Abstract
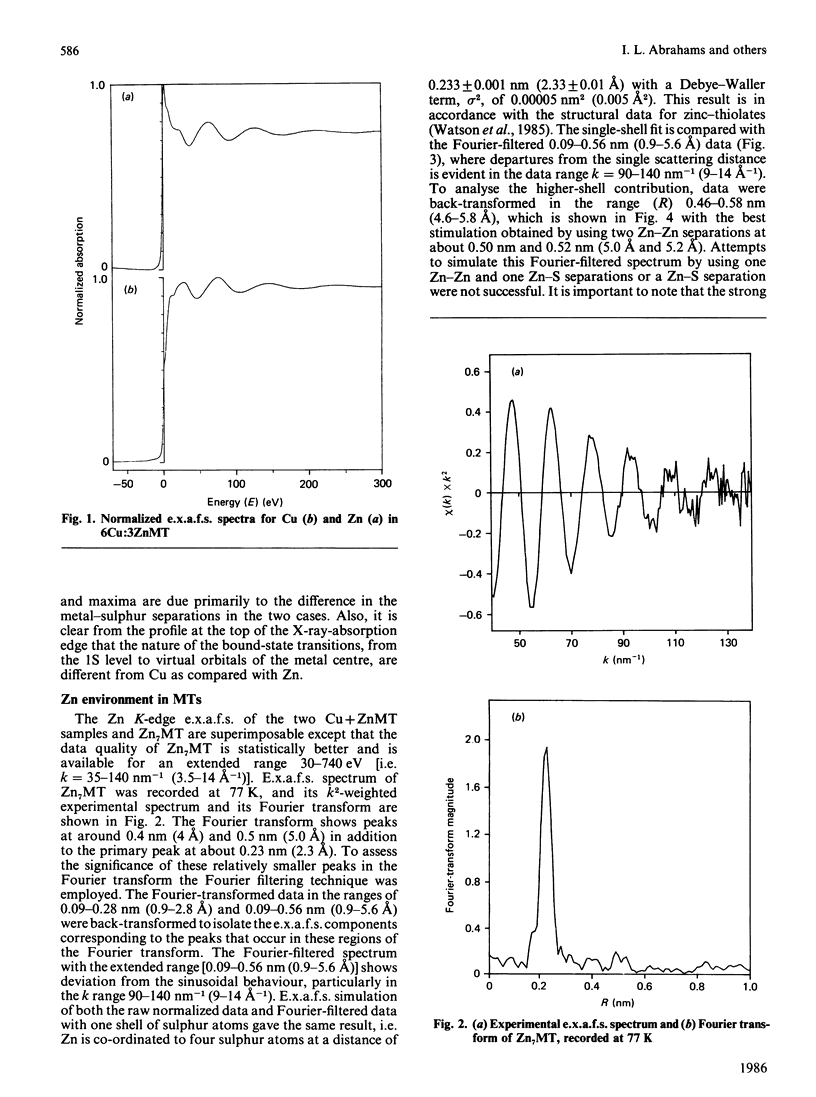
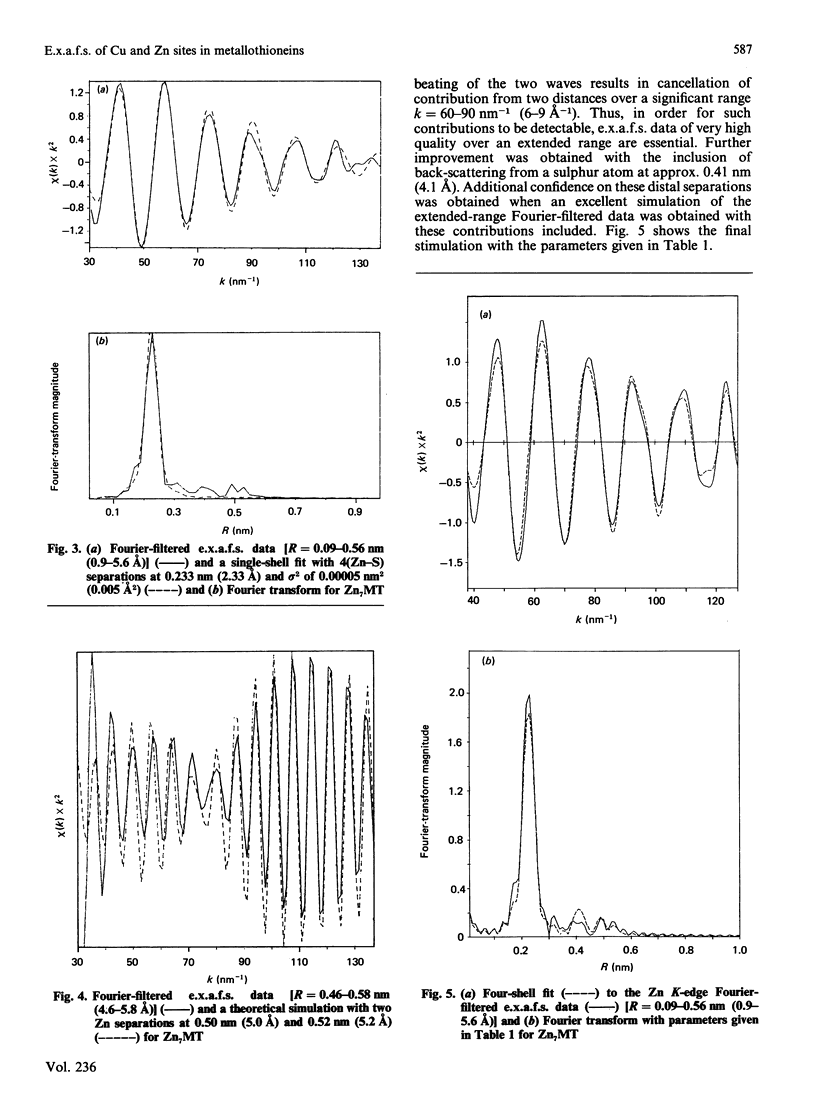
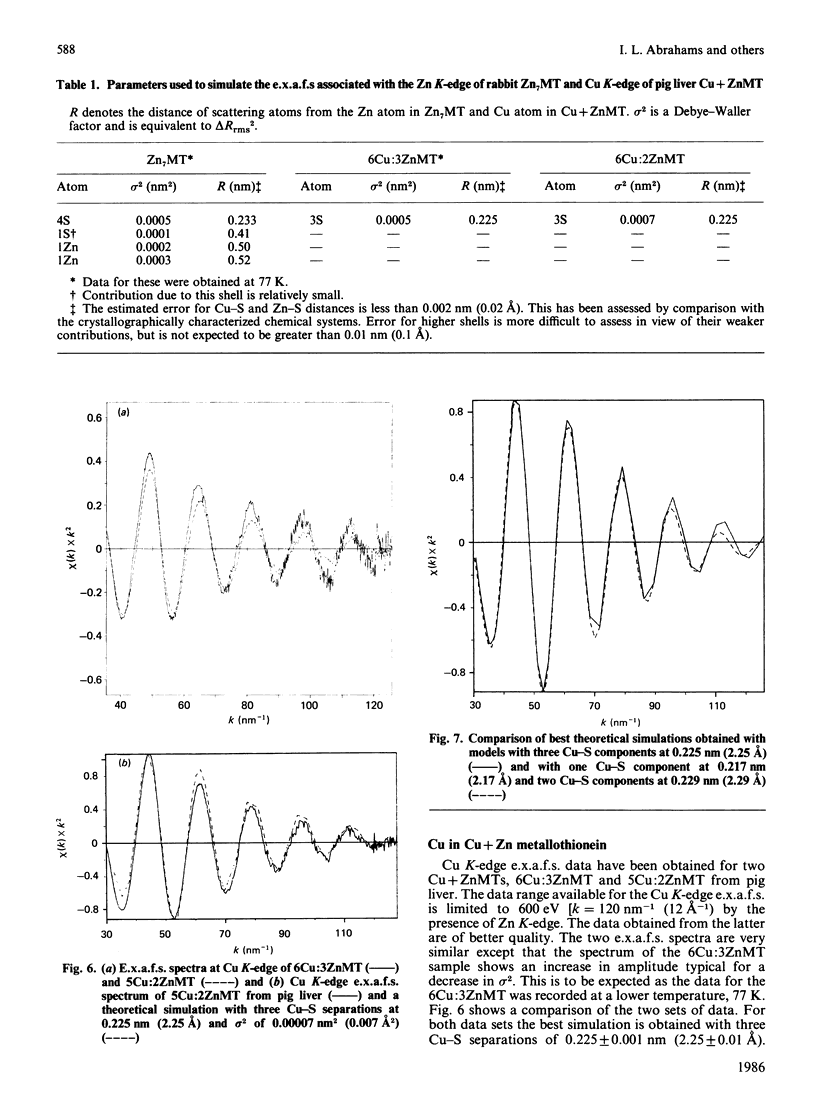
Zn-metallothionein 1 from rabbit liver was investigated by means of Zn K-edge extended X-ray-absorption fine structure (e.x.a.f.s.). Also, the Cu and Zn K-edge e.x.a.f.s. were measured for two samples of mixed Cu Zn-metallothionein 2, with Cu/Zn ratios of 5:2 and 6:3, from pig liver. Detailed simulation of the Cu sites shows a primary co-ordination with three sulphur atoms, presumably from cysteine residues at 0.225 nm +/- 0.001 nm (2.25 +/- 0.01 A). The data for the Zn sites are best reproduced by four Zn-S separations at 0.233 +/- 0.001 nm (2.33 +/- 0.01 A). The Zn K-edge e.x.a.f.s. recorded for rabbit metallothionein 1 at 77 K shows, in addition to the primary co-ordination shell, evidence for two Zn-Zn separations at approx. 0.50 nm (5.0 A). This latter result provides the first information concerning the internal arrangement of zinc atoms in Zn7-metallothionein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn N. J., Hasnain S. S., Binsted N., Diakun G. P., Garner C. D., Knowles P. F. An extended-X-ray-absorption-fine-structure study of bovine erythrocyte superoxide dismutase in aqueous solution. Direct evidence for three-co-ordinate Cu(I) in reduced enzyme. Biochem J. 1984 May 1;219(3):985–990. doi: 10.1042/bj2190985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn N. J., Hasnain S. S., Diakun G. P., Knowles P. F., Binsted N., Garner C. D. An extended X-ray-absorption-fine-structure study of the copper and zinc sites of freeze-dried bovine superoxide dismutase. Biochem J. 1983 Sep 1;213(3):765–768. doi: 10.1042/bj2130765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordas J., Koch M. H., Hartmann H. J., Weser U. Tetrahedral copper-sulphur coordination in yeast Cu-thionein. An EXAFS study. FEBS Lett. 1982 Apr 5;140(1):19–21. doi: 10.1016/0014-5793(82)80511-5. [DOI] [PubMed] [Google Scholar]
- Bremner I., Davies N. T. The induction of metallothionein in rat liver by zinc injection and restriction of food intake. Biochem J. 1975 Sep;149(3):733–738. doi: 10.1042/bj1490733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. D., Hasain S. S., Bremner I., Bordas J. An EXAFS study of the zinc sites in sheep liver metallothionein. J Inorg Biochem. 1982 Jun;16(3):253–256. doi: 10.1016/s0162-0134(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Hasnain S. S., Wardell E. M., Garner C. D., Schlösser M., Beyersmann D. Extended-X-ray-absorption-fine-structure investigations of zinc in 5-aminolaevulinate dehydratase. Biochem J. 1985 Sep 15;230(3):625–633. doi: 10.1042/bj2300625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Hasnain S. S., Duke P. J., Sessler J. L., Hahn J. E. Stereochemistry of iron in deoxyhaemoglobin. Nature. 1982 Feb 11;295(5849):535–538. doi: 10.1038/295535a0. [DOI] [PubMed] [Google Scholar]
- Vasák M., Galdes A., Hill H. A., Kägi J. H., Bremner I., Young B. W. Investigation of the structure of metallothioneins by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Feb 5;19(3):416–425. doi: 10.1021/bi00544a003. [DOI] [PubMed] [Google Scholar]



