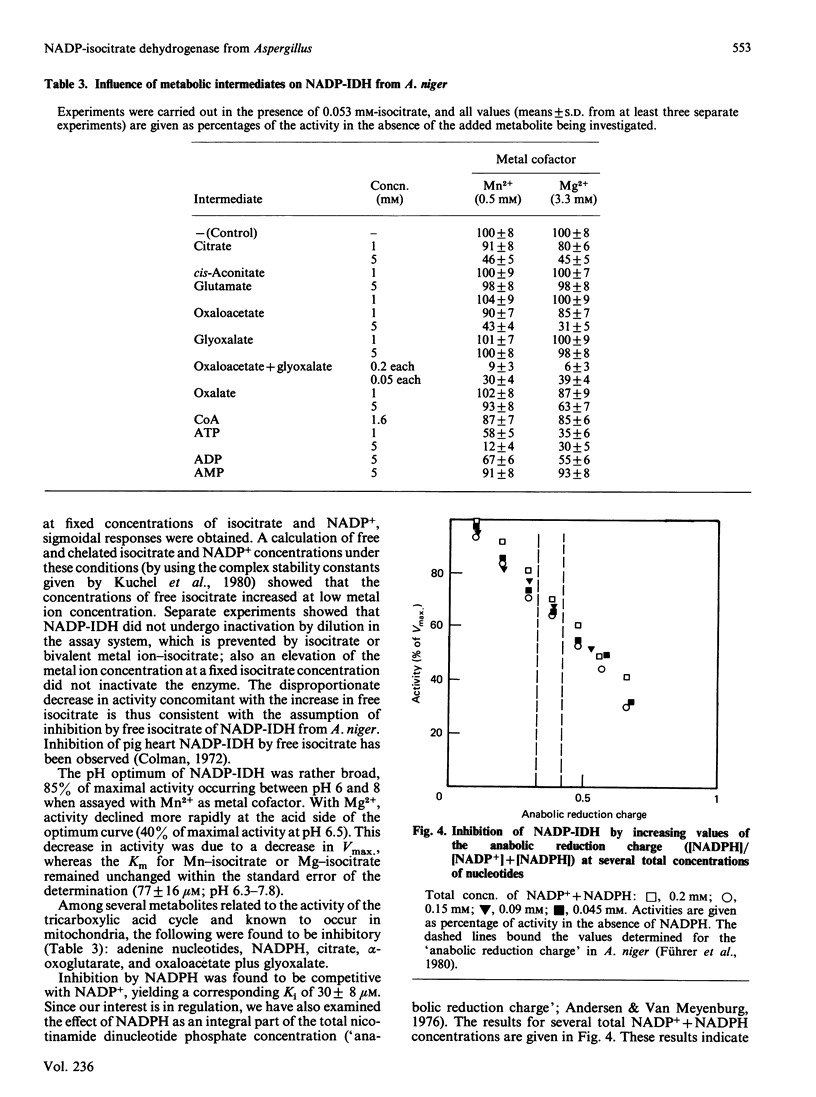
NADP-specific isocitrate dehydrogenase [threo-DS-isocitrate: NADP+ oxidoreductase (decarboxylating), EC 1.1.1.42] was purified 200-300-fold from the citric acid-accumulating fungus Aspergillus niger. The enzyme consists of a single polypeptide chain with a molecular mass of 60 +/- 4 kDa and has a pI of 5.9 +/- 0.2. Only a single enzyme protein was found, although the enzyme appears to occur both in the mitochondrion and in the cytoplasm. Growth on organic acids as carbon sources or on NO3- as nitrogen source led to increased activities, whereas the presence of amino acids led to lower activities. The enzyme exhibits hyperbolic kinetics with respect to its substrates isocitrate and NADP+. Mn2+ and Mg2+ are obligatory for enzyme activity. The enzyme is inhibited by its products alpha-oxoglutarate and NADPH. Among various metabolites, ATP and citrate appear to inhibit the enzyme at a concentration considered to occur intracellularly. In both cases, however, the mechanism is a removal of the metal ion cofactor from the substrates. It is concluded that under physiological conditions, where the Mg2+ content is around 10 mM, the observed inhibition by ATP or citrate is of poor regulatory significance.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4151–4156. [PubMed] [Google Scholar]
- Balamir A. NADP-linked isocitrate dehydrogenase from beef liver: a new method of purification and the effect of metal ion cofactor on its stability. Biochem Med. 1983 Apr;29(2):194–206. doi: 10.1016/0006-2944(83)90040-6. [DOI] [PubMed] [Google Scholar]
- Barratt D. G., Cook R. A. Role of metal cofactors in enzyme regulation. Differences in the regulatory properties of the Neurospora crassa nicotinamide adenine dinucleotide specific isocitrate dehydrogenase depending on whether Mg2+ or Mn2+ serves as divalent cation. Biochemistry. 1978 Apr 18;17(8):1561–1566. doi: 10.1021/bi00601a032. [DOI] [PubMed] [Google Scholar]
- Barrera C. R., Jurtshuk P. Characterization of the highly active isocitrate (NADP+) dehydrogenase of Azotobacter vinelandii. Biochim Biophys Acta. 1970 Dec 16;220(3):416–429. doi: 10.1016/0005-2744(70)90273-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cantore M. L., Passeron S. Kinetic properties, solubilization, and molecular characterization of Mucor rouxii adenylate cyclase. Arch Biochem Biophys. 1982 Nov;219(1):1–11. doi: 10.1016/0003-9861(82)90127-8. [DOI] [PubMed] [Google Scholar]
- Carlier M-F, Pantaloni D. Nicotinamide adenine dinucleotide phosphate linked isocitrate dehydrogenase. Catalytic activation by the reduced coenzyme product of the reaction. Biochemistry. 1976 Apr 20;15(8):1761–1766. doi: 10.1021/bi00653a026. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. NADP-linked isocitrate dehydrogenase from beef liver. Purification, quaternary structure and catalytic activity. Eur J Biochem. 1973 Aug 17;37(2):341–354. doi: 10.1111/j.1432-1033.1973.tb02993.x. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Rôle des ions métalliques dans l'activité de l'isocitrate déshydrogénase de foie de boeuf. Caractérisation des formes du substrat. Biochimie. 1976;58(1-2):27–33. doi: 10.1016/s0300-9084(76)80353-7. [DOI] [PubMed] [Google Scholar]
- Charles A. M. Properties of an NADP plus-specific isocitric dehydrogenase from Thiobacillus novellus. Can J Biochem. 1970 Jan;48(1):95–103. doi: 10.1139/o70-016. [DOI] [PubMed] [Google Scholar]
- Colman R. F. Role of metal ions in reactions catalyzed by pig heart triphosphopyridine nucleotide-dependent isocitrate dehydrogenase. II. Effect on catalytic properties and reactivity of amino acid residues. J Biol Chem. 1972 Jan 10;247(1):215–223. [PubMed] [Google Scholar]
- Dalziel K., McFerran N., Matthews B., Reynolds C. H. Transient kinetics of nicotinamide-adenine dinucleotide phosphate-linked isocitrate dehydrogenase from bovine heart mitochondria. Biochem J. 1978 Jun 1;171(3):743–750. doi: 10.1042/bj1710743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R. S., Colman R. F. Influence of substrates and coenzymes on the role of manganous ion in reactions catalyzed by pig heart triphosphopyridine nucleotide-dependent isocitrate dehydrogenase. Biochemistry. 1976 Sep 7;15(18):4034–4041. doi: 10.1021/bi00663a018. [DOI] [PubMed] [Google Scholar]
- Farrell H. M., Jr Purification and properties of NADP+:isocitrate dehydrogenase from lactating bovine mammary gland. Arch Biochem Biophys. 1980 Oct 15;204(2):551–559. doi: 10.1016/0003-9861(80)90067-3. [DOI] [PubMed] [Google Scholar]
- Fatania H. R., Dalziel K. Intracellular distribution of NADP-linked isocitrate dehydrogenase, fumarase and citrate synthase in bovine heart muscle. Biochim Biophys Acta. 1980 Aug 1;631(1):11–19. doi: 10.1016/0304-4165(80)90048-3. [DOI] [PubMed] [Google Scholar]
- Führer L., Kubicek C. P., Röhr M. Pyridine nucleotide levels and ratios in Aspergillus niger. Can J Microbiol. 1980 Mar;26(3):405–408. doi: 10.1139/m80-067. [DOI] [PubMed] [Google Scholar]
- Habison A., Kubicek C. P., Röhr M. Partial purification and regulatory properties of phosphofructokinase from Aspergillus niger. Biochem J. 1983 Mar 1;209(3):669–676. doi: 10.1042/bj2090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. L., Becker R. R. Isolation and some properties of the triphosphopyridine nucleotide isocitrate dehydrogenase from Bacillus stearothermophilus. J Biol Chem. 1970 Jun;245(12):3186–3194. [PubMed] [Google Scholar]
- Ingebretsen O. C. Properties of the nicotinamide adenine dinucleotide phosphate-specific isocitrate dehydrogenase from Blastocladiella emersonii. J Bacteriol. 1975 Oct;124(1):65–72. doi: 10.1128/jb.124.1.65-72.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Hynes M. J. The regulation of NADP-linked isocitrate dehydrogenase in Aspergillus nidulans. J Gen Microbiol. 1982 Jan;128(1):23–28. doi: 10.1099/00221287-128-1-23. [DOI] [PubMed] [Google Scholar]
- Kubicek C. P., Röhr M. Regulation of citrate synthase from the citric acid-accumulating fungus, Aspergillus niger. Biochim Biophys Acta. 1980 Oct;615(2):449–457. [PubMed] [Google Scholar]
- Kuchel P. W., Reynolds C. H., Dalziel K. Determination of the stability constants of Mn2+ and Mg2+ complexes of the components of the NADP-linked isocitrate dehydrogenase reaction by electron spin resonance. Eur J Biochem. 1980 Sep;110(2):465–473. doi: 10.1111/j.1432-1033.1980.tb04888.x. [DOI] [PubMed] [Google Scholar]
- La Nauze J. M. Aconitase and isocitric dehydrogenases of Aspergillus niger in relation to citric acid production. J Gen Microbiol. 1966 Jul;44(1):73–81. doi: 10.1099/00221287-44-1-73. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meixner-Monori B., Kubicek C. P., Habison A., Kubicek-Pranz E. M., Röhr M. Presence and regulation of the alpha-ketoglutarate dehydrogenase multienzyme complex in the filamentous fungus Aspergillus niger. J Bacteriol. 1985 Jan;161(1):265–271. doi: 10.1128/jb.161.1.265-271.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Dilts R. P., Jr Purification and characterization of glucose-6-phosphate dehydrogenase from Aspergillus parasiticus. Arch Biochem Biophys. 1984 Jan;228(1):113–119. doi: 10.1016/0003-9861(84)90052-3. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., Scrutton M. C. The sub-cellular localisation and regulatory properties of pyruvate carboxylase from Rhizopus arrhizus. Eur J Biochem. 1985 Feb 15;147(1):119–128. doi: 10.1111/j.1432-1033.1985.tb08727.x. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., Scrutton M. C. The sub-cellular localisation of pyruvate carboxylase and of some other enzymes in Aspergillus nidulans. Eur J Biochem. 1983 Jul 1;133(3):551–560. doi: 10.1111/j.1432-1033.1983.tb07499.x. [DOI] [PubMed] [Google Scholar]
- RAMAKRISHNAN C. V., STEEL R., LENTZ C. P. Mechanism of citric acid formation and accumulation in Aspergillus niger. Arch Biochem Biophys. 1955 Mar;55(1):270–273. doi: 10.1016/0003-9861(55)90564-6. [DOI] [PubMed] [Google Scholar]
- Reeves H. C., Daumy G. O., Lin C. C., Houston M. NADP + -specific isocitrate dehydrogenase of Escherichia coli. I. Purification and characterization. Biochim Biophys Acta. 1972 Jan 20;258(1):27–39. doi: 10.1016/0005-2744(72)90964-3. [DOI] [PubMed] [Google Scholar]
- Reynolds C. H., Kuchel P. W., Dalziel K. Equilibrium binding of coenzymes and substrates to nicotinamide-adenine dinucleotide phosphate-linked isocitrate dehydrogenase from bovine heart mitochondria. Biochem J. 1978 Jun 1;171(3):733–742. doi: 10.1042/bj1710733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self C. H., Parker M. G., Weitzman D. J. The isocitrate dehydrogenases of Acinetobacter lwoffi. Studies on the regulation of a nicotinamide-adenine dinucleotide phosphate-linked isoenzyme. Biochem J. 1973 Feb;132(2):215–221. doi: 10.1042/bj1320215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr M. L., Thompson V. W., Cleland W. W. The kinetics of pig heart triphosphopyridine nucleotide-isocitrate dehydrogenase. I. Initial velocity, substrate and product inhibition, and isotope exchange studies. J Biol Chem. 1974 May 10;249(9):2920–2927. [PubMed] [Google Scholar]