Abstract
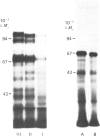
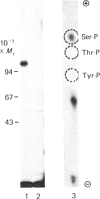
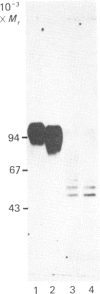
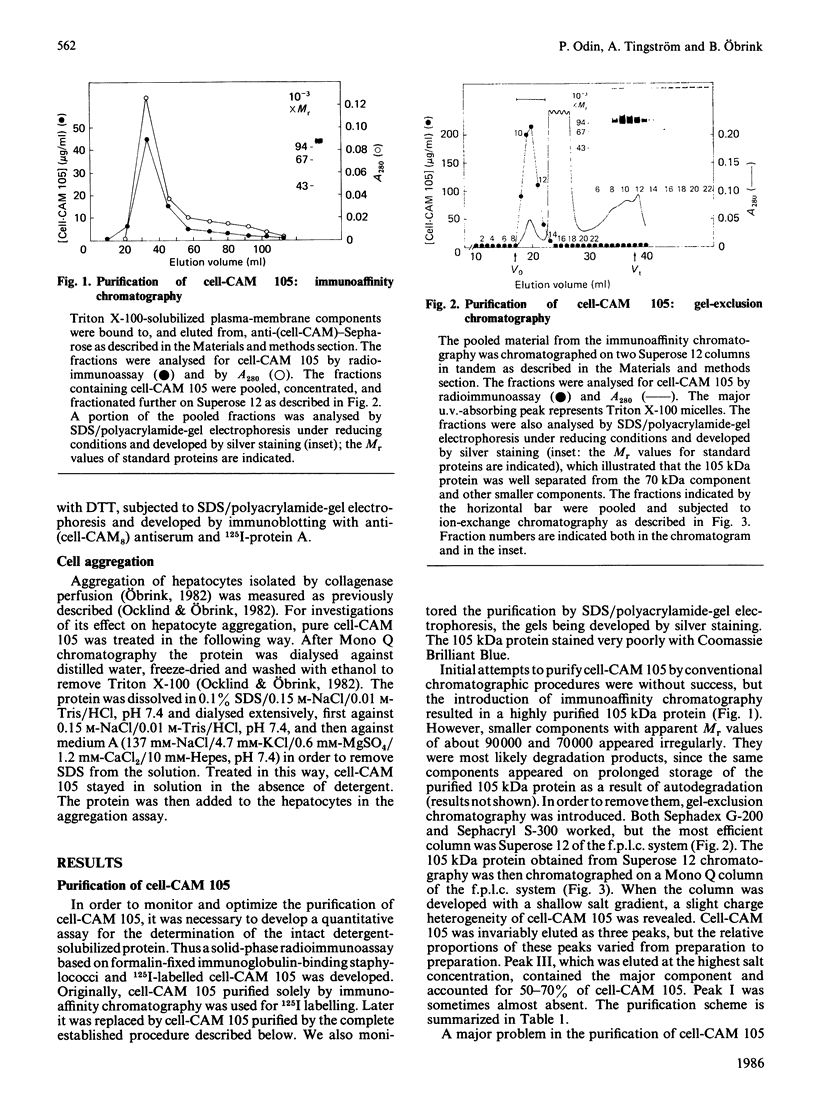
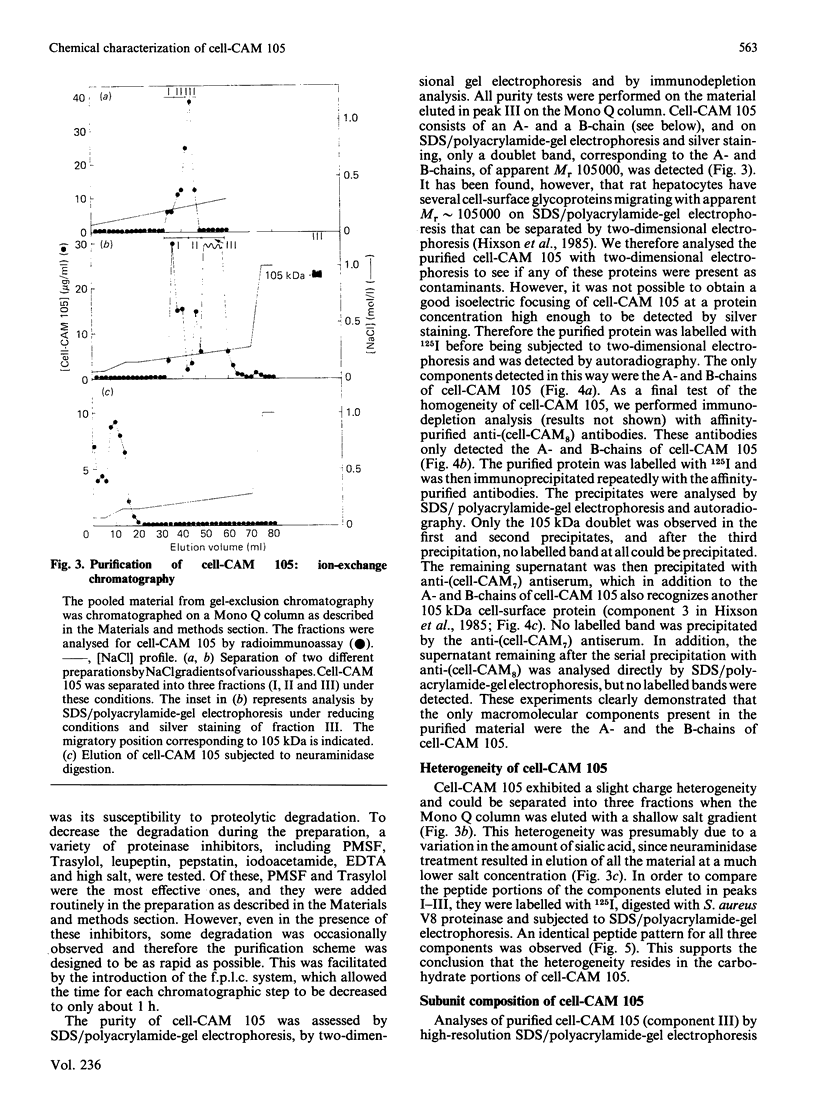
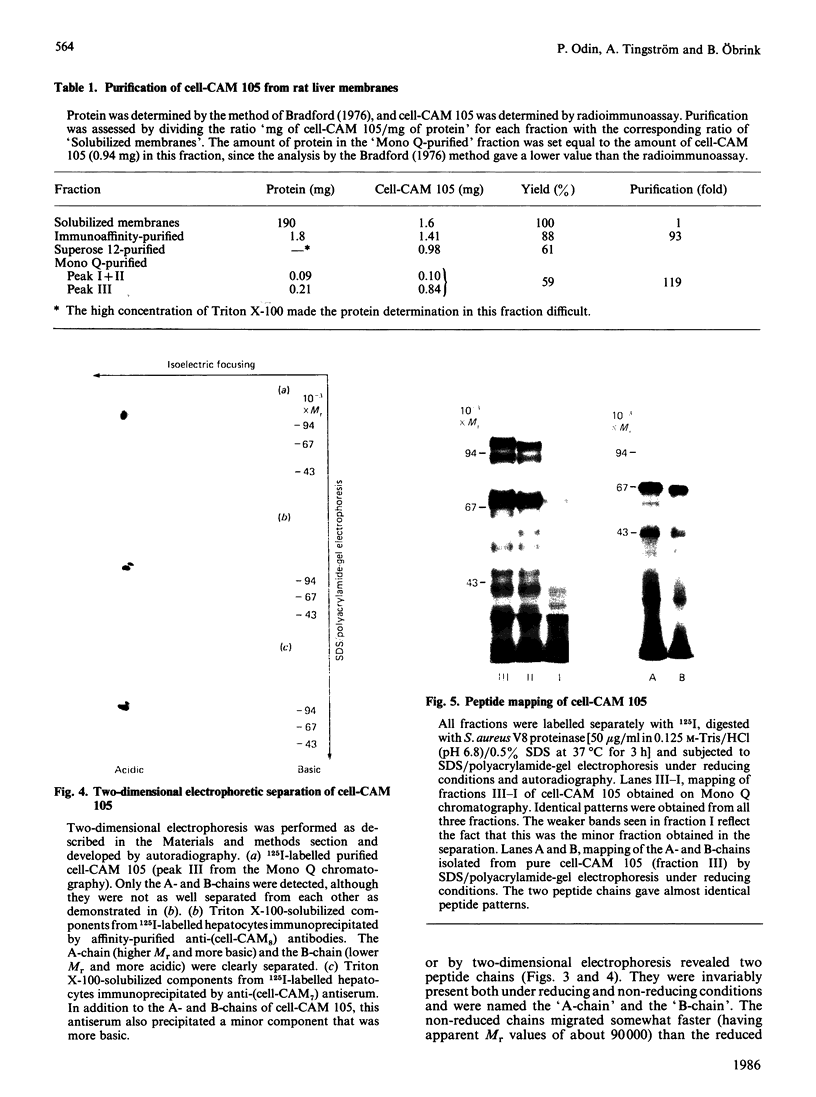
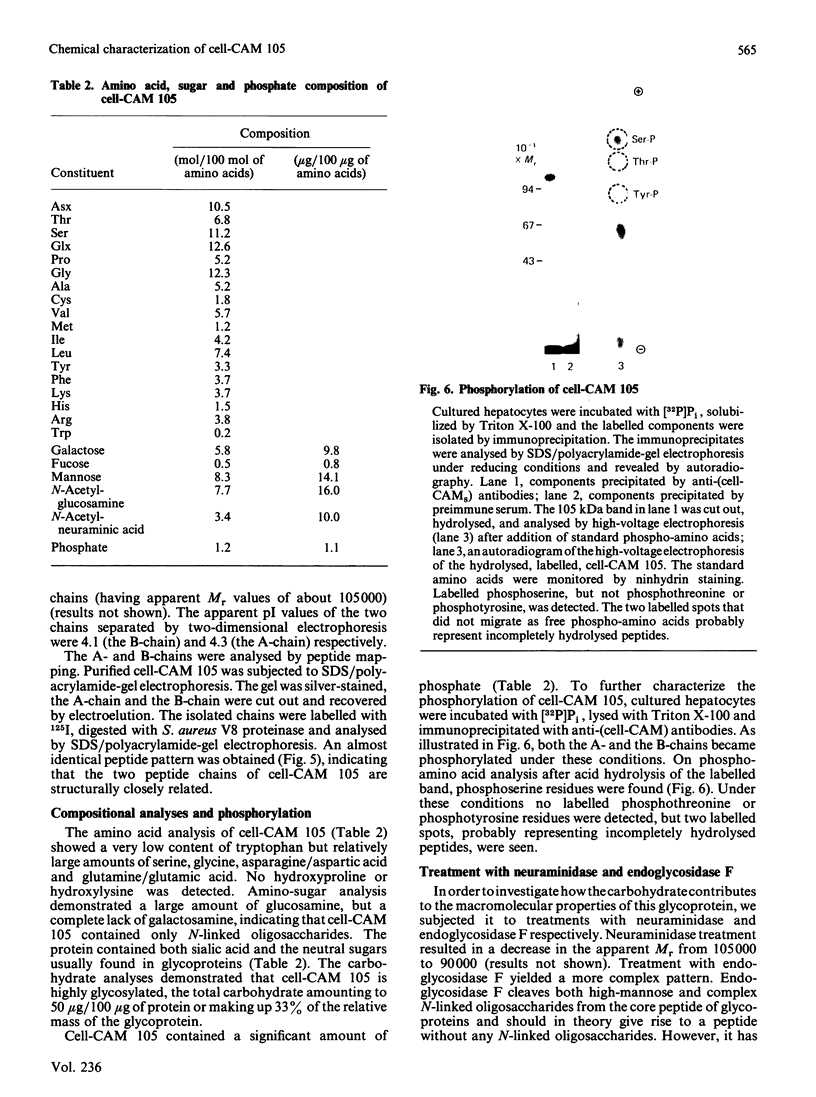
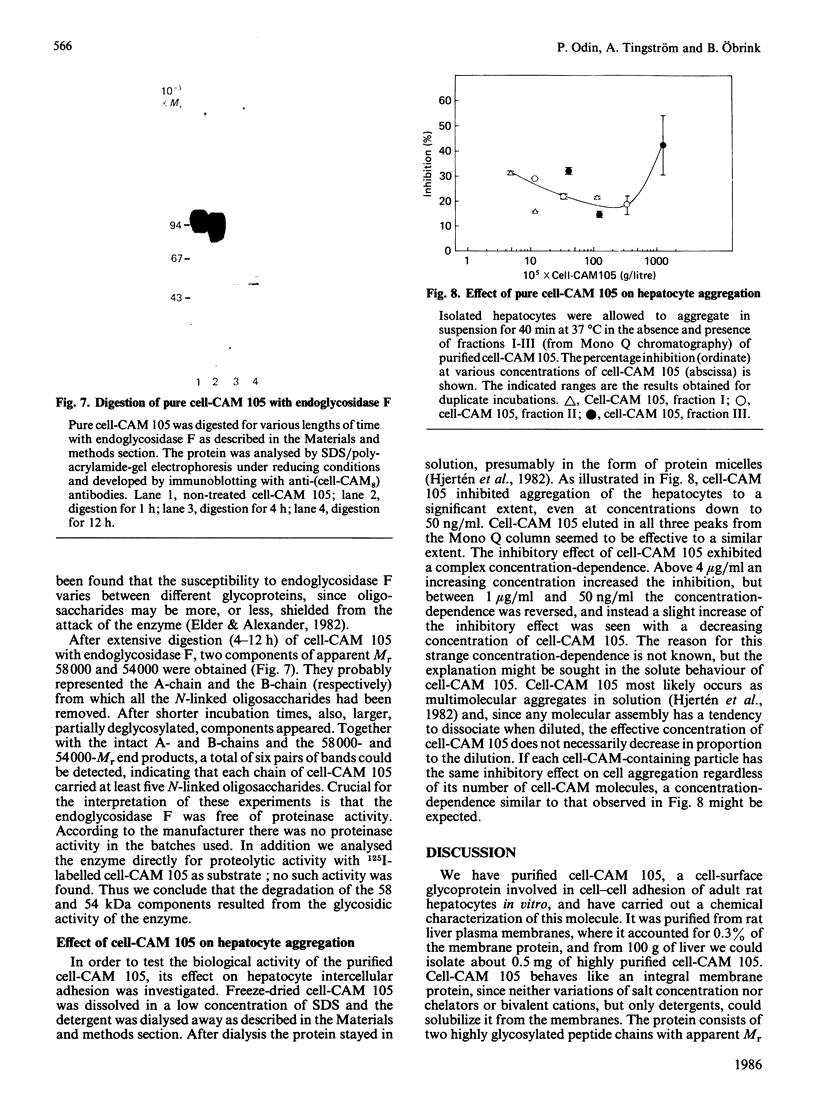
Cell-CAM 105, a glycoprotein that is involved in recognition and adhesion between isolated rat hepatocytes in vitro, was purified to homogeneity by a combination of immunoaffinity chromatography, gel-exclusion chromatography and ion-exchange chromatography. Electrophoretic, compositional and enzymic analyses were performed and the glycoprotein was shown to consist of two peptide chains, of apparent Mr 110,000 and 105,000 respectively, that are glycosylated to similar extents. Carbohydrate analyses demonstrated the presence of sialic acid, galactose, mannose, fucose and glucosamine, but no galactosamine, indicating that only N-linked oligosaccharides occurred. The total content of carbohydrate amounted to 33%. Peptide mapping indicated that the two peptide chains were structurally very similar. After incubation of cultured hepatocytes with [32P]Pi, phosphorylated cell-CAM 105 could be isolated. Both peptide chains were labelled and phospho-amino-acid analysis demonstrated that serine residues had become phosphorylated. A significant feature of cell-CAM 105 was a susceptibility to autolytic degradation that was difficult to inhibit. The major degradation products had apparent Mr 90,000 and 70,000, respectively. The effect of purified cell-CAM 105 on cell-cell adhesion of re-aggregating hepatocytes was studied. A significant inhibition was observed, indicating that the protein is directly involved in intercellular adhesion of these cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Edelman G. M., Cunningham B. A. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerton G. L., Wardrip N. J., Hedrick J. L. A gel eluter for recovery of proteins separated by polyacrylamide gel electrophoresis. Anal Biochem. 1982 Oct;126(1):116–121. doi: 10.1016/0003-2697(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Edelman G. M. Two antigenically related neuronal cell adhesion molecules of different specificities mediate neuron-neuron and neuron-glia adhesion. Proc Natl Acad Sci U S A. 1984 Jan;81(1):267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Hirn M., Pierres M., Deagostini-Bazin H., Hirsch M., Goridis C. Monoclonal antibody against cell surface glycoprotein of neurons. Brain Res. 1981 Jun 15;214(2):433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- Hixson D. C., McEntire K. D., Obrink B. Alterations in the expression of a hepatocyte cell adhesion molecule by transplantable rat hepatocellular carcinomas. Cancer Res. 1985 Aug;45(8):3742–3749. [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof B. A., Vollmers H. P., Goodman S. L., Birchmeier W. Cell-cell interaction and polarity of epithelial cells: specific perturbation using a monoclonal antibody. Cell. 1983 Dec;35(3 Pt 2):667–675. doi: 10.1016/0092-8674(83)90099-5. [DOI] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Jørgensen O. S., Delouvée A., Thiery J. P., Edelman G. M. The nervous system specific protein D2 is involved in adhesion among neurites from cultured rat ganglia. FEBS Lett. 1980 Feb 25;111(1):39–42. doi: 10.1016/0014-5793(80)80756-3. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kruse J., Keilhauer G., Faissner A., Timpl R., Schachner M. The J1 glycoprotein--a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature. 1985 Jul 11;316(6024):146–148. doi: 10.1038/316146a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Obrink B. Epithelial cell adhesion molecules. Exp Cell Res. 1986 Mar;163(1):1–21. doi: 10.1016/0014-4827(86)90554-9. [DOI] [PubMed] [Google Scholar]
- Obrink B. Hepatocyte--collagen adhesion. Methods Enzymol. 1982;82(Pt A):513–529. doi: 10.1016/0076-6879(82)82083-1. [DOI] [PubMed] [Google Scholar]
- Ocklind C., Forsum U., Obrink B. Cell surface localization and tissue distribution of a hepatocyte cell-cell adhesion glycoprotein (cell-CAM 105). J Cell Biol. 1983 Apr;96(4):1168–1171. doi: 10.1083/jcb.96.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklind C., Obrink B. Intercellular adhesion of rat hepatocytes. Identification of a cell surface glycoprotein involved in the initial adhesion process. J Biol Chem. 1982 Jun 25;257(12):6788–6795. [PubMed] [Google Scholar]
- Ocklind C., Odin P., Obrink B. Two different cell adhesion molecules--cell-CAM 105 and a calcium-dependent protein--occur on the surface of rat hepatocytes. Exp Cell Res. 1984 Mar;151(1):29–45. doi: 10.1016/0014-4827(84)90353-7. [DOI] [PubMed] [Google Scholar]
- Peyriéras N., Hyafil F., Louvard D., Ploegh H. L., Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen F. G., Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984 Jan;3(1):1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richa J., Damsky C. H., Buck C. A., Knowles B. B., Solter D. Cell surface glycoproteins mediate compaction, trophoblast attachment, and endoderm formation during early mouse development. Dev Biol. 1985 Apr;108(2):513–521. doi: 10.1016/0012-1606(85)90054-5. [DOI] [PubMed] [Google Scholar]
- Sorkin B. C., Hoffman S., Edelman G. M., Cunningham B. A. Sulfation and phosphorylation of the neural cell adhesion molecule, N-CAM. Science. 1984 Sep 28;225(4669):1476–1478. doi: 10.1126/science.6474186. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- Vestweber D., Ocklind C., Gossler A., Odin P., Obrink B., Kemler R. Comparison of two cell-adhesion molecules, uvomorulin and cell-CAM 105. Exp Cell Res. 1985 Apr;157(2):451–461. doi: 10.1016/0014-4827(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Yoshida-Noro C., Suzuki N., Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984 Jan;101(1):19–27. doi: 10.1016/0012-1606(84)90112-x. [DOI] [PubMed] [Google Scholar]