Abstract
3D bioprinting has developed tremendously in the last couple of years and enables the fabrication of simple, as well as complex, tissue models. The international space agencies have recognized the unique opportunities of these technologies for manufacturing cell and tissue models for basic research in space, in particular for investigating the effects of microgravity and cosmic radiation on different types of human tissues. In addition, bioprinting is capable of producing clinically applicable tissue grafts, and its implementation in space therefore can support the autonomous medical treatment options for astronauts in future long term and far‐distant space missions. The article discusses opportunities but also challenges of operating different types of bioprinters under space conditions, mainly in microgravity. While some process steps, most of which involving the handling of liquids, are challenging under microgravity, this environment can help overcome problems such as cell sedimentation in low viscous bioinks. Hopefully, this publication will motivate more researchers to engage in the topic, with publicly available bioprinting opportunities becoming available at the International Space Station (ISS) in the imminent future.
Keywords: additive manufacturing, biofabrication, low Earth orbit – LEO, space
The article discusses opportunities and challenges of different bioprinting technologies when applied in microgravity and possible applications in space. Those can include the fabrication of tissue models for investigation of effects of space conditions on multicellular constructs and clinically applicable tissue grafts. As bioprinters are installed now in the International Space Station, experiments can be performed to validate the findings.
1. Introduction
Since Yuri Gagarin completed one orbit of the Earth back in 1961 on board the Vostok 1 capsule, human space flight has seen tremendous progress and achievements, always supported by major technological developments. The establishment of the International Space Station (ISS) in 1998 in low Earth orbit (LEO) represented an important milestone for space exploration allowing for scientific research in biology, physics, astronomy, and other fields, to be conducted under microgravity conditions. Today and after more than 400 space flights and 65 missions, the ISS remains instrumental in supporting human exploration missions to the Moon and Mars providing a base for staging, maintaining, and testing exploration capabilities in LEO. The human quest for the reaches of the universe, trying to find answers to fundamental questions underpinning our existence and place in the cosmos, will inevitably cause space missions to become longer and to travel further into deep space, eventually culminating with the establishment of permanent human‐tended bases on other planets.[ 1 ]
Space and spaceflight have never been so present in our daily lives as they are now, and it is not difficult to imagine that in the not‐too‐distant future, travel to space might even become common for nonastronauts. But why space, and why now? The answer is not straightforward and the benefits that space can produce in our lives are just being realized. By unveiling the secrets of the universe, we hope to develop a better understanding of our world on Earth, pushing the boundaries of science and knowledge to develop new technology that will help foster new industries with significant socioeconomic impact for our human society. This is true for many sectors of our industry, but in particular for the health & life sciences sector which is undoubtedly one of the major users of the space environment.[ 2 ] With growing investment and commitment from both public and private sectors, the pace of R&D is also expected to increase, enabling to overcome a number of open challenges associated with human exploration of space, starting with political, technological, and medical issues.
Medical challenges are particularly relevant for the success of costly missions within deep space (e.g., towards Mars), where the health of the astronauts can be impacted by a number of space stressors, including radiation and microgravity. This raises serious health threatening risks such as bone fractures (caused by loss of bone density), cardiovascular incidents or even an increased risk for specific cancers.[ 3 ] On site, medical care is often compromised by the lack of storage space or specialized equipment, and a fast return to Earth for emergency medical assistance or surgical intervention is clearly impossible in deep space missions due to the long traveling distances and the need to wait for specific planetary constellations. This poses extra pressure on the space crew, already reliant on limited resources, to carry out efficient medical procedures in a self‐autonomous manner.
Additive manufacturing (AM) has been identified as a production principle that provides opportunities for flexible fabrication of objects for utilization in space and that could increase the autonomy of the crew during missions.[ 4 , 5 ] It is in this context that also 3D bioprinting technologies attracted attention of the space agencies for the generation of tissues and organs for application in tissue engineering and regenerative medicine.[ 1 , 3 , 6 ] Bioprinting allows for the computer‐controlled shaping of cells, organoids, biomaterials, and relevant biomolecules, commonly layer‐by‐layer, into 3D models capable of replicating the complexity of our tissues and organs, or at least coming close to those.[ 7 , 8 ] These models can eventually be used to study the impact of space stressors (i.e., microgravity and radiation) on the physiology of human tissues and organs, improving our understanding of space‐induce biological mechanisms on mammalian cell function and supporting the development of more efficient therapies for long‐term crewed missions.[ 6 ] Additionally, the development of medical therapies for the treatment of age‐related or chronic human diseases on Earth could also benefit from experiments conducted under microgravity.[ 9 ] The latter is responsible for inducing changes in human systems (e.g., loss of bone density), that in many ways resemble those associated with the onset of different human diseases developing on Earth (e.g., osteoporosis), but at a much faster rate.[ 10 ] Therefore, studies underpinning human ageing, disease or the testing of medical therapies that would normally take years to develop, could be accelerated with important benefits for patients with life‐threatening conditions. The above benefits, combined with the possibility of manufacturing customized artificial tissues and in future maybe even functional organs for transplantation, have led the European Space Agency (ESA) to consider 3D bioprinting as a key technology to enable future deep space exploration and human settlements.
Much has already been done for terrestrial applications where we now have a wide range of natural and synthetic polymers that can be modified using a range of moieties and strategies for improved printability and cell function. These are reviewed in detail elsewhere and can certainly be used as working basis for the design of new polymeric inks for space bioprinting.[ 7 , 11 , 12 ]
Recently, there have also been several projects supported by ESA and NASA for bioprinting research to take place on the ISS. Indeed, a microgravity environment may improve print fidelities of soft tissue structures due to the unneeded use of high viscosity materials and support structures to be able to withstand the force of gravity. Currently making use of this phenomenon is the 3D BioFabrication Facility (BFF) being part of the U.S. National Lab and developed by US‐based company Techshot (acquired by Redwire in 2021).[ 13 ] The BFF aims to print high resolution tissue analogues by extrusion‐based bioprinting that can be maintained on the ISS for up to two months within specially designed bioreactor cassettes before being sent back to Earth for analysis. So far, completed projects using the BFF have focused on bioprinting meniscal and cardiac tissue,[ 14 , 15 ] however, scientific publications about these studies are still missing. Furthermore, a handheld 3D bioprinter has been developed by German company OHB for the German Aerospace Center, DLR in collaboration with ESA and delivered to the ISS; the so‐called Bioprint FirstAid device intends to utilize ready‐made autologous cell‐laden bioinks to print dressings to cover superficial skin wounds.[ 16 ]
Here, after introducing the concept of bioprinting and its relevance for space research, we discuss specific boundary conditions that may impact the successful translation of the technology including limited resources and the need to avoid open systems. We proceed with a brief review of the available bioprinting techniques highlighting challenges and opportunities for these in microgravity. The recent global pandemic has reinforced the need for better and more efficient systems for online monitoring of patient's health. Much of this technology is already employed in the space domain and is discussed here along with remote control systems and bioreactors for tissue maturation. We conclude with a brief overview of the challenges and opportunities for the healthcare space sector and discuss future prospects for bioprinting in space.
2. Relevance of Bioprinting for Applications in Space
Human spaceflight and exploration is at a turning point: while long‐duration crewed missions to the ISS, the golden standard for more than two decades, are opening up to commercial players (e.g., Axiom, Blue Origin, SpaceX, Virgin Galactic), space agencies are focusing on more complex missions to the moon[ 17 ] and eventually to Mars. From a medical standpoint, these latter missions are much more complex because they venture deeper into space and will expose crew to more extreme and longer time periods to space stressors, both exogenous (such as, but not limited to variable gravity levels, space radiation[ 18 ]) and endogenous (e.g., psychosocial challenges due to isolation and confinement[ 19 , 20 ]). Furthermore, these missions will be operationally and logistically very challenging, as they will endure delayed or no premature return scenarios in the case of emergencies, including those that are medically‐related, and will only allow for very limited medical capabilities in‐mission.[ 21 , 22 ] This differs very strongly from the current modus operandi on the ISS where medical contingencies are treated as first‐aid interventions, but surgeries would be performed on Earth (which would be reachable within less than a day). As such, future deep space missions will need a high self‐sustainability on‐board and a high autonomy of the crew of the mission. It also infers that preparation in advance on ground needs to be thoroughly considered; however, this preparation can only be done to a certain extent as a mitigation strategy for each potential scenario cannot be foreseen. In addition, it also implies that injuries considered as less severe on Earth (e.g., wounds, smaller burns, and bone fractures) or even smaller medical events can be inflated in severity and threaten the health of the crew, as well as impact the mission detrimentally.[ 21 , 22 , 23 ] Therefore, in situ opportunities for medical treatment could tremendously help to support these long‐term human exploration missions to the Moon and eventually to Mars.
To address some of these challenges on deep space missions, the European Space Agency (ESA) is further looking into 3D bioprinting as an enabling technology. ESA has already done a considerate quantity of work on looking into further expanding this technology for use in space.[ 1 , 3 ] As a follow‐up, ESA is currently building a facility for bioprinting and 3D cell maturation in LEO. Once operational, this “3D Biosystem” will allow for fundamental research into the impact of key space stressors, such as microgravity and space radiation on a variety of cells and tissues, which will help to better understand and quantify the cumulative impact of microgravity and space radiation. Bioprinting can be used to make 3D cell constructs similar in structure to specific organs or tissues. In this way, the impact of spaceflight factors on processes occurring in these tissue models, which are otherwise difficult to investigate directly in animal or human subjects, can be studied in detail. Bioprinting will allow for testing more complex and 3D tissue models, which exceed conventional cell cultures in mimicking the native human tissues.[ 24 ] Furthermore, bioprinting can also be used to investigate the response of certain cell and tissue constructs to specific pharmacological countermeasures.[ 25 ] As such, bioprinting will allow for bridging between some of the current knowledge gaps.
After this initial phase and recognizing future long exploration missions into deep space, bioprinting could eventually be an enabling technology to support medical treatment in crew members. The application of bioprinting in relation to wound healing and skin tissue repair (e.g., in burns) has been described in recent publications.[ 23 , 24 ] In a following stage, these technologies have, for example, the potential to print personalized grafts or specific implants for the treatment of tissue injuries.[ 23 , 24 ]
The expansion of bioprinting into the food production realm has had further developments in recent years. As such, bioprinting could potentially be exploited in manufacturing food sources during space flight. The food‐tech start‐up Aleph Farms, partnered with company 3D Bioprinting Solutions, is intending to do just that by culturing bioprinted bovine muscle cells on the ISS.[ 26 ]
Lastly, 3D bioprinting is not limited to mammalian or human cells, but can also make use of other cell types, e.g., microalgae or cyanobacteria.[ 27 ] These could be implemented across a variety of applications, such as bioreactors and life support systems (e.g., for oxygen production and waste treatment[ 28 ]), as well as food and secondary metabolites production (e.g., vitamins). This topic has not been widely explored yet, but also from this angle, 3D bioprinting could be a very interesting technology for future deep space missions. Additional research is needed to, e.g., figure out which nonmammalian cell species would be most suited for this application, as well as which cell species would tolerate immobilization into the bioink's hydrogel and would be able to withstand the printing process.
3. Specific Boundary Conditions in Space
Spaceflight is still connected to a number of challenges and limitations that complicate any translation of technologies that have already been established on Earth. This includes the limited capacities for bringing payload into space (both concerning volume and weight) and the enormous costs related to this delivery. In addition, the storage conditions during launch and commonly occurring delays make it difficult to bring sensitive or fragile components into space.[ 23 ] This is especially a problem for the upload of live cells, which is perhaps one of the most limiting factors in the establishment of bioprinting at the ISS. To solve this challenge, cells that have undergone cryo‐preservation could instead be launched; however, this leads to the necessity of a number of manual process steps after arrival before such cells then could be further used, which would require an excess of crew time that is not readily available. To overcome this limitation, the long‐term storability of premixed bioinks has been investigated very recently with interesting first results: it appears to be possible to store such cell‐laden inks for several weeks at 5 °C without complete loss of cell viability.[ 29 ]
Whereas many experiments in physics or astronomy can be highly automated, this yet remains a big challenge in cell culture and tissue engineering. Respective attempts on ground led to large, very complex and difficult to control devices,[ 30 ] which cannot be established in the restricted environment of a spaceship. However, compared to the manual fabrication of 3D cell constructs using the principles of tissue engineering, several processes in bioprinting are already performed in a semi‐autonomous manner, which would help towards reducing crew time. Strict safety regulations apply in space flight, making it very complicated – if not impossible – to utilize many substances, like common fixatives, in space that are routinely used in cell‐based research and bioprinting. Again, alternative protocols and bioinks need to be developed that allow for the long‐term storage of viable cells to supersede bioink preparation on board the ISS.
Logically, microgravity is the largest challenge in handling liquids, which is the case for all steps of a bioprinting process, i.e., during cell isolation, bioink preparation, the printing process itself, and further maturation of the 3D cell constructs in culture media. In addition, fluids in general will behave differently and phenomena such as gas exchange, crucial for cell survival during cultivation, are strongly altered.[ 31 ] Both need to be considered in constructing 3D cultivation devices for use in microgravity. However, as discussed below in further detail, microgravity may also offer distinct advantages for different bioprinting technologies. Amongst others, less viscous bioinks might become applicable in extrusion‐based bioprinting with sedimentation of cells or solid bioink additives, like calcium phosphate particles, not able to occur. In contrast, this altered fluid behavior might lead to the stable entrapment of air bubbles, and the domination of surface tension forces can impair the shape fidelity and lead to unintended deformation during or after the printing process.[ 32 ] Therefore, in‐depth research and a careful selection of most suitable bioink viscosities for space is necessary. However, this is a challenging feat; many microgravity simulation devices on Earth, namely clinostats, random positioning machines or rotating wall vessels, are suitable for cultivating bioprinted constructs, yet are too small to host a standard‐sized bioprinter.[ 33 , 34 ] Other test systems, including parabolic flights or drop‐towers, can accommodate such hardware; however, the time periods in which microgravitational conditions can apply are too short for a detailed and in‐depth analysis of all relevant phenomena of the bioprinting process.[ 34 ] As such, the scientific community is eagerly awaiting for the opportunity to investigate the full bioprinting process in detail under real space conditions onboard the ISS.
In preparation for long‐term and far‐distant crewed space missions, the opportunities offered by bioprinting to provide clinically applicable tissue substitutes for the treatment of severely injured or ill astronauts shall be further explored and developed. However, a number of fundamental problems related to the clinical application of bioprinted tissues are not yet solved, even on Earth.[ 3 ] This includes, beside others, (1) the very high cell numbers required for the fabrication of volumetric human tissues, (2) the complexity of many relevant tissues concerning the arrangement of different cell types on several length scales, (3) the mechanical stability of the fabricated constructs, and (4) the necessity to provide a functional and hierarchical arteriovenous system that can be microsurgically connected to the host. In addition, improved nondestructive analytical methods for assessing the quality and properties of the bioprinted tissue constructs, both directly after fabrication and during further maturation, are required. Moreover, performing surgeries in space is in general connected to a number of specific challenges.[ 35 ] Another aspect becomes notably relevant for long‐term and far‐distant space missions: the need to produce and recycle as much as possible on‐site as the capacity for carrying materials along such journeys will be strictly limited. Therefore, it should be explored whether bioinks can be made from biopolymer components isolated from organisms, like algae or plants, that can be cultivated in space.
However, as it is generally accepted that long‐term crewed space missions, and especially those outside the LEO, are connected with higher health risks for the astronauts the development of improved and autonomous treatment options is a pressing need.[ 36 ] It is anticipated that bioprinting technologies will contribute to these advancements.
4. Bioprinting Technologies Based on Material Deposition
4.1. Extrusion‐Based Bioprinting
Extrusion‐based bioprinting (EBB)[ 37 ] technologies dispense continuous filaments of cell‐laden hydrogel materials, called bioinks, through nozzles using a piston, a screwing system, or pneumatic pressure as the driving force. For creating a 3D object, EBB technologies usually follow a layer‐by‐layer approach by printing planar layers on top of each other. Nowadays, EBB systems are the most popular approaches in the biofabrication realm thanks to their user‐friendliness, the possibility to process numerous bioinks characterized by a wide range of physicochemical properties, and to build sophisticated, heterogenous constructs containing multiple cell types and biomaterials.
The EBB research field is quite varied in embracing different extrusion strategies, each offering specific advantages and suffering of certain limitations. These strategies include direct writing, coaxial extrusion, and deposition in a coagulation or support bath (Figure 1 ).[ 38 ]
Figure 1.
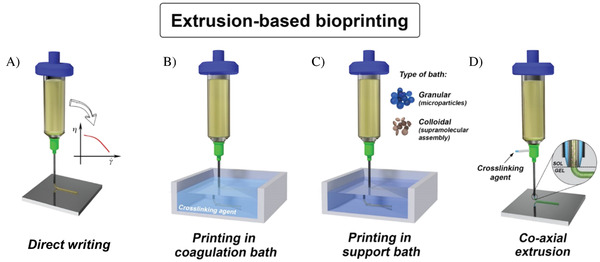
Different opportunities to use EBB. Adapted with permission under the temrs of the CC–BY license.[ 39 ] Copyright 2018, the Author(s). Published by IOP Publishing. A) Direct writing. B) Printing in coagulation bath. C) Printing in support bath. D) Co–axial extrusion.
To provide good shape fidelity, the bioink needs to possess specific rheological properties, such as shear thinning behavior, in which the viscosity drops sharply under high shear, i.e., when the material is extruded through the printing nozzle.[ 40 ] After, or sometimes during, the printing process the bioinks are further stabilized by crosslinking. For this, either photochemical reactions or ionic mechanisms, as in the case of alginate as the base bioink component, can be employed. Liquid crosslinkers can be applied during extrusion by using coaxial nozzles; however, these can also be utilized to produce strands consisting of two different bioinks (different materials and/or cell types) arranged in a core/shell fashion.[ 39 ]
In the context of microgravity conditions, direct writing and coaxial extrusion may represent the first choices, as the presence of a support bath could introduce additional operational problems. Furthermore, the direct writing approach shares multiple similarities with fused deposition modelling (FDM), the AM technology based on the extrusion of thermoplastic filaments, which has been already successfully tested on the ISS.[ 41 ] Nevertheless, EBB strategies requiring a support bath may be useful on Earth to simulate a microgravity environment, as during the extrusion process the net force acting on the bioprinted structures is the difference between the gravity and the buoyant force.
From an experimental standpoint, EBB in microgravity conditions may require troubleshooting specific problems, generally of minor impact on Earth, during both the pre‐processing and processing phases. Yet, at the same time these strategies may benefit from the reduced gravity force. For instance, during the preprocessing phase, the resuspension of cells within the biomaterial ink is often accompanied by the formation of a significant amount of sub‐millimeter size bubbles. While on Earth this issue is solved by simply exploiting gravity – bubbles tend to travel upward in the bioink making their removal relatively easy – in a microgravity environment bubble removal may represent a daunting task. A possible solution for this challenge may be the centrifugation of the bioink or the formulation of ready‐to‐use bioinks prepared on Earth to be shipped to space under cooling conditions. This option may additionally help in extending the bioink shelf‐life, avoiding extensive cell culture and manual mixing procedures in space prior to the bioprinting process.[ 29 ] Alternatively, one may design the bioink fluidic path within the bioprinter to have bubble traps along the way, a common solution now found in some microfluidic systems.[ 42 ] On a positive note, microgravity may help in significantly reducing the problems connected with cell sedimentation in the bioink cartridge, an issue causing cell density fluctuations in the bioprinted samples[ 43 ] on Earth, especially when low‐viscous bioinks are used. In general, it should be possible to utilize less viscous inks for EBB in space as gravity will not lead to deformation or collapse of the printed structures. Moreover, it may help in printing more sophisticated geometries, e.g., nonplanar[ 44 ] or overhanging structures, likely without the need to coprint additional support materials. To this aim, new toolpath generators specifically designed for microgravitational‐EBB may be needed in the future. However, the dominance of surface tension forces in the absence of gravity might lead to problems related to the tendency of fluids to form spherical objects;[ 32 ] these might be especially relevant in the case of low viscous inks.
Printability via EBB technologies is a complex property that depends on the extrusion strategy, the geometry to be printed, and the characteristics of the ink.[ 45 ] According to the dimension of the filament, volumetric (related to viscosity and density) or surface phenomena (related to surface tension) can be prevalent. In the case of direct writing with commonly used viscoelastic hydrogels on Earth, volumetric forces have a higher impact: the scaffold tends to collapse due to gravity, thus a hydrogel with a relevant yield stress is needed, yet may interfere with the extrusion process or can impair cell viability. Under microgravitational conditions, the printability window should be reanalyzed, hence the possibility of achieving high print fidelities with even lower viscosity hydrogels.
Another aspect that should be carefully considered is the wettability of the printing bed. In fact, while on Earth this feature is practically neglected (due to the high Ohnesorge number), in microgravity conditions it may play a crucial role in the adhesion of the first deposited layer and the 3D bioprinted structure as a whole. Minimizing this issue may be critical to achieve high printing accuracy. In this context, it may also be beneficial to gain a better understanding of the influence of process parameters (such as printing speed, layer height, nozzle size, etc.) over the net force acting on the sample during the bioprinting process.[ 46 ] To this aim, advanced bioprinting simulation tools should be developed to help researchers rapidly identify the optimal process parameters and the printability window. In an effort to demonstrate on Earth that EBB in exceptional gravitational forces is possible, multiple layers of a common alginate‐based bioink, as well as an adhesive calcium phosphate bone cement, were extruded against Earth's gravity using a printer suspended upside‐down.[ 3 ]
Regarding the bioink supply to the extrusion nozzle, it is not yet clear whether the use of volumetric feed control systems (e.g., syringe pumps) should be preferred in microgravity to pressure‐driven flow systems or vice versa. In fact, while volumetric systems can accurately process a wide range of bioinks, these systems are bulky and limited in dispensable volumes (up to a few tens of milliliters). On the other hand, precisely controlling the extrusion flow rate with pressure‐driven flow controllers is more challenging, requiring a careful calibration for each bioink formulation. However, pressure‐driven systems are relatively compact – a feature highly appreciated in space missions – and capable of processing larger bioink volumes. To be able to analyze and control the extrusion process remotely it would be advantageous to equip EBB devices operating in microgravity with video cameras so that both the nozzle and the bioprinted object can be monitored and the printing parameters, if needed, adjusted.
In summary, although there are still some challenges to overcome, the EBB shows a high potential to be used in space. In the near future, launching the first ESA 3D bioprinter to the ISS will provide a better understanding of this biofabrication process in a microgravity environment.
4.2. Inkjet/Drop‐on‐Demand Bioprinting
Bioprinting technologies differ greatly in their resolution and scale production capabilities, which affects the size, quality, and fidelity of the printed structures. Here, very high precision (single cell deposition) and high resolution (tenth of cells or nanoliter drops) are reached using laser‐based systems[ 47 , 48 ] and inkjet‐based techniques, respectively, to produce small structures. On the contrary, medium‐resolution and large‐volume geometries are attained using extrusion‐based bioprinters. Inkjet printing occupies here an interesting position since it links its two sister technologies in terms of size capability (Figure 2A). These three bioprinting technics also differ in the viscosity of the printed material: laser‐based systems being able to eject only adherent cells or spheroids,[ 49 ] inkjet printing handling only low viscosity cell suspensions (up to 20–40 mPa s),[ 50 , 51 ] and extrusion printing being suitable to almost all possible viscosities (up to 3 × 105 Pa s).[ 52 ]
Figure 2.
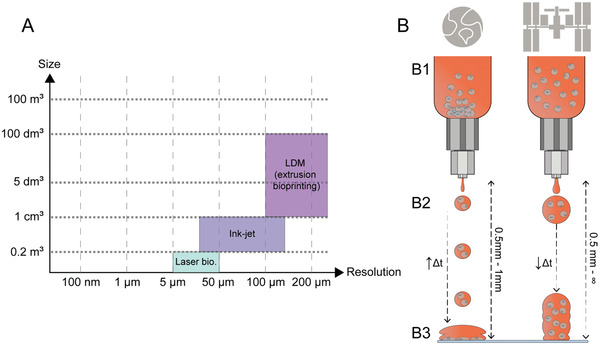
A) Resolution and size of the objects produced by liquid deposition modeling (LDM, also referred to as extrusion bioprinting or EBB), inkjet printing, and laser bioprinting. B) Differential behavior of inkjet bioprinting expected on Earth (left) and space (right). Differences are expected due to microgravity at (B1) the cell (B2) droplet and (B3) construct levels.
The ISS welcomed its first residents in November 2000 who exclusively used the inkjet printing technology for 3D printing in space (Epson 800 inkjet printer initially).[ 53 ] Microgravity is then a condition that has been already demonstrated suitable for inkjet deposition of liquid inks and what is more, 3D printing of cells with high viability was originally demonstrated [ 54 ] with biohacked inkjet office printers.[ 55 ] Hence, the gap is closed between space bioprinting and inkjet technology.
When it comes to bioprinting and particularly tissue engineering on Earth, inkjet printing has been mainly used for the production of thin tissues, such as the retina, or covering external cellularized layers, such as endothelium and epithelium.[ 56 , 57 , 58 ] Indeed, these tissue layers are known to be composed almost exclusively of cells. Their biofabrication thus only relies on the positioning of cells onto or into a more complex system, which might be a scaffold or another tissue. This is where inkjet‐based technologies are the most advantageous, being able to deposit precise volumes of cell suspension (e.g., down to 300 pL using SCIENION inkjet printer) with a precision of 5 µm and no dead volume.[ 59 ]
In the meantime, gravity on Earth still limits inkjet printing capabilities with well‐known drawbacks that could be leveraged with microgravity (Figure 2B)
Material Viscosity—Since materials used for inkjet (bio)printing have low viscosities, cells easily settle in the liquid phase and generate heterogeneities in the bioink. Likewise, the range of cell concentrations usable for inkjet printing is limited and high cell density results in clogging of the printing nozzle. An attempt to cope with this issue was proposed by constantly agitating the reservoir, perpetually resuspending cells.[ 60 ] Here, microgravity will be an asset since the dispersion of the cell population will remain homogenous, even in a large volume of low viscosity liquid. Consequently, high cell density might be compatible with inkjet printing in space.
Static Yield Stress—The static yield stress of a low‐viscosity material is also intrinsically low. The capacity of the jetted material to generate large parts in the Z direction is then very limited since the material is collapsing under its own weight.[ 61 , 62 ] This can be partially solved on Earth by in situ reticulation of the jetted material during the printing process, either by using photochemistry [ 63 ] or soft chemistry.[ 64 ] In space, microgravity is expected to reduce the force applied by a layer due to its own weight, enabling the production of larger parts than on Earth while keeping the viscosity and static yield stress as low as possible.
Surface Tension—The behavior of fluids in microgravity is driven by surface tension forces, and liquids can achieve sizes much larger than on Earth.[ 65 ] Therefore, drops of higher volume could be extruded from similar valve opening time than on Earth.[ 66 ] Together with the lower static yield stress, larger structures could be built‐up with inkjet printing in microgravity than on Earth, changing our conception of the size capability for the technology.
Working Distance—Inkjet printing is a near‐field method on Earth, with the trajectory deviation and geometry variations of the droplet being a function of the distance between the jetting nozzle and the target support. A classical distance of 0.5–1 mm is considered optimal on the ground.[ 67 ] In microgravity conditions, it is hard to predict what would be the droplet's behavior and unusual comportments or performances that may arise from using experimental conditions that would be unsuitable on Earth. For example, less energy might be necessary to eject droplets in microgravity, leading to a slower droplet speed and higher cell viability. Inkjet bioprinting of cells in standard viscosity bioinks should thus be performed through unusual printing settings in microgravity; this will have to be documented and compared to the behavior of the same material on Earth.
Thus, microgravity has the potential of changing how we use inkjet printing for tissue engineering. However, other aspects of specific fluid behavior in microgravity could conversely create limitations
Liquid‐free Displacement—As often depicted in space station footage, liquids are free to move in microgravity. In the case of a droplet jetting system such as inkjet bioprinting, the droplet missing the target area could fly away endlessly into the laboratory until it impacts a surface. Clearly, this should be avoided and new hardware may have to be created to keep the jetted material under control. An interesting secondary effect is that flying droplets might also be able to be harvested far from the inkjet nozzle, opening the path to new suspended culture systems in which each droplet is a separated microbioreactor.
Liquid Motion—Liquid motion is much slower in microgravity. The inkjet printing process could thus be slower than on Earth, with a lower frequency of drop ejection to avoid liquid accumulation at the exit of the printing nozzle.[ 66 ]
Liquid Shape—On Earth, gravity distorts the shape of liquids, but not in microgravity where they tend to take up a shape of having minimal surface area; therefore, drops retain a spherical shape under microgravity conditions.[ 65 ] Consequently, homogeneously covering a surface might not be as trivial in microgravity.
Cell Adhesion—As demonstrated by the cosmonaut Nikolai Budarin, who photographed a small bubble of air suspended within a droplet of water in April 2003, there is no buoyancy in microgravity.[ 68 ] Conversely, there is no settling, and adherent cells may be incapable of reaching the surface of an object once the ejected droplet reaches the target.
4.3. Melt Electrowriting
Melt electrowriting (MEW) is an electrohydrodynamic AM process that enables the fabrication of 3D constructs from fibers with diameters in the lower micrometer range. The high resolution of MEW is used to generate scaffolds that reveal unique cell‐material interactions and guide cellular orientation, differentiation, and cellular invasion by design of the fiber deposition patterns.
In MEW, the material, in most cases a thermoplastic,[ 69 ] is molten and thus liquefied in a heated reservoir and fed to the nozzle from which it is extruded with a constant feed rate. An electric potential between the nozzle and the collector is used to stabilize and stretch the extruded material on its way to the collector.[ 69 ] This enables the reduction of fiber diameters in one to two orders of magnitude compared to the nozzle diameter and results in fibers of diameters as small as 820 nm.[ 70 ] Most publications report fiber diameters between 10 and 50 µm.[ 71 ] 3D constructs are generated by collecting the material onto a moving, computer‐controlled collector in a layer‐by‐layer fashion (Figure 3A). The shape of the deposited structures is preserved by temperature‐induced solidification of the thermoplastic polymer, and the material properties can be altered after printing if the material enables post‐process modification like light‐induced crosslinking.[ 72 ] Due to the distance between nozzle and collector needing to be high enough to prevent unwanted electrical discharge, MEW is not a direct writing technique and deposition patterns must be adjusted for optimal shape fidelity. In addition, straight fibers can only be collected above the critical translation speed (CTS), which defines the speed at which fiber deposition transfers from sinusoidal to straight, as depicted in Figure 3B.[ 73 ] Increasing the speed above the CTS is commonly used[ 74 ] to further decrease the fiber diameter, but the lag between material deposition on the collector and the nozzle tip must be considered when programming the print patterns to achieve accurate shape fidelity on critical shapes like corners and edges. The process is continuous, and the constructs are, in most cases, made from a single fiber that is deposited along a closed pattern. MEW is not limited to flat collectors but can also be performed on nonplanar surfaces[ 75 ] and on cylindrical collectors that enable the production of tubular constructs.[ 76 ]
Figure 3.
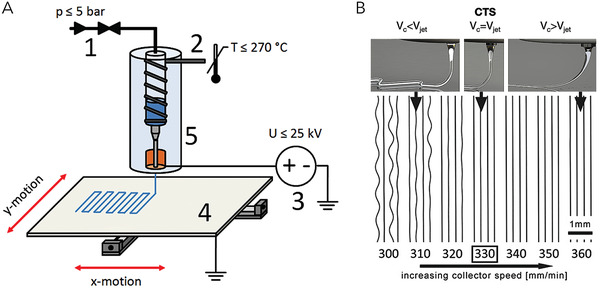
A) Schematic overview of the MEW process; B) Concept of the critical translation speed (CTS) and relation to collector speed v c and speed of the jet v jet. (A) Adapted with permission under the terms of the CC–BY license.[ 70 ] Copyright 2015, the Authors. Published by IOP Publishing. (B) Adapted with permission under the terms of the CC–BY license.[ 74 ] Copyright 2018, the Authors. Published by Wiley–VCH GmbH.
In terms of biomedical applications, most of the work performed so far utilizes the special cues that the micrometer‐sized fibers of MEW constructs and their deposition patterns (examples of which are depicted in Figure 4 ), defining the pore's design, have upon the cells. Due to the advanced control of fiber deposition and the resolution, MEW enables to study the biophysical cues of scaffolds and especially their pore geometry on the fate of cells without the need for external biochemical cues. Recent studies revealed that fiber geometry influences the orientation of cells and that fibers act as guiding structures (see Figure 4). It was also demonstrated that highly aligned fibers influence the fate of stem cells in terms of their differentiation,[ 77 ] while also defining the polarization of immune cells.[ 78 ] Scientists working with MEW are utilizing these results and increasingly starting to apply those for more complex tissue models and biomedical applications, such as cocultures, to recreate tissue niches for modeling and replicating in vitro the tumor microenvironment, and, in general, for advanced humanized in vitro and in vivo models.[ 79 , 80 ]
Figure 4.
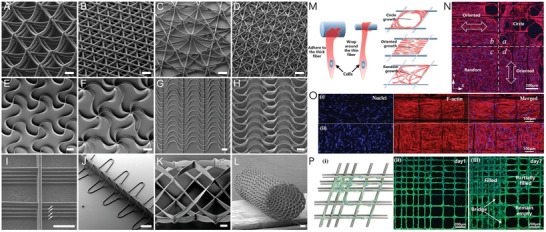
A–L) Deposition patterns used to influence fiber deposition and to generate different constructs with defined pores and properties; structure shown in (L) was collected on a tubular collector; scale bars = 100 µm (A)–(D), 500 µm (E)–(H), 50 µm (I), 200 µm (J)–(L); M–P) Influence of pattern and fiber deposition on cell orientation and filling of pores. (A−L) Reproduced under terms of the CC‐BY license. [ 71 ] Copyright 2019, the Authors. Published by Wiley–VCH GmbH. (M–P) Reproduced with permission.[ 81 ] Copyright 2019, Elsevier.
Furthermore, MEW can be combined with other AM processes [ 82 ] to generate hybrid constructs. The most familiar is the combination of MEW and cell‐laden hydrogels to influence the mechanical properties of soft hydrogels. Pioneering work in this area[ 83 ] showed that the combination of gel and MEW fibers can drastically influence the compressive strength of composites compared to MEW‐only or hydrogel‐only samples. It was also shown that the design of the structures has great influence on the properties of MEW fiber reinforced hydrogels.[ 84 , 85 ] Studies with very soft hydrogels, which are difficult to handle and transfer between cell cultures due becoming too fragile and resultingly damaged, demonstrate that MEW can be used to improve handling properties without influencing the mechanical microenvironment of the gels that are sensed by the cells.[ 86 ]
To date, one of the biggest challenges in MEW is the limited availability of materials that can be processed with the technology. Polycaprolactone is the current gold standard material and delivers the best quality prints. Yet, the material selection is increasing, and new materials and material blends are showing great potential. These materials have been reviewed recently but more alternatives are emerging, including processing bioinks in the presence of an electrical potential to reduce the strand diameter of deposited cell‐laden material, termed “cell electrowriting”.[ 69 , 72 , 87 ] So far, the thickness of the constructs that can be fabricated with MEW is limited as residual charges remain on the fibers, especially when the amount of stacked layers increases. These residual charges diminish the stacking accuracy and lead to defects. It has been demonstrated that adjusting the electric field density by increasing the voltage with increasing construct height can enable the fabrication of centimeter‐thick specimens via MEW, which may be a solution to this process inherent limitation.[ 88 ]
A key opportunity of MEW in microgravity is that the electrical potential that stabilizes and stretches the jet allows printing in different orientations without the need for gravity to act as a source to enable pattern deposition. As depicted in Figure 5 , it was already shown that printing cannot only be performed with the nozzle on the top and the collector on the bottom in a vertical setup, but also horizontally without any negative influence on print quality.[ 89 ] Therefore, it should be possible to create similar constructs with MEW under microgravity conditions in space as established on Earth.
Figure 5.
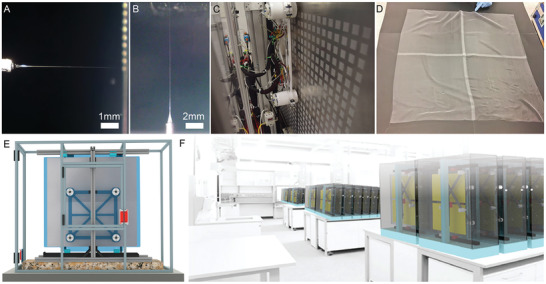
Demonstration of horizontal and vertical MEW printing. The minimal effect of gravity on the MEW jet during printing shown in A) in horizontal and B) upside‐down orientation followed by the translation into C) a scale‐up MEW printer capable of fabricating 1024 scaffolds per print and D) a single 80 cm × 80 cm fabric. E) Image showing a CAD model of the scale‐up prototype configuration with 8 print heads evenly spaced on a horizontal configuration to a large translating collector and F) a computer rendering envisioning how the small footprint permits multiple systems to operate in unison. Reproduced under terms of the CC‐BY license.[ 71 ] Copyright 2019, the Authors Published by WileyVCH GmbH.
5. Lithography‐Based Bioprinting Technologies
5.1. Stereolithography, Digital Light Processing, and Multiphoton Lithography
Stereolithography (SLA) is one of the most widely used light‐based AM technologies. The 1980s SLA patent by Chuck Hull is considered to be seminal for the development of the whole AM industry.[ 90 ] The history of the technology has many more facets and is described in great detail in one of the early Wohlers reports.[ 91 ] Over the years, countless variations of the technology evolved and developed, and SLA has become a valuable tool not only for prototyping but also in AM. It is still one of the most widespread approaches for the production of polymeric parts, which has been boosted by applications such as the manufacturing of hearing aids and dental aligners.[ 92 ] SLA is based on irradiating the surface of a photoreactive resin in a precise pattern by scanning over it with an ultraviolet (UV) laser. Thereby, photoinitiator molecules are excited by the UV light and form radicals that locally induce polymerization and solidification of the resin. After completing polymerization of the first layer on the building platform, the platform is moved downwards and the surface is covered with fresh resin, allowing for the curing of a new layer (Figure 6A). Following this layer‐by‐layer approach, complex structures with a high freedom of design can be built.
Figure 6.
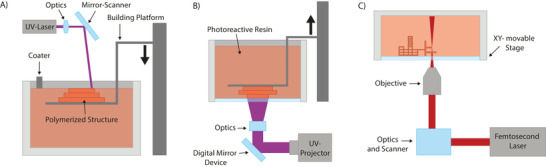
A) SLA, B) DLP, C) MPL. Generally, all three technologies can be executed in top‐down or inverted configurations; the latter is usually employed for 3D bioprinting. In addition, with the dawn of novel light sources and photoinitiators, wavelengths in the visible range are increasingly used. Reproduced with permission.[ 95 ] Copyright 2021, Elsevier.
In contrast to SLA's vectorized approach, digital light processing (DLP)[ 93 ] uses a light engine which exposes the whole layer in one shot (raster approach). The light path of the engine is modulated with the help of an array of micrometer‐sized controllable mirrors, known as a digital micromirror device. Each individual mirror can be repositioned rapidly and either reflects the light through the focusing optics or onto a heat sink, with each mirror thereby representing one pixel. The resin is typically illuminated from below through a transparent vat and the building platform is pulled upwards after the fabrication of a layer (Figure 6B). Since the whole image is projected at once instead of scanning the pattern, the throughput is generally higher.
Akin to SLA, multiphoton lithography (MPL)[ 94 ] is based on a laser being scanned through a photoreactive resin. However, the fundamental difference is that MPL takes advantage of two‐photon absorption (2PA) to excite the photoinitiator molecules. By using a femtosecond laser in the near‐infrared region to activate the photoinitiator instead of a single photon, two or more photons must be absorbed almost simultaneously by the same molecule. For this to occur, high photon densities are needed, which is provided in the focal spot of a pulsed femtosecond laser. 2PA is quadratically dependent on the light intensity and occurs only in a confined area around the focal spot. By moving the laser focus with a high spatial and temporal control through the photoresist, sophisticated high‐resolution structures can be built (see Figure 6C).
Given that an overview of the state of the art of lithography‐based AM technologies is not at the core of this publication, we would like to refer the readers to some of the reviews addressing this subject.[ 95 , 96 , 97 , 98 ]
Light‐based 3D printing technologies are particularly versatile regarding the freedom of design and the scales at which structures can be built, reaching from submicrometer‐sized features to decimeter‐sized objects. The achievable resolution of the printing techniques is determined by parameters such as the optics used, the photochemistry of the material, or the desired object size. The resolution provided by SLA and DLP is usually in the range of several tens of micrometers, while MPL can provide features on the submicrometer scale, but is restricted to a smaller achievable object size. Light‐based 3D printing techniques have become a valuable tool in 3D bioprinting[ 99 , 100 ] and particularly in 3D high‐definition (HD) bioprinting, which describes approaches to reproduce fine features at cellular and subcellular scales (<50 µm), while providing reasonable printing volumes.[ 101 ] Figure 7 shows an overview of HD‐bioprinting techniques, demonstrating throughput against resolution. Examples for HD bioprinting include the fabrication of scaffolds for cell culture, simple organ models, or even in situ printing within living organisms. Furthermore, light‐based 3D printing technologies enable the production of structures across transparent tissue culture labware. In particular, MPL allows for printing directly within a microfluidic chip, which reduces the number of assembly steps, avoids sterility issues, and enables the fabrication of constructs noninvasively.[ 102 ]
Figure 7.
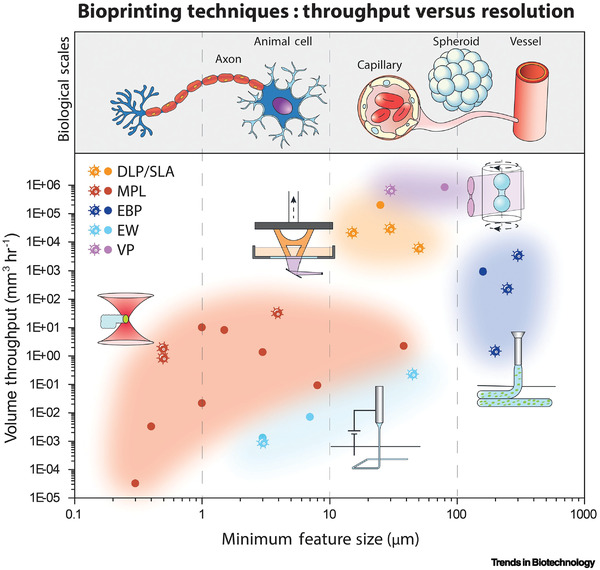
Graphical representation of the capabilities of several bioprinting techniques discussed in this work, regarding the throughput and resolution: digital light processing (DLP), stereolithography (SLA), extrusion bioprinting (EBP), melt electrowriting (MEW), multiphoton lithography (MPL), and volumetric printing (VP). With both MPL and EW, features within the scale of cells and smaller can be produced, allowing it to control the microenvironment at the cellular level. However, these techniques are limited in their throughput to a few mm3 h−1. DLP, SLA, VP, and EBP enable the realization of constructs of several cm3 with a minimal feature size in the range of capillaries, cell spheroids, or blood vessels. Acell‐shaped datapoint indicates studies that involved cell encapsulation during printing. Adapted with permission.[ 101 ] Copyright 2023, Elsevier.
One of the general bottlenecks of lithography‐based technologies that is particularly relevant for 3D bioprinting is the realization of multimaterial/multicellular constructs. Different solutions in this direction were demonstrated over the years with SLA and DLP.[ 103 , 104 ] They can also be used with MPL, but for smaller components it is more reasonable to use microfluidic systems.[ 105 ]
The following aspects must be considered to apply these technologies for microgravitational environments
Material Handling—SLA and DLP rely on sequential deposition of even material layers. Most systems still use active layer levelling, with gravity taking care of the excessive material on Earth. Specialized solutions will have to be considered in space. Control of shape, uneven liquid surface or preventing the slurry/bioink from climbing the walls of the container should be considered in microgravity.[ 106 ] Since cell culture in space is likely to be performed in closed systems, MPL is perhaps the most directly suited technology in this regard since it is already operated within chambers. The latter capability was also demonstrated to provide unique advantages for producing tissue models within closed containers, such as microfluidic chips.
Cell Sedimentation—Common to most bioprinting technologies, cell sedimentation within the bioink due to gravity will naturally be alleviated in microgravity. This will also allow for using low viscosity bioinks, which will help towards solving the potential outlined material handling issues.
Universality—These techniques are perhaps most inclusive with regards to “universality” and coverable resolution range (see Figure 7). The material portfolio is quite rich, especially when it comes to classical AM applications. The same device could be used to produce components necessary for cell culture, e.g., microfluidic chips, and the tissue construct of interest. In space, it is likely that only a limited machine park might be available – potential maintenance of the system and spare part availability for different technologies will be an important consideration.
Medical Applications—Microgravity and space radiation can have major effects on human tissue. Therefore, understanding the health‐related implications is significant to provide suitable medical assistance and expand the understanding on space travel.[ 107 ] Tissue chips are a promising toolset to develop space biomedicine and study microgravity‐related medical effects.[ 108 , 109 ] As previously discussed, light‐based 3D printing technologies and, in particular, MPL have proven to be versatile techniques for the production of such chip‐based systems.[ 110 , 111 ]
5.2. Volumetric Bioprinting and Related Technologies
Conventional 3D (bio)printing technologies typically rely on the sequential spatial deposition of discrete building blocks, e.g., in the form of extruded fibers of photopolymerized hydrogel precursors, in a layer‐by‐layer fashion. While versatile, this approach requires extended printing times for constructs of clinically relevant sizes. This can induce stress for the cells, which can even hamper the functionality of centimeter‐scale objects.[ 82 , 112 ] In an effort to overcome this hurdle, volumetric bioprinting (VBP) was recently introduced, which enables the fabrication of large cell‐laden constructs in the timescale of tens of seconds, regardless of their size and architectural complexity.[ 113 ] This is an important advantage that could prove highly valuable for upscaling the production of both cell‐free objects and biological constructs also in environment with limited machinery and resources, such as in orbital stations, during space flight and space exploration. This technology relies on the combination of a light source (either a laser or a noncollimated source), a spatial light modulator (i.e., a digital micromirror device, DMD), and a photoresin (usually preladen with cells) placed in a cylindrical vat on a rotating stage. As the vat rotates, light (typically in the visible portion of the spectrum, i.e., cell‐friendly) is then shaped by the DMD into filtered back‐projections of the object to be printed, according to a tomographic reconstruction algorithm. Each projection is delivered into the vat at a given rotation angle, and sequential combination of the light patterns results in anisotropic 3D light dose distribution within the volume of photoresin. Therefore, even though the whole vat is illuminated, the light field exceeds the threshold of crosslinking of the bioresin only where the print needs to be formed, causing the whole object to photocrosslink at once in a layerless, volumetric fashion.[ 114 , 115 ] The remaining unreacted bioresin can be flushed to retrieve the object and used for subsequent biomedical or tissue culture applications. Initially developed for printing cell‐free resins derived from conventional stereolithographic processes,[ 114 , 115 ] an increasing array of hydrogel‐based bioresins are being introduced, also including unmodified, pristine proteins.[ 116 , 117 ] To date, applications in engineering cartilage,[ 113 ] bone,[ 118 ] muscle,[ 119 ] and liver,[ 120 ] have been described. Notably, the contactless and nozzle‐free nature of this technology allows to process mechanically fragile biological structures in a shear‐stress free manner, an advantage that has implications, for instance, in organoid research,[ 120 ] and could therefore facilitate the implementation of these powerful models also in space research. Recently, multiple schemes for volumetric printing of multimaterial constructs, in which multiple cell types can even be compartmentalized into specific regions of the constructs, were demonstrated.[ 121 , 122 ] Potential applications include mimicking interfaces across different tissues (i.e., muscle‐to‐tendon), and multi‐walled tissues (i.e., blood vessels).
The accuracy of volumetric printing and related processes is dependent on a variety of factors related to the optical properties (light absorptivity, refractive index, and light scattering of the resin and cells), physicochemical properties (viscosity, degree of substitution, etc.) and photocrosslinking kinetics (step‐growth or chain‐growth polymerization) of the bioresin. Research in terrestrial environments has highlighted how printing quality and fabrication time can be optimized by the selection of highly efficient photocrosslinking mechanisms, such as thiol‐ene‐based photoclick chemistry,[ 119 ] by the addition of compounds that mitigate light scattering from the embedded cells[ 120 ] or via software corrections that modify the light patterns delivered to the vat.[ 123 , 124 ]
Although printing via tomographic reconstruction is currently the fastest volumetric AM technology available, other technological solutions belonging to this family are also being studied such as holographic printing, which involves static light projections from multiple directions to cause localized increases in light doses for crosslinking.[ 125 ] Alternatively, light sheet stereolithography and Xolography use two light sources at different wavelengths to overlap in a given voxel of interest in order to activate a photoinitiator to crosslink the resin.[ 126 , 127 ] Recently, filamented light (FLight) biofabrication, which is based on optically modulated light projections without rotation of the printing vial, has emerged as a powerful technique for the fabrication of aligned tissue constructs such as muscles, vessels and tendons.[ 128 ] Here, the speckle patterns of lasers induce longitudinal microfilaments within the photocrosslinkable polymers, which act as excellent topographical cues for guiding cell alignment and extracellular matrix organization. Similar to existing techniques for multimaterial volumetric printing, FLight can also allow for the changing of material across the length or the cross‐section, thereby enabling complex multicellular tissues (e.g., vascularized muscle) or interfaces (e.g., myotendinous junctions) to be fabricated.[ 128 ] Of note, these microfilaments, although advantageously utilized in FLight biofabrication, are considered printing artifacts within volumetric printing and can be mitigated within VBP using refractive index tuning, or by using a uniform background illumination after printing.[ 129 ]
5.2.1. Challenges and Opportunities of VBP and Related Technologies in Microgravity
Apart from enabling rapid fabrication, VBP and the related methods could, in fact, benefit from microgravitational effects. In terrestrial environments, the resin for VBP needs to be either highly viscous or thermo‐reversibly gelated to prevent sedimentation of the cells or of the crosslinked polymer during printing,[ 114 , 115 ] which results in loss of shape fidelity and limits the available options of the photoresins that can be used for printing. For instance, most volumetric bioprinting efforts so far have used gelatin as the main polymer component in the resin,[ 113 , 118 , 119 , 120 ] as it undergoes thermo‐reversible gelation at lower‐than‐physiological temperatures.[ 130 ] This also restricts the printing temperatures for the resin formulations (generally vials need to be <10 °C for successful printing). In space, microgravity would actually allow for the printing with low viscosity resins and nonthermally gelating systems, alleviating the concerns of sedimentation of cells and of the printed constructs. Another aspect with terrestrial printing relates to the exothermic nature of photocrosslinking, which imparts localized temperature gradients resulting in convective flows which may impair resolution by dislocating the resin from its original crosslinking location.[ 131 ] In microgravity, there is minimal presence of convective flows, which might allow better print resolution, provided that the temperature increase is small to prevent any adverse effects on the cells or the chemical structure of the matrix.
As with other printing technologies, VBP will require re‐imagining several aspects related to the process and materials for printing in space. The resin, if not thermoreversibly crosslinked, may float in microgravity. In this case, the containers need to be completely packed with the resin and appropriately capped to prevent leakage (Figure 8 ). The cells can be pre‐mixed with the resin containing cryoprotectants prior to lift‐off, and the resins stored at −80 °C until they are used. Existing studies on cryo‐bioprinting have demonstrate that the high cell viabilities could be achieved upon recovery within the printing resins after long‐term storage.[ 132 , 133 ] Alternatively, for short duration space flights lasting a few hours, cells could be premixed with the resin and the resin stored at 4 °C until used.[ 113 ] As the biofabricated tissue constructs are often fragile, it will be beneficial to redesign the print vials such that they allow for easy resin filling without spillage, and can be directly integrated within perfusion reactor system for maturation of the tissues within the spacecraft (Figure 8). In this case, inlet and outlet ports with one‐way valves should be added within the printing vials for adding resin prior to printing, or removing uncrosslinked resin through perfusion of a biological buffer solution (e.g., phosphate‐buffered saline, PBS) followed by perfusion of cell culture media. Here, the nozzle tip for the resin filling would be inserted through the one‐way valve. As the plunger is pushed to inject the resin into the reservoir, a second syringe is placed at the exit port of the vat to apply a vacuum and extract air out. For the perfusion of PBS or media, the nozzle tips connected to the peristaltic pumps will be inserted through the one‐way valves to allow for media perfusion.
Figure 8.
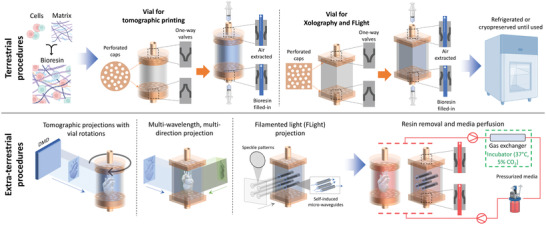
Concept of integrated vials and perfusion system involving terrestrial procedures – Resin constitution and printing vial preparation for tomographic projections, Xolography or FLight biofabrication, and extra‐terrestrial procedures – printing of the tissue constructs and media circulation within a pressurized reactor system. Note: After preparation, the resin may be stored under normal refrigeration at 4 °C or cryopreserved (using cryo‐protectants).
As every resource may be valuable within a space flight, recycling the PBS and media by removing uncrosslinked resin (via filtering; a vacuum is freely available in space and can be harnessed), cellular debris and metabolic byproducts. In addition, symbiotic cellular coculture systems can reduce nutritional demands of cells,[ 134 , 135 , 136 ] or the usage of cells capable of growth in serum‐free media can improve cost effectiveness and feasibility of the entire process.[ 135 , 136 , 137 ]
5.2.2. Recent Successes for VBP and Related Technologies in Space
The SpaceCAL (UC Berkeley, USA) initiative built a compact enclosure with five computed axial lithography‐based setups connected in parallel to make scale‐up volumetric printing possible in space flights. This apparatus has been already successfully evaluated in Zero‐Gravity Flights,[ 138 ] thus demonstrating the suitability of volumetric printing to work in microgravity. Bioprinting applications in space with this system are now being explored also by the Lawrence Livermore National Laboratory, with a first focus on printing cartilage‐mimicking constructs with low stiffness methacrylated gelatin (GelMA)‐based resins (≈100 Pa compression modulus) on the ISS, with flights planned to the station within the next three years in collaboration with the company SpaceTango and NASA.[ 139 ] At the same time, Xolo has also brought their light‐sheet lithography printer on a parabolic flight, first demonstrating its use for printing nonhydrogel‐based resins. Overall, the active interest and involvement of space agencies, research institutes, and industry and commercial actors within collaborative projects relating to volumetric printing in orbital stations demonstrates the promise of this new technology. Together, they should pave the way for further engineering of this technique to expand the toolbox available for AM and 3D bioprinting in space research and exploration.
6. Online Monitoring, Bioreactors, and Remote Control
As clearly discussed in all the previous sections, space conditions induce several significant challenges to successful 3D bioprinting, which might translate into high defective rates and risks of job failure.[ 140 ] Furthermore, the need of reducing the amount of materials to be sent onboard, combined with the mandatory requirement to minimize scraps, waste and sacrificial constructs, imposes a paradigm shift towards first‐time‐right, zero‐defect bioprinting.[ 3 ] Eventually, process automation and data‐assisted support should aid nonexpert operators, who will possibly run bioprinting and postprocess maturation in space missions. In this scenario, novel solutions to support in situ process optimization, monitoring and control via sensorized platforms should be combined and integrated with novel solutions for automatic control and optimize tissue maturation in bioreactors.
Recently, many approaches for in situ monitoring and control of bioprinting processes have been proposed in the literature, exploiting different sensing solutions including cameras in the visible range,[ 141 , 142 ] laser scanners,[ 143 , 144 , 145 ] and optical coherence tomography (OCT) for a better reconstruction of internal features.[ 146 , 147 , 148 ] An additional opportunity is the utilization of thermal cameras for monitoring the temperature to support extrusion of sensitive materials[ 149 ] or inkjet bioprinting.[ 150 , 151 ] All the proposed solutions focused on the inspection of the bioprinted construct (layer‐wise analysis) or the extruder conditions (layer‐height analysis) (both Figure 9 ) to achieve first‐time‐right printing and possibly develop a digital platform implementing data mining to support monitoring (i.e., detection of unexpected events), feedback control (i.e., acting on process parameters to maintain process stability) or combining a virtual simulation of the process towards digital twin solutions (Figure 9).
Figure 9.
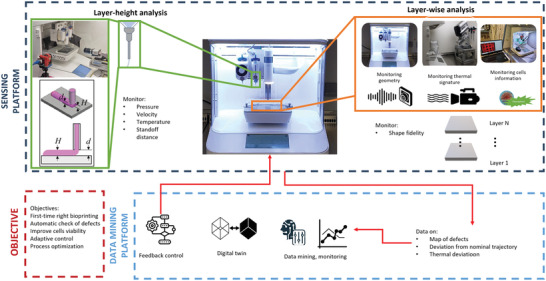
In situ data mining for intelligent bioprinting (system proposed by Politecnico di Milano).
As cell viability is difficult to observe in situ and inline via external sensors (cameras and thermal cameras), some authors have proposed functionalized bioinks to act as in situ sensors to assess different aspects of cell metabolism[ 152 ] or to control oxygen and nutrient concentrations through the addition of luminescent optical sensor nanoparticles.[ 153 ] All these seminal solutions are laying the foundations towards a future perspective that envisions a fully sensorized and intelligent bioprinting platform (Figure 9) that can automatically control and adapt the 3D bioprinting process to external conditions, unexpected events, or hard‐to‐bioprint materials. This will represent a standard rather than an exception in space application. Along this line, in situ monitoring of the bioprinting process can act as an enabling solution for in situ bioprinting,[ 154 ] where sensors can be used to adapt the process to the printing environment, reminiscent to a dynamically morphing organ.
In space, the printing environment must be considered regarding the following steps of bioprinted tissue cultivation. While on Earth, a bioprinted tissue is subjected to complex cultivation sequences, including mostly manual steps inside a sterile biosafety cabinet (i.e., tissue transfer and feeding).[ 155 , 156 ] In space, such types of sequences could strongly limit the deployment of tissue bioprinting strategies. Accordingly, implementing an all‐in‐one integrated and specifically designed bioprinting and cultivation device is strategic and essential. Here, the ideal foreseen device is a cultivation bioreactor, which could welcome both a freeform and sterile bioprinting and subsequent tissue culture protocol (Figure 10 ). Such a device should, (i) reduce the footprint of the overall process, (ii) include a confined/watertight chamber to maintain sterility of the bioprint and further immerse the construct within nutritive fluids, and (iii) allow for cultivation in a regulated manner. Indeed, in microgravity it will become mandatory to maintain, control, and regulate the main environmental parameters impacting live cells, namely temperature, dissolved oxygen availability, pH, and osmotic pressure. Regulating such parameters relies on heat and mass transfer, liquid–gas interface, bubbling, and mixing. In microgravitational environments, all such physical behavior is affected,[ 65 , 157 , 158 , 159 ] thus rendering it impossible to directly transfer tools from Earth for space. While none of the available common cell culture bioreactors[ 160 ] can fit the constraints imposed by microgravity and space, several dedicated equipment and installations have been deployed to the ISS to provide an adequate cell culture environment.[ 161 ] For instance, the ESA Microgravity Science Glovebox (MSG), the Multiple Orbital Bioreactor with Instrumentation and Automated Sampling (MOBIAS), and the BioCulture System of NASA have been proven to maintain appropriate conditions to provide relevant biological studies both for 2D/3D shaped cell culture applications. Finally, nondestructive online analytics will also be necessary during these cultivation phases to describe specific tissue maturation and acquisition of biological functions, such as cell growth, microstructure organization, extracellular matrix remodeling, and barrier functions. These analytical tools should aim to describe a tissue's cellularity, internal microstructuration (porosity, mechanical properties, biomatrix composition), and cellular environment (local pH and oxygen conditions). In fact, none of the standard analytical methods used on Earth can currently be adopted for the longitudinal and nondestructive monitoring of large cellularized 3D structures. Cytometry, cell counting or tissue histology, commonly used for the description of cell populations, proliferation profiles and 3D cell organization, are unable be used as analytical techniques within space. Here, in vivo imaging tools could be advantageously exploited, such as magnetic resonance imaging (MRI) or echography[ 162 , 163 ] that is currently in place on the ISS, and spectroscopic analyses like Raman spectroscopy.[ 164 , 165 , 166 ] We expect such work will rapidly advance the development of tools not yet available on Earth for the longitudinal and nondestructive monitoring of grown tissues at scales and depth ranges beyond that of millimeters.
Figure 10.
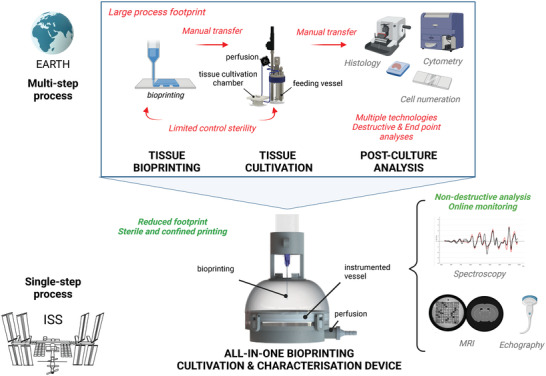
Adapting postprinting maturation and tissue characterization to space. All‐in‐one integrated and specifically designed bioprinting and cultivation instrumented device.
7. Conclusions
Additive manufacturing has developed into a powerful class of technologies for space flight, while 3D bioprinting is also attracting growing interest of the international space agencies. Bioprinting allows for the manufacturing of 3D cell and tissue constructs that can be utilized in space to investigate the specific effects of stressors, such as microgravity and cosmic radiation. In addition, bioprinting techniques possess the opportunity to fabricate clinically applicable human tissues that might contribute to the autonomous medical treatment options for injured astronauts on future long‐term and far‐distant space missions.
The specific limitations connected to space flight are challenging for the establishment of the bioprinting process chain. However, microgravity is believed to also provide significant advantages for several bioprinting methods compared to conventional conditions applying on Earth. This includes the opportunity to use low viscous bioinks in extrusion‐based bioprinting that will prevent the sedimentation of cells or other particulate matter both in bioinks and resins. Consequently, the scientific community is excited about the possibility to investigate bioprinting processes and the development of bioprinted cell constructs under real space conditions at the ISS once the ESA has commissioned the construction of respective hardware. For effective utilization of such devices, equipment with cameras and sensors is required to allow the scientists on ground to follow and control the experimental procedures. With such devices becoming functional, life science in space will make a remarkable step forward.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
All authors contributed equally to this work. The authors want to thank several members of the European Space Research and Technology Centre (ESTEC) of the European Space Agency ESA, namely Dr. Christiane Hahn, Dr. Andreas Schoen, Dr. Tommaso Ghidini, and Pierfilippo Manieri for stimulating discussions and valuable insights, as well as Dr. Markus Braun from the German Space Agency at DLR for the same. The authors acknowledge the contributions of Prof. Dr. Nieves Cubo Mateo (Nebrija University, Madrid, Spain) to the development of the topic. Special thanks go to Sophia Read for the thorough review of the manuscript with regard to its linguistic quality. The manuscript has been conceptualized by the ESA Topical Team on “3D Bioprinting of living tissue for utilization in space exploration and extraterrestrial human settlements”, coordinated by M.G.
Open access funding enabled and organized by Projekt DEAL.
Van Ombergen A., Chalupa‐Gantner F., Chansoria P., Colosimo B. M., Costantini M., Domingos M., Dufour A., De Maria C., Groll J., Jungst T., Levato R., Malda J., Margarita A., Marquette C., Ovsianikov A., Petiot E., Read S., Surdo L., Swieszkowski W., Vozzi G., Windisch J., Zenobi‐Wong M., Gelinsky M., 3D Bioprinting in Microgravity: Opportunities, Challenges, and Possible Applications in Space. Adv. Healthcare Mater. 2023, 12, 2300443. 10.1002/adhm.202300443
References
- 1. Ghidini T., J. Thorac. Dis. 2018, 10, S2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Why Space? The Opportunity for Health and Life Science Innovation, UK Space Life and Biomedical Sciences Association, 2020, http://www.ukspacelabs.co.uk/documents/space-life-science-paper.pdf. [Google Scholar]
- 3. Cubo‐Mateo N., Podhajsky S., Knickmann D., Slenzka K., Ghidini T., Gelinsky M., Biofabrication 2020, 12, 043001. [DOI] [PubMed] [Google Scholar]
- 4. Zocca A., Wilbig J., Waske A., Günster J., Widjaja M. P., Neumann C., Clozel M., Meyer A., Ding J., Zhou Z., Tian X., Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100018. [Google Scholar]
- 5. Makaya A., Pambaguian L., Ghidini T., Rohr T., Lafont U., Meurisse A., CEAS Space J. 2023, 15, 69. [Google Scholar]
- 6. Moroni L., Tabury K., Stenuit H., Grimm D., Baatout S., Mironov V., Trends Biotechnol. 2022, 40, 398. [DOI] [PubMed] [Google Scholar]
- 7. Fonseca A. C., Melchels F. P. W., Ferreira M. J. S., Moxon S. R., Potjewyd G., Dargaville T. R., Kimber S. J., Domingos M., Chem. Rev. 2020, 120, 11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Groll J., Boland T., Blunk T., Burdick J. A., Cho D.‐W., Dalton P. D., Derby B., Forgacs G., Li Q., Mironov V. A., Moroni L., Nakamura M., Shu W., Takeuchi S., Vozzi G., Woodfield T. B. F., Xu T., Yoo J. J., Malda J., Biofabrication 2016, 8, 013001. [DOI] [PubMed] [Google Scholar]
- 9. Sharma A., Clemens R. A., Garcia O., Taylor D. L., Wagner N. L., Shepard K. A., Gupta A., Malany S., Grodzinsky A. J., Kearns‐Jonker M., Mair D. B., Kim D.‐H., Roberts M. S., Loring J. F., Hu J., Warren L. E., Eenmaa S., Bozada J., Paljug E., Roth M., Taylor D. P., Rodrigue G., Cantini P., Smith A. W., Giulianotti M. A., Wagner W. R., Stem Cell Rep. 2022, 17, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giulianotti M. A., Low L. A., Pharm. Res. 2019, 37, 1. [DOI] [PubMed] [Google Scholar]
- 11. Li J., Wu C., Chu P. K., Gelinsky M., Mater. Sci. Eng., R 2020, 140, 100543. [Google Scholar]
- 12. Jungst T., Smolan W., Schacht K., Scheibel T., Groll J., Chem. Rev. 2016, 116, 1496. [DOI] [PubMed] [Google Scholar]
- 13.“Redwire Launching Upgraded 3D Bioprinter to Space Station to Investigate New Treatment to Aid Military Service Members, Expands Crop Production Research and Materials Testing on Orbit,” can be found under https://redwirespace.com/newsroom/redwire‐launching‐upgraded‐3d‐bioprinter‐to‐space‐station‐to‐investigate‐new‐treatment‐to‐aid‐military‐service‐members‐expands‐crop‐production‐research‐and‐materials‐testing‐on‐orbit/ (accessed: December 2022).
- 14. Rogers A., “Redwire Cardiac Bioprinting Investigation – BioFabrication Facility‐Cardiac,” can be found under https://www.nasa.gov/mission_pages/station/research/experiments/explorer/Investigation.html?#id=8741 (accessed: December 2022).
- 15. Gaston J., “BFF Assembled Next‐gen Development of Collagenous Allograft Meniscal Prosthetics aboard the International Space Station,” can be found under https://www.nasa.gov/mission_pages/station/research/experiments/explorer/Investigation.html?#id=8274 (accessed: December 2022).
- 16.“3D‐printed bio‐plaster – Matthias Maurer conducts Bioprint FirstAid experiment on the ISS,” can be found under https://www.dlr.de/content/en/articles/news/2022/01/20220131_3d‐printed‐bio‐plaster.html (accessed: December 2022).
- 17. Horneck G., Facius R., Reichert M., Rettberg P., Seboldt W., Manzey D., Comet B., Maillet A., Preiss H., Schauer L., Dussap C. G., Poughon L., Belyavin A., Reitz G., Baumstark‐Khan C., Gerzer R., Adv. Space Res. 2003, 31, 2389. [DOI] [PubMed] [Google Scholar]
- 18. Thirsk R. B., Ann ICRP 2020, 49, 182. [DOI] [PubMed] [Google Scholar]
- 19. Krittanawong C., Singh N. K., Scheuring R. A., Urquieta E., Bershad E. M., Macaulay T. R., Kaplin S., Dunn C., Kry S. F., Russomano T., Shepanek M., Stowe R. P., Kirkpatrick A. W., Broderick T. J., Sibonga J. D., Lee A. G., Crucian B. E., Cells 2023, 12, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doarn C. R., Polk J. D., Shepanek M., Neurol. India 2019, 67, 190. [DOI] [PubMed] [Google Scholar]
- 21. Robertson J. M., Dias R. D., Gupta A., Marshburn T., Lipsitz S. R., Pozner C. N., Doyle T. E., Smink D. S., Musson D. M., Yule S., J. Surg. Res. 2020, 246, 305. [DOI] [PubMed] [Google Scholar]
- 22. Yule S., Robertson J. M., Mormann B., Smink D. S., Lipsitz S., Abahuje E., Kennedy‐Metz L., Park S., Miccile C., Pozner C. N., Doyle T., Musson D., Dias R. D., Hum Factors 2022, 00187208211067575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puhl C., Caplin N., Fogtman A., Van Ombergen A., Front. Bioeng. Biotechnol. 2022, 10, 958515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cubo‐Mateo N., Gelinsky M., Front. Bioeng. Biotechnol. 2021, 9, 720217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagenbuchner J., Nothdurfter D., Ausserlechner M. J., Essays Biochem. 2021, 65, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.“Space Meat – The 3D Printed Future of Astronaut Food | Aleph Farms,” can be found under https://www.aleph‐farms.com/aleph‐zero (accessed: December 2022).
- 27. Krujatz F., Dani S., Windisch J., Emmermacher J., Hahn F., Mosshammer M., Murthy S., Steingröwer J., Walther T., Kühl M., Gelinsky M., Lode A., Biotechnol. Adv. 2022, 58, 107930. [DOI] [PubMed] [Google Scholar]
- 28. Gensler M., Leikeim A., Möllmann M., Komma M., Heid S., Müller C., Boccaccini A. R., Salehi S., Groeber‐Becker F., Hansmann J., PLoS One 2020, 15, e0242615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Windisch J., Reinhardt O., Duin S., Schütz K., Rodriguez N. J. N., Liu S., Lode A., Gelinsky M., Adv. Healthcare Mater. 2023, 2300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kikuchi T., Kino‐oka M., Wada M., Kobayashi T., Kato M., Takeda S., Kubo H., Ogawa T., Sunayama H., Tanimoto K., Mizutani M., Shimizu T., Okano T., Regen. Ther. 2018, 9, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun S., Wang C., Bi Y., Li N., Lü D., Chen Q., Chen J., Long M., Rev. Sci. Instrum. 2019, 90, 075114. [DOI] [PubMed] [Google Scholar]
- 32. Nijhuis J., Schmidt S., Tran N. N., Hessel V., Front. Space Technol. 2022, 2, 779696. [Google Scholar]
- 33. Kiss J. Z., Wolverton C., Wyatt S. E., Hasenstein K. H., van Loon J. J. W. A., Front. Plant Sci. 2019, 10, 1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferranti F., Del Bianco M., Pacelli C., Appl. Sci. 2021, 11, 68. [Google Scholar]
- 35. Panesar S. S., Ashkan K., Br. J. Surg. 2018, 105, 1234. [DOI] [PubMed] [Google Scholar]
- 36. Patel Z. S., Brunstetter T. J., Tarver W. J., Whitmire A. M., Zwart S. R., Smith S. M., Huff J. L., NPJ Microgravity 2020, 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moroni L., Boland T., Burdick J. A., De Maria C., Derby B., Forgacs G., Groll J., Li Q., Malda J., Mironov V. A., Mota C., Nakamura M., Shu W., Takeuchi S., Woodfield T. B. F., Xu T., Yoo J. J., Vozzi G., Trends Biotechnol. 2018, 36, 384. [DOI] [PubMed] [Google Scholar]
- 38. Chiesa I., Maria C. D., Lapomarda A., Fortunato G. M., Montemurro F., Gesù R. D., Tuan R. S., Vozzi G., Gottardi R., Biofabrication 2020, 12, 025013. [DOI] [PubMed] [Google Scholar]
- 39. Costantini M., Colosi C., Święszkowski W., Barbetta A., Biofabrication 2018, 11, 012001. [DOI] [PubMed] [Google Scholar]
- 40. Groll J., Burdick J. A., Cho D.‐W., Derby B., Gelinsky M., Heilshorn S. C., Jüngst T., Malda J., Mironov V. A., Nakayama K., Ovsianikov A., Sun W., Takeuchi S., Yoo J. J., Woodfield T. B. F., Biofabrication 2018, 11, 013001. [DOI] [PubMed] [Google Scholar]
- 41. Prater T., Werkheiser N., Ledbetter F., Timucin D., Wheeler K., Snyder M., Int. J. Adv. Manuf. Technol. 2019, 101, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang M., Sun N., Luo Y., Lai X., Li P., Zhang Z., Biomicrofluidics 2022, 16, 031503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ding H., Tourlomousis F., Chang R. C., J. Manuf. Sci. Eng. 2018, 140, 051014. [Google Scholar]
- 44. De Maria C., Chiesa I., Morselli D., Ceccarini M. R., Bittolo Bon S., Degli Esposti M., Fabbri P., Morabito A., Beccari T., Valentini L., Adv. Funct. Mater. 2021, 31, 2105665. [Google Scholar]
- 45. Bonatti A. F., Chiesa I., Vozzi G., De Maria C., Int. J. Bioprint. 2021, 24, e00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Percoco G., Arleo L., Stano G., Bottiglione F., Addit. Manuf. 2021, 38, 101791. [Google Scholar]
- 47. Guillotin B., Guillemot F., Trends Biotechnol. 2011, 29, 183. [DOI] [PubMed] [Google Scholar]
- 48. da Silva G. R., Junior A. d. S. C., Saliba J. B., Berdugo M., Goldenberg B. T., Naud M. C., Ayres E., Oréfice R. L., Cohen F. B., Int. J. Artif. Organs 2011, 34, 198. [DOI] [PubMed] [Google Scholar]
- 49. Hakobyan D., Médina C., Dusserre N., Stachowicz M.‐L., Handschin C., Fricain J.‐C., Guillermet‐Guibert J., Oliveira H., Biofabrication 2020, 12, 035001. [DOI] [PubMed] [Google Scholar]
- 50. Zhou Z., Cantu L. R., Chen X., Alexander M. R., Roberts C. J., Hague R., Tuck C., Irvine D., Wildman R., Addit. Manuf. 2019, 29, 100792. [Google Scholar]
- 51. Gudapati H., Parisi D., Colby R. H., Ozbolat I. T., Soft Matter 2020, 16, 10506. [DOI] [PubMed] [Google Scholar]
- 52. Hölzl K., Lin S., Tytgat L., van Vlierberghe S., Gu L., Ovsianikov A., Biofabrication 2016, 8, 032002. [DOI] [PubMed] [Google Scholar]
- 53. Ulanoff L., “After nearly 20 years, the Space Station is getting a printer upgrade,” can be found under https://www.mashable.com/article/nasa‐updates‐international‐space‐station‐printer, n.d.
- 54. Boland T., Jr W. C. W., Xu T., US7051654B2, 2006.
- 55. Mironov V., Boland T., Trusk T., Forgacs G., Markwald R. R., Trends Biotechnol. 2003, 21, 157. [DOI] [PubMed] [Google Scholar]
- 56. Masaeli E., Forster V., Picaud S., Karamali F., Nasr‐Esfahani M. H., Marquette C., Biofabrication 2020, 12, 025006. [DOI] [PubMed] [Google Scholar]
- 57. Kang D., Park J. A., Kim W., Kim S., Lee H.‐R., Kim W.‐J., Yoo J.‐Y., Jung S., Adv. Sci. 2021, 8, 2004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen E. P., Toksoy Z., Davis B. A., Geibel J. P., Front. Bioeng. Biotechnol. 2021, 9, 664188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berthuy O. I., Muldur S. K., Rossi F., Colpo P., Blum L. J., Marquette C. A., Lab Chip 2016, 16, 4248. [DOI] [PubMed] [Google Scholar]
- 60. Liu J., Shahriar M., Xu H., Xu C., Biofabrication 2022, 14, 045020. [DOI] [PubMed] [Google Scholar]
- 61. Courtial E.‐J., Perrinet C., Colly A., Mariot D., Frances J.‐M., Fulchiron R., Marquette C., Addit. Manuf. 2019, 28, 50. [Google Scholar]
- 62. Lopez A., Marquette C. A., Courtial E.‐J., Prog. Addit. Manuf. 2021, 6, 53. [Google Scholar]
- 63. Gao G., Yonezawa T., Hubbell K., Dai G., Cui X., Biotechnol. J. 2015, 10, 1568. [DOI] [PubMed] [Google Scholar]
- 64. Teo M. Y., Kee S., RaviChandran N., Stuart L., Aw K. C., Stringer J., ACS Appl. Mater. Interfaces 2020, 12, 1832. [DOI] [PubMed] [Google Scholar]
- 65. Meseguer J., Sanz‐Andrés A., Pérez‐Grande I., Pindado S., Franchini S., Alonso G., Eur. J. Phys. 2014, 35, 055010. [Google Scholar]
- 66. Smith A. W., “Looking at Liquid Motion in Microgravity,” can be found under https://www.issnationallab.org/looking‐at‐liquid‐motion‐in‐microgravity/ (accessed: December 2022).
- 67. Castrejon‐Pita J. R., Baxter W. R. S., Morgan J., Temple S., Martin G. D., Hutchings I. M., At. Sprays 2013, 23, 541. [Google Scholar]
- 68.“NASA – The Physics of Space Gardens,” can be found under https://www.nasa.gov/audience/forstudents/5‐8/features/space_gardens_feature.html (accessed: December 2022).
- 69. Kade J. C., Dalton P. D., Adv. Healthcare Mater. 2021, 10, 2001232. [Google Scholar]
- 70. Hochleitner G., Jüngst T., Brown T. D., Hahn K., Moseke C., Jakob F., Dalton P. D., Groll J., Biofabrication 2015, 7, 035002. [DOI] [PubMed] [Google Scholar]
- 71. Robinson T. M., Hutmacher D. W., Dalton P. D., Adv. Funct. Mater. 2019, 29, 1904664. [Google Scholar]
- 72. Pien N., Bartolf‐Kopp M., Parmentier L., Delaey J., De Vos L., Mantovani D., Van Vlierberghe S., Dubruel P., Jungst T., Macromol. Mater. Eng. 2022, 307, 2200097. [Google Scholar]
- 73. Hochleitner G., Youssef A., Hrynevich A., Haigh J. N., Jungst T., Groll J., Dalton P. D., BioNanomaterials 2016, 17, 159. [Google Scholar]
- 74. Hrynevich A., Elçi B. Ş., Haigh J. N., McMaster R., Youssef A., Blum C., Blunk T., Hochleitner G., Groll J., Dalton P. D., Small 2018, 14, 1800232. [DOI] [PubMed] [Google Scholar]
- 75. Luposchainsky S., Jörissen S., Nüchter A., Dalton P. D., Macromol. Mater. Eng. 2022, 307, 2200450. [Google Scholar]
- 76. Jungst T., Muerza‐Cascante M. L., Brown T. D., Standfest M., Hutmacher D. W., Groll J., Dalton P. D., Polym. Int. 2015, 64, 1086. [Google Scholar]
- 77. Eichholz K. F., Hoey D. A., Acta Biomater. 2018, 75, 140. [DOI] [PubMed] [Google Scholar]
- 78. Tylek T., Blum C., Hrynevich A., Schlegelmilch K., Schilling T., Dalton P. D., Groll J., Biofabrication 2020, 12, 025007. [DOI] [PubMed] [Google Scholar]
- 79. Wagner F., Holzapfel B. M., McGovern J. A., Shafiee A., Baldwin J. G., Martine L. C., Lahr C. A., Wunner F. M., Friis T., Bas O., Boxberg M., Prodinger P. M., Shokoohmand A., Moi D., Mazzieri R., Loessner D., Hutmacher D. W., Biomaterials 2018, 171, 230. [DOI] [PubMed] [Google Scholar]
- 80. Włodarczyk‐Biegun M. K., Villiou M., Koch M., Muth C., Wang P., Ott J., del Campo A., ACS Biomater. Sci. Eng. 2022, 8, 3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xie C., Gao Q., Wang P., Shao L., Yuan H., Fu J., Chen W., He Y., Mater. Des. 2019, 181, 108092. [Google Scholar]
- 82. de Ruijter M., Ribeiro A., Dokter I., Castilho M., Malda J., Adv. Healthcare Mater. 2019, 8, 1800418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Visser J., Melchels F. P. W., Jeon J. E., van Bussel E. M., Kimpton L. S., Byrne H. M., Dhert W. J. A., Dalton P. D., Hutmacher D. W., Malda J., Nat. Commun. 2015, 6, 6933. [DOI] [PubMed] [Google Scholar]
- 84. Vernon M. J., Lu J., Padman B., Lamb C., Kent R., Mela P., Doyle B., Ihdayhid A. R., Jansen S., Dilley R. J., De‐Juan‐Pardo E. M., Adv. Healthcare Mater. 2022, 11, 2201028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Castilho M., van Mil A., Maher M., Metz C. H. G., Hochleitner G., Groll J., Doevendans P. A., Ito K., Sluijter J. P. G., Malda J., Adv. Funct. Mater. 2018, 28, 1803151. [Google Scholar]
- 86. Janzen D., Bakirci E., Wieland A., Martin C., Dalton P. D., Villmann C., Adv. Healthcare Mater. 2020, 9, 1901630. [DOI] [PubMed] [Google Scholar]
- 87. Castilho M., Levato R., Bernal P. N., de Ruijter M., Sheng C. Y., van Duijn J., Piluso S., Ito K., Malda J., Biomacromolecules 2021, 22, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wunner F. M., Wille M.‐L., Noonan T. G., Bas O., Dalton P. D., De‐Juan‐Pardo E. M., Hutmacher D. W., Adv. Mater. 2018, 30, 1706570. [DOI] [PubMed] [Google Scholar]
- 89. Wunner F. M., Eggert S., Maartens J., Bas O., Dalton P. D., De‐Juan‐Pardo E. M., Hutmacher D. W., 3D Print. Addit. Manuf. 2019, 6, 82. [Google Scholar]
- 90. Hull C. W., US4575330A 1986.
- 91. Wohlers T., Early Research & Development – Rapid Prototyping, Tooling & Manufacturing State of the Industry – Wohlers Report 2005, Wohlers Associates, 2005, https://wohlersassociates.com/product/wohlers-report-2005/.
- 92. Liaw C.‐Y., Guvendiren M., Biofabrication 2017, 9, 024102. [DOI] [PubMed] [Google Scholar]
- 93. Zhao Z., Tian X., Song X., J. Mater. Chem. C 2020, 8, 13896. [Google Scholar]
- 94. Stampfl J., Liska R., Ovsianikov A., Multiphoton Lithography: Techniques, Materials and Applications, Wiley‐VCH, Weinheim, Germany: 2016. [Google Scholar]
- 95. Zheng Z., Eglin D., Alini M., Richards G. R., Qin L., Lai Y., Engineering 2021, 7, 966. [Google Scholar]
- 96. Ligon S. C., Liska R., Stampfl J., Gurr M., Mülhaupt R., Chem. Rev. 2017, 117, 10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Huang J., Qin Q., Wang J., Processes 2020, 8, 1138. [Google Scholar]
- 98. Bagheri A., Jin J., ACS Appl. Polym. Mater. 2019, 1, 593. [Google Scholar]
- 99. Lim K. S., Galarraga J. H., Cui X., Lindberg G. C. J., Burdick J. A., Woodfield T. B. F., Chem. Rev. 2020, 120, 10662. [DOI] [PubMed] [Google Scholar]
- 100. Li H., Dai J., Wang Z., Zheng H., Li W., Wang M., Cheng F., Aggregate 2023, 4, e270. [Google Scholar]
- 101. Zandrini T., Florczak S., Levato R., Ovsianikov A., Trends Biotechnol. 2023, 41, 604. [DOI] [PubMed] [Google Scholar]
- 102. Dobos A., Gantner F., Markovic M., Hoorick J. V., Tytgat L., Vlierberghe S. V., Ovsianikov A., Biofabrication 2020, 13, 015016. [DOI] [PubMed] [Google Scholar]
- 103. Zorlutuna P., Jeong J. H., Kong H., Bashir R., Adv. Funct. Mater. 2011, 21, 3642. [Google Scholar]
- 104. Grigoryan B., Sazer D. W., Avila A., Albritton J. L., Padhye A., Ta A. H., Greenfield P. T., Gibbons D. L., Miller J. S., Sci. Rep. 2021, 11, 3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mayer F., Richter S., Westhauser J., Blasco E., Barner‐Kowollik C., Wegener M., Sci. Adv. 2019, 5, eaau9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dou R., Tang W., Hu K., Wang L., J. Eur. Ceram. Soc. 2022, 42, 3968. [Google Scholar]
- 107. Fang Y., Guo Y., Liu T., Xu R., Mao S., Mo X., Zhang T., Ouyang L., Xiong Z., Sun W., Chin. J. Mech. Eng., Addit. Manuf. Front. 2022, 1, 100011. [Google Scholar]
- 108. Mu X., He W., Rivera V. A. M., De Alba R. A. D., Newman D. J., Zhang Y. S., Life Sci. Space Res. 2022, 35, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Silvani G., Basirun C., Wu H., Mehner C., Poole K., Bradbury P., Chou J., Adv. Ther. 2021, 4, 2100106. [Google Scholar]
- 110. Weisgrab G., Ovsianikov A., Costa P. F., Adv. Mater. Technol. 2019, 4, 1900275. [Google Scholar]
- 111. Ho C. M. B., Ng S. H., Li K. H. H., Yoon Y.‐J., Lab Chip 2015, 15, 3627. [DOI] [PubMed] [Google Scholar]
- 112. Li J., Zhao C., Xu Y., Song L., Chen Y., Xu Y., Ma Y., Wang S., Xu A., He F., Bioact Mater 2023, 22, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bernal P. N., Delrot P., Loterie D., Li Y., Malda J., Moser C., Levato R., Adv. Mater. 2019, 31, 1904209. [DOI] [PubMed] [Google Scholar]
- 114. Kelly B. E., Bhattacharya I., Heidari H., Shusteff M., Spadaccini C. M., Taylor H. K., Science 2019, 363, 1075. [DOI] [PubMed] [Google Scholar]
- 115. Loterie D., Delrot P., Moser C., Nat. Commun. 2020, 11, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xie M., Lian L., Mu X., Luo Z., Garciamendez‐Mijares C. E., Zhang Z., López A., Manríquez J., Kuang X., Wu J., Sahoo J. K., González F. Z., Li G., Tang G., Maharjan S., Guo J., Kaplan D. L., Zhang Y. S., Nat. Commun. 2023, 14, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Soliman B. G., Longoni A., Wang M., Li W., Bernal P. N., Cianciosi A., Lindberg G. C. J., Malda J., Groll J., Jungst T., Levato R., Rnjak‐Kovacina J., Woodfield T. B. F., Zhang Y. S., Lim K. S., Adv. Funct. Mater. 2023, 33, 2210521. [Google Scholar]
- 118. Gehlen J., Qiu W., Schädli G. N., Müller R., Qin X.‐H., Acta Biomater. 2023, 156, 49. [DOI] [PubMed] [Google Scholar]
- 119. Rizzo R., Ruetsche D., Liu H., Zenobi‐Wong M., Adv. Mater. 2021, 33, 2102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bernal P. N., Bouwmeester M., Madrid‐Wolff J., Falandt M., Florczak S., Rodriguez N. G., Li Y., GröÔbacher G., Samsom R.‐A., van Wolferen M., van der Laan L. J. W., Delrot P., Loterie D., Malda J., Moser C., Spee B., Levato R., Adv. Mater. 2022, 34, 2110054. [DOI] [PubMed] [Google Scholar]
- 121. Chansoria P., Rütsche D., Wang A., Liu H., D'Angella D., Rizzo R., Hasenauer A., Weber P., Ibrahim N. B. M., Korshunova N., Zenobi‐Wong M., bioRxiv 2022, 10.1101/2022.11.29.518318. [DOI] [Google Scholar]
- 122. Größbacher G., Bartolf‐Kopp M., Gergely C., Bernal P. N., Florczak S., de Ruijter M., Groll J., Malda J., Jüngst T., Levato R., Adv. Mater. 2023, 10.1002/adma.202300756. [DOI] [PubMed] [Google Scholar]
- 123. Madrid‐Wolff J., Boniface A., Loterie D., Delrot P., Moser C., Adv. Sci. 2022, 9, 2105144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bhattacharya I., Toombs J., Taylor H., Addit. Manuf. 2021, 47, 102299. [Google Scholar]
- 125. Shusteff M., Panas R., Henriksson J., Kelly B. E., Browar A., Fang N. X., Spadaccini C. M., presented at 27th Annual Solid Freeform Fabrication Symposium – an Additive Manufacturing Conference, 2016, p. 1183.
- 126. Regehly M., Garmshausen Y., Reuter M., König N. F., Israel E., Kelly D. P., Chou C.‐Y., Koch K., Asfari B., Hecht S., Nature 2020, 588, 620. [DOI] [PubMed] [Google Scholar]
- 127. Hahn V., Rietz P., Hermann F., Müller P., Barner‐Kowollik C., Schlöder T., Wenzel W., Blasco E., Wegener M., Nat. Photonics 2022, 16, 784. [Google Scholar]
- 128. Liu H., Chansoria P., Delrot P., Angelidakis E., Rizzo R., Rütsche D., Applegate L. A., Loterie D., Zenobi‐Wong M., Adv. Mater. 2022, 34, 2204301. [DOI] [PubMed] [Google Scholar]
- 129. Rackson C. M., Toombs J. T., De Beer M. P., Cook C. C., Shusteff M., Taylor H. K., McLeod R. R., Opt. Lett. 2022, 47, 1279. [DOI] [PubMed] [Google Scholar]
- 130. Chansoria P., Asif S., Polkoff K., Chung J., Piedrahita J. A., Shirwaiker R. A., ACS Biomater. Sci. Eng. 2021, 7, 5175. [DOI] [PubMed] [Google Scholar]
- 131. Belk M., Kostarev K. G., Volpert V., Yudina T. M., J. Phys. Chem. B 2003, 107, 10292. [Google Scholar]
- 132. Luo Z., Tang G., Ravanbakhsh H., Li W., Wang M., Kuang X., Garciamendez‐Mijares C. E., Lian L., Yi S., Liao J., Xie M., Guo J., Zhou Z., Zhang Y. S., Adv. Mater. 2022, 34, 2108931. [DOI] [PubMed] [Google Scholar]
- 133. Ravanbakhsh H., Luo Z., Zhang X., Maharjan S., Mirkarimi H. S., Tang G., Chávez‐Madero C., Mongeau L., Zhang Y. S., Matter 2022, 5, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Haraguchi Y., Kagawa Y., Sakaguchi K., Matsuura K., Shimizu T., Okano T., Sci. Rep. 2017, 7, 41594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lindroos B., Boucher S., Chase L., Kuokkanen H., Huhtala H., Haataja R., Vemuri M., Suuronen R., Miettinen S., Cytotherapy 2009, 11, 958. [DOI] [PubMed] [Google Scholar]
- 136. Chaturvedi V., Dye D. E., Kinnear B. F., van Kuppevelt T. H., Grounds M. D., Coombe D. R., PLoS One 2015, 10, e0127675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Brunner D., Frank J., Appl H., Schöffl H., Pfaller W., Gstraunthaler G., ALTEX – Altern. Anim. Exp. 2010, 27, 53. [DOI] [PubMed] [Google Scholar]
- 138. Waddell T., Toombs J., Reilly A., Schwab T., Castaneda C., Mohnot P., Shan I., Taylor H., Proc. Int. Aeronautical Congress , Paris, October, 2022. [Google Scholar]
- 139.Thomas, “NASA funds LLNL to demonstrate ‘replicator’ 3D printer to produce cartilage in space,” can be found under https://www.llnl.gov/news/nasa‐funds‐llnl‐demonstrate‐replicator‐3d‐printer‐produce‐cartilage‐space (accessed: December 2022).
- 140. Gelinsky M., J. 3D Print. Med. 2020, 4, 1. [Google Scholar]
- 141. Jin Z., Zhang Z., Shao X., Gu G. X., ACS Biomater. Sci. Eng. 2021, 10.1021/acsbiomaterials.0c01761. [DOI] [PubMed] [Google Scholar]
- 142. Gugliandolo S. G., Margarita A., Santoni S., Moscatelli D., Colosimo B. M., Proc. CIRP 2022, 110, 219. [Google Scholar]
- 143. Armstrong A. A., Alleyne A. G., Johnson A. J. W., Biofabrication 2020, 12, 045023. [DOI] [PubMed] [Google Scholar]
- 144. Armstrong A. A., Norato J., Alleyne A. G., Johnson A. J. W., Biofabrication 2019, 12, 015017. [DOI] [PubMed] [Google Scholar]
- 145. Armstrong A. A., Pfeil A., Alleyne A. G., Wagoner Johnson A. J., Int. J. Bioprint. 2021, 21, e00126. [Google Scholar]
- 146. Wang L., Xu M., Zhang L., Zhou Q., Luo L., Biomed. Opt. Express 2016, 7, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wang L., Xu M., Luo L., Zhou Y., Si P., Sci. Rep. 2018, 8, 2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yang S., Wang L., Chen Q., Xu M., Addit. Manuf. 2021, 47, 102251. [Google Scholar]
- 149. Moncal K. K., Ozbolat V., Datta P., Heo D. N., Ozbolat I. T., J. Mater. Sci. Mater. Med. 2019, 30, 55. [DOI] [PubMed] [Google Scholar]
- 150. Ogunsanya M., Isichei J., Parupelli S. K., Desai S., Cai Y., Procedia Manuf. 2021, 53, 427. [Google Scholar]
- 151. Lies B. T., Cai Y., Spahr E., Lin K., Qin H., Procedia Manuf. 2018, 26, 29. [Google Scholar]
- 152. Fedi A., Vitale C., Giannoni P., Caluori G., Marrella A., Sensors 2022, 22, 1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Trampe E., Koren K., Akkineni A. R., Senwitz C., Krujatz F., Lode A., Gelinsky M., Kühl M., Adv. Funct. Mater. 2018, 28, 1804411. [Google Scholar]
- 154. Zhu Z., Ng D. W. H., Park H. S., McAlpine M. C., Nat. Rev. Mater. 2021, 6, 27. [Google Scholar]
- 155. Pourchet L. J., Thepot A., Albouy M., Courtial E. J., Boher A., Blum L. J., Marquette C. A., Adv. Healthcare Mater. 2017, 6, 1601101. [DOI] [PubMed] [Google Scholar]
- 156. Pragnere S., El Kholti N., Gudimard L., Essayan L., Marquette C., Petiot E., Pailler‐Mattei C., J. Mech. Behav. Biomed. Mater. 2022, 134, 105365. [DOI] [PubMed] [Google Scholar]
- 157. Vega‐Martínez P., Rodríguez‐Rodríguez J., van der Meer D., Soft Matter 2020, 16, 4728. [DOI] [PubMed] [Google Scholar]
- 158. Gaponenko Y., Shevtsova V., Microgravity Sci. Technol. 2008, 20, 307. [Google Scholar]
- 159. Kim Y.‐K., Park S.‐H., Lee J.‐H., Choi G.‐H., J. Astron. Space Sci. 2015, 32, 81. [Google Scholar]
- 160. Mainardi V. L., Rubert M., Sabato C., de Leeuw A., Arrigoni C., Dubini G., Candrian C., Müller R., Moretti M., Acta Biomater. 2022, 153, 374. [DOI] [PubMed] [Google Scholar]
- 161. Zhang J., Wehrle E., Rubert M., Müller R., Int. J. Mol. Sci. 2021, 22, 3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sarty G. E., Obenaus A., Can. Aeronaut Space J. 2012, 58, 60. [Google Scholar]
- 163. Law J., Macbeth P. B., McGill J. Med. MJM Int. Forum Adv. Med. Sci. Stud. 2011, 13, 59. [PMC free article] [PubMed] [Google Scholar]
- 164. Meksiarun P., Andriana B. B., Matsuyoshi H., Sato H., Sci. Rep. 2016, 6, 37068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Koljenovic S., Schut T. C. B., Wolthuis R., de Jong B., Santos L., Caspers P. J., Kros J. M., Puppels G. J., J. Biomed. Opt. 2005, 10, 031116. [DOI] [PubMed] [Google Scholar]
- 166. Desroches J., Jermyn M., Pinto M., Picot F., Tremblay M.‐A., Obaid S., Marple E., Urmey K., Trudel D., Soulez G., Guiot M.‐C., Wilson B. C., Petrecca K., Leblond F., Sci. Rep. 2018, 8, 1792. [DOI] [PMC free article] [PubMed] [Google Scholar]


