Abstract
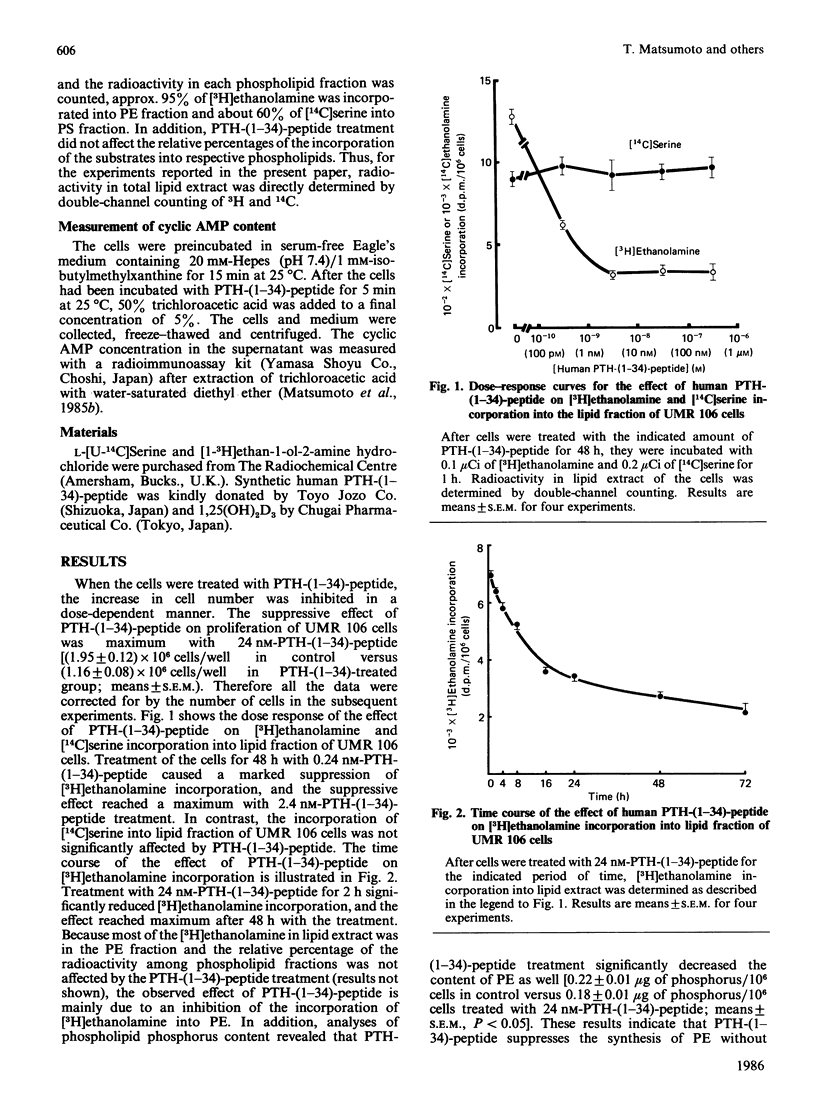
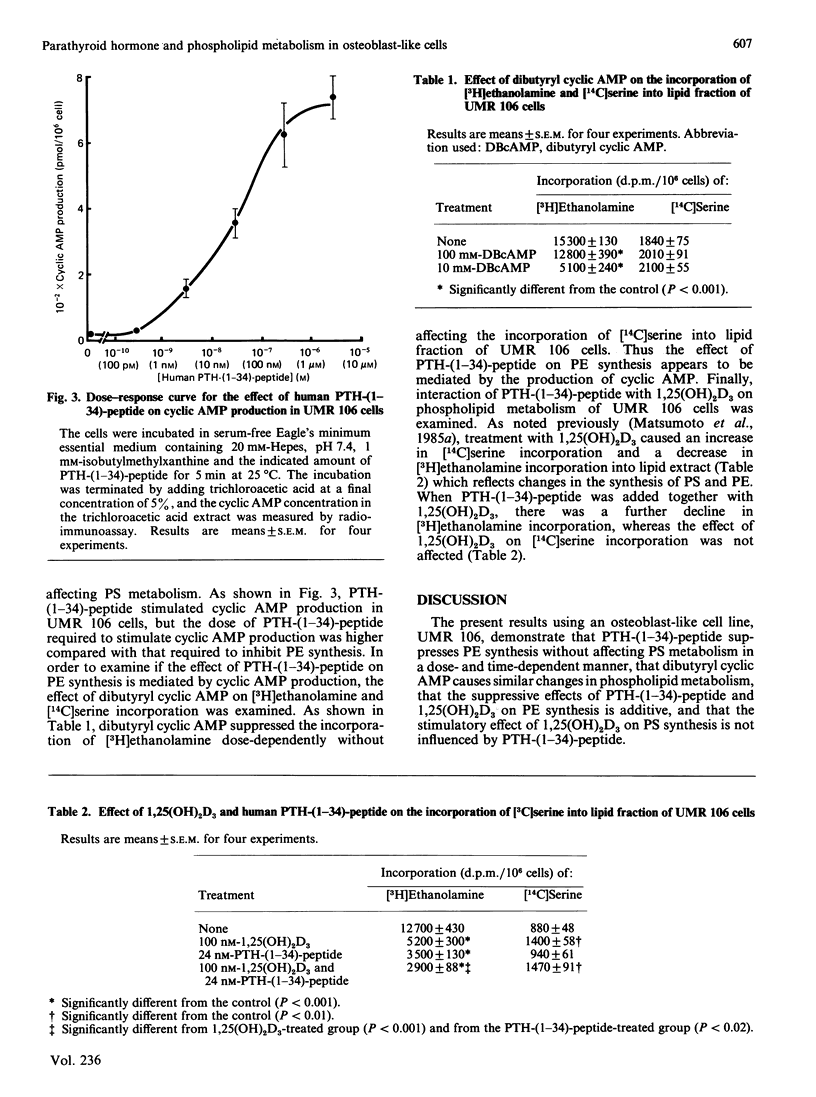
Previous results have shown that 1,25-dihydroxycholecalciferol [1,25(OH)2D3] enhances the synthesis of phosphatidylserine (PS) and suppresses the synthesis of phosphatidylethanolamine (PE) in osteoblast-like rat osteogenic sarcoma UMR 106 cells [Matsumoto, Kawanobe, Morita & Ogata (1985) J. Biol. Chem. 260, 13704-13709]. In the present study, the effect of parathyroid hormone (PTH) on phospholipid metabolism is examined by using these cells. Treatment of UMR 106 cells with human PTH-(1-34)-peptide suppresses the synthesis of phosphatidylethanolamine in a dose- and time-dependent manner without affecting the synthesis of PS. The maximal effect on PE synthesis is obtained with 2.4 nM-human PTH-(1-34)-peptide when the cells are treated for 48 h or longer. In addition, when human PTH-(1-34)-peptide is added together with the maximal dose of 1,25(OH)2D3, there is a further decline in PE synthesis, whereas the stimulation of PS synthesis by 1,25(OH)2D3 is not altered. Because methylation of PE is suggested to affect hormone receptor-adenylate cyclase coupling, the observed change in PE metabolism by PTH and 1,25(OH)2D3 may be, at least in part, involved in the development of desensitization phenomenon to PTH in these cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boskey A. L., Posner A. S. The role of synthetic and bone extracted Ca-phospholipid-PO4 complexes in hydroxyapatite formation. Calcif Tissue Res. 1977 Oct 20;23(3):251–258. doi: 10.1007/BF02012794. [DOI] [PubMed] [Google Scholar]
- Catherwood B. D. 1,25-Dihydroxycholecalciferol and glucocorticosteroid regulation of adenylate cyclase in an osteoblast-like cell line. J Biol Chem. 1985 Jan 25;260(2):736–743. [PubMed] [Google Scholar]
- Hamilton J. A., Lingelbach S., Partridge N. C., Martin T. J. Regulation of plasminogen activator production by bone-resorbing hormones in normal and malignant osteoblasts. Endocrinology. 1985 Jun;116(6):2186–2191. doi: 10.1210/endo-116-6-2186. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Livesey S. A., Kemp B. E., Re C. A., Partridge N. C., Martin T. J. Selective hormonal activation of cyclic AMP-dependent protein kinase isoenzymes in normal and malignant osteoblasts. J Biol Chem. 1982 Dec 25;257(24):14983–14987. [PubMed] [Google Scholar]
- Majeska R. J., Holwerda D. L., Wuthier R. E. Localization of phosphatidylserine in isolated chick epiphyseal cartilage matrix vesicles with trinitrobenzenesulfonate. Calcif Tissue Int. 1979 Mar 13;27(1):41–46. doi: 10.1007/BF02441159. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Rodan G. A. Alkaline phosphatase inhibition by parathyroid hormone and isoproterenol in a clonal rat osteosarcoma cell line. Possible mediation by cyclic AMP. Calcif Tissue Int. 1982 Jan;34(1):59–66. doi: 10.1007/BF02411210. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D3 on phospholipid metabolism in chick duodenal mucosal cell. Relationship to its mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3354–3360. [PubMed] [Google Scholar]
- Matsumoto T., Kawanobe Y., Morita K., Ogata E. Effect of 1,25-dihydroxyvitamin D3 on phospholipid metabolism in a clonal osteoblast-like rat osteogenic sarcoma cell line. J Biol Chem. 1985 Nov 5;260(25):13704–13709. [PubMed] [Google Scholar]
- Matsumoto T., Kawanobe Y., Ogata E. Regulation of 24,25-dihydroxyvitamin D-3 production by 1,25-dihydroxyvitamin D-3 and synthetic human parathyroid hormone fragment 1-34 in a cloned monkey kidney cell line (JTC-12). Biochim Biophys Acta. 1985 Jun 30;845(3):358–365. doi: 10.1016/0167-4889(85)90199-5. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Wolf A. M., Arnaud C. D. Bone cells in culture: morphologic transformation by hormones. Science. 1976 Jun 25;192(4246):1340–1343. doi: 10.1126/science.1273593. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Alcorn D., Michelangeli V. P., Ryan G., Martin T. J. Morphological and biochemical characterization of four clonal osteogenic sarcoma cell lines of rat origin. Cancer Res. 1983 Sep;43(9):4308–4314. [PubMed] [Google Scholar]
- Partridge N. C., Opie A. L., Opie R. T., Martin T. J. Inhibitory effects of parathyroid hormone on growth of osteogenic sarcoma cells. Calcif Tissue Int. 1985 Sep;37(5):519–525. doi: 10.1007/BF02557835. [DOI] [PubMed] [Google Scholar]
- Peck W. A., Klahr S. Cyclic nucleotides in bone and mineral metabolism. Adv Cyclic Nucleotide Res. 1979;11:89–130. [PubMed] [Google Scholar]
- Peck W. A., Kohler G. Hormonal and nonhormonal desensitization in isolated bone cells. Calcif Tissue Int. 1980;32(2):95–103. doi: 10.1007/BF02408528. [DOI] [PubMed] [Google Scholar]
- Podbesek R., Edouard C., Meunier P. J., Parsons J. A., Reeve J., Stevenson R. W., Zanelli J. M. Effects of two treatment regimes with synthetic human parathyroid hormone fragment on bone formation and the tissue balance of trabecular bone in greyhounds. Endocrinology. 1983 Mar;112(3):1000–1006. doi: 10.1210/endo-112-3-1000. [DOI] [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Silve C. M., Hradek G. T., Jones A. L., Arnaud C. D. Parathyroid hormone receptor in intact embryonic chicken bone: characterization and cellular localization. J Cell Biol. 1982 Aug;94(2):379–386. doi: 10.1083/jcb.94.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C. S., Heersche J. N., Murray T. M., Parsons J. A. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982 Feb;110(2):506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- Wong G. L. Induction of metabolic changes and down regulation of bovine parathyroid hormone-responsive adenylate cyclase are dissociable in isolated osteoclastic and osteoblastic bone cells. J Biol Chem. 1979 Jan 10;254(1):34–37. [PubMed] [Google Scholar]
- Wuthier R. E. A review of the primary mechanism of endochondral calcification with special emphasis on the role of cells, mitochondria and matrix vesicles. Clin Orthop Relat Res. 1982 Sep;(169):219–242. [PubMed] [Google Scholar]


