Abstract
The most recent strategies available for upcycling agri‐food losses and waste (FLW) into functional bioplastics and advanced materials are reviewed and the valorization of food residuals are put in perspective, adding to the water–food–energy nexus. Low value or underutilized biomass, biocolloids, water‐soluble biopolymers, polymerizable monomers, and nutrients are introduced as feasible building blocks for biotechnological conversion into bioplastics. The latter are demonstrated for their incorporation in multifunctional packaging, biomedical devices, sensors, actuators, and energy conversion and storage devices, contributing to the valorization efforts within the future circular bioeconomy. Strategies are introduced to effectively synthesize, deconstruct and reassemble or engineer FLW‐derived monomeric, polymeric, and colloidal building blocks. Multifunctional bioplastics are introduced considering the structural, chemical, physical as well as the accessibility of FLW precursors. Processing techniques are analyzed within the fields of polymer chemistry and physics. The prospects of FLW streams and biomass surplus, considering their availability, interactions with water and thermal stability, are critically discussed in a near‐future scenario that is expected to lead to next‐generation bioplastics and advanced materials.
Keywords: biopolymers, circularity, food losses, food waste, functional materials, sustainable plastics, upcycling
The state‐of‐the‐art strategies to upcycle agri‐food losses and wastes (FLW) into advanced materials are reviewed. The right accent is put on the feasible means of effectively deconstructing and reassembling, synthesizing, or engineering FLW‐derived monomeric, polymeric, and colloidal building blocks targeting multifunctional, sustainable bioplastics. The food–materials–energy nexus is put in perspective as far as the next‐generation bioplastics fitting the circular bioeconomy.
1. Introduction
In the quest to achieve a circular bioeconomy, bioresources such as renewable and/or recycled streams are considered for their sustainable utilization along with strategies for end‐of‐life disposal and/or recirculation. Bioplastics or bioplastic‐forming compounds derived from biomass have been demonstrated as viable options that can meet the demands of product manufacturing and functions.[ 1 ] In parallel, an increasing concern is building up as massive volumes of biomass are generated from agro‐industrial operations and consumption, best exemplified by food loss and waste, thereafter referred to as FLW. Some of the FLW biomass can be utilized, for instance, as nutrients for livestock; however, the associated economic and environmental costs remain as important barriers for such use, for example, considering feed quality control, stream management, and others. Therefore, the transformation into “green” materials is as an emerging option that utilizes residual biomass and streams in the food supply chain.
The complex and heterogenous chemical make‐up of FLW‐derived biomass is a challenge but can also offer great opportunities, e.g., if appropriate fractionation tactics are applied. FLW offers an unparalleled potential for upcycling into materials sourced from monomeric, polymeric, and colloidal building blocks (Figure 1 ). Additionally, FLW can be utilized without extensive purification or separation (bulk waste), and as sources of nutrients for biotechnological routes. Beyond packaging, FLW versatility allows upcycling into advanced materials, suitable for biomedical devices, sensors, actuators, and energy storage and conversion devices. In this review, we critically discuss the recent breakthroughs in FLW valorization, associated sustainability aspects, and the prospects to meet the fabrication demands toward advanced, functional materials, and devices. Our contribution is expected to serve as a basis to catalyze new efforts in developing next‐generation bioplastics from agri‐food side streams. Indeed, taking the stratosphere as a boundary, there is no throwing out, and FLW streams should be regarded as useful resources in our efforts to achieve circularity. We put into perspective the interplay between FLW composition and building block origin, considering the different stages that exist during the lifetime of the given bioresource, as a waste or residual stream or as a product loss or purge. We analyze the properties of the resulting building blocks based on those of the precursor FLW and targeted materials. We discuss proposed strategies to obtain bioplastics from FLW, the steps that lead to efficient processing, as well as the approaches needed to meet the performance gap that exists between bioplastics and conventional plastics. Lastly, we present the main challenges in the utilization of FLW as a widespread source of materials, also considering aspects related to socioeconomical, environmental, and sustainability impacts.
Figure 1.
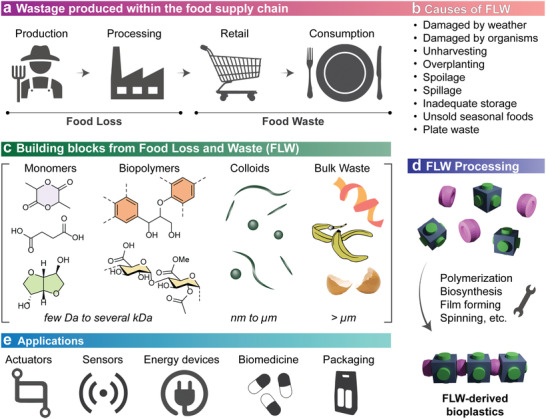
Illustrative landscape covering the utilization of food losses and waste (FLW) to produce bioplastics aimed to fulfill advanced applications. a) Food wastage is divided into loss and waste, depending on its origins. While losses take place during production and processing, waste is sourced at the retail and consumption stages. b) Several technical and behavioral reasons lead to FLW, spanning from agricultural losses from environmental conditions to human activities, for example, waste from eatery and catering utensils. c) A wide variety of building blocks can be obtained from FLW. Monomers (such as dilactide, succinic acid, and isosorbide), biopolymers (e.g., lignin and cellulose), colloids (with morphologies such as nanospheres, nanofibers, and nanocrystals), and bulk waste (e.g., unpurified peels and shells), all of which can be collected as FLW. Note: The polyaromatic structure shown corresponds to G‐type lignin with a β‐aryl ether (β‐O‐4) linkage. d) Processing of FLW‐derived building blocks, e.g., to synthesize bioplastics, can use several routes, including polymerization, self‐assembly, spinning, and others. e) FLW‐based bioplastics are finding application in traditional materials (for example, packaging) as well as advanced materials and systems, as noted.
2. Losses and Waste Associated with the Food Industry and Opportunities as Bioplastics
2.1. Food Losses and Waste in Numbers
A 2011 estimate from the Food and Agriculture Organization (FAO) indicated that roughly a third of the total food produced in the world was lost or wasted (around 1.3 Gt annually).[ 2 ] More recently, the FAO and the United Nations (UN) Environment Programme developed more precise estimates by the adoption of two main metrics, namely, the “Food Loss Index” (for losses in the production or supply chain, before retail) and “Food Waste Index” (for waste by retailers and consumers).[ 3 ] The key difference between these categories (food loss and food waste) considers the point along the supply chain from which the food item is removed, with losses taking place at earlier stages, and waste occurring later, usually influenced by consumer practices and behavior.[ 4 ] Initial estimates of the Food Loss Index indicated that ≈14% of food is lost before retailing. Meanwhile, the Food Waste Index involves more complex accounting. The largest volume of losses (≈48% of the total) is under the category of “cereals and pulses”, followed by “roots, tubers, and oil‐bearing crops” (≈32%), and fruits and vegetables (≈12%).[ 3 ] In developing countries, the absence (or insufficiency) of strategies to preserve perishable crops, associated with the first stages of the supply chain, represents a major challenge. By comparison, the waste generated at the final stages is a dominant contribution to losses in high‐income countries.[ 5 , 6 ]
2.2. What Is Wrong with Food Losses and Waste?
Food losses and waste (FLW) bring adverse economic consequences such as those associated with the nutrient and food life cycle cost as well as waste disposal. Such factors undermine the business structure and raise prices, producing social pressure and lowering food security, both representing major impacts to the poorest segments of society.[ 6 , 7 , 8 ] FLW have been recognized as a global challenge due to their environmental impacts.[ 9 , 10 ] In fact, meeting the increasing agricultural needs, in an environmentally sustainable manner, is part of the “Grand Challenge” of achieving food security. Such challenge includes “sustainability” (with particular reference to environmental aspects) as the fifth component dimension following those related to “availability”, “accessibility”, “utilization”, and “stability.”[ 11 ] Considering these challenges, the major footprints derived from FLW include the a) carbon footprint associated with the emission of greenhouse gases throughout the food's life cycle (mainly during the primary production phase, such as enteric fermentation by ruminants, manure management, and the use of fertilizers); b) land footprint, including the surface of land used for food production; and c) water footprint or water used at all stages of the supply chain. Overall, there is an increased need to consider new strategies to prevent and valorize FLW. For instance, in 2015 the UN defined seventeen Sustainable Development Goals in the “2030 Agenda for Sustainable Development,” whose objectives were intended to guide UN actions over the next 15 years. According to Target 12.3, the per capita global food waste should be halved by 2030 together with a considerable reduction of food loss. Therefore, reducing FLW while limiting energy consumption, environmental and social impacts are critical to meet the challenge of feeding the world in a sustainable manner.[ 3 , 12 ] Such FLW mitigation has evolved from initiatives such as food charity and campaigns for consumer awareness, as well as strategies that include the reutilization of by‐products, within given industrial processes,[ 13 ] adding operational improvements,[ 14 ] or applying machine learning for optimization of stream and process integration.[ 15 ]
Although FLW minimization is crucial in feeding more people and in reducing the environmental impacts of the food chain, there are unavoidable losses resulting from food production and processing. Hence, FLW valorization is a complementary approach that allows the recovery or upcycling of resources for a variety of purposes, including those associated with energy,[ 15 , 16 ] animal feed, chemicals,[ 17 ] and materials.[ 18 ] Since the transformations needed for FLW valorization, on their own, might involve additional environmental impacts, one needs to consider the feasibility of each valorization initiative in a holistic manner, for example, by using life cycle assessments (LCA) and other approaches to support decision‐making. The fact that a product/material has a renewable origin does not necessarily mean that it has a better environmental performance, for example, compared to conventional options. Thus, a case‐based evaluation is always required.[ 19 ]
FLW streams usually contain valuable components, as shown in Table 1 , which should be regarded as resources that will help to meet the major global challenge associated with the transition from a fossil‐based to a bio‐based economy. In this context, the term “food by‐products,” has been adopted to indicate that biomass converted into marketable products.[ 20 ] The complementary concepts of bioeconomy and circular economy have been presented as sustainable alternatives to the dominant economic development model following the “take‐make‐dispose” plan.[ 21 ] The bioeconomy is based on the transformation of renewable resources into end‐products and materials; meanwhile, the circular economy proposes the transformation of the current linear supply chain into a circular model, focused on optimizing resource efficiency and processes by reusing and recycling products, thus enabling a closed‐loop, ideally leading to a waste‐free system. Such concepts are designed to counterbalance the socioeconomic and environmental shortcomings that exist under the current linear model. Hence, generally, food losses are usually preferred for use over food waste, as the former ones are abundant, spatially concentrated (facilitating collection), and usually less deteriorated than the latter, which is normally produced at the end of the food supply chain.[ 20 ] The abundant food waste, on the other hand, is fragmented (from households and commercial establishments), heterogeneous in composition, and inconsistent as far as generation rate and volume, making handling difficult.[ 22 , 23 , 24 ]
Table 1.
Examples of FLW sources, their major components, and potential for material production. Also included are the fractions/components proposed for use in bioplastics, according to the recent literature, as cited
| FLW | Annual generation [Mt] | Useful components/fractions a) | Fractions used for bioplastics |
|---|---|---|---|
| Apple pomace | 24[ 61 ] | Pectin (3–14%),[ 62 ] lignin (15–23%),[ 62 ] cellulose (7–44%),[ 62 ] phenolics (0.5%)[ 63 ] | Cellulose;[ 64 ] bulk apple pomace [ 65 , 66 ] |
| Banana peels | 34.7[ 67 , 68 ] | Starch (30%),[ 69 ] cellulose (18.7%), pectin (14.2%), lignin (16.8%),[ 70 ] phenolics (≈1%)[ 71 ] | Pectin,[ 72 ] cellulose,[ 72 , 73 ] phenolics[ 74 ] |
| Brewer's spent grains | 39[ 75 ] | Arabinoxylans (22–29%),[ 76 , 77 ] cellulose (17.9%),[ 77 ] lignin (12–28%),[ 77 , 78 ] protein (15– 30%)[ 75 , 78 ] | Arabinoxylans,[ 79 ] feruloylated arabinoxylo‐oligosaccharides,[ 79 ] protein[ 80 ] |
| Coconut fiber | 23[ 81 ] | Cellulose (31.6%), hemicelluloses (25.5%), lignin (35.1%)[ 82 ] | Cellulose,[ 83 ] phenolics[ 84 ] |
| Coffee husks | 1.8[ 67 , 85 ] | Cellulose (29.2%), lignin (22.3%)[ 85 ], phenolics (≈0.3%)[ 86 ] | Cellulose,[ 87 , 88 ] phenolics[ 88 ] |
| Corn gluten meal | 1.1 only in USA[ 89 ] | Proteins (zein, 41–48%; glutelin, 17–20%)[ 90 ] | Zein[ 91 , 92 , 93 ] |
| Corn stover | 1,836[ 67 , 94 ] | Cellulose (32.7%), hemicelluloses (31.1%), lignin (10.1%)[ 94 ] | Cellulose[ 95 ] |
| Grape pomace | 10[ 96 ] | Pectin (32%),[ 97 ] cellulose (16.4%),[ 98 ] phenolics (<4%)[ 99 ] including anthocyanins (0.5%)[ 100 ] | Cellulose,[ 101 ] phenolics,[ 102 , 103 ] anthocyanins;[ 104 ] bulk grape pomace[ 105 ] |
| Mango stones (kernels + shells) + peels | 22.5[ 106 ] | Stones: cellulose (55%),[ 107 ] lignin (23.8%),[ 107 ] starch (33%).[ 108 ] Peels: pectin (16–27%),[ 109 ] cellulose (8.2%),[ 35 ] lignin (6.4%),[ 35 ] phenolics (1.5%)[ 110 ] | Starch,[ 60 , 111 ], cellulose,[ 111 ] fat,[ 60 ] phenolics;[ 35 , 60 , 112 ] bulk mango peel [ 35 ] |
| Oil palm empty fruit bunch (OPEFB) | 54[ 67 , 113 ] | Cellulose (24–65%), hemicelluloses (21–33%), lignin (14–30%)[ 114 ] | |
| Olive pomace | 2[ 118 ] | Lignin (43.2%), hemicelluloses (22.3%), cellulose (12.5%), lipids (16.6%)[ 119 ] | Bulk olive pomace[ 120 , 121 , 122 ] |
| Orange peels/pomace | 15.6[ 123 ] | Cellulose (30–38%),[ 124 ] pectin (11.5%)[ 125 ] | Bulk orange pomace (sugars removed)[ 126 ] |
| Pineapple leaf | 76.4[ 127 ] | Cellulose (70–85%), hemicelluloses (6–19%), lignin (4–15%)[ 128 ] | Cellulose;[ 129 , 130 ] pineapple leaf pulp[ 131 ] |
| Potato peels | 0.07– 0.14[ 132 ] | Starch (44.8%), cellulose (34.3%), lignin (4.3%)[ 133 ] | Bulk potato peels[ 134 , 135 ] |
| Rice husk | 800[ 136 ] | Cellulose (25–35%), lignin (26–31%), silica (15–17%)[ 136 ] | Cellulose,[ 87 , 88 , 137 ] phenolics[ 88 ] |
| Rice straw | 650– 975[ 136 ] | Cellulose (37%), lignin (14%)[ 138 ] | Cellulose,[ 139 , 140 , 141 ] hemicelluloses,[ 140 ] lignin;[ 140 ] bulk rice straw[ 142 ] |
| Spent coffee grounds (SCG) | 6[ 143 ] | Hemicelluloses (30–40%, mainly mannans and arabinogalactans),[ 143 ] cellulose (12.4%),[ 144 ] lignin (23.9%),[ 144 ] proteins (13.6%),[ 145 ] phenolics (≈2%)[ 145 ] | (Total) polysaccharides,[ 146 , 147 ] hemicelluloses,[ 148 ] cellulose;[ 149 ] bulk SCG;[ 150 ] SCG extract (with caffeine, chlorogenic acids, and fatty acids)[ 151 ] |
| Sugarcane bagasse | 533[ 67 , 152 ] | Cellulose (42.2%), hemicelluloses (27.6%), lignin (21.6%)[ 153 ] | Cellulose,[ 154 ] hemicelluloses;[ 155 ] bulk sugarcane bagasse fibers[ 156 , 157 , 158 ] |
| Tomato pomace | 5.4–9.0[ 159 ] | Cellulose (13.9%),[ 160 ] pectin (28%),[ 161 ] phenolics (0.1%),[ 162 ] lipidic fraction (25%)[ 163 ] including cutin (20%)[ 162 ] | Lipidic fraction[ 163 , 164 , 165 ] |
| Wheat straw | 807[ 67 , 166 ] | Cellulose (32.6),[ 167 ] hemicelluloses (29.9%, mainly arabinoxylans),[ 167 , 168 ] lignin (≈20%)[ 169 ] | Hemicelluloses,[ 167 , 168 , 170 ] cellulose;[ 168 ] alkali‐treated wheat straw[ 171 ] |
| Meat (beef, pork, chicken) by‐products | 201 (for a meat: residue ratio of 1.5)[ 172 ] | Collagen: ≈30% in hides, [ 173 ] 25% in bones,[ 174 ] 70–80% in tendons.[ 175 ] Keratin: 90% in chicken feathers[ 176 ] | Collagen,[ 177 , 178 ] gelatin,[ 179 , 180 , 181 ] keratin[ 182 , 183 , 184 ] |
| Crustacean shells | 6–8[ 185 ] | Chitin: 15–40%[ 185 , 186 ] | Chitin,[ 187 , 188 , 189 ] chitosan[ 188 , 190 , 191 ] |
| Fish waste | 76[ 192 ] | Collagen/gelatin: up to 70% in skins[ 193 ] | Collagen,[ 194 , 195 ] gelatin,[ 180 , 196 , 197 ] gelatin hydrolysate[ 198 , 199 ] |
| Milk whey | 180[ 200 ] | Protein (12%)[ 201 ] | Whey protein isolate[ 202 ] |
If provided in the literature, the actual component content (from chemical evaluations) is provided on a dry basis. Otherwise, extraction yields are provided instead (shown in italics).
Given that competitive and environmentally friendly alternatives are needed to replace fossil resources, including energy, chemicals, and materials, several efforts have been applied for FLW valorization. The latter uses a variety of approaches, which depend on several factors, mainly related to the nature of the raw materials as well their chemical composition and physical structure. FLW have been traditionally examined for the production of biofuels[ 25 , 26 , 27 ] and energy recovery[ 28 , 29 ] and, to a lesser extent, to synthesize biobased materials, the subject of this review, given the promising prospects of such efforts.
2.3. Bioplastics—Volume, Properties, and Prospects
We emphasize the utilization of FLW to produce biobased polymeric materials, here loosely termed as “bioplastics” and considered in more detail in other sections of this review. Bioplastics can either replace traditional, non‐renewable counterparts or create new solutions to current technological challenges, thus improving the sustainability and circularity aspects of material manufacturing. The annual plastic production from fossil sources is ≈360 Mt worldwide, which poses a threat to the environment, for example, if one assumes that 0.1% of this amount ends as microplastics, accounting for hundreds of thousands of tons. Such highly mobile and persistent particulate materials would bioaccumulate in plants and animals (see recent reports on microplastics[ 30 , 31 , 32 ]). In 2017, 115 Mt of plastics were used only for packaging,[ 33 ] mainly single‐use plastics of short service life (a year, on average). These materials persist for centuries in the environment, with serious ecological impacts, especially on marine ecosystems.[ 34 ] To compound the global concerns related to the consequences of persistent materials, it is worth noting that the packaging market (particularly food packaging) is continuously growing, following the ever increasing demand for convenience foods and the rising urban population.[ 35 ] Bioplastics represent only a small fraction (≈1%) of the total plastic production, with packaging being the main application (more than 53%, representing 1.14 million ton in 2019).[ 33 ] The use of bioplastics, however, has diversified into other segments such as those related to the biomedical (e.g., tissue engineering),[ 36 , 37 , 38 ] transportation (e.g., automotive parts),[ 39 , 40 , 41 ] and construction (e.g., thermal insulation materials).[ 42 , 43 ] It is worth mentioning that the use of food packaging is rather essential to protect foodstuff (from spoiling agents, mechanical damage, dehydration, among others). Hence, the main goal should be to minimize FLW while using long‐lasting materials, considering circularity and persistence of the natural resources within the economic cycle. At any rate, it is clear that the use of non‐biodegradable plastics for single use is a great concern, even if derived from biomass.
The broad term “bioplastic” comprises three distinct polymer categories:[ 44 ] a) bio‐based and biodegradable (e.g., polylactide—PLA—and thermoplastic starch—TPS); b) bio‐based and nonbiodegradable (e.g., the “drop‐in” replacements of conventional plastics, such as bio‐based polyethylene—PE); and c) nonrenewable and biodegradable (such as poly(ε‐caprolactone)—PCL). In this Review, we indicate “bioplastics” referring to bio‐based materials obtained from FLW, irrespective whether being biodegradable or not (although biodegradability is desirable for short‐term applications). The term “bio‐based plastic” relates to the ‘rate or kinetics of renewability’ of the raw material used in its manufacture. A widely neglected fact is that virtually everything that comes from nature is renewable, although at different timescales. Thinking of sustainability, it is reasonable to target raw materials that can be restored in nature, following a timescale that is comparable to the intended lifetime of the bioplastic‐based material, where the environmental fate should also be included. Furthermore, if one looks into the ‘thermodynamics of renewability', it becomes evident that the terms “circularity” and “close‐loop” are idealized, as there must be some energy input (sun irradiation, at least) involved in the process. This energy input, in turn, is demanded at given extent and can derive from clean (e.g., sunlight, wind, hydropower, geothermal) or less clean (e.g., burning oil, gas, and coal) sources. There is consensus in achieving an environment that largely benefits from energy inputs that should be minimized and as clean as possible, considering resource depletion and emissions.
Most current bioplastics are first‐generation, i.e., produced from carbohydrate‐rich plants that, at least in some instances, could instead be used as food or animal feed (e.g., corn, sugarcane, soybean, wheat, and potato), which leads to disagreements around food versus nonfood applications. On the other hand, second‐generation bioplastics are derived from feedstocks that are not intended for food use (including wood cellulose and FLW). A third generation of bioplastics, still in development, involves the direct production of plastics (or their building blocks) from living organisms.[ 45 ] So, FLW utilization to obtain materials is compatible with both the second‐ and third‐generation bioplastics. Regardless, bioplastics are presented as sustainable materials compared to those produced from fossil sources. However, due to information gaps, the evaluation of the global sustainability performance has been mainly focused on a few aspects, particularly global warming potential (GWP). In this regard, it is telling that substitution of ≈65.8% of all conventional plastics by bioplastics would save 241 to 316 Mt of CO2‐equivalent per year,[ 46 ] signifying a great impact on GWP.
Albeit more environmentally advantageous, most bioplastics lack in their properties, for instance, to match those of petroleum‐based plastics.[ 44 ] “Drop‐in” replacements of conventional plastics provide bio‐based alternatives with the same chemical structures (as well as properties and applications); however, associated costs need to be reduced given the relatively smaller production scale and processing capacity, as well as the higher raw material costs.[ 44 ] Most bioplastics, on the other hand, do not share the chemical structure typical of conventional plastics, and might present challenges in processability. These are barriers preventing bioplastics to enter the traditional, highly competitive markets. For instance, as far as performance, biopolymers may have a glass transition temperature (T g) that is too close to their degradation temperature,[ 44 ] thus limiting common heat processing, such as extrusion, compression, injection molding, and melt spinning. Therefore, processing of bioplastics does not typically follow that of synthetic counterparts, and requires engineering adaptations or new methods, such as continuous casting (see Section 5). Moreover, given their brittleness, bioplastics might require plasticizers,[ 44 , 47 ] i.e., to increase elongation at break or to accommodate plastic deformations.[ 44 , 48 , 49 ] Compositing, the addition of crosslinking agents[ 50 , 51 ] or nanofillers[ 52 , 53 ] are used to tailor the mechanical strength of bioplastics but usually compromise elongation.[ 50 , 54 ] Nanofillers may improve the thermal and barrier properties of bioplastics,[ 55 , 56 ] while blending with other polymers can be cost‐effective to enhance the properties according to the intended use.[ 44 ] For instance, materials for food packaging must fulfill safety demands associated with biological, chemical, or physical stability, for example, during storage. Moreover, antimicrobial properties in food packaging materials are useful to minimize microbial spoilage[ 57 , 58 ] (e.g., in cheese, fruits, and vegetables). Additional properties include antioxidant, and UV‐absorbing activity, relevant to lipid oxidation (e.g., in vegetable oils and edible nuts).[ 59 , 60 ]
3. Food Losses and Waste as Precursors of Biocolloids and Advanced Bioplastics
3.1. Biocolloids for Materials Assembly
The use of biocolloids for assembling materials has mostly involved highly pure systems, such as those derived from dissolving wood fibers, cotton, and, more recently, micro‐ and nanocelluloses. However, given the large volumes of FLW available, they can be considered as a source of biocolloids. In this section, we review the isolation of nanocelluloses, nanochitins, carbon dots, and biogenic silica from plant‐ (e.g., fruit peels, stems, and bagasse) and animal‐based FLW (e.g., crab shells). We discuss extraction yields and biocolloid properties as far as the recalcitrance and chemical composition of the source biomass.
3.1.1. Nanocelluloses
Wood is currently the major source of nanocelluloses, i.e., cellulose nanofibrils (CNF) and nanocrystals (CNC). It has been estimated that around 40,000 metric tons of nanocelluloses were produced in 2018, and forecasted to grow ≈30% yearly, surpassing 250 000 metric tons by 2025.[ 203 ] Nanocelluloses derived from woody biomass represented a market value of ≈USD 300 million in 2020 and are expected to double by 2025. North America, Northern Europe, and Japan are major nanocellulose markets. We suggest that sourcing nanocelluloses from FLW would rapidly increase the worldwide market value, given the possibility of creating globally reaching, decentralized units that produce nanocelluloses in smaller scales, using waste available locally, e.g., from agriculture and food chains. Under this scenario, regions with consolidated agriculture‐based economies, such as Western Europe and Latin America, could become important players in the nanocellulose market. Along the food chain, nanocelluloses can be sourced from the primary processing (e.g., fruit tree cuttings)[ 204 ] to the final consumption (e.g., peels and bagasse).[ 205 , 206 , 207 , 208 ] Some high‐quality nanocelluloses have been successfully isolated from bagasse,[ 207 ] peels,[ 209 ] stems,[ 210 ] or leaves[ 211 ] of a variety of fruits,[ 205 , 206 , 209 ] nuts,[ 212 ] vegetables,[ 208 ] and cereals.[ 213 , 214 ] In addition to the typical nanocelluloses (high‐aspect ratio CNC and CNF, Figure 3b), cellulose nanospheres (with enhanced surface activity compared to CNC and CNF) have been produced from corncob waste.[ 215 ] These nanospheres (Figure 3c), comprising cellulose II crystal structures, have been reported to feature a soft particle shell structure,[ 216 , 217 , 218 ] which is suitable for adsorption and biosensing.[ 218 ]
Figure 3.

Formation of high‐quality, well‐defined biocolloids from various FLW. a) FLW biocolloids can be obtained into different sizes, aspect ratios, and 3D complexities. Examples include: b) CNF from apple pomace (Adapted with permission.[ 281 ] Copyright 2019, Elsevier B.V). c) Cellulose nanoparticles from corncob (Adapted with permission.[ 215 ] Copyright 2020, The Authors, under exclusive license to Springer Nature Limited). d) ChNC from crab shell (Adapted with permission.[ 260 ] Copyright 2020, Elsevier B.V.). e) Carbon QD from mixed FLW (Adapted with permission.[ 275 ] Copyright 2014, American Chemical Society). and f) Hierarchically structured silica particles from pineapple peels (Adapted under the terms of the CC BY 4.0 license.[ 209 ] Copyright 2018, The Authors, published by Springer Nature).
Small‐scale and high‐value markets of FLW‐sourced nanocelluloses could become prominent in the future bioeconomy. In this regard, however, there is a differentiation between food losses and waste, namely, the isolation of nanocelluloses from food waste is considered more challenging than from food losses. Food waste, e.g., peels and bagasse, are usually enriched with nutrients, including low‐molecular weight (MW) sugars[ 219 ] that favor the growth of unwanted microorganisms (e.g., fungi). Thus, transportation and storage of food waste must be considered along with the incorporation of preservatives. On the other hand, wood, the prevalent source of nanocelluloses, is associated with a relatively low total yield. For example, if one considers the production of Kraft pulp, the most common route for wood processing, the yield from wood to Kraft pulp ranges from 45% to 60%, depending on the process parameters and extent of lignin removal.[ 220 ] Wood pulp can be converted into CNF in high yields; in contrast, CNC yield from the same source is clearly lower, ≈20–40%, depending on the process used.[ 221 , 222 ] The yield of nanocelluloses produced from FLW varies widely according to the source, based on their chemical composition and the stage from which the material is resourced, along the supply chain (Figure 2 ). Biomass from early stages of the supply chain, such as fruit tree pruning (e.g., branches and stems), includes lignified tissues that are chemically comparable to wood.[ 223 ] Therefore, the isolation and associated yields of nanocelluloses from branches and stems are analogous to those of wood. Isolation typically involves pulping (with NaOH and Na2S, as in the Kraft process), bleaching (ClO2, H2O2, ozone, and others), mechanical defibrillation, and acid hydrolysis.
Figure 2.
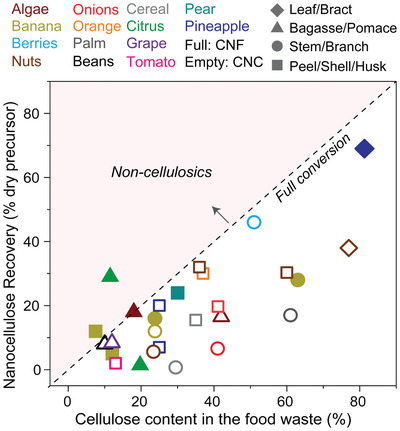
Extraction of nanocelluloses, represented by nanofibrils (CNF) and nanocrystals (CNC), from vegetal FLW. Primary biocolloid sources include leaves, bagasse, branches, and peels of a variety of edible vegetal species (note: here we include algae, which is not part of the plant kingdom). The data shown in the figure were collected from the available literature, discussed in the text.[ 101 , 129 , 130 , 204 , 205 , 206 , 207 , 209 , 210 , 212 , 213 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 ] The plot includes the percent nanocellulose recovery as a function of the cellulose content in the FLW source.
Compared to wood, bagasse and peels are relatively richer in cellulose and low‐MW carbohydrates. Also, they include less lignified tissues.[ 219 ] However, the higher cellulosic fraction does not necessarily imply a higher nanocellulose recovery, for example, when compared to woody branches and stems (Figure 2). This is related to the fact that part of the cellulosic matter is degraded during the fractionation into nanocellulose. A low nanocellulose recovery yield in such cases results from the poor structural integrity, for example, in peel/pomace biomass, owing to i) the inherent chemical composition (rich in low‐MW carbohydrates) and ii) microstructural disintegration of the biomass during food processing (e.g., thermomechanical methods) to extract pulp or juice. Processing and associated yields also follow biomass recalcitrance, which in turn depends on chemical factors such as the degree of polymerization of cellulose, lignin, and non‐cellulosic polysaccharides, protein content and the presence of acetyl groups, syringyl/guaiacyl (S/G) ratio of lignin, as well as physical factors such as the degree of crystallinity and surface accessibility.[ 224 , 225 ] Biomass that is rich in noncellulosic carbohydrates is not as recalcitrant compared to lignified biomass, with well‐developed plant cell walls,[ 226 ] which require more severe conditions for disassembling into single fibers. Unfortunately, selective processes to solubilize pectins or hemicelluloses, without attacking the cellulose scaffold, are not fully developed in either case.[ 223 , 227 ] Hence, most efforts to isolate nanocelluloses from fruit peels and bagasse are designed to produce CNC (see Figure 2 comparing CNF and CNC production from different sources and respective yields), given that acid hydrolysis (typically used to obtain CNC) degrades the amorphous (and low‐MW) carbohydrates, leaving behind the cellulose crystals. On the other hand, CNF production from peels and bagasse involves large amounts of low‐MW sugars, unless stepwise purification is considered, affecting colloidal and cohesive properties as well as susceptibility to biodegradation, given the low MW of the sugars.[ 228 ]
3.1.2. Nanochitins
Unlike nanocelluloses, whose major source includes nonfood biomass (wood and others), nanochitin is primarily extracted from wastes from the fishing industry[ 244 , 245 ] along with other emerging sources, such as insects.[ 246 , 247 ] Shellfish residuals represent an important source of chitin biocolloids, given the significant volume of marine waste available, for example, considering that only 40% of crab mass is meat.[ 185 ] Over 90% of the recent literature addresses the isolation of chitin from the exoskeleton (shell) of shellfish (e.g., crab, lobster, prawn etc.). Currently, shellfish waste is a common source of chitin biocolloids, in addition to chitosan (see Section 3.2.1), following processing conditions that lead to the isolation of both short and long nanofibers as well as nanocrystals.[ 248 ] Here we discuss the composition‐processing‐property correlations that exist for chitin colloids derived primarily from shellfish, most relevant in the context of FLW upcycling.
The exoskeleton of shellfish varies with species, but includes 20–30 wt% of chitin, 30–40 wt% proteins, 20–50 wt% inorganic salts (calcium carbonate and phosphates), as well as a smaller fraction of lipids (up to 14 wt%).[ 185 ] Therefore, the isolation of chitin is a stage‐wise chemical process that comprises deproteination, demineralization, and purification with organic solvents. Proteins can be removed from the chitin plywood structure primarily by dilute alkaline solution (1–10% w/v NaOH) at temperatures ranging from 65 to 100 °C, and using times from 30 min up to 72 h.[ 249 ] Processing time and temperature can be lowered by increasing alkali concentration and vice‐versa. The solubilized protein can be further recovered by precipitation, lowering the pH of the solution to its isoelectric value. Demineralization procedures have been optimized considering calcium carbonate (CaCO3) as the main exoskeleton inorganic salt. The main goal is to transform the insoluble CaCO3 into a soluble calcium form (for instance, CaCO3 + 2HCl → CO2 + CaCl2 + H2O) that can be washed out, thus exposing the chitin building blocks. This step is usually carried out at room temperature using dilute solutions of HCl (1–10 wt%); however, other acids (HNO3, HCOOH, H2SO4, and CH3COOH) have been also employed.[ 249 ] The final step for chitin purification usually involves extraction with organic solvents, to remove carotenoids and lipids, yielding pure chitin biomass that can be further processed as nanofibers (ChNF) or nanocrystals (ChNC) (Figure 3d). Although deproteination and demineralization involve simple chemical reactions, they are bottlenecks in the production of chitin biocolloids, as they consume significant chemicals and water. Deproteination takes place at very high pH, whereas demineralization is carried out in very acidic conditions. A typical chitin yield is 15–20 wt%,[ 250 , 251 , 252 ] or as low as 3 wt% in the case of cuttlefish bones.[ 253 ] Thus, many efforts are being directed to optimize the reaction conditions for chitin extraction and to match high‐valued applications.
At least five different types of nanochitins can be produced from the exact same source, only by taking different deconstruction routes.[ 254 ] Surface deacetylation, acid hydrolysis, and TEMPO‐mediated oxidation can be used to generate positively or negatively charged nano‐sized fibers or crystals as well as zwitterionic crystals.[ 254 ] Surface deacetylation (with, e.g., NaOH), which converts the chitin acetyl groups into primary amines, is a common process that facilitates the defibrillation of chitin flakes into single fibrils (ChNF), driven by weakened hydrophobic interactions and increased electrostatic repulsion.[ 255 ] The process to obtain ChNF from deacetylated chitin is then analogous to the preparation of CNF, for example, by mechanical (microfluidization) or sonochemical (ultrasound) processing, to mention only a few. Controlled deacetylation can lead to the formation of ChNF with aspect ratios going from 15 to over 60,[ 248 ] featuring colloidal stability with zeta potential (ζ) values near +80 mV.[ 255 ] On the other hand, TEMPO‐oxidation induces negative charges (ζ = −50 mV) in the chitin backbone, e.g., by selective reaction at the C6 group, similarly to cellulose, thus yielding individualized chitin fibrils, with diameters as low as 10 nm.[ 256 , 257 ] As it is observed for cellulose, hydrolysis of chitin's amorphous regions yields nanocrystals that are ≈150–400 nm long and twisted along the main axis;[ 255 ] they assemble into liquid crystals in suspension,[ 258 ] and chiral nematic order upon consolidation.[ 259 ] Upon TEMPO‐oxidation of deacetylated ChNF, zwitterionic ChNC are obtained by regioselective treatment, with oxidation taking place at C6 and deacetylation at C2.[ 254 ] With such treatments, a variety of high‐aspect ratio biocolloids are generated, with tailored characteristics (length, diameter, charge density and type, and crystallinity).
The purified nanochitin yield (from pure chitin) is ≈75–90% for ChNF[ 260 ] and up to 50% for ChNC.[ 261 ] However, the yield drops significantly if FLW are used as starting material (5–25% for ChNF and 10% for ChNC from shellfish‐based FLW).[ 250 , 251 , 252 ] For instance, one kilogram of wet prawn residues yields only 90 g of dry matter after removal of meat tissue. Therefore, highly efficient and green isolation processes (e.g., urea‐based hydrothermal treatment and others) still yield a low total ChNF < 5%.[ 252 ] Considering the low yield and harsh conditions often applied for isolation, efforts are in development to isolate biocolloids, including enzymatic routes to modify (deacetylate) chitin,[ 262 , 263 ] fractionation with acidic deep eutectic solvents,[ 260 ] and ball‐milling pretreatments.[ 264 ] Process engineering simulation has been used to assess the techno‐economic feasibility of ChNF and ChNC isolation from purified chitin. Water consumption can reach 34% of the total process costs for ChNC and ≈7 € kg–−1 for ChNFs and 12 € kg−1 for ChNC were determined in the year 2018.[ 251 ] Hence, so far, biomedicine, pharma as well as other high‐value‐added products are the most prominent markets expected for such biocolloids.
3.1.3. Biogenic Mineral Particles and Carbon Quantum Dots
Residues obtained at the beginning of the food production‐consumption chain, i.e., agricultural residues, have potential for the isolation of biogenic silica. Rice and wheat biomass are the main sources of biogenic silica, accounting for up to 20 wt% of their dry biomass. Given the volume of rice and wheat produced worldwide, associated residual biomass is a considerable source of SiO2 colloids, which is relevant to the widespread utilization of silica colloids in advanced materials. Examples include 3D printing inks[ 265 , 266 ] and porous particle carriers for pharmaceuticals[ 267 ] and agrochemicals.[ 268 , 269 ] The production of silica colloids from biorefinery platforms of waste biomass is a timely topic within the bioeconomy, especially when compared to traditional routes used to produce synthetic silica (mining, energy‐demanding chemical reactions, and toxic reactants).[ 270 ] Biogenic silica displays a self‐similar nanostructure, with primary SiO2 units (<10 nm), but aggregating into porous, submicron, and kinetically stable supracolloids. For instance, the specific surface area (SSA) of biogenic SiO2 derived from rice can reach up to 500 m2 g‐1, with a low pore tortuosity, yielding a highly percolating nanostructure,[ 268 ] differently from the highly directional, templated mesoporous silica (e.g., MCM‐41).
The isolation of biogenic silica from Si‐accumulating biomasses is simple and scalable. The process comprises a hydrolysis step with dilute acid (e.g., HCl or H2SO4 at 2% w/v) that is used to digest the cellulosic matter and to disassemble the plant microstructure, followed by calcination at temperatures ranging from 500 to 700 °C. The hydrolysis step is important to extract (leach out) other alkali and earth alkaline metals from the plant. Their presence decreases extensively the T g of the mineral mixture, thus causing the sintering of the primary mineral nanoparticles and closure of their pores. Interestingly, some fruits, such as pineapples, contain silica in their peels.[ 209 ] It has been demonstrated that rosette‐like biogenic silica microparticles (Figure 3f) can be obtained after the extraction of CNC from pineapple peels. The silica recalcitrance to all reactants involved allows the recovery of the particles at the end of the process, as an insoluble matter that can be collected.[ 209 ] Complex 3D particles find use in many applications where the colloidal interactions between particles or between particles and surfaces need to be controlled.[ 271 ]
Other types of particles that can be derived from FLW, from the end of the production‐consumption line, are carbon quantum dots (CQD). CQD are very small carbon nanoparticles (usually < 10 nm in diameter; Figure 3e) that display prominent photoluminescence given quantum effects, relevant to optoelectronic, biomedical, and sensing platforms.[ 272 ] Many processing technologies (e.g., microwave, plasma, ultrasonic, solvothermal) have been developed to produce CQD from carbon sources; however, those related to FLW mostly comprise (hydro)solvothermal approaches. Solvothermal routes, using mostly water as a solvent, aim to oxidize the FLW at temperatures ranging from 150 to 300 °C for 2–36 h, thus yielding —COOH functionalized nanoparticles with quantum yield from 20 up to 60%, depending on the source.[ 273 ] Contrarily to what is required for the isolation of nanofibers or particles, FLW are highly degraded as they serve only as carbon source for the bottom‐up production of CQD. Hence, fast‐food and mixed waste from restaurants (which contain complex mixtures of protein, carbohydrates, lipids etc.),[ 274 , 275 , 276 ] soft drinks,[ 277 ] chewing gums,[ 278 ] animal cartilage[ 279 ] and skins,[ 273 ] fruit bagasse and peels,[ 280 ] among others, have been used to produce CQD.[ 280 ] Production yields, if put in perspective with other food‐sourced biocolloids, are relatively low (≈0.2 wt% from the initial wet FLW);[ 275 ] however, the possibility to use basically any FLW, under given conditions, align with the high value of CQD applications and pushes forward efforts to up‐scale and implement CQD platforms.[ 275 ]
3.2. Soluble Biopolymers for Material Assembly
Colloidal matter, as discussed in Section 3.1, is mostly obtained from the residues of water‐insoluble polysaccharides (Figure 4a). Regarding nanocellulose, the precursors are generally purified and upconverted to cellulose‐rich fractions, which are then further processed into nanomaterials. During this purification, noncellulosic components are removed, mainly water‐soluble biopolymers, including heteropolysaccharides, proteins, and polyphenols. The structure and composition of these biopolymers in FLW are diverse and are mostly dependent on the FLW source. Interestingly, the season of the plant harvesting or the ripening state of fruits can strongly affect the composition and quantity of polysaccharides and sugars,[ 282 ] which are factors that need to be understood to devise suitable extraction processes and to unlocking the full potential of biopolymers from FLW.[ 61 ] In this section, we review the isolation of a group of water‐soluble biopolymers from given FLW sources, with a focus on plant‐based fractions used to isolate polysaccharides and polyphenolics. We also consider FLW for the valorization of protein fractions.
Figure 4.
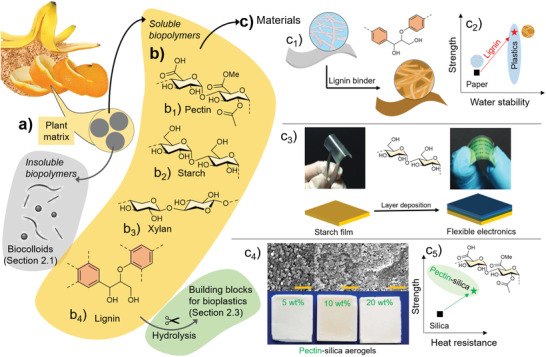
The major fractions extracted from plant‐derived FLW include a,b) polysaccharides and polyphenols. a) Soluble polysaccharides are extracted from the complex plant biomatrix and the most common ones include b1) pectin, b2) amylose (starch), and b3) xylan (hemicellulose). Apart from these polymers, nonsoluble fractions can be processed into biocolloids (Section 3.1) and polyphenols, e.g., b4) lignin. c) These biopolymers can be either hydrolyzed into monomeric buildings blocks for bioplastic synthesis (Section 3.3) or directly applied in materials. c1) Lignin can be used as a binder in paper and to develop c2) strong and water‐resistant biodegradable bioplastics (Adapted with permission.[ 294 ] Copyright 2020, John Wiley & Sons). c3) Starch can be processed into flexible and transparent substrates to prepare biodegradable disposable electronics (Reproduced with permission.[ 295 ] Copyright 2019, John Wiley & Sons). c4) Pectin can be used to reinforce silica aerogels to prepare c5) super‐insulating materials with high compressive strength and low heat conductivity (respective aerogels were prepared at pH 1.5) (Adapted with permission.[ 296 ] Copyright 2015, John Wiley & Sons).
Water‐soluble biopolymers are obtained as soluble fractions derived from complex FLW matrices, with hemicelluloses as the most prominent extractable polysaccharides from plants,[ 283 ] as well as starch,[ 284 ] and pectin (Figure 4b).[ 285 ] In addition, water‐soluble polysaccharides from animals can be obtained in the form of hyaluronic acid and chitosan.[ 286 ] Besides, the extraction of polyphenols, mostly from water‐soluble lignins and tannins, has received increased attention due to their abundance and the fact that they have been largely underutilized.[ 287 , 288 ] Also, valuable proteins can be extracted from FLW, including collagen,[ 289 , 290 ] gelatin,[ 291 , 292 ] as well as whey and soybean proteins.[ 293 ] In short, most of the current, commercially available biopolymers could be sourced from FLW, to optimize the usage of resources within the food supply chain. In addition, due to their diverse structures, new types of biopolymer derivatives are available, which could be important to enabling the mission of the bioeconomy.
3.2.1. Isolation of Polysaccharides and Polyphenols
Hemicelluloses
Hemicelluloses are some of the most abundant renewable plant material,[ 297 ] with an annual production in nature in the range of 60 Gt.[ 298 ] According to their primary structure, there are three main groups of hemicelluloses, namely, xylans (Figure 4b 3), mannans, and β‐glucans. The major sources of hemicelluloses from FLW derive from sugar and soybean production,[ 299 ] but also from spent coffee grounds[ 300 ] and other plant‐based FLW residues (Table 1). In most cases, a preliminary removal of extractives and nonpolymeric sugars is required, considered as impurities.[ 283 , 301 ] Hemicelluloses are mostly extracted by alkaline treatments using diluted aqueous NaOH or KOH solutions, but other treatments, for example, based on H2O2, have also been reported.[ 302 ] After solubilization, hemicelluloses are isolated by precipitation from the soluble phase. Different hemicellulose fractions can be separated based on their solubility through acidification or precipitation in ethanol/water mixtures.[ 283 , 301 ]
Hemicelluloses in sugarcane bagasse (SCB), mainly arabinoxylans, can be extracted with alkaline aqueous solutions (NaOH concentration 1–8 wt%, 3 h at 50 °C),[ 302 ] with a mass yield of 25% (i.e., ≈75% of original hemicellulose present in SCB). Owing to their good film‐forming capability, a main opportunity for xylans and other hemicelluloses is the synthesis of films and coatings (see Section 4).[ 303 , 304 ] Xylans from SCB have been extracted using a H2O2‐treatment at higher yields, up to 95% of total xylan content in SCB.[ 302 ] The amount of lignin could be controlled in this treatment, from 5 to 14 wt%, by varying the extraction temperature. Xylans could be as well extracted from cereal grain flours.[ 295 , 296 ] Due to their high protein and starch content, pretreatments are required. Starch can be hydrolyzed by enzymatic means, whereas soluble proteins can be removed with enzymes, adsorbed by clay or coagulated by heat treatment.[ 305 , 306 ] β‐Glucans have been isolated from such sources as well, and separated from xylans based on their solubility in ethanol/water mixtures.[ 306 ] Apart from the application of hemicelluloses and starch in film and coatings, they can be also used to prepare nanoparticles for drug delivery applications.[ 307 ] Additionally, their high water‐binding capability makes them promising materials for other biomedical applications as well, e.g., as wound dressings[ 308 ] or scaffolds for bone tissue engineering.[ 309 ]
Polyphenols
Lignins (Figure 4b4) can be extracted from FLW following two main avenues: 1) by solubilization during pulping, for example, of sugarcane bagasse via Kraft or soda processes.[ 310 , 311 ] In this case, lignin is precipitated by acidification, mostly due to the protonation of charged carboxylate groups.[ 312 ] Alternatively, lignin can be isolated from 2) a lignin‐rich residue by enzymatic digestion of residual polysaccharides or solubilization in organic solvents and subsequent precipitation by acidification from the concentrated residue.[ 313 ]
In contrast to xylan extraction, lignin isolation from SCB requires harsher alkaline conditions (aqueous 40 wt% NaOH solution and heating under stirring at 90 °C for 4 h).[ 314 ] Thereby, a total lignin yield of 20% has been reported, with low hemicellulose content (<0.5 wt%). Partial substitution of water with ethanol in this treatment can be used to increase further the lignin purity. Extracted SCB lignin has been valorized as a coating material for fresh fruits (limes) and shown to display superior properties compared to technical Kraft lignin, exhibiting antimicrobial properties and reducing weight and color losses of the coated fruits.[ 314 ] Solubilization of SCB lignins is also achieved at slightly alkaline conditions (0.5 to 1.5% w/v NaOH) by using relatively higher temperatures, 130 to 170 °C.[ 315 ] This treatment was successfully applied in the pilot scale and the dissolved lignin was recovered by acid‐induced precipitation.[ 312 ] Higher lignin yields have been achieved via acid‐catalyzed glycerol treatment, enabling the recovery of 63% of the initial lignin in SCB at a high purity of 90%.[ 313 ]
Lignin solutions can be transformed into nano‐ and microparticles by aerosolization or solvent exchange routes.[ 316 ] The size of lignin particles can be controlled by the processing conditions,[ 317 ] and their surface chemistry can be tailored by selecting the lignin source material.[ 318 ] Nanoparticles prepared with different SCB lignins were used in the stabilization of Pickering emulsions and encapsulation of curcumin as bioactive compound, where the more hydrophobic organosolv lignin showed superior stabilization performance compared to that of soda SCB lignin.[ 319 ] Lignins have also been used as binder to overcome limited wet strength of celluloses, thereby strong lignin‐cellulose bioplastics were obtained that surpassed conventional cellulose paper and some fossil‐based plastics (Figure 4c1,c2).[ 294 ]
Tannins represent important polyphenolic compounds. They contribute to the sensory properties in food (particularly mouthfeel and color) as well as stability due to their antioxidant and antibacterial effects.[ 320 , 321 ] Tannins are classified as hydrolysable tannins and flavonoids.[ 322 ] Hydrolysable tannins mostly consist of water‐soluble gallic acid esters, gallotannins, and ellagitannins (MW of 500–5000 g mol−1),[ 320 ] and can be extracted from various FLW.[ 288 , 320 , 321 ] Conventionally, hydrolysable tannins are extracted with mixtures of water and organic solvents,[ 320 ] including acetone:water (8:2, v:v), which was used with grape seeds to extract phenols and flavonoids, with strong anti‐oxidant properties.[ 321 ] The extraction of hydrolysable tannins can be also accomplished with aqueous solvents, e.g., in alkaline media (1–5 wt% NaOH).[ 321 , 323 ] The NaOH concentration can be selected depending on the FLW source. For example, a high yield of hydrolysable tannins (4 mg g−1) was obtained from chestnut peels using 1 wt% of NaOH. Due to the strong interactions of tannins with other biopolymers, they have strong adhesive properties.[ 324 ] Tannins have been used together with isocyanates in wood adhesives,[ 325 ] and can increase the wet shear strength (by 91%) of soy protein isolate (SPI)‐based adhesives.[ 326 ] Finally, tannins can be readily processed into colloids of different morphologies (cuboid, platelet, needle‐like, rhomboid, and others) by adjusting the base and pH used in the morphogenesis process.[ 327 ]
Starch
Given the competition with food applications, starch extracted from cereals and tubers is a less preferred source for material development. Yet, significant amounts of starch can be potentially extracted from FLW to replace other sources.[ 284 , 328 ] Chemically, starch is a mixture of linear amylose (Figure 4b2) and branched amylopectin. Native starch granules are usually non‐soluble in cold water, but starch gelatinization at temperatures of 60–70 °C causes granule swelling, loss of crystallinity, and dissolution of amylose.[ 329 ] Several FLW sources of starch are available (Table 1), including waste associated with the processing of fruits such as pineapple, mango, banana, and others.[ 284 ] Indeed, pineapple production generates large amounts of FLW, as plants have to be replanted every other year and each pineapple plant produces ≈6−8 kg waste as leaves, stems, and roots.[ 330 ] Starch was successfully extracted from pineapple stems by mixing the stems with water (1:1, w:w), grounding, and separating the starch slurry by filtration. The starch slurry (in case no suspension is obtained, starch can be precipitated overnight by cooling[ 331 ]) was further washed prior to drying.[ 330 ] The extracted starch had a high purity (98%) with a process mass yield of 9 wt%, amounting to ≈80% of the starch content in the initial biomass. Starch can be also extracted from banana peels by homogenization with 0.03 wt% aqueous sodium bisulfite solution.[ 332 , 333 ] Sodium bisulfite was used as a reducing agent to enhance, among others, protein solubility (dependent on the biomass solid content in this process, also higher amounts of bisulfite are required).[ 331 ] The precipitate was further purified with aqueous 0.2 wt% NaOH solution, water, and ethanol, yielding starch with a granular size of ≈17 µm, an amylose content of 21–26 wt%, and a gelatinization temperature of ≈70 °C.[ 333 ] Banana peels contain ≈30 wt% starch and 36 Mt peel waste is produced annually,[ 69 ] representing a major source of starch. Separation of starch from other soluble fractions, such as proteins, is possible at the industrial scale and achieved through hydrocyclones that separate two phases by densities.[ 334 ] Starches extracted from fruit FLW have unique features and are expected to expand the possible range of uses in industry.[ 284 ] Starch can be processed by various conventional processing techniques, such as extrusion or injection molding and offers an opportunity for the preparation of biodegradable bioplastics (see Section 5).[ 335 ] Starch can be as well processed by casting to obtain, e.g., flexible and biodegradable films for food packaging or as substrate for organic transient electronics (Figure 4c3).[ 295 ]
Pectins
FLW are valuable sources of pectins, which are compounds of diverse structure and properties, enabling utilization in a wide range of applications.[ 336 ] Pectins consist primarily of galacturonic acid, which occurs either in free carboxyl or methoxyl ester form. Homogalacturonan is the major type of pectin in the cell wall, representing 60% of total pectin amount,[ 337 ] which is partly methyl esterified at C6 position and/or O‐acetylated at C2‐OH and/or C3‐OH (Figure 4b1). The structure of pectins is highly complex and contains up to 17 different monosaccharides and 20 different types of linkages.[ 338 ] The ratio of esterified to nonesterified galacturonic acids defines the gelation properties, and pectins with low amount of ester groups, i.e., low‐methoxyl pectins, form strong hydrogels with Ca2+.[ 337 ] In contrast, high‐methoxyl pectins form stronger and tougher films due to their higher cohesive strength.[ 339 ] A high amount of pectins in plants is calcium‐bound, meaning that they are rendered insoluble due to ionic complexation between the carboxylate group and Ca2+.[ 340 ] This is why pectin extraction usually involves treatment with a chelating agent to complex Ca2+ for isolation of water‐soluble pectins or an acidic treatment to break such complexes.[ 341 ] Common chelating agents used for extraction include cyclohexanediamine tetraacetic acid and ethylenediamine tetraacetic acid (EDTA).[ 337 ] The remaining pectins are then extracted in alkaline media, usually with aqueous Na2CO3 solution.[ 340 ] Alternatively, high‐methoxyl pectins are extracted using dilute and hot acid, which is preferentially used over alkaline extraction because no depolymerization reactions via ß‐elimination occur.[ 337 ]
Several FLW contain high amounts of pectins (Table 1), up to 25 wt% in case of orange peels and pumpkin (pulp and peel) or even around 32 wt% in grape pomace;[ 97 ] onion hulls and endive roots contain significant amounts of ≈10 wt%.[ 336 ] It has been reported that pectin structures in these streams are hardly affected during food processing and storage, except for a visible reduction of methyl ester and acetyl groups.[ 341 ] A high‐yield extraction of pectins is usually accompanied by time‐consuming protocols to remove starch by solvent‐extraction with DMSO and/or enzyme treatment. However, there are straightforward avenues to isolate pectic polysaccharides, for instance by using 0.5 m HCl as extractant, achieving high yields (≈20%) by applying pressure, heating or microwave treatments.[ 341 ] Such isolated pectins have been further purified by a cascade of ethanol washing and filtration steps, which remove non‐polymeric sugars. The purified polysaccharides featured higher MW and gelling power compared to commercial pectin.
Conventional applications of pectins include those as texturizing and stabilizing agents in food and cosmetics, but their properties make them also suitable for the preparation of high value‐added materials. For instance, pectins from orange peels have been used to produce resistive memory devices as transient electronics.[ 342 ] The SSA of pectin aerogels has been found to be tunable, from 300 to 600 m2 g−1.[ 343 ] Pectins have been tested as important additives in silica aerogels, to obtain mechanically robust, thermal superinsulators.[ 296 ] Incorporation of pectins lowered the heat conductivities, down to 14 mW m−1 K−1, and increased the compressive strength of silica aerogels (Figure 4c4,c5).
Other Polysaccharides
Hyaluronic acid is a high value‐added polysaccharide with a broad range of medical applications,[ 344 ] with worldwide market estimated to be over US $1 billion.[ 345 ] It is composed of repeating disaccharide units of β‐1, 3‐N‐acetyl glucosamine and β‐1, 4‐glucuronic acid. Due to the high market potential, recent efforts have focused on extracting hyaluronic acids from fish FLW, mostly from fish eyeballs.[ 286 , 346 ] Apart from hyaluronic acid, also chitosan can be extracted from seafood waste. Chitosan is generally prepared by deacetylating chitin, as previously discussed in Section 3.1.2, to degrees exceeding 50% degree of deacetylation;[ 347 ] and is performed in industrial scale under acidic conditions.[ 348 ] Chitosan in the fully deacetylated state is composed of β‐1,4‐glucosamine units. Hence, it can be extracted from the same FLW as chitin, e.g., marine chitinous waste,[ 349 , 350 ] as well as insect exuviae.[ 351 ] Chitosan has versatile applications, for example, for bone tissue engineering.[ 352 ] In solution, chitosan is pH sensitive and can form, in combination with a crosslinkable polymer, double network hydrogels of high strength and toughness.[ 353 ] Chitosan gels can be also further processed into robust aerogels, with high SSA, for application as high‐performance thermal insulator.[ 354 , 355 ]
3.2.2. Isolation of Proteins
Collagen and Gelatin
Extraction of protein from FLW is already commercially implemented. Gelatin, which is obtained through hydrolysis of collagen, is currently extracted in large volumes from pork and cattle by‐products,[ 359 ] as well as from the fishery industry,[ 360 ] satisfying a worldwide demand of gelatin estimated at ≈0.6 Mt per year (2019).[ 361 ] Gelatin is commercially prepared either by alkaline or acidic treatment of animal tissue; the obtained alkali‐ or acid‐degraded collagen is then heat‐treated causing dissociation of collagen to various products, loosely termed as gelatin.[ 362 ] Both alkali and acid treatments influence and loosen the intrinsic fibrillar quaternary structure of collagen (Figure 5a), yielding a collagen that is more prone to hydrolysis reactions at elevated temperatures.[ 363 , 364 ] Depending on the animal tissue, specific pretreatments are required to remove other components, such as non‐collagenous proteins, fats, or inorganics.[ 289 ] Collagen has been extracted by acid treatment with 0.5 m acetic acid for mass yields of 11% and 2% from bigeye snapper skin and bone, respectively. In analogous procedures, collagen has been extracted at higher yields, 15% and 20%, from silver carp and shark skin, respectively.[ 290 , 365 ] Endeavors to further increase the yield by a subsequent pepsin treatment found no success, showing that simple acid treatment is sufficient for removing the most of extractable collagen.[ 365 ] These extracts are due to their high purity valuable alternatives to commercial collagen,[ 290 ] and can be further processed into gelatin by heat‐induced denaturation, i.e., gelatinization.[ 291 , 292 ]
Figure 5.
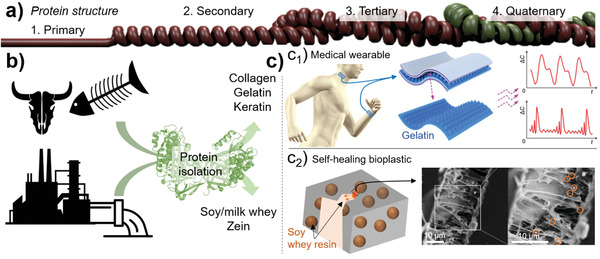
Extraction of soluble proteins from FLW. a) The properties of proteins are structurally dependent and in the native state they occur as tertiary or quaternary structures (Reproduced with permission.[ 356 ] Copyright 2019, American Chemical Society). Their extraction is usually associated with a protein deconstruction into lower structural order, e.g., from tertiary to secondary. b) Valuable protein fraction such as collagen, gelatin, milk whey, and keratin can be obtained from animal‐based FLW, while soy whey and zein can be extracted from crop‐derived FLW. c) Such proteins can be used to obtain advanced materials such as c1) gelatin‐based wearable tactile sensors to monitor physiological signals or physical motions (Adapted with permission.[ 357 ] Copyright 2020, John Wiley & Sons) and c2) soy whey‐loaded microcapsules (orange spheres) incorporated into PLA to introduce self‐healing properties (Adapted with permission.[ 358 ] Copyright 2016, John Wiley & Sons).
Collagen, as one of the main components in the extracellular matrix of mammalians, can be used in biomedical applications, such as in scaffolds for bone tissue engineering.[ 366 ] As collagen can be processed into flexible and transparent films, it is also a promising substrate for electronics, such as artificial synaptic devices.[ 367 ] Gelatin offers temperature‐sensitive and reversible gelation behavior, explaining its application in food, but also in inks for additive manufacturing. In particular, a UV‐crosslinkable gelatin methacrylate is widely used to prepare hydrogels for medical applications,[ 368 ] but can be also used as a substrate for tactile sensors in medical wearables (Figure 5c1).[ 357 ] The processing of biopolymers and their promise in emerging applications are further discussed in Sections 5 and 6, respectively.
Soy and Milk Whey Proteins
Efforts have been devoted to replace collagen and gelatin with plant‐derived proteins, such as soy whey or soy protein isolates (SPI),[ 369 , 370 ] as well as with whey proteins from the dairy industry.[ 371 ] Soybean protein represents a valuable alternative to animal counterparts, as it is one of the main components in waste from soy industries,[ 372 ] amounting to 40 Mt per year.[ 373 ] Soybean proteins have been isolated from waste liquors via acid extraction.[ 374 ] These liquors contain also mono‐ and oligosaccharides as well as other non‐proteinaceous components, which are separated from by ultrafiltration. Isolation of valuable soy protein fractions increases the sustainability and efficiency of the processing plants.
Soy whey accumulates in the aqueous waste of tofu production and is usually disposed in the sewage, polluting water bodies.[ 293 ] The recovery of proteins from soybean wastewaters is key to further improve the efficiency and competitiveness of soybean products and reducing water pollution and ecological footprint.[ 375 , 376 ] Whey can be also generated as animal FLW from the dairy industry. Similar to the soybean industry, whey wastewater from dairy streams is currently disposed in sewage, fields, or used as animal feed, which is associated with high costs, but also environmental concerns due to pollution of water bodies.[ 371 , 377 ] Besides the suggested application as food additives, these protein fractions are suitable precursors for high value‐added materials. Taking advantage of its biodegradability, biocompatibility, and film‐forming capability, milk whey has been used in coatings and films for food packaging,[ 378 ] as well as in advanced drug delivery systems.[ 379 ] Soy whey is commonly used for materials applications and has excellent water‐binding capability, which makes it suitable for wound dressings.[ 380 ] Due to their amphiphilic properties, they can stabilize emulsions and act as active components in resins, given their chemical reactivity, enabling the production of reactive microcapsules in self‐healing PLA composites (Figure 5c2).[ 358 ] The reader is referred to Section 6 to gain further insights about applications in bioplastics.
Other Proteins
FLW are sources of a variety of additional proteins that are used in materials manufacturing. Other than those previously addressed, keratin and zein are relevant proteinaceous biopolymers extracted from FLW by solubilization. Zein is a prolamine that is abundant in corn and contains large amounts of hydrophobic amino acids. For this reason, it is insoluble in pure water but it has been isolated by using mixtures of water and aliphatic alcohols, including ethanol.[ 381 ] Although zein is the main storage protein in corn, it occurs exclusively in the edible endosperm; hence, its application in materials competes with food options. The water insolubility of zein makes it useful in moisture‐resistant bioplastics, such as water‐barrier packaging, coatings, capsules, and pouches. Examples of FLW serving as protein sources include dry milled corn, corn gluten meal, and distiller's dried grains with solubles.[ 382 ]
Keratin is a fibrous protein present in epithelial cells of mammals, birds, and reptiles. Abundant keratinaceous FLW particularly those derived from butchery, including swine and bovine hair, as well as poultry feathers. The latter represents ≈24 wt% of the produced poultry meat, in which the feather waste alone accounts for 3.1 Mt annually in the European Union.[ 383 ] Chicken feathers (containing 90–92 wt% keratin) have been used to extract keratin and to further assembly solid‐state materials, such as fibers,[ 176 ] 3D cryogels,[ 384 ] and nanocomposite films.[ 385 ] Like cellulose and chitin, keratin plays a role as a structural element, enabled by the tightly packed 3D network formed by disulfide bonds among cysteine residues, which render keratin sources quite recalcitrant. Keratin extraction from FLW, particularly chicken feathers, have involved a) combined pretreatments with sodium bisulfite, sodium hydroxide, and heat (87 °C),[ 386 ] or b) processing with urea, sodium dodecyl sulfate, and cysteine at 70 °C and pH 10.5, to facilitate keratin dissolution by full cleavage of the original disulfide bonds.[ 176 ] These procedures lead to protein yields in the range of 65–67 wt%.
3.3. Bioplastic Building Blocks via Synthetic Routes
In this section, FLW are presented as sources of monomers for polymerization into bioplastics. Albeit these polymers do not occur naturally, they are classified as carbon sinks. This particularly applies to durable bioplastics, suitable for long‐term applications, and synthesized from biorenewables. Regardless, even if not classified as carbon sinks, these biopolymers fit the circular bioeconomy framework and present a number of advantages compared to fossil feedstock‐derived counterparts.[ 387 ] Note that even if originated from readily available, renewable monomers, a bioplastic is not necessarily biodegradable. Biobased and biodegradable features are interchangeably used, which is a mistake. Moreover, “green” labels should be used appropriately, because polymerization processes may not be as clean as the production or isolation of the precursor monomers.[ 388 ]
Although varying remarkably in composition, agricultural waste is in general dominated by organic compounds, including film‐forming carbohydrates, proteins, and lipids. Biopolymers may be directly extracted or produced from breaking down waste components into mono/oligosaccharides, amino acids, and fatty acids/glycerol. These small molecules, in turn, can serve as precursors for the synthesis of macromolecules, after chemical modification, or after biotechnological conversion into any polymerizable monomer. While the topic used of polymerization reactions is outside the scope of this review, the next subsections focus on FLW‐derived monomeric precursors used for polymer synthesis.
3.3.1. Monomers Directly Isolated from FLW
Molecules isolated from FLW that serve as monomers for the polymerization of bioplastic‐forming polymers, without chemical conversion, are somewhat uncommon and include terpenes and fatty acids. Biomass‐derived monoterpenes (e.g., α‐pinene and limonene) have been extensively exploited for bioplastic synthesis.[ 389 ] As far as food sources, d‐limonene (4‐isopropenyl‐1‐methylcyclohexene) stands out as a by‐product of citrus peels and with the limonene enantiomer representing a surplus biomass, accounting for 3 wt%[ 390 ] of the generated citrus peel waste. d‐Limonene has been exploited as a polymerizable monomer to produce, via plasma polymerization, optically transparent thin films with smooth surfaces (Figure 6 ).[ 391 ] Even if d‐limonene meets the bifunctionality criterion needed for polymerization via its double bonds, free‐radical homopolymerization is prevented by the relatively high stability and steric hindrance of the formed allylic radical.[ 392 ] Related bioplastics are hence more commonly produced from polymerizable limonene oxide (LO), limonene dioxide (LDO), and limonene dicarbonate (LDC), as addressed in Section 3.3.2.
Figure 6.
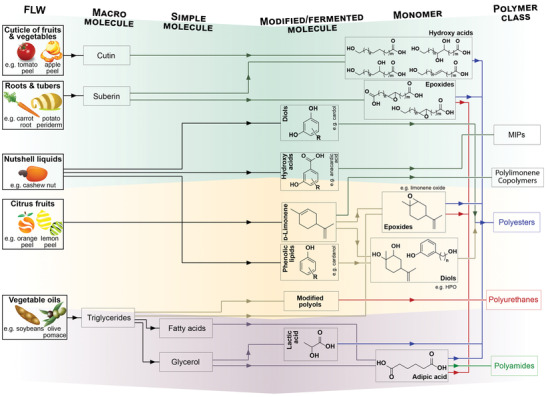
Synthetic routes to produce bioplastic‐forming polymers can use monomers directly isolated (green background and related pathways), chemically modified (orange background and pathways), or produced by fermentation (purple background and pathways) of nonsugary FLW.
Cutin, a component of plant cuticles and an important by‐product of the tomato industry, is also found in the periderm of pepper, apple, watermelon as well as in leaves.[ 393 ] Cutin is a biopolyester consisting of esterified bi‐ and trifunctional fatty acids. Its monomers can be extracted by alkaline hydrolysis, using, for example, potassium methanolate[ 394 ] and particularly 10,16‐dihydroxyhexadecanoic acid (10,16‐DHPA) from agroresidual tomato waste and other naturally occurring fatty polyhydroxyacids. As such, they have been polymerized into aliphatic polyesters.[ 395 , 396 ] A polyaleurate was synthesized using 9,10,16‐trihydroxyhexadecanoic (aleuritic) acid as a monomer.[ 397 ] Suberin, a biopolyester analogous to cutin, is found in the organs (e.g., roots and tubers) and periderm layers (e.g., cork and bark) of vascular plants. Despite being of lower occurrence in FLW, at least when compared to cutin, suberin has been depolymerized by ester cleavage (i.e., hydrolysis, trans‐esterification, or reductive cleavage) into a complex mixture of aliphatic alcohols, (ω‐hydroxy)fatty acids, α,ω‐dicarboxylic acids, and aromatics. Some of these molecules serve as monomers for the polymerization of polyurethanes and polyesters.[ 398 , 399 , 400 ] Biopolyols derived from olive stones (an abundant by‐product of the olive oil industry) have been demonstrated as macromonomers for polyurethanes and polyesters upon oxypropylation,[ 401 ] similarly to what has been shown for sugar beet pulp[ 402 , 403 ] to produce (poly)urethanes.[ 404 ]
Finally, cardol and anacardic acid, derived from cashew nutshell liquid (CNSL), can be polymerized without chemical modification,[ 405 ] though in the presence of co‐monomers. We note that, contrary to the case of the molecules introduced in this section, most of the industrially relevant monomers deriving from FLW are polymerized after chemical modification.
3.3.2. Monomers Obtained from Chemical Modification of FLW
Some molecules isolated from FLW are polymerized only at low yields or cannot be polymerized at all, unless chemically modified. Here we introduce this latter case. As discussed previously, CNSL, the pericarp fluid in cashew nuts, is a major loss from the cashew industry and accounts for ≈20 wt% of the raw cashew nut, comprising typically 10–65% of cardanol, up to 65% of anacardic acid, and 15–20% of cardol, depending chiefly on the extraction method (Figure 6, where R stands for a hydrocarbon chain, with 15 carbons, either saturated or unsaturated).[ 405 ] In the context of polymer‐based systems, CSNL has found use as surfactants, in linings, paints, adhesives, and laminates.[ 405 ] Cardol has been co‐polymerized as such into polyesters using 8‐(3‐hydroxyphenyl) octanol (HPO), which can be produced from cardanol.[ 405 ] The literature on the polymerization of unmodified CNSL‐derived monomers is rather scarce. For polyurethane production, specifically, CNSL‐derived cardanol has been converted into polymerizable diols by reaction with aminophenol.[ 406 ] Finally, anacardic acid isolated from CNSL as well as its acrylate (anacardanyl acrylate) and methacrylate (anacardanyl methacrylate) are derivatives that serve as comonomers for radical polymerization in the presence of ethylene glycol dimethacrylate or divinylbenzene, for instance, to produce molecularly imprinted polymer structures.[ 407 ] This is another example of the polymerization of unmodified FLW‐derived molecules, but is not as efficient as the radical polymerization of d‐limonene.
The olefin groups in d‐limonene can be chemically processed into more reactive monomers, most commonly the epoxide, limonene oxide, or limonene diols.[ 408 ] Sustainable pathways toward novel limonene hydroxy‐acids have also been recently established.[ 409 ] These reactive limonene derivatives can be polymerized into polycarbonates, polyurethanes, and polyesters, as reviewed in the literature.[ 408 , 409 ] An advantage of limonene oxides is that resulting polycarbonates can be obtained via ring‐opening copolymerization with CO2, which may also originate from FLW. Thereby the polymerization process can be fully integrated into processes that include CO2 generation from waste streams.[ 408 , 410 ] CO2 itself can be also converted into a range of polymerizable organic monomers (e.g., carbonates, carbamates, and ureas) or copolymerized with other FLW‐derived comonomers into polycarbonates, polyurethanes, polyureas, and polyesters.[ 410 ]
Lignocellulosic biomass has been introduced as source of biocolloids (Section 3.1) and as precursor of soluble biopolymers (Section 3.2). These macromolecular building blocks can be converted into polymerizable monomers, e.g., upon hydrolytic degradation followed by fermentation (see Section 3.3.3) and/or chemical modification. Fructose, glucose, and other hexoses, such as galactose and mannose, give rise to an important platform chemical, namely, 5‐hydroxymethylfurfural (5‐HMF), through isomerization and dehydration reactions.[ 411 ] In the context of bioplastics, 5‐HMF can be converted into 2,5‐furan dicarboxylic acid (2,5‐FDA), a “drop‐in” replacement of terephthalic and isophthalic acids for the syntheses of polyesters such as PET, polyamides, and polyurethanes, being considered as one of the main sleeping giants that are envisaged for the next‐generation bioplastics.[ 412 ] 5‐HMF can react into adipic acid for polymerization into polyamides (e.g., Nylon‐6,6), polyester, or polyurethane resins, and can plasticize polyvinyl chloride (PVC) and polyvinyl butyral (PVB).[ 413 ] Adipic acid can be also produced directly from glucose via glucaric acid as an intermediate[ 413 ] or microbial fermentation (Section 3.3.3).
Furfural is another important platform chemical derived from lignocellulosics, particularly hemicelluloses. When oxidized, furfural leads to fumaric acid, which in turn can be hydrogenated to succinic acid or further into 1,4‐butanediol, both used as polyester monomers either via transesterification or polycondensation. This route to synthesize polyesters has been demonstrated using corn cob and other FLW, producing poly(butylene succinate), and poly(butylene terephthalate) (PBT), if polymerized in combination with terephthalic acid.[ 414 ] As mentioned earlier, terephthalic acid can be replaced by bio‐based 2,5‐FDA or produced directly from FLW‐derived muconic acid.
Lignocellulosic and starchy biomass are also indirect sources of another important platform molecule, namely isosorbide. This sugar derivative is a diol and belongs to the class of dianhydrohexitols, which have a wide range of applications, e.g., as monomers in the syntheses of polycarbonates, polyesters, and polyurethanes.[ 415 ] As other diol monomers derived from furfural, isosorbide can be copolymerized with terephthalic acid into polyethylene terephthalate (PET). Isosorbide can also replace bisphenol A (widely studied as an endocrine disruptor, potentially carcinogenic)[ 416 ] in the manufacture of polycarbonates.[ 417 ]
Glucose, as precursor for isosorbide, 5‐HMF, adipic acid, and others, can be considered as the most important platform chemical within the synthetic bio‐based polymer framework. Apart from chemical derivatization, reactive monomers can be also obtained by biotechnological conversion of glucose and other fermentable molecules.
3.3.3. Monomers from FLW Fermentation
Simple sugars obtained from FLW can be converted, via fermentation or similar processes, either i) directly into bioplastic‐forming polymers (see Section 3.2) or ii) into platform molecules that can be then polymerized (Figure 7 ). Regardless of the route, biopolymers from FLW must be first broken down into fermentable substrates by a suitable pretreatment, which can be either mechanical, thermal, chemical, or biological/enzymatic.[ 24 ] A combination of these treatments is typically used to render biopolymers more accessible to enzyme cocktails, making the overall process to reactive monomers more efficient.
Figure 7.
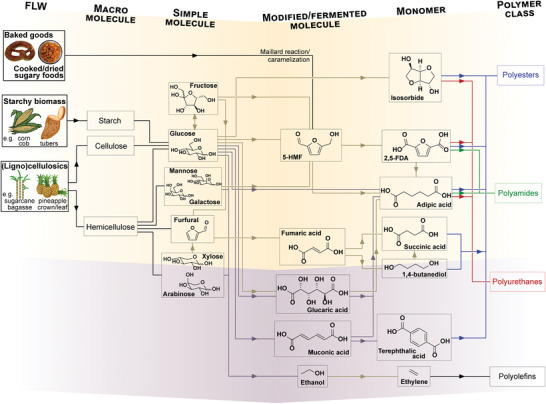
Synthetic routes applied to produce bioplastics from monomers obtained from sugary FLW, either by chemical modification (orange background and pathways) or by fermentation (purple background and pathways).
Adipic acid, introduced in Section 3.3.2, can be synthesized through the fermentation of glucose, but also from glycerol and fatty acids (derived from vegetable oils) by using bacteria such as Escherichia coli.[ 418 , 419 ] Apart from these pathways, adipic acid is more often obtained from cis,cis‐muconic acid (derived from glucose fermentation) or chemical pathways via glucaric acid, as introduced in Section 3.3.2.[ 418 ] Regardless of being produced chemically or microbially, the processes to adipic acid still require optimization with regard to yield and efficiency, i.e., to make them competitive against those used in the context of oil‐based sources.
Lactic acid can be found in nature, synthesized chemically from petroleum‐derived feedstocks, or yet biotechnologically obtained from biomass. Lactide, produced by dehydration of lactic acid, can undergo ring‐opening polymerization into PLA. While natural and synthetic routes lead to mixtures of L(+) and D(‐) enantiomers, which can be copolymerized into amorphous PLA, microbial fermentation enables the production of enantiopure lactic acid, which in turn may be homopolymerized into semicrystalline PLA.[ 420 ] Nevertheless, the biotechnological production of lactic acid via fermentation is associated with high costs. This bottleneck can be overcome by using waste biomass streams as source of fermentable sugars for lactic acid bacteria. Suitable FLW include but are not limited to SCB, corncob residue, municipal food waste, banana peel, molasses, apple pomace, and corn steep liquor. Required pretreatments and details on the respective fermentation processes are summarized in a recent review.[ 421 ]
Sugars are not the only nutritional requirements of lactic acid bacteria. Nitrogen, minerals, and vitamins are also needed, with the carbon‐to‐nitrogen ratio being a main factor influencing the lactic acid yield.[ 420 ] FLW are beneficial for providing a complex composition with all required nutrients, replacing expensive high‐purity sugars as carbon source, as well as yeast extract or peptone as source of nitrogen. Examples of such FLW precursors include fish waste hydrolysate, corn steep liquor, wheat bran, and silkworm larvae.[ 420 ] Although relevant in virtually all facets discussed in this contribution, exploiting FLW is not per se advantageous in all aspects. The typical high costs involved in the pretreatment of FLW‐derived biomass, e.g., to render it less recalcitrant, needs to be considered. These costs are often associated with processes to turn FLW into fermentable sugars, representing a major bottleneck for biotechnological downstream processes. To enable a proper comparison, we emphasize that a comprehensive evaluation, including technoeconomic and environmental analyses should be carried out.
Ethanol is widely produced from sugars, both using sugary or starch‐rich crops (1G ethanol) and through hydrolysis of lignocellulosic biomass (2G).[ 388 ] FLW as sources of sugars and starch can make 1G ethanol more appealing, decreasing the competition with food. However, these routes bear also challenges, as other fruit components, such as d‐limonene, can act as fermentation inhibitors.[ 25 ] The limonene fraction can be valorized as monomer or monomer precursor, as discussed in Sections 3.3.1–3.3.2. Alternatively, other polysaccharide‐rich waste streams from FLW, which can be processed in sugar hydrolysates, can be used to produce 2G ethanol, e.g., pulping residues from bagasse,[ 422 ] crop husks,[ 423 ] or other agricultural residues.[ 424 ] In general, lignocelluloses, such as straw, are liquefied by enzymes to produce soluble sugar, a lignin fraction, and nonsoluble residues, with the possibility for the latter two to be burnt for energy generation or for valorizing the isolation of biocolloids (Section 3.1) or biopolymers (Section 3.2). The produced ethanol can be turned into ethylene by dehydration, to enable the production of bio‐derived polyethylene.[ 388 , 425 ] This process is conducted by catalytic systems, which are very sensitive to impurities; hence, biobased ethylene is only currently produced from 1G ethanol from sugarcane. Compatibilization of this process for more sustainable 2G ethanol is rather challenging and requires further process optimization and the development of more appropriate catalyst systems. Obviously, the produced ethylene has the same properties as those derived from petroleum and could be directly used as biobased replacement.[ 426 ] For example, the copolymer ethylene‐vinyl acetate (EVA) can be produced from bio‐based ethanol (and commercialized under trademarks such as I'm green by Braskem). Relying on LCA, each kilogram of bio‐based polyethylene and EVA captures is claimed to sink ≈3.1 and 2.5 kg of CO2 from the atmosphere, respectively.[ 427 ]
3.4. Nutrient Source for Microbial Biopolymers
Bioengineering and biotechnology are powerful tools within the current material development landscape. They have been utilized to modify or manipulate living organisms toward energy‐efficient fabrication, also contributing to a better use of natural resources through bottom‐up routes rather than contrasting with deconstruction‐reconstruction platforms. Remarkable examples include the preparation of materials from bacterial cellulose (BC) and fungal mycelium. Additionally, polyhydroxyalkanoates (PHA) are excellent examples of bioplastics derived from microbial bioengineering. These three materials (BC, mycelium, and PHA) are prepared from similar precursors, such as carbohydrates, proteins, and lipids. Therefore, FLW can be (and have been) explored as non‐expensive, circular sources of nutrients for biosynthesis. In the next sections, we discuss state‐of‐the‐art uses of BC, micellar, and PHA materials, and the integration of FLW in such efforts.
3.4.1. Bacterial Cellulose
Certain types of bacteria, especially those from the genus Komagataeibacter, are able to synthesize extracellular cellulose from monomeric glucose under aerobic conditions. Secreted BC comprises high‐aspect ratio CNF of high chemical purity, as well as high degree of polymerization and crystallinity.[ 428 , 429 ] For a long time, the aerobic conditions required for BC growth have limited production at macroscopic air interfaces, which typically result in cellulose pellicles forming at the surface of the glucose‐rich culture media, utilized as a nutrient source. Even with geometric restrictions imposed by the inherent production of BC as pellicles (i.e., planar materials), its high strength and water retention capacity have led to many possible applications. For instance, BC pellicles have been directly utilized for, e.g., wound dressing[ 430 , 431 ] and uranium removal.[ 432 ] Further engineering of such materials has led to aligned, highly strong (tensile strength ≈800 MPa) BC filaments,[ 433 ] as well as optically transparent (transmittance over 80%) and strong (tensile strength ≈1 GPa) films.[ 434 ] More recently, 3D complex and branched structures, e.g., similar to those found in the lungs, have been formed from BC using biofabrication (Figure 8a)[ 435 , 436 ] and 3D printing (Figure 8b).[ 437 ] Other advanced applications have been introduced,[ 438 , 439 , 440 ] such as the immobilization of BC‐producing bacteria on surfaces to allow the production of lubricant coatings.[ 440 ]
Figure 8.
Advanced materials produced from bacterial cellulose (BC) and opportunities for upcycling FLW as nutrient source for microbial development. a) Complex BC materials have been produced by utilizing superhydrophobic templates (Reproduced with permission.[ 435 , 436 ] Copyright 2018, The Royal Society of Chemistry and Copyright 2020, American Chemical Society) and b) 3D printed culture media containing bacteria (Adapted with permission under the terms of the CC BY‐NC 4.0 license.[ 437 ] Copyright 2017, The Authors, some rights reserved; exclusive licensee AAAS). c) FLW can be upcycled via close‐loop biorefinery platforms into nutrient‐rich media that fulfill the requirements for BC production (Adapted with permission.[ 445 ] Copyright 2019, Elsevier B.V.). d) Demonstration of high‐quality BC pellicles from overripe banana (Adapted with permission.[ 447 ] Copyright 2020, Elsevier B.V.).
The nutrient sources for BC production are still expensive because they are chemically defined or synthetic in their origin, hindering industrial implementation. To biosynthesize cellulose, the microorganisms require carbon, hydrogen, oxygen, nitrogen, and minor elements such as iron and zinc, meanwhile, the production can be boosted in the presence of vitamins and hormones.[ 428 , 429 ] Sugars are the main source of carbon, whereas yeast and polypeptone provide nitrogen and vitamins.[ 441 , 442 ] Many efforts have focused on the implementation of alternative sources of such compounds, as an attempt to make BC production economically feasible and to incentivize a widespread utilization for the preparation of sustainable materials.[ 443 , 444 , 445 ] Interestingly, the composition requirements of the culture media can be fulfilled by utilizing FLW as a whole, purified, or supplemented, i.e., to meet the nutrient requirements. The full library of FLW as nutrient sources for BC growth has been recently revised.[ 441 , 442 ] Some remarkable examples include the production of high‐quality BC from mixed kitchen waste, rich in starch (Figure 8c),[ 446 ] low‐MW sugar‐rich overripe banana (Figure 8d),[ 447 ] waste from breweries (rich in yeast and sugars),[ 448 ] as well as citrus beverage industrial waste.[ 449 , 450 , 451 ] The yields of BC production from those FLW have reached values comparable to well‐defined culture media (such as the Hestrin and Schramm, HS system), ranging from 3 to 10 g of dried BC per liter of the medium.[ 441 , 452 ] Related efforts highlight the creation of closed‐loop, zero‐waste biorefineries that would not only be more profitable but would also incentivize the production of more advanced materials from sustainable colloids. We envision that an alliance between materials science and biorefinery efforts, especially for upcycling FLW, can lead to more widespread use of BC to manufacture materials needed in our daily lives.
3.4.2. Fungal Mycelium
Elongated chitinous fiber‐like structures, i.e., hyphae, constitute the vegetative growth of filamentous fungi. The biomass comprising branching hyphae networks is collectively called mycelium. Most of the known fungal mycelia are natural fiber‐like composites built from rigid chitin and branched, flexible glucan segments, thus featuring a unique chitin natural composite that is not found in other sources of chitin biocolloids such as shellfish (Section 3.1.2).[ 453 ] As a source of building blocks, fungal mycelia offer several interesting features relevant in the context of the bioeconomy and sustainable materials. Such features include the fast‐growing character compared to that of other multicellular tissues; the utilization of low‐cost substrates as growth media; the possibility to control the final product shape by templated growth; the non‐seasonal dependency for growth; the potential of altering properties depending on the feeding media; and (especially importantly) biodegradability.[ 453 , 454 , 455 , 456 ] Considering such advantages over many synthetic and biobased materials, several biotechnology companies have been developing and are now marketing fungi‐derived materials (Figure 9a).
Figure 9.
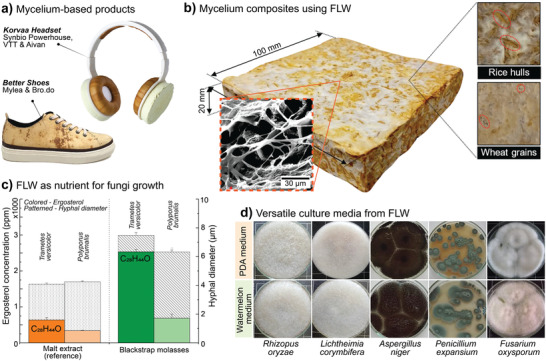
Opportunities for the utilization of FLW in the formation of materials from mycelia. a) Synbio Powerhouse, VTT, and Aivan developed the Korvaa Headset concept (www.aivan.fi), which contains mycelium‐based foams as one of its components (Reproduced with permission). Mylea, in partnership with Bro.do, designed and fabricated the Better Shoes (www.mycl.bio), which contain leather‐like mycelium‐based materials (Reproduced with permission, copyright 2020, MYCL). b) Thermally stable composites have been prepared from Trametes versicolor using FLW culture media rich in silica, such as wheat and rice hulls (Adapted with permission.[ 461 ] Copyright 2018, John Wiley & Sons). c) Comparison of FLW (blackstrap molasses) and reference malt extract as a source for mycelium production, biomarked by the hyphae diameter and ergosterol production (Adapted with permission.[ 466 ] Copyright 2019, Elsevier B.V.). d) Watermelon‐based FLW was shown to fulfill the nutrient requirements to growth a series of mycelium‐producing fungi (Adapted with permission.[ 467 ] Copyright 2020, Elsevier B.V).
Given the potential of fungal mycelia for biofabrication, several efforts aim to push forward the property boundaries of mycelia‐based materials as far as performance and tunability. For instance, it has been demonstrated that manipulation of the feeding substrate can tune the properties of the resulting fungal mycelium.[ 457 ] In this regard, two fungi (Ganoderma lucidum and Pleurotus ostreatus) were fed with a traditional culture media based on potato and dextrose (PBD) and a version of it enriched by amorphous cellulose. Whereas PBD induced structural changes in the proteins of the cell wall as well as the content of lipids, cellulose induced higher concentration of chitin. These are results of specific responses of the enzymatic machinery of the microbes that were able to selectively break down polysaccharides for energy, and also incorporated them into their vegetative tissues. When grown in cellulose media, the mycelia produced were more rigid and presented higher Young's moduli, for example, compared to that produced from the PBD substrate.[ 457 ] The mechanical properties of the mycelium are tethered to their composition, i.e., chitin/glucan ratio, whose variations can lead to elastic (tensile strength of 200 MPa and 4% elongation) or plastic (tensile strength of 50 MPa and 15% elongation) materials.[ 458 ] The interplay between fungi species, feeding substrate, and posttreatments is a powerful mean to tailor the properties of the resulting mycelia.[ 459 , 460 ] Moreover, it is possible to tailor the wetting behavior of nanopapers made from mycelium and following given posttreatments (e.g., HCl or H2O2). The latter treatments reduce the lipid content of the mycelium, thus yielding more hydrophilic systems (water contact angle, WCA = 80–90°), when compared to those treated with alkali (WCA > 100°).[ 460 ] Purification of the mycelium typically leads to chitin/glucan recovery yields ranging from 15 to ≈30%,[ 459 , 460 ] which are comparable to chitin from crustaceans.
As noted, the culture medium for mycelium development is based on polysaccharides, thus opening opportunities for FLW upcycling into nutrients towards biofabrication strategies. FLW can be compounded as a whole with fungal spores to allow the growth of the composite foam mycelium, or they can be processed into soluble sugars that serve as a more traditional culture medium—analogous to PBD, for instance. Non‐purified, food‐related biomasses have been mixed with fungal spores for the preparation of composite materials. In a procedure, a biomass‐fungi mixture was inoculated at relative humidity (70–80%) and temperature (25–30 °C) conditions, for optimum mycelium growth,[ 457 ] and the resulting biomass was thermally treated to inactivate the microbial colonies, thus stopping the growth. Interestingly, silica‐rich biomass such as wheat and rice hulls were used to prepare highly thermally stable composites with Trametes versicolor (Figure 9b), a known white‐rot fungus that biodegrades lignin.[ 461 ] The chitinous, N‐rich biomass has been confirmed to improve fire resistance of mycelium‐based materials.[ 462 ]
Because of such potential for FLW valorization through the preparation of sustainable materials, several efforts have been devoted to studying the growth kinetic of commercially attractive fungi (such as P. ostreatus, Trametes versicolor, and Polyporus brumalis) and by using different FLW as nutrient sources, such as SCB and wheat bran. A comparison with sawdust, a commonly employed non‐purified biomass used in mycelium composites, was carried out.[ 463 ] It was demonstrated that P. ostreatus mycelium grew much faster in the presence of either wheat or SCB (1.5 cm d−1, in vitro measurements) when compared to wood sawdust (0.66 cm d−1), resulting in mycelium microstructures that were independent of the growth rate.[ 463 ] This is a result of sugars being more easily available in wheat (e.g., starch) and sugarcane (e.g., hemicellulose) when compared to wood (e.g., cellulose). Fast consolidation protocols are ideal when working with living organisms because they limit cross‐contamination with airborne microbes, such as Aspergillus niger. Fungal mycelium is often characterized as the binder component in such biomass‐mycelium composites. Sugarcane molasses, coconut husk, and rice bran are other FLW examples suitable for the preparation of mycelium‐based materials.[ 464 ] More degraded biomasses obtained at the end of the supply chain, such as coffee grounds, banana peels, and eggshells, have also been composited with fungal mycelia for the preparation of biobricks.[ 465 ] Furthermore, although rice, wheat, and sugarcane residues cannot sustain the same growth rates of commercial wheat grain‐based culture media, liquid agricultural by‐products, such as blackstrap molasses, induce very high mycelium growth even compared to pure malt extract.[ 466 ] Both ergosterols, a fungal biomarker, content, and hyphae diameter were significantly higher when utilizing sugarcane molasses (Figure 9c).
Processed FLW have been proposed to replace traditional potato‐dextrose‐agar (PDA) or PBD culture media for microbial development. For instance, watermelon peel was subjected to a series of (isolated or combined) high‐energy blending, boiling, and filtration treatments to solubilize macro (proteins, lipids, and sugars) and micronutrients (minerals) to further allow its utilization as culture medium for mycelium development.[ 467 ] The chemical composition of the treated watermelon waste was similar to that of PDA, which resulted in a similar growth rate for a variety of fungi, from different families. Watermelon waste presented, however, higher lipid and protein content, which may induce other properties in the final mycelium (Figure 9d).[ 467 ] Mycelium recovery yields reached ≈4 g L−1 depending on the fungi. The growth can be further boosted (+ 250%) by enriching the media with dextrose. By optimizing the conditions, fungal growth from FLW can easily reach 50 g L−1.[ 468 ]
3.4.3. Polyhydroxyalkanoates
A series of bacteria produce and accumulate polyhydroxyalkanoates (PHA) in their cells, used as an energy storage mechanism during periods of low nutrient availability.[ 469 ] The ability of such microbes to intracellularly biosynthesize PHA is of great interest within the bioeconomy landscape, especially as the nutrient source for biosynthesis can rely on FLW, rather than pure sugars and fatty acids. PHA and other microbial bioplastics have been recently reviewed.[ 469 ] Here we give few examples of how FLW can be used for PHA production. For instance, the proper utilization of whey, as a liquid by‐product of cheese production, is considered a major industrial challenge. Since cheese‐making process involves whey proteins undergoing chemical modifications and decrease their water holding capacity and stability to shear or temperature, they lose value in the dairy industry because they cannot produce creamy textures. Hence, efforts have focused on the utilization of whey as a carbon source for PHA production.[ 470 ] Engineered E. coli has shown remarkable production capacity, yielding ≈5 g L−1 polyhydroxybutyrate (PHB). Meanwhile, waste vegetable oils from both household and industrial sources are major waste with no evident recycling potential. However, the production of PHA from wasted oils is a viable alternative to avoid the accumulation and contamination of landfills with fatty acids, which do not breakdown easily by the environmental microfauna. Corn, sunflower, and rapeseed oils have served as carbon sources to produce PHB, mostly by Cupriavidus necator. Total mass of cell production was measured to be relatively high, about 11 g L−1, with PHB recovery at ≈70% (on a dry cell weight basis). Several other FLW sources, such as fruit peels and bagasse, coir pith, olive pomace, spent coffee, rice husk, wheat straw, and many others have served similar purposes.[ 471 , 472 ] The interplay between the carbon source (FLW), pretreatments, and microbes have led to a wide variety of biodegradable polyesters with controllable chain length and copolymeric sequences, such as poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate) (PHBV).
4. Bulk FLW as Source of Advanced Bioplastics
While most studies related to the utilization of FLW for material development use isolated fractions, bulk FLW have also been considered (including bagasse, pomaces, peels/skins, seeds etc.), e.g., without isolation/extraction steps and usually in the form of micronized powders. Since bulk FLW (in contrast to isolated fractions) are inherently variable in composition, it has been mostly used for packaging films, whose basic requirements are related to processability, tensile and barrier properties, as well as safety, especially when used as biomaterials or primary (direct contact) packaging for foods, beverages, and drugs (Figure 10 ).
Figure 10.

Utilization of bulk FLW for the production of bioplastics: a) FLW components and their advantages/disadvantages in film formation. b) Films formed from bulk FLW by processing b1) carrots, b2) parsley, b3) radicchio, and b4) cauliflower (Adapted with permission.[ 485 ] Copyright 2018, The Royal Society of Chemistry).
The use of bulk FLW is closely associated with the integral use of biomass, although there are some key challenges, which need to be overcome. The large varietal, seasonal as well as regional changes in FLW composition make it difficult to accurately predict the final properties of the obtained materials. Dissolving some raw materials in common solvents, particularly lignocellulosics, may be another challenge. Solvents capable of co‐solubilizing given fractions have been used, such as dimethyl sulfoxide (DMSO)‐based solvents and ionic liquids.[ 473 ] Trifluoroacetic acid (TFA), another powerful solvent, is an organic acid recyclable by distillation, capable of co‐solubilizing cellulose and other organic matter from plant‐based residues.[ 474 , 475 ] TFA disrupts hydrogen as well as covalent bonds, induces partial cellulose depolymerization, and reduces its crystallinity, besides hydrolyzing ester linkages in the lignin structure.[ 474 , 476 ] The formation of cohesive structures from such systems may be hindered by the tendency of self‐cracking upon drying, which may result from strong self‐interactions of lignin and hemicellulose (especially when they are in excess, hindering miscibility with other components).[ 477 , 478 ] For example, films prepared from SCB dissolved in DMSO/LiCl showed cracking, which was associated with hemicellulose structure and composition.[ 473 ] The main hemicelluloses in SCB are arabinoxylans, whose film‐forming capability depends on their structure: a high Xyl/Ara ratio, compared to a rather linear xylan chain structure, contributes to the mechanical properties of films,[ 155 , 479 ] while the arabinan side branches are important to prevent intermolecular xylan chain aggregation/crystallization, which might impair film formation.[ 155 ] Arabinoxylans with less arabinose are more likely to aggregate and become insoluble.[ 480 ] Additionally, lignin and glucan may also be helpful to prevent self‐aggregation of xylans,[ 155 ] although an excessive lignin content may hinder film formation.[ 479 ] Hemicellulose extraction with high alkali concentration leads to a low degree of substitution (≈0.10–0.15) and removal of acetyl groups, lignin, and glucose, resulting in cracks.[ 155 ] The cracks in SCB films have been avoided by replacing water with a solvent comprising acetone:water (9:1, v:v) in the coagulation bath or by freezing‐induced crosslinking of the wet film prior to drying.[ 473 ] Although most FLW contain film‐forming components (usually polysaccharides and/or proteins), some of them lead to a poor tensile strength (compared to the typical tensile strength of low‐density polyethylene, polypropylene, and PET are 10, 21–40, and 50–80 MPa, respectively).[ 481 ] One example is the mixed fruit‐vegetable waste flour with high polysaccharide concentration and non‐film forming components, such as mono‐ and disaccharides as well as acids that impair tensile properties.[ 482 ] Such limitation may be compensated through the addition of supporting film‐forming components, such as hydroxypropyl methylcellulose (HPMC) and microfluidized cellulosic fibers (reinforcement fillers) which have been tested with carrot processing waste.[ 483 ] While carrot waste alone was not able to form a cohesive self‐standing film, an optimal formulation (with 33/14/53 carrot waste/HPMC/cellulose fiber weight ratio) yielded a biocomposite film with suitable properties for application in food packaging (tensile strength of 30 MPa, modulus of 2 GPa), although with a relatively low elongation (2.8%). Pomelo peel flour (containing 33% pectin) was added with sodium alginate (at 5 wt% on PPF),[ 484 ] to produce films with tensile strength of up to 18.5 MPa and elongation 12–15%. Finally, because bulk FLW are heterogeneous as far as the components, which likely present different optical properties (refractive index and light absorption/scattering), they usually produce opaque rather than transparent materials. This might be undesirable for applications in which the product requires visualization by the consumers. On the contrary, the same composition might be suitable in materials that are susceptible to UV‐triggered oxidation.
The utilization of bulk FLW is economical and offers environmental advantages. The first (and more obvious) one is that the yields are higher (nothing is extracted), and less residues are generated. Moreover, no chemical extraction is involved, and the use of harsh chemicals is avoided (or at least minimized), implying a lower processing time, reduced costs, and lower environmental impacts. Finally, some active components present in FLW compositions (such as phenolic compounds) can be explored to endow the resulting materials with active properties. As an example, radicchio waste films exhibited high antioxidant activity (more than 90% inhibition of ABTS radical), which was ascribed to the high anthocyanin content.[ 485 ]
Some FLW powders have been used for film preparation, e.g., by simple processing involving only physical steps such as water dispersion, heating, and separation techniques, such as filtration and/or centrifugation to remove larger particles. This process was used for a mixed fruit‐vegetable flour.[ 482 ] An ultrasound treatment to potato peel and sweet lime pomace powders was reported to enhance the tensile properties of the resulting films, namely, by breaking down biopolymer particles, making the film structures more uniform.[ 135 ] In other studies, different chemical pretreatments have been applied to FLW. Some of them are described below:
Ammonium hydroxide and orthophosphoric acid were used to reduce interactions between polymer chains in mixed fruit‐vegetable powder, enabling the formation of cohesive films.[ 486 ] The material itself presented a reasonable elongation (≈30%) but poor tensile strength (27 kPa) and modulus (3 kPa), which made them unsuitable for an application as a self‐standing film (Table 2 ). On the other hand, its use as a coating for fresh‐cut carrots was shown to be promising in reducing weight loss (due to its water vapor barrier, reducing dehydration on storage), and preventing whitening in the produce.
Trifluoroacetic acid (TFA) at 3% was applied in aged cocoa pod husks as well as parsley and spinach waste for times ranging from 3 d to 2 weeks.[ 474 ] The suspensions were then centrifuged and cast into films with various tensile properties (Table 2). For instance, those from cocoa pod husks were the strongest/stiffest (tensile strength 30 MPa, modulus ≈1.1 GPa, elongation 10%), which was ascribed to their high cellulose and triglyceride contents (the triglycerides being esters, biopolymer precursors), while those from rice hulls (tensile strength 7 MPa, modulus ≈0.85 GPa) were quite brittle (elongation 3%) due to the high silica content. Those from spinach and parsley stems (tensile strength of 1 and 5 MPa respectively, both with modulus ≈0.1 GPa) yielded the highest elongation (60% and 45%, respectively). It was suggested that a wide range of tensile properties could be achieved by combing these FLW at different ratios.
Immersion in a 5% HCl solution at 40 °C for 12 h was used to partially hydrolyze the amorphous regions of cellulose of vegetable waste powders (from carrot, cauliflower, parsley, and radicchio).[ 485 ] After subsequent dialysis, the dispersions were cast and dried to produce films. Carrot waste films, which contained the highest cellulose concentration, presented the highest tensile strength (38 MPa) and modulus (1.3 GPa). Interestingly, HCl‐treated carrot waste did not require the addition of supporting film‐forming components as in a previous study with untreated carrot waste.[ 483 ] The HCl pretreatment was suggested as a more eco‐friendly alternative[ 485 ] compared to TFA treatment.[ 474 ] Unfortunately, since the starting materials were not the same in these studies, any comparison of the effect of treatment on the film properties would only be but speculative;
Dewaxing and fractionation: Although a bulk FLW was not actually used, rice straw was explored as whole precursor following an alkaline treatment for dewaxing.[ 140 ] Thereby, a cellulose‐rich solid phase and a hemicellulose/lignin‐rich aqueous solution were separated, and the latter was used as a film matrix, while the former was used as a reinforcement (with sorbitol as plasticizer). The addition of the cellulose‐rich phase at 30 wt% on the hemicellulose/lignin matrix increased both tensile strength (from 6.4 to 23.4 MPa) and modulus (from 0.17 to 0.70 GPa), while the elongation was kept at ≈11–12%.
Table 2.
Composition and mechanical properties (σ, tensile strength; ε, elongation at break; E, Young's modulus) of bioplastics produced from bulk FLW
| FLW material | Additional components a) | Processing b) | Tensile properties | Refs. |
|---|---|---|---|---|
| Mixed fruit‐vegetable flour | – | Casting | σ = 0.27 MPa; ε ≈ 30%; E = 3 kPa | [ 482 ] |
| Apple pomace | Glycerol (7%) | Casting | σ = 3.3–16.5 MPa; ε = 11– 55% | [ 65 ] |
| Glycerol (43%) | Compression molding (8 MPa, 20 min, 100 °C) | σ = 3.0–5.8 MPa; ε = 0.9– 1.5%; E = 0.4–0.6 GPa | ||
| Carrot waste | HPMC (optimum: 42%), cellulose fibers (optimum: 160%) | Casting (bench mode) | σ = 30 MPa; ε = 2.8%; E = 2.0 GPa | [ 483 ] |
| Casting (continuous mode) | σ = 7 MPa; ε = 0.6%; E = 1.6 GPa | |||
| – | Casting (in a HCl 5% solution) | σ = 38 MPa; ε < 6%; E = 1.3 GPa | [ 485 ] | |
| Cocoa pod husk | – | Casting (in TFA) | σ = 30 MPa; ε = 10%; E ≈ 1.1 GPa | [ 474 ] |
| Parsley stems | – | Casting (in TFA) | σ = 5 MPa; ε = 45%; E ≈ 0.1 GPa | [ 474 ] |
| Pomelo peel | Alginate (5%), glycerol (17%), tea polyphenols (0–20%) | Casting | σ = 14.5–18.5 MPa; ε = 12– 15% | [ 484 ] |
| Potato peel | Glycerol (30%) | Casting (after high‐pressure homogenization at 138 MPa) | σ ≈10 MPa; ε = 5–7%; E ≈ 0.4 GPa | [ 489 ] |
| Rice hulls | – | Casting (in TFA) | σ = 7 MPa; ε = 3%; E ≈ 0.85 GPa | [ 474 ] |
| Rice straw: hemicellulose/lignin matrix (HL) and CNF reinforcement (0–30% on HL) | Sorbitol (30% on HL) | Casting | σ = 6.4–23.4 MPa; ε = 10– 12%; E = 0.17–0.70 GPa | [ 140 ] |
| Spinach stems | – | Casting (in TFA) | σ = 1 MPa; ε = 60%; E < 0.1 GPa | [ 474 ] |
| Tea waste (spent tea leaves) | Citric acid (6% on tea waste) | Casting | σ = 6.2 MPa; ε = 13% | [ 490 ] |
Content of additional components provided as weight % on a dry FLW basis
Unless otherwise stated, “casting” refers to bench casting using water as a solvent.
Bulk waste may also be used as additives in the production of other composite films. For instance, wheat bran (at 2.7 wt% on starch) promoted an increase in the T g of the glycerol‐rich phase (from ≈‐60 to ≈–55 °C) when added as filler to cassava starch films. It also broadened and decreased the intensity of this transition, which suggested an improved glycerol dispersion. With an increase in wheat bran content, the storage modulus increased, the loss tangent decreased, and the water vapor permeability of the composites decreased by up to ≈70%.[ 487 ] The formation of related composite materials is especially appealing since the global average price of wheat bran is much lower than that of cassava starch (2020 prices of US$ 0.13–0.20 kg−1 and 0.26–0.42 kg−1, respectively).[ 488 ]
FLW may be added with non‐film forming components (such as glycerol[ 484 ] or sorbitol[ 140 ] as plasticizers and CaCl2 as crosslinker to pectin[ 135 ]), which may further enhance the physical properties of the final material. Other nonfilm forming components may incorporate active properties, such as tea polyphenols, as has been also exemplified by the antioxidant and antibacterial properties of pomelo peel flour/alginate films.[ 484 ] However, since most FLW (especially plant‐derived ones) are sources of phenolics and terpenoids (which are produced by plants as secondary metabolites with biological activity), their use as matrices may result in associated benefits. This makes the bulk‐waste approach especially interesting for a myriad of applications, for example, in the active packaging of products (especially foods) susceptible to microbial deterioration and/or oxidative changes.
5. Processing of Advanced Bioplastics from FLW
A major subject of research concerns the transformation of long‐lasting products from the agrifood chain, for example, to develop bioplastic‐based materials. In such cases, the manufacturing pathway is crucial and plays a role in virtually all properties of the resulting materials, which should also consider trade‐offs between performance and economic viability. The use of underexploited FLW or low‐value by‐products for bioplastic production replacing expensive, high‐purity fermentation media (see Section 3.4) is envisaged to make the manufacturing process to weight more heavily than the raw materials themselves, at least as far as total product costs. Additionally, the processing ought to be as clean as possible (ideally as clean as the biobased starting material) for the product to bear a minimum environmental footprint. An optimum cost‐performance‐sustainability balance in bioplastics produced from FLW is necessary through the processing‐related features, as discussed in this section.
Most of the current polymer processing facilities rely upon heating to both compound and shape objects in the molten state. Any attempts to switch from fossil‐based plastics to bioplastics would benefit from using the same infrastructure, namely, by reducing the capital costs. Compared to conventional plastics, nevertheless, most FLW‐derived feedstocks have limited processability and will undergo degradation prior to melting, as is the case for most polysaccharides and proteins. Thermoplastic starch (TPS), however, is one of the few exceptions as it is widely processed via screw extrusion, once its native granular structuring is disrupted thermomechanically after plasticization.[ 491 ] The same applies to plastics synthesized from biobased monomers, which are identical (except for the age/origin of the carbon) to their fossil‐derived counterparts. Yet, consolidating bioplastics from most of the above‐discussed sources may be challenging as far as shape complexity and yield, as these are typically processed from solution or dispersion.
Bioplastics processed via wet routes typically develop from aqueous systems owing to i) sustainability concerns and ii) the inherent hydrophilicity of most biopolymers obtained from FLW. In addition to water, other solvents can originate from side streams from the agri‐food chain. d‐Limonene, introduced earlier as a bioplastic‐forming monomer, stands out as a powerful solvent. It performs better from the sustainability perspective when compared to, e.g., toluene, as indicated by the milder LCA data of the former.[ 492 ] Importantly, much is said, though generically, about the low environmental impact of naturally occurring compounds. A proper comparison with compounds posing well‐known environmental impacts (e.g., halogenated carbon solvents) should involve a rather comprehensive assessment, i.e., through Environment Health & Safety (EHS), LCA, or other suitable quantitative methodology. As a solvent, d‐limonene enables the processing of polystyrene (PS), particularly from waste expanded PS (EPS) packaging, into nano/microparticles, through an emulsification‐diffusion method (wherein an EPS solutions in d‐limonene is the dispersed phase of an emulsion having water as nonsolvent and continuous phase and a polymeric particle stabilizer),[ 493 ] and nanofibers via electrospinning[ 494 ] and solution blow spinning.[ 495 ]
In addition to the methods above, FLW‐based materials have been obtained via a wide range of techniques. The unit operations used may be as simple as drying and top‐down milling into fine powder, as demonstrated for pomegranate peel and orange pomace.[ 496 ] Interestingly, when devoid of phenolic compounds, these particles serve as adsorbents for environmentally toxic phenolics from olive mill wastewaters. For instance, apple pomace was micronized similarly and served as an antioxidant stabilizer for food Pickering emulsions.[ 497 ] The processing techniques are continually engineered to approach 0D to 3D FLW‐based materials with tailored morphologies and functionalities.
Olive pomace extract has been shaped by bottom‐up processing using supercritical‐assisted atomization with maltodextrin, also potentially coming from any starchy FLW, into 0D nanoparticles.[ 498 ] This approach is relevant as it is applicable to thermolabile compounds, such as numerous FLW components. It further allows encapsulation of active molecules (e.g., polyphenols) and prevents oxidation through the use of warm nitrogen instead of hot air, as in case of conventional spray drying. Additionally, FLW nanoparticles can be formed by solvent displacement, as is the case of the apple peel nanoparticles.[ 499 ] Ethanolic extracts were added to varying amounts of water as an antisolvent under stirring, the precipitating nanoparticles serving as antioxidant fillers in chitosan/gelatin composite films. A similar regeneration method (i.e., solubilization and controlled coagulation) led to Pickering‐stabilizing nanoparticles featuring a zein core (see Section 3.2) with a pectin shell isolated from hawthorn wine pomace.[ 500 ]
FLW are also shapeable via spinning into 1D fiber‐like constructs, i.e., diameter/width (from nm to µm) much smaller than length. For instance, FLW‐derived building blocks have been forced to flow through one or multiple spinnerets, mostly in dispersion or solution but also, if applicable, in the molten state (melt spinning). Single or multiple filaments can be fabricated depending on the system setup, most of which operate as continuous and scalable processes. In the case of bio‐based single yarns, wet and dry spinning have been used, wherein consolidation is driven by solvent (or dispersant) removal either by replacement by a poor solvent in a coagulation bath (wet process, Figure 11a 1) or by drying (dry process, Figure 11 a2). Importantly, in these processes, the flow restriction imposed at the spinneret is likely to orient the macromolecular chains parallel to the flow direction. This will largely depend on the process conditions (markedly flow rate, spinneret dimensions, dopant viscosity, among others) and, if retained in the resulting filaments, the orientation will have a pronounced positive impact on the mechanical performance.[ 501 ]
Figure 11.

Emerging processing of 1D, 2D‐, and 3D FLW‐based bioplastics. Generic scheme of a1) wet and a2) dry spinning, wherein the solvent or FLW suspending medium is exchanged with a nonsolvent in a coagulation bath or is evaporated, respectively (Adapted under the terms of the CC BY 4.0 International license.[ 528 ] Copyright 2017, American Chemical Society). b1) Solution blow spinning apparatus used to fabricate FLW nanofibrous mats following high‐throughput, gas‐driven spinning (Adapted with permission.[ 507 ] Copyright 2020, American Chemical Society). b2) A tape casting apparatus is shown to continuously produce films by knife‐coating a FLW‐containing slurry onto a conveying substrate followed by the fast removal of the solvent or suspension medium (Adapted with permission.[ 529 ] Copyright 2015, The Authors). c1) Coaxial setup used to 3D‐print FLW‐containing inks with induced gelation by an added crosslinker (Adapted under the terms of the CC BY 3.0 license.[ 530 ] Copyright 2018, IOP Publishing Ltd). c2) Biofabrication of 3D bacterial cellulose nanofibrils (BCNF) by using superhydrophobic PTFE particles, supplying air to the bacteria within a FLW‐containing medium, followed by purification (Adapted with permission.[ 435 ] Copyright 2018, The Royal Society of Chemistry).
Although low‐ and high‐aspect ratio—0D and 1D—constructs have unique features owing to their remarkably high SSA, when it comes to applications as bioplastics, they often require a ligand or binder for an improved cohesiveness. In low‐aspect ratio building blocks, cohesion is achievable with FLW, physically, when combined with high‐aspect ratio biocolloidal binders,[ 266 ] when embedded within a bioplastic‐forming polymer matrix,[ 502 ] or when chemically treated, for example, by crosslinking (e.g., with genipin derived from the peel or unripe fruits of Genipa americana or with citric or tannic acids). Welding through sintering has been also used with FLW that is infusible while heat sensitive. This is similar to synthetic bioplastics that can melt or joint by welding techniques, e.g., ultrasonic, vibration, spin, and laser welding. Otherwise, solvent welding is a good choice to partially solubilize the surface of biocolloids that are then fused, while preserving their native cores and inherent multi‐level assembly, which are features that are important for their performance.[ 503 , 504 ] These fusing strategies for assembled building blocks can be extended to higher‐order constructs, e.g., for bioplastic sealing.
Adhesive bonding is a straightforward means for joining bulky materials. Notably, anisotropic biocolloidal building blocks, suitable in the production of bioplastics, for instance, CNC (potentially extracted from FLW, see Section 3.1.1), have been demonstrated to provide outstanding in‐plane adhesiveness between solid surfaces. This is owing to their confined evaporation‐induced nematic assembly and resulting superstructuring.[ 505 ] In addition to crosslinkers and adhesives, various other property modifiers originate from FLW, including polyol plasticizers (e.g., glycerol, sorbitol, and xylitol) as well as lipids (e.g., soybean and linseed oils, and some fatty acids).[ 506 ]
As far as surface area and mechanical robustness, an interesting observation involves the interplay between the high SSA of nanofibers and the physical cohesion of entangled networks, in which supramolecular interactions also participate. Differently from single filaments, high throughput spinning techniques are efficient in producing 2D nanofibrous mats. Among these processes, electrospinning has been reviewed in the context of (bio)polymer nanofibers, together with other spinning techniques.[ 507 ] Electrospinning of FLW components has been used to produce fibrous mats from rice husk,[ 508 ] okara and oil palm trunk and frond,[ 509 ] corncob and wheat straw,[ 510 ] PHBV resources from cheese whey, olive oil mill wastewater, products of fermentation of fruits,[ 511 , 512 ] and nanochitin.[ 245 ] Electrospinning and its variants involve high‐voltage power and require an electrically conductive collector; thus, other spinning techniques are more versatile, portable, and cheaper. Among these, solution blow spinning (Figure 11b1), for instance, allows the development of fiber mats with similar dimensions and morphologies to those produced by electrospinning, but often at higher yields and by using a wide variety of substrates, including biological tissues.[ 507 ] Solution blow spinning allows processing materials with low dielectric constant and/or electrical conductivity, which is typical of FLW. This is feasible because the process relies on a pressurized gas to enable nanofiber formation from polymer solutions, as opposed to the needed high voltage potential typical of electrospinning. Solution blow spinning has been used to produce nanofibrous mats from waste EPS using a FLW‐derived solvent comprising 97% d‐limonene.[ 495 ] The variables affecting yield, morphology, alignment, and others are many, but include those associated with the spinning solution (e.g., solvent type, surface tension, solid content, and MW), the setup (e.g., nozzle geometry, nozzle‐to‐collector distance, and collecting surface), processing parameters (e.g., feeding rate and gas pressure), and environmental conditions (e.g., temperature and relative humidity), as detailed elsewhere.[ 507 ]
While high‐SSA fibrous mats are suitable for a range of advanced applications, from (bio)sensing[ 513 , 514 , 515 ] to regenerative medicine,[ 507 , 516 ] the performance as barriers is rather poor. Continuous 2D (monolithic) films analogs to fibrous mats can be considered for the latter purpose, wherein no apparent empty voids exist but rather a solid material structure. Films are by far the most investigated form of FLW bioplastics. From the barrier standpoint and for a given material, films perform better than fibrous mats. On the other hand, solid films display a reduced SSA, which may impair applications that rely on sorption phenomena. As far as processing, fusible FLW‐based polymers are typically shaped in the molten state via melt blowing, flat‐die co‐extrusion, or calendering. As discussed previously, most FLW‐derived polymers do not melt without extensive thermal degradation and are then transformed into films from solution or suspensions, following casting or filtration. Alternative processes include spin coating and compression molding, among others. Abundant literature is available describing bench‐scale systems used to cast FLW films by placing a film‐forming solution or dispersion onto a given mold, allowing the solvent or dispersant to evaporate, followed by the removal of the dry film, which is facilitated if a non‐interacting casting surface is used.[ 502 ] This approach is quite difficult to scale up and several efforts exist to produce continuous casting approaches, for example, by combining elements of tape casting (knife coating, doctor blading) and infrared‐accelerated and convective drying (Figure 11b2).[ 517 ] As an example, a small‐scale continuous lamination setup easily produced 1.56 m2 h−1 of films from carrot processing waste.[ 483 ] The main difference among the different processes is the required operational area, as the continuous apparatus would occupy 4 m2, while bench casting would require at least 37.4 m2 for the same daily yield. To double the production, the area needed would need to be increased by 27.5 and 100% under the continuous and bench‐scale modes, respectively. Overall, upscaling makes bench casting unpractical in pilot and industrial settings. The energy input, in turn, is highly dependent on the material (e.g., convective coefficients of heat and mass transfer and shrinkage kinetics) and on the process (e.g., time, temperature, and wet layer thickness).[ 518 ] Besides carrot's waste, other FLW‐based films have been produced, including yellow passionfruit rind.[ 519 ] Similarly to spinning techniques,[ 520 ] though to a lesser extent, continuous casting has been demonstrated to induce alignment and mechanical anisotropy in CNF films.[ 521 ]
3D bioplastic objects can be obtained from thermoplastic FLW by injection molding and thermoforming; however, additive manufacturing has emerged as a most promising route. While the unit operations involved in classic processing are being combined with advanced additive manufacturing, such as melt electrowriting,[ 522 ] 3D printing is becoming increasingly popular and has been used to shape FLW materials, including hemicelluloses from corncob.[ 523 ] Given its relatively low melting point, good printability, and shape fidelity, PLA from FLW‐derived lactic acid (Section 3.3.3) is often reported for the manufacture of 3D products. However, 3D shaping is not limited to thermoplastic source, since FLW can be also processed from solution or multiphase systems (foams, emulsions, and dispersions) via direct ink writing, extrusion, biofabrication, and foaming. FLW‐containing inks can be 3D‐printed either as shear‐thinning gels (which easily flow upon extrusion and “solidify” under no shear) that further crosslink upon chemical or physical treatments (Figure 11 c1). Biofabrication using BC and FLW as carbon source was already discussed in Section 3.4.1. Because it is produced under aerobic conditions, as a pellicle accumulating at the interface of the FLW‐containing medium and air, BC has long been exploited as pseudo‐2D hydrogels or films. Recently, superhydrophobic surfaces have been introduced to template or guide the biofabrication process to produce 3D morphologies, following the concepts of liquid marbles and plastrons (Figure 11 c2).[ 435 , 436 ] These strategies have been combined with 3D printing, within a PTFE microparticle bed, an ink containing both BC‐producing bacteria and nutrients needed for their metabolism.[ 438 ] This approach can be taken as a 4D printing method leading to constructs that over time go through changes in composition, properties, and shape. Finally, highly porous foams and light‐weight materials such as aerogels, xerogels, and cryogels represent an important class of 3D FLW‐based materials. They are suitable for application as filters, adsorbents, insulators, scaffolds, among others. Such systems are produced by drying (supercritical, oven, and freeze casting) as well as sacrificial and porogen templating, as summarized previously in the literature.[ 524 , 525 ] Notably, fibers from pineapple leaves were converted into highly adsorbent aerogels.[ 127 ] Scaffolds for cell culturing and biosensing were produced from decellularized vegetable waste such as broccoli stalk[ 526 ] and other decellularized plants.[ 527 ]
6. Emerging Applications of FLW Bioplastics
The last decade has witnessed a fast development in the area of biodegradable and/or bio‐based materials as precursors for electronic devices, sensors, actuators, and flexible electronics, among others.[ 531 , 532 , 533 , 534 , 535 , 536 ] This is mainly because such materials can reduce the waste streams associated with recycling or disposal of devices along with the minimization of health risks, e.g., by avoiding hazardous waste streams typical of conventional routes. In this context, the term “transient electronics” has been employed, which refers to electronic materials, components, and circuits that dissolve or decompose by hydrolysis or composting in given environments in a programmed fashion, as is the case for some biodegradable bioplastics.[ 536 ] For instance, the use of paper‐based sensors[ 533 , 537 , 538 , 539 , 540 , 541 , 542 ] using low‐cost platforms has gained popularity, once they can be integrated with microfluidics and lab‐on‐a‐chip devices, associated with low‐cost production and scalability, with potential to decentralize and disseminate analytical procedures for food, environmental, and medical sensing. Specifically, biobased flexible sensors have gained a great impact due to their potential for application in biomedical devices, e.g., to monitor health‐related parameters including glucose levels in blood, lactate, Na+ and K+, as well as pH, and electrical conductivity changes caused by disease‐specific metabolic processes.[ 534 , 543 , 544 , 545 , 546 , 547 , 548 ]
FLW‐based materials have been successfully employed as proof‐of‐concept in electronic devices, sensors, organic electronics, and slow‐release systems, among others. Special attention has been given to those employing nanocellulose and related by‐products (Section 3.1.1), given the advantageous properties of nanocellulose‐based materials, namely, high tensile strength, biocompatibility, biodegradability in soil, film‐forming ability, and flexibility.[ 549 ] Altogether, these features render cellulose highly suitable for designing flexible and wearable sensors and electronic devices,[ 550 , 551 , 552 ] in addition to membrane hydrogels or functional thin films.
SCB is an example of FLW successfully employed in the fabrication of chemical sensors. Specifically, SCB residues were combined with a carbon paste electrode to develop an electrochemical sensor for detecting toxic heavy metals (Pb and Cd) in the concentration ranges of 100–600 and 500–1200 µg L−1, yielding limits of detection of 10 and 171 µg L−1, respectively.[ 553 ] An interesting approach was proposed combining cellulose extracted from banana stem residue with carboxylated multiwalled carbon nanotubes to produce conductive composites.[ 554 ] The flexible and low‐cost electrode was successfully employed in methanol sensing. Other types of chemical sensors have been developed using side streams from seafood. For instance, nanochitin extracted from squid pens (Section 3.1.2) has been composited with zinc oxide nanoparticles and polyaniline into disposable and flexible sensors for selective monitoring of ethanol for a detection limit of only 17 ppm.[ 555 ]
FLW can also be a rich and abundant source of carbon for designing active materials for sensors and devices with suitable optical and electrical properties. For instance, due to their strong photoluminescence, carbon dots (CDs) (quasi‐spherical carbon nanoparticles with diameters less than 10 nm) and graphene quantum dots (GQDs) (small fragments of graphene with lateral dimensions smaller than 100 nm in a single or a few layers) have shown a great potential for fabricating biopolymer‐based devices and chemical sensors, and both (CDs and GQD) can be obtained from various waste, including FLW.[ 556 , 557 , 558 , 559 ] In this direction, the synthesis of CDs (Section 3.1.3) using leftovers from cat feedstock and the sandwich was reported[ 274 , 276 ] in which the N‐doped core and multifunctional groups on the surface of the CDs resulted in strong fluorescence and high quantum yield, 28% (Figure 12 1a). Moreover, Fe3+ could quench the fluorescence of FLW‐driven CDs selectively, with no interference of other metal ions, indicating that FLW can indeed be a source of high‐value bioproducts to be used in optochemical sensors (Figure 12a) as well as in cell imaging (Figure 12b).
Figure 12.
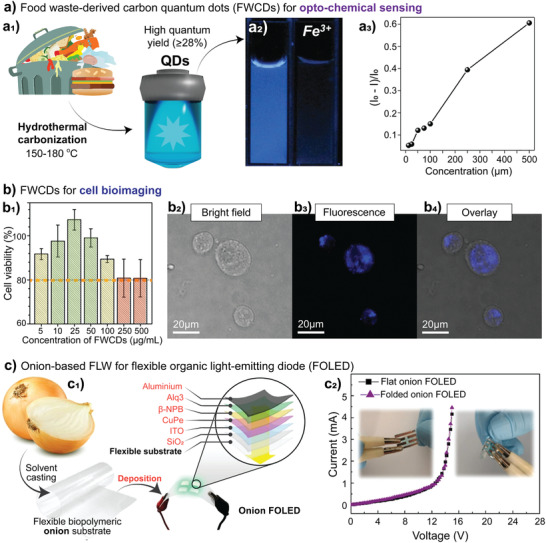
Emerging FLW applications: a) An optical chemical sensor from a1) FLW‐derived carbon dots (FWCD) suspended in distilled water and in Fe3+ solution (indicated by Fe3+) a2) irradiated with UV light at 365 nm. a3) Stern‐Volmer plot of FWCD solution at various Fe3+ concentrations (Adapted with permission.[ 274 ] Copyright 2019, Elsevier B.V). b) FWCDs for cell bioimaging demonstrate the b1) viability of HCT116 cells depending on the concentration of FWCDs. Microscopic images along light sources by b2) bright field and b3) fluorescence as well as b4) merged (overlay) image. (Adapted with permission.[ 274 ] Copyright 2019, Elsevier B.V). c‐c1) Flexible organic light‐emitting diode (FOLED) fabricated by solvent casting on top of an onion substrate and its c2) current density as a function of applied voltage in the flat and folded states (Adapted with permission.[ 560 ] Copyright 2019, American Chemical Society).
Bread waste has been employed as starting material to produce high‐quality, single‐layer graphene, at a much lower price compared to standard commercial counterparts,[ 559 ] demonstrating a high potential to upcycle FLW into advanced nanomaterials for sensing application. However, not only pristine graphene can be obtained from FLW, but also graphene‐based composites. For instance, a nitrogen and sulfur codoped aminated lignosulfonate/GQDs composite was directly synthesized using citric acid monohydrate and by‐products of sulfite‐pulping (sodium lignosulfonate), yielding a GQD‐based composite of superior chemical activity and photoluminescence.[ 561 ] Such features enabled this type of material to be used in silver ion sensing (concentration range from 0.005 × 10−6 to 500 × 10−6 m). Additionally, the material presented low toxicity and showed to be biocompatible with adenocarcinomic human alveolar basal epithelial cells (A549 cells), and therefore, could be potentially useful to image such cells.
Fruit and vegetable waste has been employed in the design of several types of sensors. For instance, grape stalk was proposed as a reducing agent and stabilizer in the synthesis of metallic nanoparticles, which were then used in the fabrication of screen‐printed electrodes for the simultaneous voltammetric determinations of Pb(II) and Cd(II).[ 562 ] More recently, screen‐printed electrodes were fabricated onto BC,[ 563 , 564 , 565 ] with advantageous features to be used as wearable sensors, including biocompatibility and superior mechanical resistance. Although in these cases BC was not directly obtained from food waste,[ 563 , 564 , 565 ] its production via fermentation of FLW (Section 3.4.1) could become a viable option to decrease the cost and allow scalability of such screen‐printed electrodes for sensors. Advances in this direction are expected in the near future.
In addition to sensors, vegetable parts and biomass residues can be employed to design organic electronic devices. In this context, a green and ecological biosubstrate fabricated with onion pulp (Allium cepa L.) was employed to develop flexible organic light‐emitting diodes (FOLEDs).[ 560 ] For this purpose, the onion substrates were produced using a simple, rapid, and highly reproducible solvent casting method (see Section 5). Indium tin oxide (ITO) and SiO2 thin films were deposited onto the onion‐based biosubstrates to obtain conductive, yet flexible, transparent anodes, on top of which FOLED was produced, as schematically illustrated in Figure 12 c1. The as‐developed flexible substrate displayed optical transparency, while optimization of ITO films allowed suitable electrical properties for FOLED fabrication using copper(II) phthalocyanine, N,N‐di(naphthalen‐2‐yl)‐N,N‐diphenyl‐benzine (β‐NPB), and tris(8‐hydroxyquinolinato)aluminum as organic layers, exhibiting a maximum luminance of about 2062 cd m−2 at 16.6 V. Additionally, flexibility tests with the onion FOLED and current–voltage (I–V) measurements were performed for both the flat and folded states (over a 3 mm diameter cylinder), as shown in Figure 12 c2. Only a very small difference between the I−V curves was observed before and after folding, indicating that the device operated reliably even if folded, which in turn suggests the potential of using vegetable parts for optoelectronic devices. FLW (and in this specific case, onion waste) can be potentially applied in such devices as well, provided the elementary building blocks are identical (or at least similar) in composition to the non‐waste counterparts.
Starch (Section 3.2.1) from FLW is a biopolymer with promise in designing biodegradable flexible substrates for organic electronics (Figure 13a). Ecofriendly and biodegradable starch paper was formed by starch gelatinization using PVA to improve its mechanical properties.[ 295 ] The biobased paper presented remarkable stability under cyclic operation, and resisted nonpolar solvents, which is a key factor for the manufacture of organic electronics that use processes based on nonpolar media. As a proof of concept, flexible organic transistors were fabricated from pentacene, dinaphtho[2,3‐b:2′,3′‐f]thieno[3,2‐b]thiophene, and poly(dimethyltriarylamine) and using both vacuum and solution processes (Figure 13 a1). The starch‐based paper was tested for biodegradability with fungi for up to 24 d (Figure 13 a2).
Figure 13.
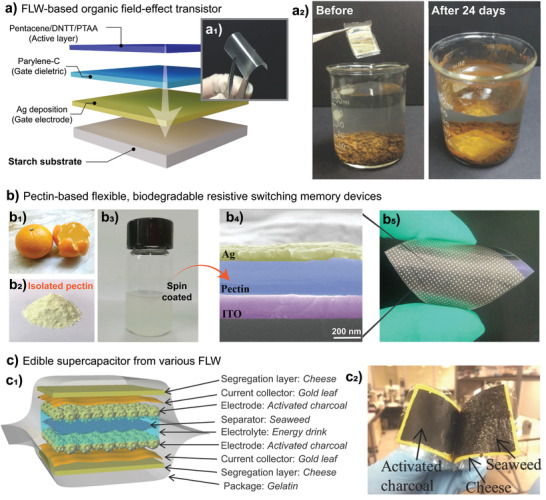
Emerging FLW application: Biodegradable and flexible organic field‐effect transistor (OFET) using starch papers. a) Schematic diagram showing the OFET fabrication process and a1) image of the flexible starch paper prepared with added PVA. a2) Results from the biodegradability tests conducted by immersing starch paper in fishbowl water before and after 24 d (Adapted with permission.[ 295 ] Copyright 2017, John Wiley & Sons). b) Biodegradable resistive switching memory based on b1) orange peel‐derived b2) pectin and b3) spin‐casting solution deposited onto b4) ITO and leading to the b5) Ag/pectin/ITO memory device (Adapted with permission.[ 342 ] Copyright 2018, John Wiley & Sons). c) Edible supercapacitors: c1) schematic structural illustration and c2) actual opened supercapacitor displaying the activated charcoal electrode, seaweed separator, cheese segregation layer, and gelatin package (Adapted with permission.[ 566 ] Copyright 2016, John Wiley & Sons).
Pectin (Section 3.2.1) extracted from natural orange peel was processed into transient electronics, specifically, resistive switching memory devices (Ag/pectin/indium tin oxides, Figure 13b). The as‐fabricated device operated at low voltages (≈1.1 V), fast switching rates (<70 ns) and long retention time (>100 s). Additionally, although being composed by pectin, the device performance was maintained after more than 100 bending cycles (Figure 13 b5), which is a crucial feature for the intended application. Additionally, owing to the good solubility of pectin, which bears abundant carboxylic groups, the flexible memory device could be dissolved under mild conditions in deionized water (10 min).
Chiral biopolymers, including those derived from FLW, are suitable for designing photonic devices. As an example, a hierarchical nanocellulose structure was produced with a helicoidal organization assembled with chiral rigid CNC and longer CNF (Section 3.1.1) isolated from hydrolyzed wood pulp.[ 567 ] The chiral nanocellulose material presented high iridescence and good mechanical properties, enabling a novel strategy to transform wood pulp residues into high‐value‐added polysaccharide materials for photonic applications. The same potential is valid for CNC extracted from other sources (Section 3.1.1), not originally intended for photonic applications.[ 101 , 281 , 568 ]
Supercapacitors are devices that store energy and are used in the fabrication of batteries and energy harvesting devices, e.g., to produce efficient, continuous, reliable, and affordable energy supply.[ 569 , 570 , 571 ] Traditional supercapacitors are usually based on heavy metals that can be poisonous and cause hazardous effects to human health and the environment. Such adverse effects raise concerns in the case of electronic devices that are implanted or administered orally to monitor cellular and organ functions or for drug delivery. To overcome this limitation, innovative solutions relying on edible supercapacitors have been developed.[ 566 ] For example, Figure 13 c1 shows a schematic structural illustration of an edible supercapacitor based on activated charcoal that was used as the electrode, cheese as the segregation layer, seaweed as the separator, isotonic beverage as polyelectrolyte, gold leaf as current collector, and gelatin (Section 3.2.2) as a package. Figure 13 c2 displays an opened supercapacitor and highlights its components. The edible supercapacitor inactivated E. coli in in vitro experiments, indicating its potential in the treatment of foodborne bacterial diseases. Such supercapacitor could power a commercial USB camera, prompting the use of such devices for ingestible biomedical products. In addition to food, FLW materials can be employed for designing nonedible supercapacitors. For instance,[ 572 ] soybean shell, a major by‐product of soybeans (Section 3.2), was successfully converted into porous carbon with high SSA via hydrothermal carbonization, whose porosity was beneficial for KOH activation. The high SSA porous carbon (2523 m2 g−1) reached a specific capacitance of 301 F g−1 in 6 m KOH electrolyte at a current density of 0.1 A g−1. Considering the good stability and suitable energy density of these carbons, this type of FLW is a promising electrode material for electric double‐layer capacitors.
Another remarkable advance entails rechargeable Zn–MnO2 batteries that were prepared from biomass waste (reed straw, found in the ocean).[ 573 ] The obtained 3D electrodes exhibited a honeycomb‐like cellular structure with open channels of varied features. Such high SSA and complex channel structure allowed a high MnO2 loading combined with low‐resistance pathways that are favorable for electrolyte diffusion and ion transport. As a result, the fabricated battery enabled a capacity of 370 mAh g−1 at low MnO2 content, as well as improved cycling stability compared to standard batteries of this type.[ 573 ] The battery was operated at high temperature, from –20 to 100 °C. It was also integrated with a flexible solar cell to yield a self‐sustained power bar, demonstrating that biomass can be a sustainable alternative to conventional batteries at much lower cost. Another exciting development was the use of waste pomegranate peels to recover pectin (Section 3.2) and punicalagin (a rich source of phenolic compound), the leftovers of which were then carbonized via pyrolysis in presence of potassium hydroxide and heating, yielding hard carbon (nongraphitizable carbon).[ 574 ] The obtained carbon was then successfully tested as electrode in a sodium battery, providing a constant charge–discharge cycle performance of 180 mAh g−1 at a C/2 rate and 100% columbic efficiency after 100 cycles.[ 574 ]
Other FLW applications include the synthesis of additives or fillers (e.g., metallic nanoparticles). These products can be combined with biobased plastics to develop smart packaging materials.[ 575 , 576 ] Aqueous extracts from watermelon rind were reported to function as both reducing and stabilizing agents for the biosynthesis of palladium nanoparticles, which presented catalytic properties suitable for industrial applications.[ 577 ] Silver nanorods were synthesized from AgNO3 in the presence of industrial milk waste, containing lactose and whey as reducing and stabilizing agents, respectively.[ 578 ] The silver nanostructures were non‐cytotoxic (to three human cell lines) and could be used to extend the milk shelf‐life by controlling microbial growth.[ 578 ] Such waste‐originated nanoparticles can be combined with biopolymers into bioplastics for smart packaging applications.
FLW have been used as precursor for the development of smart adsorption systems for pollutant remediation and slow‐release system for agricultural application. Superabsorbers fabricated from coco peat powder were modified with maleic anhydride, followed by grafting of poly(acrylic acid) and then applied in controlled release of soil nutrients, combining irrigation and fertilizer applications.[ 579 ] A superswelling biopolymer hydrogel for retention and slow‐release of water was developed through the copolymerization of acrylic acid using pulping red liquor (lignosulphonate and polysaccharide), which in principle can also be extended to FLW.[ 580 ] The waste of yerba mate plant (Ilex paraguariensis), an infusion traditionally drunk in South America, and alginate were employed as biodegradable matrix to encapsulate urea. The system functioned as a nitrogen source in soil at pH 7.5, with potential as a low‐cost and environmentally friendly fertilizer.[ 581 ]
In summary, the recent literature illustrates the many types of devices, including sensors, capacitors, active nanoparticles, and slow‐release systems, that can be fabricated by using FLW. Although most of the devices reported so far are proposed as a proof‐of‐concept exercise, in terms of fabrication, it is still of paramount importance to consider FLW as a future raw material source to fabricate added‐value devices that would address resource sufficiency and, at the same time, reduce streams associated with recycling or disposal of waste. Standardization and quality control of FLW for such applications are expected to enable scalable industrial processes.
7. Prospects of Food Waste Valorization
In closing, it is worth considering the several aspects associated with FLW utilization. First, there is a need to recognize the massive production of food side streams, posing a great negative impact on our environment, in terms of pollution, land use, and waste. Such factors will eventually add pressure in efforts to find rational use of associated streams. Hence, there is a critical demand to reduce FLW. As discussed in this review, a highly significant source of food waste is associated with fruits and vegetables, with losses of about one third of their weight. Such FLW contain high levels of vitamins, minerals, dietary fibers, and proteins, all of which can be ideally converted back into food. However, this conversion is challenging due to typical food standards, which render most FLW unsuitable. Alternatively, FLW can be processed into high‐added value materials. They include those for (food) packaging and advanced components in sensors, capacitors and drug delivery systems, among others. Due to the large and increasing volume of FLW, there is a genuine interest on the side of food producers to valorize such streams. The valorization of FLW in non‐food materials, coupled with a more efficient food production and supply chain, will ultimately be necefssary to meet the requirements of a growing human population, increasing the efficiency of food production. This can be further combined with state‐of‐the‐art nanotechnologies, as illustrated by the examples discussed about the use of renewable nanoparticles, to increase yields and improve nutritional quality. In these efforts, sustainability is a major driver to convert waste products into commercially valuable materials.
The issue of waste disposal cost should be considered. The adoption of new alternatives, different from those derived from fossil carbon, is challenged by the available processing routes and the excellent control in transforming petrochemical streams. This is specially the case considering the processes that are in place, ideally suited for such feedstock types. Thus, in the short‐term, biobased plastics can become competitive if regulations and policies favor or promote uses in addition or in substitution to those derived from petroleum sources. This can include ‘disposal costs’ to plastic waste, incorporating suitable factors to quantify sustainability as far as end‐of‐life, environmental fate, and environmental impacts. The increased bans of single‐use plastics signal an opportunity for developments in this area. The adoption of biobased alternatives, including those from FLW, can be incentivized by environmental labeling and also by promoting the use of locally available resources; however, these efforts are not sufficient unless cost‐performance factors become competitive compared to the currently available options.
For FLW to be used in their various forms, supply chains need to be in place given that current practices are designed for disposal and landfilling, both preventing the possibility for reutilization. Thus, FLW separation and use must be implemented with updated systems and practices, e.g., to manage waste disposal at the community and city levels. Such operations, beyond composting, require stream separation, which is a major challenge unless it is applied at the source. Related biomass resources need to be associated with a market value/price and their availability needs to be secured to become input streams for further processing. In such cases, the reduction of food waste, e.g., by following the optimization of food chains, might be more sustainable than reusing excess or residual biomass. The highest potential in the future is perhaps for food losses in industrial processes, such as SCB, protein‐rich waste waters, peels, and husks, among others.
There is no doubt that FLW resources are abundant and diversified and are suitable alternatives that can reduce costs compared to traditional, expensive precursors used in manufacturing. However, current inefficiencies in waste processing and the global food system need attention. FLW can play an important role in decreasing the waste streams associated with recycling or disposal of traditional electronic garbage. However, the lack of current FLW standardization, as far as quality and composition, can be an obstacle in the short term, namely, for FLW to find practical use in advanced materials. Related efforts are within reach in the mid and long term.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
D.S.C. thanks the financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grants no. 2017/12174‐4 and 2018/22214‐6), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), MCTI‐SisNano (CNPq/402.287/2013‐4), and Rede Agronano‐EMBRAPA. The authors also acknowledge funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (ERC Advanced Grant Agreement No. 788489, “BioElCell”). O.J.R. is grateful to the Canada Excellence Research Chair initiative and the Canada Foundation for Innovation (CFI).
Biographies
Caio G. Otoni is assistant professor of Materials Engineering at the Federal University of São Carlos (UFSCar, Brazil), where he also received his Ph.D. in materials science and engineering (2017) in a dual affiliation with Embrapa Instrumentation. Food engineer by training (2013; UFV, Brazil), he was a postdoctoral research fellow with Prof. Watson Loh at UNICAMP, Brazil (2017–2020), in a dual affiliation with LNNano/CNPEM, and with Prof. Orlando Rojas at Aalto University, Finland (2019). Prof. Otoni has also been with ARS/USDA (2010‐2011). His research is centered at biobased multifunctional materials from nanocelluloses and other biopolymeric colloids.

Henriette M. C. Azeredo is a researcher at the Brazilian Agricultural Research Corporation (EMBRAPA) since 2001. She worked as a visiting scientist at the ARS/WRRC/USDA (Albany, CA, USA) and the Quadram Institute (Norwich, UK). Her research is mostly focused on bionanocomposite edible and biodegradable films and coatings from food by‐products for food packaging applications. In 2019 and 2020, she was named among Clarivate Analytics highly cited researchers in the agricultural sciences category.

Bruno D. Mattos is a wood engineer (2012; UFPel, Brazil) and holds a Master (2014; UFPel, Brazil) and Ph.D. (2018; UFPR, Brazil) in materials science and engineering. Dr. Mattos has been with the University of Basque Country, Spain (2013) and Embrapa Florestas, Brazil (2014–2018). Currently, he holds a postdoctoral researcher position in the Department of Bioproducts and Biosystems at Aalto University, Finland (since 2018). Dr. Mattos’ research is devoted to wood nanotechnologies, nano‐enabled agriculture, and multifunctional materials from biocolloids.

Marco Beaumont is an Erwin Schrödinger Fellow and affiliated with University of Natural Resources and Life Sciences, Vienna (BOKU) in Austria and Queensland University of Technology in Australia. He studied chemistry at University of Freiburg (Germany) and obtained his Ph.D. degree in chemistry of renewables from BOKU University in 2017. From 2017 to 2020, he has held postdoctoral positions at Aalto University with Prof. Orlando Rojas and BOKU University with Prof. Thomas Rosenau. His current research interest is the establishment of new chemical concepts to control shape, physical properties, and surface chemistry of biocolloids.

Daniel S. Correa holds a B.Sc. in materials engineering (2004) from Federal University of São Carlos (UFSCar) and a Ph.D. in materials science and engineering (2009) from São Paulo University (USP), Brazil. He was a visiting scholar at Friedrich‐Schiller‐University (Germany) (2003) and at Harvard University (USA) (2007). During 2009–2010, he worked as a postdoctoral fellow at IFSC‐USP. He is currently a full researcher at the Nanotechnology National Laboratory for Agriculture (LNNA) at Embrapa Instrumentation and holds appointments as collaborating professor at post‐graduation programs at UFSCar. His research group is focused on functional nanomaterials for agriculture, environment, and biomedicine.

Orlando J. Rojas is a professor and Canada Excellence Research Chair at the University of British Columbia, with joint appointments with the Departments of Chemical and Biological Engineering, Wood Science, and Chemistry. He is director of the BioProducts Institute and visiting professor in Aalto University, Finland. Dr. Rojas is the recipient of the 2018 Anselme Payen and 2015 Tappi Nanotechnology awards. He is an elected Fellow of the American Chemical Society and the Finnish Academy of Science and Letters. His recent grants include the prestigious ERC‐Advanced. He is PI of the FinnCERES Flagship and leads several projects under the Boreal Alliance initiative.

Otoni C. G., Azeredo H. M. C., Mattos B. D., Beaumont M., Correa D. S., Rojas O. J., The Food–Materials Nexus: Next Generation Bioplastics and Advanced Materials from Agri‐Food Residues. Adv. Mater. 2021, 33, 2102520. 10.1002/adma.202102520
Contributor Information
Caio G. Otoni, Email: caio.otoni@ufscar.br.
Orlando J. Rojas, Email: orlando.rojas@ubc.ca.
References
- 1. Li T., Chen C., Brozena A. H., Zhu J. Y., Xu L., Driemeier C., Dai J., Rojas O. J., Isogai A., Wågberg L., Hu L., Nature 2021, 590, 47. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration (FDA), Global food losses and food waste, http://www.fao.org/3/a-i2697e.pdf (accessed: June 2021).
- 3.Food and Drug Administration (FDA), The state of food and agriculture: Moving forward on food loss and waste reduction, http://www.fao.org/3/ca6030en/ca6030en.pdf (accessed: June 2021).
- 4. Kibler K. M., Reinhart D., Hawkins C., Motlagh A. M., Wright J., Waste Manage. 2018, 74, 52. [DOI] [PubMed] [Google Scholar]
- 5. Roodhuyzen D. M. A., Luning P. A., Fogliano V., Steenbekkers L. P. A., Trends Food Sci. Technol. 2017, 68, 37. [Google Scholar]
- 6.High Level Panel of Experts on Food Security and Nutrition (HLPE), Food losses and waste in the context of sustainable food systems, http://www.fao.org/3/a-i3901e.pdf (accessed: June 2021).
- 7. Muth M. K., Birney C., Cuéllar A., Finn S. M., Freeman M., Galloway J. N., Gee I., Gephart J., Jones K., Low L., Meyer E., Read Q., Smith T., Weitz K., Zoubek S., Sci. Total Environ. 2019, 685, 1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaboud G., Daviron B., Global Food Secur. 2017, 12, 1. [Google Scholar]
- 9. Teigiserova D. A., Hamelin L., Thomsen M., Sci. Total Environ. 2020, 706, 136033. [DOI] [PubMed] [Google Scholar]
- 10. Read Q. D., Brown S., Cuéllar A. D., Finn S. M., Gephart J. A., Marston L. T., Meyer E., Weitz K. A., Muth M. K., Sci. Total Environ. 2020, 712, 136255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shafiee‐Jood M., Cai X., Environ. Sci. Technol. 2016, 50, 8432. [DOI] [PubMed] [Google Scholar]
- 12. Vázquez‐Rowe I., Laso J., Margallo M., Garcia‐Herrero I., Hoehn D., Amo‐Setién F., Bala A., Abajas R., Sarabia C., Durá M. J., Fullana‐i‐Palmer P., Aldaco R., Int. J. Life Cycle Assess. 2020, 25, 1197. [Google Scholar]
- 13. Cruz M. G., Bastos R., Pinto M., Ferreira J. M., Santos J. F., Wessel D. F., Coelho E., Coimbra M. A., J. Cleaner Prod. 2018, 193, 652. [Google Scholar]
- 14. Martin‐Rios C., Demen‐Meier C., Gössling S., Cornuz C., Waste Manage. 2018, 79, 196. [DOI] [PubMed] [Google Scholar]
- 15. Camaréna S., J. Cleaner Prod. 2020, 271, 122574. [Google Scholar]
- 16. Nguyen D. D., Yeop J. S., Choi J., Kim S., Chang S. W., Jeon B.‐H., Guo W., Ngo H. H., Waste Manage. 2017, 66, 161. [DOI] [PubMed] [Google Scholar]
- 17. Yu I. K. M., Attard T. M., Chen S. S., Tsang D. C. W., Hunt A. J., Jérôme F., Ok Y. S., Poon C. S., ACS Sustainable Chem. Eng. 2019, 7, 2821. [Google Scholar]
- 18. Xu C., Nasrollahzadeh M., Selva M., Issaabadi Z., Luque R., Chem. Soc. Rev. 2019, 48, 4791. [DOI] [PubMed] [Google Scholar]
- 19. Correa J. P., Montalvo‐Navarrete J. M., Hidalgo‐Salazar M. A., J. Cleaner Prod. 2019, 208, 785. [Google Scholar]
- 20. Galanakis C. M., Trends Food Sci. Technol. 2012, 26, 68. [Google Scholar]
- 21. Maina S., Kachrimanidou V., Koutinas A., Curr. Opin. Green Sustainable Chem. 2017, 8, 18. [Google Scholar]
- 22. Antonopoulou G., Ntaikou I., Pastore C., di Bitonto L., Bebelis S., Lyberatos G., Appl. Energy 2019, 242, 1064. [Google Scholar]
- 23. Engelberth A. S., Curr. Opin. Green Sustainable Chem. 2020, 26, 100385. [Google Scholar]
- 24. Tsang Y. F., Kumar V., Samadar P., Yang Y., Lee J., Ok Y. S., Song H., Kim K.‐H., Kwon E. E., Jeon Y. J., Environ. Int. 2019, 127, 625. [DOI] [PubMed] [Google Scholar]
- 25. Choi I. S., Lee Y. G., Khanal S. K., Park B. J., Bae H.‐J., Appl. Energy 2015, 140, 65. [Google Scholar]
- 26. Pavi S., Kramer L. E., Gomes L. P., Miranda L. A. S., Bioresour. Technol. 2017, 228, 362. [DOI] [PubMed] [Google Scholar]
- 27. Edwiges T., Frare L., Mayer B., Lins L., Triolo J. M., Flotats X., Mendonça Costa M. S. S., Waste Manage. 2018, 71, 618. [DOI] [PubMed] [Google Scholar]
- 28. Di Maria F., Sordi A., Cirulli G., Micale C., Appl. Energy 2015, 150, 9. [Google Scholar]
- 29. Eriksson M., Spångberg J., Waste Manage. 2017, 60, 786. [DOI] [PubMed] [Google Scholar]
- 30. Mitrano D. M., Wohlleben W., Nat. Commun. 2020, 11, 5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Q., Xu E. G., Li J., Chen Q., Ma L., Zeng E. Y., Shi H., Environ. Sci. Technol. 2020, 54, 3740. [DOI] [PubMed] [Google Scholar]
- 32. Du J., Xu S., Zhou Q., Li H., Fu L., Tang J., Wang Y., Peng X., Xu Y., Du X., Environ. Sci. Pollut. Res. 2020, 27, 11494. [DOI] [PubMed] [Google Scholar]
- 33.Statista, Global plastic production 1950–2018, https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/#:∼:text=In 2018%2C the global production,quarter of the global production (accessed: June 2021).
- 34. Chen X., Yan N., Mater. Today Sustainable 2020, 7‐8, 100031. [Google Scholar]
- 35. Torres‐León C., Vicente A. A., Flores‐López M. L., Rojas R., Serna‐Cock L., Alvarez‐Pérez O. B., Aguilar C. N., LWT 2018, 97, 624. [Google Scholar]
- 36. Soares R. M. D., Siqueira N. M., Prabhakaram M. P., Ramakrishna S., Mater. Sci. Eng. C 2018, 92, 969. [DOI] [PubMed] [Google Scholar]
- 37. Yang E., Miao S., Zhong J., Zhang Z., Mills D. K., Zhang L. G., Polym. Rev. 2018, 58, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He M., Wang X., Wang Z., Chen L., Lu Y., Zhang X., Li M., Liu Z., Zhang Y., Xia H., Zhang L., ACS Sustainable Chem. Eng. 2017, 5, 9126. [Google Scholar]
- 39. Oliver‐Ortega H., Julian F., Espinach F. X., Tarrés Q., Ardanuy M., Mutjé P., J. Cleaner Prod. 2019, 226, 64. [Google Scholar]
- 40. Akampumuza O., Wambua P. M., Ahmed A., Li W., Qin X.‐H., Polym. Compos. 2017, 38, 2553. [Google Scholar]
- 41. Bouzouita A., Samuel C., Notta‐Cuvier D., Odent J., Lauro F., Dubois P., Raquez J.‐M., J. Appl. Polym. Sci. 2016, 133, 43402. [Google Scholar]
- 42. Torres‐Rivas A., Palumbo M., Haddad A., Cabeza L. F., Jiménez L., Boer D., Appl. Energy 2018, 224, 602. [Google Scholar]
- 43. Muthuraj R., Lacoste C., Lacroix P., Bergeret A., Ind. Crops Prod. 2019, 135, 238. [Google Scholar]
- 44. Zhao X., Cornish K., Vodovotz Y., Environ. Sci. Technol. 2020, 54, 4712. [DOI] [PubMed] [Google Scholar]
- 45. Brizga J., Hubacek K., Feng K., One Earth 2020, 3, 45. [Google Scholar]
- 46. Spierling S., Knüpffer E., Behnsen H., Mudersbach M., Krieg H., Springer S., Albrecht S., Herrmann C., Endres H.‐J., J. Cleaner Prod. 2018, 185, 476. [Google Scholar]
- 47. Bocqué M., Voirin C., Lapinte V., Caillol S., Robin J.‐J., J. Polym. Sci., Part A: Polym. Chem. 2016, 54, 11. [Google Scholar]
- 48. Kumar A., Tumu V. R., Ray Chowdhury S., R. R. S. V. S., Int. J. Biol. Macromol. 2019, 121, 588. [DOI] [PubMed] [Google Scholar]
- 49. Zhao X., Venoor V., Koelling K., Cornish K., Vodovotz Y., J. Appl. Polym. Sci. 2019, 136, 47334. [Google Scholar]
- 50. Azeredo H. M. C., Waldron K. W., Trends Food Sci. Technol. 2016, 52, 109. [Google Scholar]
- 51. Garavand F., Rouhi M., Razavi S. H., Cacciotti I., Mohammadi R., Int. J. Biol. Macromol. 2017, 104, 687. [DOI] [PubMed] [Google Scholar]
- 52. Kaur M., Arshad M., Ullah A., ACS Sustainable Chem. Eng. 2018, 6, 1977. [Google Scholar]
- 53. Sadasivuni K. K., Saha P., Adhikari J., Deshmukh K., Ahamed M. B., Cabibihan J.‐J., Polym. Compos. 2020, 41, 32. [Google Scholar]
- 54. El Achaby M., El Miri N., Hannache H., Gmouh S., Ben youcef H., Aboulkas A., Int. J. Biol. Macromol. 2018, 117, 592. [DOI] [PubMed] [Google Scholar]
- 55. Travalini A. P., Lamsal B., Magalhães W. L. E., Demiate I. M., Int. J. Biol. Macromol. 2019, 139, 1151. [DOI] [PubMed] [Google Scholar]
- 56. Li H., Shi H., He Y., Fei X., Peng L., Int. J. Biol. Macromol. 2020, 164, 4104. [DOI] [PubMed] [Google Scholar]
- 57. Haghighi H., Leugoue S. K., Pfeifer F., Siesler H. W., Licciardello F., Fava P., Pulvirenti A., Food Hydrocolloids 2020, 100, 105419. [Google Scholar]
- 58. Ahmed J., Mulla M., Jacob H., Luciano G., Almusallam B. T. B. A., Food Packag. Shelf Life 2019, 21, 100355. [Google Scholar]
- 59. Janani N., Zare E. N., Salimi F., Makvandi P., Carbohydr. Polym. 2020, 247, 116678. [DOI] [PubMed] [Google Scholar]
- 60. Melo P. E. F., Silva A. P. M., Marques F. P., Ribeiro P. R. V., Souza Filho M. S. M., Brito E. S., Lima J. R., Azeredo H. M. C., Food Hydrocolloids 2019, 95, 487. [Google Scholar]
- 61. Van Dyk J. S., Gama R., Morrison D., Swart S., Pletschke B. I., Renewable Sustainable Energy Rev. 2013, 26, 521. [Google Scholar]
- 62. Dhillon G. S., Kaur S., Brar S. K., Renewable Sustainable Energy Rev. 2013, 27, 789. [Google Scholar]
- 63. Fernandes P. A. R., Le Bourvellec C., Renard C. M. G. C., Nunes F. M., Bastos R., Coelho E., Wessel D. F., Coimbra M. A., Cardoso S. M., Food Chem. 2019, 294, 9. [DOI] [PubMed] [Google Scholar]
- 64. Wang T., Zhao Y., Carbohydr. Polym. 2021, 253, 117225. [DOI] [PubMed] [Google Scholar]
- 65. Gustafsson J., Landberg M., Bátori V., Åkesson D., Taherzadeh M. J., Zamani A., Polymers 2019, 11, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Picard M. C., Rodriguez‐Uribe A., Thimmanagari M., Misra M., Mohanty A. K., Waste Biomass Valorization 2020, 11, 3775. [Google Scholar]
- 67. Food and Agriculture Organization of the United Nations (FAO) , FAOSTAT – Food and Agriculture Data, http://www.fao.org/faostat/en/#data (accessed: June 2021).
- 68. Sial T. A., Khan M. N., Lan Z., Kumbhar F., Ying Z., Zhang J., Sun D., Li X., Process Saf. Environ. Prot. 2019, 122, 366. [Google Scholar]
- 69. Vu H. T., Scarlett C. J., Vuong Q. V., J. Funct. Foods 2018, 40, 238. [Google Scholar]
- 70. Oliveira T. Í. S., Rosa M. F., Cavalcante F. L., Pereira P. H. F., Moates G. K., Wellner N., Mazzetto S. E., Waldron K. W., Azeredo H. M. C., Food Chem. 2016, 198, 113. [DOI] [PubMed] [Google Scholar]
- 71. Vu H. T., Scarlett C. J., Vuong Q. V., Drying Technol. 2017, 35, 1141. [Google Scholar]
- 72. Oliveira T. Í. S., Rosa M. F., Ridout M. J., Cross K., Brito E. S., Silva L. M. A., Mazzetto S. E., Waldron K. W., Azeredo H. M. C., Int. J. Biol. Macromol. 2017, 101, 1. [DOI] [PubMed] [Google Scholar]
- 73. Pelissari F. M., Andrade‐Mahecha M. M., Sobral P. J. A., Menegalli F. C., J. Colloid Interface Sci. 2017, 505, 154. [DOI] [PubMed] [Google Scholar]
- 74. Zhang W., Li X., Jiang W., Int. J. Biol. Macromol. 2020, 154, 1205. [DOI] [PubMed] [Google Scholar]
- 75. Lynch K. M., Steffen E. J., Arendt E. K., J. Inst. Brew. 2016, 122, 553. [Google Scholar]
- 76. Coelho E., Rocha M. A. M., Moreira A. S. P., Domingues M. R. M., Coimbra M. A., Carbohydr. Polym. 2016, 139, 167. [DOI] [PubMed] [Google Scholar]
- 77. López‐Linares J. C., García‐Cubero M. T., Lucas S., González‐Benito G., Coca M., Chem. Eng. J. 2019, 368, 1045. [Google Scholar]
- 78. Fernández‐Delgado M., Plaza P. E., Coca M., García‐Cubero M. T., González‐Benito G., Lucas S., Ind. Crops Prod. 2019, 130, 409. [Google Scholar]
- 79. Moreirinha C., Vilela C., Silva N. H. C. S., Pinto R. J. B., Almeida A., Rocha M. A. M., Coelho E., Coimbra M. A., Silvestre A. J. D., Freire C. S. R., Food Hydrocolloids 2020, 108, 105836. [Google Scholar]
- 80. Proaño J. L., Salgado P. R., Cian R. E., Mauri A. N., Drago S. R., J. Sci. Food Agric. 2020, 100, 54. [DOI] [PubMed] [Google Scholar]
- 81. Swarnam T. P., Velmurugan A., Pandey S. K., Dam Roy S., Bioresour. Technol. 2016, 207, 76. [DOI] [PubMed] [Google Scholar]
- 82. Nascimento D. M., Almeida J. S., Vale M. S., Leitão R. C., Muniz C. R., Figueirêdo M. C. B., Morais J. P. S., Rosa M. F., Ind. Crops Prod. 2016, 93, 66. [Google Scholar]
- 83. Wu J., Du X., Yin Z., Xu S., Xu S., Zhang Y., Carbohydr. Polym. 2019, 211, 49. [DOI] [PubMed] [Google Scholar]
- 84. Nagarajan M., Benjakul S., Prodpran T., Songtipya P., Food Packag. Shelf Life 2015, 6, 42. [Google Scholar]
- 85. Baêta B. E. L., Cordeiro P. H. M., Passos F., Gurgel L. V. A., Aquino S. F., Fdz‐Polanco F., Bioresour. Technol. 2017, 245, 66. [DOI] [PubMed] [Google Scholar]
- 86. Neves J. V. G., Borges M. V., Silva D. M., Leite C. X. D. S., Santos M. R. C., Lima N. G. B., Lannes S. C. S., Silva M. V., Food Sci. Technol. 2019, 39, 348. [Google Scholar]
- 87. Collazo‐Bigliardi S., Ortega‐Toro R., Chiralt Boix A., Carbohydr. Polym. 2018, 191, 205. [DOI] [PubMed] [Google Scholar]
- 88. Collazo‐Bigliardi S., Ortega‐Toro R., Chiralt A., Food Packag. Shelf Life 2019, 22, 100383. [Google Scholar]
- 89.USDA, Grain crushings and co‐products production – 2019 summary, https://www.nass.usda.gov/Publications/Todays_Reports/reports/cagcan20.pdf (accessed: June 2021).
- 90. Jin J., Ma H., Wang K., Yagoub A. E.‐G. A., Owusu J., Qu W., He R., Zhou C., Ye X., Ultrason. Sonochem. 2015, 24, 55. [DOI] [PubMed] [Google Scholar]
- 91. Chen G., Dong S., Zhao S., Li S., Chen Y., Ind. Crops Prod. 2019, 129, 318. [Google Scholar]
- 92. Vahedikia N., Garavand F., Tajeddin B., Cacciotti I., Jafari S. M., Omidi T., Zahedi Z., Colloids Surf., B 2019, 177, 25. [DOI] [PubMed] [Google Scholar]
- 93. Liu Z., Cao X., Ren S., Wang J., Zhang H., Ind. Crops Prod. 2019, 130, 57. [Google Scholar]
- 94. Wang Z., He X., Yan L., Wang J., Hu X., Sun Q., Zhang H., Ind. Crops Prod. 2020, 143, 111960. [Google Scholar]
- 95. Xu J., Krietemeyer E. F., Boddu V. M., Liu S. X., Liu W.‐C., Carbohydr. Polym. 2018, 192, 202. [DOI] [PubMed] [Google Scholar]
- 96. Schieber A., Annu. Rev. Food Sci. Technol. 2017, 8, 97. [DOI] [PubMed] [Google Scholar]
- 97. Minjares‐Fuentes R., Femenia A., Garau M. C., Meza‐Velázquez J. A., Simal S., Rosselló C., Carbohydr. Polym. 2014, 106, 179. [DOI] [PubMed] [Google Scholar]
- 98. Lu P., Hsieh Y.‐L., Carbohydr. Polym. 2012, 87, 2546. [DOI] [PubMed] [Google Scholar]
- 99. Monteiro G. C., Minatel I. O., Junior A. P., Gomez‐Gomez H. A., Camargo J. P. C., Diamante M. S., Pereira Basílio L. S., Tecchio M. A., Pereira Lima G. P., LWT 2021, 135, 110053. [Google Scholar]
- 100. Putnik P., Bursać Kovačević D., Ježek D., Šustić I., Zorić Z., Dragović‐Uzelac V., J. Food Process. Preserv. 2018, 42, e13342. [Google Scholar]
- 101. Coelho C. C. S., Silva R. B. S., Carvalho C. W. P., Rossi A. L., Teixeira J. A., Freitas‐Silva O., Cabral L. M. C., Int. J. Biol. Macromol. 2020, 159, 1048. [DOI] [PubMed] [Google Scholar]
- 102. Xu Y., Scales A., Jordan K., Kim C., Sismour E., J. Appl. Polym. Sci. 2017, 134, 44438. [Google Scholar]
- 103. Saurabh C. K., Gupta S., Variyar P. S., J. Food Sci. Technol. 2018, 55, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kurek M., Hlupić L., Ščetar M., Bosiljkov T., Galić K., J. Food Sci. 2019, 84, 2490. [DOI] [PubMed] [Google Scholar]
- 105. Gowman A., Wang T., Rodriguez‐Uribe A., Mohanty A. K., Misra M., ACS Omega 2018, 3, 15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Statista, Food & Nutrition – Statistics and facts on Food & Nutrition, https://www.statista.com/markets/415/topic/468/food-nutrition/ (accessed: June 2021).
- 107. Henrique M. A., Silvério H. A., Flauzino Neto W. P., Pasquini D., J. Environ. Manage. 2013, 121, 202. [DOI] [PubMed] [Google Scholar]
- 108. Nawab A., Alam F., Haq M. A., Lutfi Z., Hasnain A., Int. J. Biol. Macromol. 2017, 98, 869. [DOI] [PubMed] [Google Scholar]
- 109. Banerjee J., Singh R., Vijayaraghavan R., MacFarlane D., Patti A. F., Arora A., Food Hydrocolloids 2018, 77, 142. [Google Scholar]
- 110. Martínez‐Ramos T., Benedito‐Fort J., Watson N. J., Ruiz‐López I. I., Che‐Galicia G., Corona‐Jiménez E., Food Bioprod. Process. 2020, 122, 41. [Google Scholar]
- 111. Silva A. P. M., Oliveira A. V., Pontes S. M. A., Pereira A. L. S., Souza Filho M. M., Rosa M. F., Azeredo H. M. C., Carbohydr. Polym. 2019, 211, 209. [DOI] [PubMed] [Google Scholar]
- 112. Maryam Adilah Z. A., Jamilah B., Nur Hanani Z. A., Food Hydrocolloids 2018, 74, 207. [Google Scholar]
- 113. Yiin C. L., Ho S., Yusup S., Quitain A. T., Chan Y. H., Loy A. C. M., Gwee Y. L., Bioresour. Technol. 2019, 290, 121797. [DOI] [PubMed] [Google Scholar]
- 114. Chang S. H., Biomass Bioenergy 2014, 62, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zailuddin N. L. I., Husseinsyah S., Procedia Chem. 2016, 19, 366. [Google Scholar]
- 116. Zailuddin N. L. I., Osman A. F., Rahman R., SPE Polym. 2020, 1, 4. [Google Scholar]
- 117. Chaiwong W., Samoh N., Eksomtramage T., Kaewtatip K., Composites, Part B 2019, 176, 107331. [Google Scholar]
- 118. Duman A. K., Özgen G. Ö., Üçtuğ F. G., Sustainable Prod. Consumption 2020, 22, 126. [Google Scholar]
- 119. Leite P., Salgado J. M., Venâncio A., Domínguez J. M., Belo I., Bioresour. Technol. 2016, 214, 737. [DOI] [PubMed] [Google Scholar]
- 120. Lammi S., Le Moigne N., Djenane D., Gontard N., Angellier‐Coussy H., Ind. Crops Prod. 2018, 120, 250. [Google Scholar]
- 121. Rizzi V., Lacalamita D., Gubitosa J., Fini P., Petrella A., Romita R., Agostiano A., Gabaldón J. A., Fortea Gorbe M. I., Gómez‐Morte T., Cosma P., Sci. Total Environ. 2019, 693, 133620. [DOI] [PubMed] [Google Scholar]
- 122. Crizel T. M., Oliveira Rios A., Alves V. D., Bandarra N., Moldão‐Martins M., Hickmann Flôres S., Food Hydrocolloids 2018, 74, 139. [Google Scholar]
- 123. Balu A. M., Budarin V., Shuttleworth P. S., Pfaltzgraff L. A., Waldron K., Luque R., Clark J. H., ChemSusChem 2012, 5, 1694. [DOI] [PubMed] [Google Scholar]
- 124. Battista F., Remelli G., Zanzoni S., Bolzonella D., ACS Sustainable Chem. Eng. 2020, 8, 6834. [Google Scholar]
- 125. Güzel M., Akpınar Ö., Food Bioprod. Process. 2019, 115, 126. [Google Scholar]
- 126. Bátori V., Lundin M., Åkesson D., Lennartsson P. R., Taherzadeh M. J., Zamani A., Polymers 2019, 11, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lim Z. E., Thai Q. B., Le D. K., Luu T. P., Nguyen P. T. T., Do N. H. N., Le P. K., Phan‐Thien N., Goh X. Y., Duong H. M., J. Environ. Chem. Eng. 2020, 8, 104524. [Google Scholar]
- 128. Fareez I. M., Ibrahim N. A., Wan Yaacob W. M. H., Mamat Razali N. A., Jasni A. H., Abdul Aziz F., Cellulose 2018, 25, 4407. [Google Scholar]
- 129. Balakrishnan P., Sreekala M. S., Kunaver M., Huskić M., Thomas S., Carbohydr. Polym. 2017, 169, 176. [DOI] [PubMed] [Google Scholar]
- 130. Dai H., Ou S., Huang Y., Huang H., Cellulose 2018, 25, 1743. [Google Scholar]
- 131. Iewkittayakorn J., Khunthongkaew P., Wongnoipla Y., Kaewtatip K., Suybangdum P., Sopajarn A., J. Mater. Res. Technol. 2020, 9, 5056. [Google Scholar]
- 132. Sampaio S. L., Petropoulos S. A., Alexopoulos A., Heleno S. A., Santos‐Buelga C., Barros L., Ferreira I. C. F. R., Trends Food Sci. Technol. 2020, 103, 118. [Google Scholar]
- 133. Ben Atitallah I., Antonopoulou G., Ntaikou I., Alexandropoulou M., Nasri M., Mechichi T., Lyberatos G., Bioresour. Technol. 2019, 289, 121614. [DOI] [PubMed] [Google Scholar]
- 134. Rommi K., Rahikainen J., Vartiainen J., Holopainen U., Lahtinen P., Honkapää K., Lantto R., J. Appl. Polym. Sci. 2016, 133, 42862. [Google Scholar]
- 135. Borah P. P., Das P., Badwaik L. S., Ultrason. Sonochem. 2017, 36, 11. [DOI] [PubMed] [Google Scholar]
- 136. Santos F., Machado G., Faria D., Lima J., Marçal N., Dutra E., Souza G., Biomass Convers. Biorefin. 2017, 7, 117. [Google Scholar]
- 137. Kargarzadeh H., Johar N., Ahmad I., Compos. Sci. Technol. 2017, 151, 147. [Google Scholar]
- 138. Ranjan A., Moholkar V. S., Fuel 2013, 112, 567. [Google Scholar]
- 139. Perumal A. B., Sellamuthu P. S., Nambiar R. B., Sadiku E. R., Appl. Surf. Sci. 2018, 449, 591. [Google Scholar]
- 140. Hu S., Gu J., Jiang F., Hsieh Y.‐L., ACS Sustainable Chem. Eng. 2016, 4, 728. [Google Scholar]
- 141. Wang Z., Qiao X., Sun K., Carbohydr. Polym. 2018, 197, 442. [DOI] [PubMed] [Google Scholar]
- 142. Bilo F., Pandini S., Sartore L., Depero L. E., Gargiulo G., Bonassi A., Federici S., Bontempi E., J. Cleaner Prod. 2018, 200, 357. [Google Scholar]
- 143. Getachew A. T., Chun B. S., J. Cleaner Prod. 2017, 142, 3719. [Google Scholar]
- 144. Ballesteros L. F., Teixeira J. A., Mussatto S. I., Food Bioprocess Technol. 2014, 7, 3493. [Google Scholar]
- 145. Campos‐Vega R., Loarca‐Piña G., Vergara‐Castañeda H. A., Oomah B. D., Trends Food Sci. Technol. 2015, 45, 24. [Google Scholar]
- 146. Ballesteros L. F., Cerqueira M. A., Teixeira J. A., Mussatto S. I., Int. J. Biol. Macromol. 2018, 106, 647. [DOI] [PubMed] [Google Scholar]
- 147. Batista M. J. P. A., Ávila A. F., Franca A. S., Oliveira L. S., Carbohydr. Polym. 2020, 233, 115851. [DOI] [PubMed] [Google Scholar]
- 148. Coelho G. O., Batista M. J. A., Ávila A. F., Franca A. S., Oliveira L. S., J. Food Eng. 2021, 289, 110083. [Google Scholar]
- 149. Kanai N., Honda T., Yoshihara N., Oyama T., Naito A., Ueda K., Kawamura I., Cellulose 2020, 27, 5017. [Google Scholar]
- 150. Mendes J. F., Martins J. T., Manrich A., Sena Neto A. R., Pinheiro A. C. M., Mattoso L. H. C., Martins M. A., Carbohydr. Polym. 2019, 210, 92. [DOI] [PubMed] [Google Scholar]
- 151. Cacciotti I., Mori S., Cherubini V., Nanni F., Int. J. Biol. Macromol. 2018, 112, 567. [DOI] [PubMed] [Google Scholar]
- 152. Morais L. C., Maia A. A. D., Yamaji F. M., Viana S. R. F., Resende P., Biomass Convers. Biorefin. 2020, 10.1007/s13399-020-01097-y. [DOI] [Google Scholar]
- 153. Rocha G. J. M., Nascimento V. M., Gonçalves A. R., Silva V. F. N., Martín C., Ind. Crops Prod. 2015, 64, 52. [Google Scholar]
- 154. Vanitjinda G., Nimchua T., Sukyai P., Int. J. Biol. Macromol. 2019, 122, 503. [DOI] [PubMed] [Google Scholar]
- 155. Xiang Z., Jin X., Huang C., Li L., Wu W., Qi H., Nishiyama Y., Cellulose 2020, 27, 7307. [Google Scholar]
- 156. Anggono J., Farkas Á. E., Bartos A., Móczó J., Antoni, Purwaningsih H., Pukánszky B., Eur. Polym. J. 2019, 112, 153. [Google Scholar]
- 157. Guna V., Ilangovan M., Hu C., Venkatesh K., Reddy N., Ind. Crops Prod. 2019, 131, 25. [Google Scholar]
- 158. Ramlee N. A., Jawaid M., Zainudin E. S., Yamani S. A. K., J. Mater. Res. Technol. 2019, 8, 3466. [Google Scholar]
- 159. Lu Z., Wang J., Gao R., Ye F., Zhao G., Trends Food Sci. Technol. 2019, 86, 172. [Google Scholar]
- 160. Torbica A., Belović M., Mastilović J., Kevrešan Ž., Pestorić M., Škrobot D., Dapčević Hadnađev T., Food Bioprod. Process. 2016, 98, 299. [Google Scholar]
- 161. Alancay M. M., Lobo M. O., Quinzio C. M., Iturriaga L. B., J. Food Meas. Charact. 2017, 11, 2119. [Google Scholar]
- 162. Heredia‐Guerrero J. A., Caputo G., Guzman‐Puyol S., Tedeschi G., Heredia A., Ceseracciu L., Benitez J. J., Athanassiou A., Mater. Today Sustainable 2019, 3–4, 100004. [Google Scholar]
- 163. Aloui H., Baraket K., Sendon R., Silva A. S., Khwaldia K., Int. J. Biol. Macromol. 2019, 139, 128. [DOI] [PubMed] [Google Scholar]
- 164. Tedeschi G., Benitez J. J., Ceseracciu L., Dastmalchi K., Itin B., Stark R. E., Heredia A., Athanassiou A., Heredia‐Guerrero J. A., ACS Sustainable Chem. Eng. 2018, 6, 14955. [Google Scholar]
- 165. Benítez J. J., Castillo P. M., del Río J. C., León‐Camacho M., Domínguez E., Heredia A., Guzmán‐Puyol S., Athanassiou A., Heredia‐Guerrero J. A., Materials 2018, 11, 2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Hernández C., Escamilla‐Alvarado C., Sánchez A., Alarcón E., Ziarelli F., Musule R., Valdez‐Vazquez I., Biofuels, Bioprod. Biorefin. 2019, 13, 1143. [Google Scholar]
- 167. Azeredo H. M. C., Kontou‐Vrettou C., Moates G. K., Wellner N., Cross K., Pereira P. H. F., Waldron K. W., Food Hydrocolloids 2015, 50, 1. [Google Scholar]
- 168. Pereira P. H. F., Waldron K. W., Wilson D. R., Cunha A. P., Brito E. S., Rodrigues T. H. S., Rosa M. F., Azeredo H. M. C., Carbohydr. Polym. 2017, 164, 317. [DOI] [PubMed] [Google Scholar]
- 169. Bian H., Gao Y., Luo J., Jiao L., Wu W., Fang G., Dai H., Waste Manage. 2019, 91, 1. [DOI] [PubMed] [Google Scholar]
- 170. Ali U., Bijalwan V., Basu S., Kesarwani A. K., Mazumder K., Carbohydr. Polym. 2017, 161, 90. [DOI] [PubMed] [Google Scholar]
- 171. Dixit S., Yadav V. L., J. CleanEr Prod. 2019, 240, 118228. [Google Scholar]
- 172.True Animal Protein Price Coalition, FAO: global meat production decline, https://www.tappcoalition.eu/nieuws/14456/fao--global-meat-production-decline-in-2019-and-2020 (accessed: June 2021).
- 173. Smith P. T., Narupai B., Tsui J. H., Millik S. C., Shafranek R. T., Kim D.‐H., Nelson A., Biomacromolecules 2020, 21, 484. [DOI] [PubMed] [Google Scholar]
- 174. Lee B. E. J., Luo L., Grandfield K., Andrei C. M., Schwarcz H. P., Micron 2019, 124, 102706. [DOI] [PubMed] [Google Scholar]
- 175. Heybeli N., Kömür B., Yılmaz B., Güler O., in Musculoskeletal Research and Basic Science (Ed: Korkusuz F.), Springer International Publishing, Cham, Switzerland: 2016, Ch. 28. [Google Scholar]
- 176. Mu B., Hassan F., Yang Y., Green Chem. 2020, 22, 1726. [Google Scholar]
- 177. Li W., Guo R., Lan Y., Zhang Y., Xue W., Zhang Y., J. Biomed. Mater. Res., Part A 2014, 102, 1131. [DOI] [PubMed] [Google Scholar]
- 178. Shi D., Liu F., Yu Z., Chang B., Goff H. D., Zhong F., Food Hydrocolloids 2019, 87, 436. [Google Scholar]
- 179. Nazmi N. N., Isa M. I. N., Sarbon N. M., Food Biosci. 2017, 19, 149. [Google Scholar]
- 180. Zhang T., Sun R., Ding M., Li L., Tao N., Wang X., Zhong J., LWT 2020, 125, 109207. [Google Scholar]
- 181. Benbettaïeb N., Kurek M., Bornaz S., Debeaufort F., J. Sci. Food Agric. 2014, 94, 2409. [DOI] [PubMed] [Google Scholar]
- 182. Ramakrishnan N., Sharma S., Gupta A., Alashwal B. Y., Int. J. Biol. Macromol. 2018, 111, 352. [DOI] [PubMed] [Google Scholar]
- 183. He M., Zhang B., Dou Y., Yin G., Cui Y., J. Appl. Polym. Sci. 2017, 134, 44680. [Google Scholar]
- 184. Sharma S., Gupta A., Kumar A., Kee C. G., Kamyab H., Saufi S. M., Clean Technol. Environ. Policy 2018, 20, 2157. [Google Scholar]
- 185. Yan N., Chen X., Nature 2015, 524, 155. [DOI] [PubMed] [Google Scholar]
- 186. Hamdi M., Hammami A., Hajji S., Jridi M., Nasri M., Nasri R., Int. J. Biol. Macromol. 2017, 101, 455. [DOI] [PubMed] [Google Scholar]
- 187. Wang Y., Fu C., Wu Z., Chen L., Chen X., Wei Y., Zhang P., Carbohydr. Polym. 2017, 174, 723. [DOI] [PubMed] [Google Scholar]
- 188. Mushi N. E., Nishino T., Berglund L. A., Zhou Q., ACS Sustainable Chem. Eng. 2019, 7, 1692. [Google Scholar]
- 189. Wang Y., Li J., Li B., J. Agric. Food Chem. 2016, 64, 5736. [DOI] [PubMed] [Google Scholar]
- 190. Liu J., Liu S., Wu Q., Gu Y., Kan J., Jin C., Food Hydrocolloids 2017, 73, 90. [Google Scholar]
- 191. Sun L., Sun J., Chen L., Niu P., Yang X., Guo Y., Carbohydr. Polym. 2017, 163, 81. [DOI] [PubMed] [Google Scholar]
- 192. Ching‐Velasquez J., Fernández‐Lafuente R., Rodrigues R. C., Plata V., Rosales‐Quintero A., Torrestiana‐Sánchez B., Tacias‐Pascacio V. G., Renewable Energy 2020, 153, 1346. [Google Scholar]
- 193. Blanco M., Vázquez J. A., Pérez‐Martín R. I., Sotelo C. G., Mar. Drugs 2017, 15, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Bhuimbar M. V, Bhagwat P. K., Dandge P. B., J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar]
- 195. Tang L., Chen S., Su W., Weng W., Osako K., Tanaka M., Process Biochem. 2015, 50, 148. [Google Scholar]
- 196. Menezes M. L. L. R., Pires N. R., Cunha P. L. R., Freitas Rosa M., Souza B. W. S., Feitosa J. P. A., Souza Filho M. S. M., Food Packag. Shelf Life 2019, 19, 7. [Google Scholar]
- 197. Etxabide A., Leceta I., Cabezudo S., Guerrero P., de la Caba K., ACS Sustainable Chem. Eng. 2016, 4, 4626. [Google Scholar]
- 198. Jamróz E., Kulawik P., Tkaczewska J., Guzik P., Zając M., Juszczak L., Krzyściak P., Turek K., Food Chem. 2021, 338, 127867. [DOI] [PubMed] [Google Scholar]
- 199. Nuanmano S., Prodpran T., Benjakul S., Food Hydrocolloids 2015, 47, 61. [Google Scholar]
- 200. Sebastián‐Nicolás J. L., González‐Olivares L. G., Vázquez‐Rodríguez G. A., Lucho‐Constatino C. A., Castañeda‐Ovando A., Cruz‐Guerrero A. E., Biofuels, Bioprod. Biorefin. 2020, 14, 1010. [Google Scholar]
- 201. Kristoffersen K. A., Afseth N. K., Böcker U., Lindberg D., de Vogel‐van den Bosch H., Ruud M. L., Wubshet S. G., Food Chem. 2020, 310, 125800. [DOI] [PubMed] [Google Scholar]
- 202. Zhang W., Chen J., Chen Y., Xia W., Xiong Y. L., Wang H., Carbohydr. Polym. 2016, 138, 59. [DOI] [PubMed] [Google Scholar]
- 203. Miller J., Tappi J. 2019, 18, 313. [Google Scholar]
- 204. Pacheco C. M., Bustos A. C., Reyes G., J. Dispersion Sci. Technol. 2020, 41, 1731. [Google Scholar]
- 205. Melo E. M., Clark J. H., Matharu A. S., Green Chem. 2017, 19, 3408. [Google Scholar]
- 206. Tibolla H., Pelissari F. M., Rodrigues M. I., Menegalli F. C., Ind. Crops Prod. 2017, 95, 664. [Google Scholar]
- 207. Cypriano D. Z., Silva L. L., Tasic L., Waste Manage. 2018, 79, 71. [DOI] [PubMed] [Google Scholar]
- 208. Szymanska‐Chargot M., Chylinska M., Gdula K., Koziol A., Zdunek A., Polymers 2017, 9, 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Corrales‐Ureña Y. R., Villalobos‐Bermúdez C., Pereira R., Camacho M., Estrada E., Argüello‐Miranda O., Vega‐Baudrit J. R., Sci. Rep. 2018, 8, 10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Meng F., Wang G., Du X., Wang Z., Xu S., Zhang Y., Composites, Part B 2019, 160, 341. [Google Scholar]
- 211. Cherian B. M., Leão A. L., Souza S. F., Thomas S., Pothan L. A., Kottaisamy M., Carbohydr. Polym. 2010, 81, 720. [Google Scholar]
- 212. Marett J., Aning A., Foster E. J., Ind. Crops Prod. 2017, 109, 869. [Google Scholar]
- 213. Longaresi R. H., Menezes A. J., Pereira‐da‐Silva M. A., Baron D., Mathias S. L., Ind. Crops Prod. 2019, 133, 232. [Google Scholar]
- 214. García A., Gandini A., Labidi J., Belgacem N., Bras J., Ind. Crops Prod. 2016, 93, 26. [Google Scholar]
- 215. Liu B., Li T., Wang W., Sagis L. M. C., Yuan Q., Lei X., Cohen Stuart M. A., Li D., Bao C., Bai J., Yu Z., Ren F., Li Y., Nat. Sustainability 2020, 3, 448. [Google Scholar]
- 216. Beaumont M., Nypelö T., König J., Zirbs R., Opietnik M., Potthast A., Rosenau T., Green Chem. 2016, 18, 1465. [Google Scholar]
- 217. Beaumont M., Rosenfeldt S., Tardy B. L., Gusenbauer C., Khakalo A., Nonappa, Opietnik M., Potthast A., Rojas O. J., Rosenau T., Nanoscale 2019, 11, 17773. [DOI] [PubMed] [Google Scholar]
- 218. Beaumont M., Solin K., Rosenfeldt S., Orelma H., Borghei M., Bacher M., Opietnik M., Rojas O. J., Small 2020, 50, 2004702. [DOI] [PubMed] [Google Scholar]
- 219. Souza C. B., Jonathan M., Saad S. M. I., Schols H. A., Venema K., Bioact. Carbohydr. Diet. Fibre 2018, 16, 90. [Google Scholar]
- 220. MacLeod M., Pap. PuuPap. Timber 2007, 89, 1 [Google Scholar]
- 221. Filson P. B., Dawson‐Andoh B. E., Schwegler‐Berry D., Green Chem. 2009, 11, 1808. [Google Scholar]
- 222. Habibi Y., Lucia L. A., Rojas O. J., Chem. Rev. 2010, 110, 3479. [DOI] [PubMed] [Google Scholar]
- 223. García A., Labidi J., Belgacem M. N., Bras J., Cellulose 2017, 24, 693. [Google Scholar]
- 224. Zoghlami A., Paës G., Front. Chem. 2019, 7, 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Li M., Pu Y., Ragauskas A. J., Front. Chem. 2016, 4, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Polo C. C., Pereira L., Mazzafera P., Flores‐Borges D. N. A., Mayer J. L. S., Guizar‐Sicairos M., Holler M., Barsi‐Andreeta M., Westfahl H., Meneau F., Sci. Rep. 2020, 10, 6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Mahanta N., Leong W. Y., Valiyaveettil S., J. Mater. Chem. 2012, 22, 1985. [Google Scholar]
- 228. Hosseinaei O., Wang S., Taylor A. M., Kim J.‐W., Int. Biodeterior. Biodegrad. 2012, 71, 29. [Google Scholar]
- 229. Harini K., Ramya K., Sukumar M., Carbohydr. Polym. 2018, 201, 329. [DOI] [PubMed] [Google Scholar]
- 230. Costa A. L. R., Gomes A., Tibolla H., Menegalli F. C., Cunha R. L., Carbohydr. Polym. 2018, 194, 122. [DOI] [PubMed] [Google Scholar]
- 231. Chen Y. W., Hasanulbasori M. A., Chiat P. F., Lee H. V., Int. J. Biol. Macromol. 2019, 123, 1305. [DOI] [PubMed] [Google Scholar]
- 232. Gao H., Duan B., Lu A., Deng H., Du Y., Shi X., Zhang L., Food Hydrocolloids 2018, 79, 473. [Google Scholar]
- 233. Jongaroontaprangsee S., Chiewchan N., Devahastin S., Carbohydr. Polym. 2018, 193, 149. [DOI] [PubMed] [Google Scholar]
- 234. Jiang F., Hsieh Y.‐L., Carbohydr. Polym. 2015, 122, 60. [DOI] [PubMed] [Google Scholar]
- 235. Sá N. M. S. M., Mattos A. L. A., Silva L. M. A., Brito E. S., Rosa M. F., Azeredo H. M. C., Int. J. Biol. Macromol. 2020, 161, 1337. [DOI] [PubMed] [Google Scholar]
- 236. Elanthikkal S., Francis T., Akhtar S., J. Nat. Fibers 2019, 1. [Google Scholar]
- 237. Hemmati F., Jafari S. M., Kashaninejad M., Barani Motlagh M., Int. J. Biol. Macromol. 2018, 120, 1216. [DOI] [PubMed] [Google Scholar]
- 238. Frost B. A., Foster E. J., J. Renewable Mater. 2020, 8, 187. [Google Scholar]
- 239. Hafemann E., Battisti R., Bresolin D., Marangoni C., Machado R. A. F., Waste Biomass Valorization 2020, 11, 6595. [Google Scholar]
- 240. Hafemann E., Battisti R., Marangoni C., Machado R. A. F., Carbohydr. Polym. 2019, 218, 188. [DOI] [PubMed] [Google Scholar]
- 241. Kallel F., Bettaieb F., Khiari R., García A., Bras J., Chaabouni S. E., Ind. Crops Prod. 2016, 87, 287. [Google Scholar]
- 242. Liu Z., Li X., Xie W., Deng H., Carbohydr. Polym. 2017, 173, 353. [DOI] [PubMed] [Google Scholar]
- 243. Rhim J.‐W., Reddy J. P., Luo X., Cellulose 2015, 22, 407. [Google Scholar]
- 244. Ifuku S., Saimoto H., Nanoscale 2012, 4, 3308. [DOI] [PubMed] [Google Scholar]
- 245. Joseph B., Mavelil Sam R., Balakrishnan P., Maria H. J., Gopi S., Volova T., Fernandes S. C. M., Thomas S., Polymers 2020, 12, 1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Soetemans L., Uyttebroek M., Bastiaens L., Int. J. Biol. Macromol. 2020, 165, 3206. [DOI] [PubMed] [Google Scholar]
- 247. Lucas A. J. S., Oreste E. Q., Costa H. L. G., López H. M., Saad C. D. M., Prentice C., Food Chem. 2020, 343, 128550.33191008 [Google Scholar]
- 248. Grande R., Bai L., Wang L., Xiang W., Ikkala O., Carvalho A. J. F., Rojas O. J., ACS Sustainable Chem. Eng. 2019, 8, 1137. [Google Scholar]
- 249. Hayes M., Carney B., Slater J., Brück W., Biotechnol. J. 2008, 3, 871. [DOI] [PubMed] [Google Scholar]
- 250. Salaberria A. M., Diaz R. H., Labidi J., Fernandes S. C. M., React. Funct. Polym. 2015, 89, 31. [Google Scholar]
- 251. Larbi F., García A., del Valle L. J., Hamou A., Puiggalí J., Belgacem N., Bras J., Carbohydr. Polym. 2018, 196, 385. [DOI] [PubMed] [Google Scholar]
- 252. Devi R., Dhamodharan R., ACS Sustainable Chem. Eng. 2018, 6, 11313. [Google Scholar]
- 253. Jung H.‐S., Kim M. H., Park W. H., ACS Biomater. Sci. Eng. 2019, 5, 1744. [DOI] [PubMed] [Google Scholar]
- 254. Tran T. H., Nguyen H.‐L., Hwang D. S., Lee J. Y., Cha H. G., Koo J. M., Hwang S. Y., Park J., Oh D. X., Carbohydr. Polym. 2019, 205, 392. [DOI] [PubMed] [Google Scholar]
- 255. Bai L., Kämäräinen T., Xiang W., Majoinen J., Seitsonen J., Grande R., Huan S., Liu L., Fan Y., Rojas O. J., ACS Nano 2020, 14, 6921. [DOI] [PubMed] [Google Scholar]
- 256. Ye W., Hu Y., Ma H., Liu L., Yu J., Fan Y., Carbohydr. Polym. 2020, 237, 116125. [DOI] [PubMed] [Google Scholar]
- 257. Ye W., Liu L., Wang Z., Yu J., Fan Y., ACS Sustainable Chem. Eng. 2019, 7, 19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Narkevicius A., Steiner L. M., Parker R. M., Ogawa Y., Frka‐Petesic B., Vignolini S., Biomacromolecules 2019, 20, 2830. [DOI] [PubMed] [Google Scholar]
- 259. Nguyen T. D., Maclachlan M. J., Adv. Opt. Mater. 2014, 2, 1031. [Google Scholar]
- 260. Yuan Y., Hong S., Lian H., Zhang K., Liimatainen H., Carbohydr. Polym. 2020, 236, 116095. [DOI] [PubMed] [Google Scholar]
- 261. Pereira A. G. B., Muniz E. C., Hsieh Y.‐L., Carbohydr. Polym. 2014, 107, 158. [DOI] [PubMed] [Google Scholar]
- 262. Ye W., Ma H., Liu L., Yu J., Lai J., Fang Y., Fan Y., Green Chem. 2019, 21, 3143. [Google Scholar]
- 263. Jiang J., Ye W., Yu J., Fan Y., Ono Y., Saito T., Isogai A., Carbohydr. Polym. 2018, 189, 178. [DOI] [PubMed] [Google Scholar]
- 264. Tran T. H., Nguyen H. L., Hao L. T., Kong H., Park J. M., Jung S. H., Cha H. G., Lee J. Y., Kim H., Hwang S. Y., Park J., Oh D. X., Int. J. Biol. Macromol. 2019, 125, 660. [DOI] [PubMed] [Google Scholar]
- 265. Huan S., Mattos B. D., Ajdary R., Xiang W., Bai L., Rojas O. J., Adv. Funct. Mater. 2019, 29, 1902990. [Google Scholar]
- 266. Mattos B. D., Tardy B. L., Greca L. G., Kämäräinen T., Xiang W., Cusola O., Magalhães W. L. E., Rojas O. J., Sci. Adv. 2020, 6, eaaz7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Li X., Han J., Wang X., Zhang Y., Jia C., Qin J., Wang C., Wu J. R., Fang W., Yang Y. W., Mater. Chem. Front. 2019, 3, 103. [Google Scholar]
- 268. Mattos B. D., Tardy B. L., Pezhman M., Kämäräinen T., Linder M., Schreiner W. H., Magalhães W. L. E., Rojas O. J., Sci. Rep. 2018, 8, 5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Kaziem A. E., Gao Y., He S., Li J., J. Agric. Food Chem. 2017, 65, 7854. [DOI] [PubMed] [Google Scholar]
- 270. Mattos B. D., Tardy B. L., Magalhães W. L. E., Rojas O. J., J. Controlled Release 2017, 262, 139. [DOI] [PubMed] [Google Scholar]
- 271. Kämäräinen T., Tardy B. L., Javan Nikkhah S., Batys P., Sammalkorpi M., Rojas O. J., J. Colloid Interface Sci. 2020, 579, 794. [DOI] [PubMed] [Google Scholar]
- 272. Du X.‐Y., Wang C.‐F., Wu G., Chen S., Angew. Chem., Int. Ed. 2020, 60, 8585. [DOI] [PubMed] [Google Scholar]
- 273. Wen X., Shi L., Wen G., Li Y., Dong C., Yang J., Shuang S., Sens. Actuators, B 2016, 235, 179. [Google Scholar]
- 274. Ahn J., Song Y., Kwon J. E., Lee S. H., Park K. S., Kim S., Woo J., Kim H., Mater. Sci. Eng. C 2019, 102, 106. [DOI] [PubMed] [Google Scholar]
- 275. Park S. Y., Lee H. U., Park E. S., Lee S. C., Lee J. W., Jeong S. W., Kim C. H., Lee Y. C., Huh Y. S., Lee J., ACS Appl. Mater. Interfaces 2014, 6, 3365. [DOI] [PubMed] [Google Scholar]
- 276. Ahn J., Song Y., Kwon J. E., Woo J., Kim H., Data Brief 2019, 25, 104038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Genc R., Alas M. O., Harputlu E., Repp S., Kremer N., Castellano M., Colak S. G., Ocakoglu K., Erdem E., Sci. Rep. 2017, 7, 11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Hu Y., Gao Z., ChemistrySelect 2019, 4, 12807. [Google Scholar]
- 279. Kim K. W., Choi T.‐Y., Kwon Y. M., Kim J. Y. H., Electron. J. Biotechnol. 2020, 47, 36. [Google Scholar]
- 280. Fan H., Zhang M., Bhandari B., hui Yang C., Trends Food Sci. Technol. 2020, 95, 86. [Google Scholar]
- 281. Szymańska‐Chargot M., Chylińska M., Pieczywek P. M., Zdunek A., Carbohydr. Polym. 2019, 210, 186. [DOI] [PubMed] [Google Scholar]
- 282. Mesa K., Serra S., Masia A., Gagliardi F., Bucci D., Musacchi S., Sci. Hortic. 2016, 211, 60. [Google Scholar]
- 283. Peng F., Ren J. L., Xu F., Bian J., Peng P., Sun R. C., J. Agric. Food Chem. 2010, 58, 1768. [DOI] [PubMed] [Google Scholar]
- 284. Kringel D. H., Dias A. R. G., Zavareze E. R., Gandra E. A., Starch/Stärke 2020, 72, 1900200. [Google Scholar]
- 285. Schiewer S., Patil S. B., Bioresour. Technol. 2008, 99, 1896. [DOI] [PubMed] [Google Scholar]
- 286. Murado M. A., Montemayor M. I., Cabo M. L., Vázquez J. A., González M. P., Food Bioprod. Process. 2012, 90, 491. [Google Scholar]
- 287. Tao J., Li S., Ye F., Zhou Y., Lei L., Zhao G., Crit. Rev. Food Sci. Nutr. 2020, 60, 2011. [DOI] [PubMed] [Google Scholar]
- 288. Aires A., Carvalho R., Saavedra M. J., Waste Manage. 2016, 48, 457. [DOI] [PubMed] [Google Scholar]
- 289. Kittiphattanabawon P., Benjakul S., Visessanguan W., Nagai T., Tanaka M., Food Chem. 2005, 89, 363. [Google Scholar]
- 290. Abdollahi M., Rezaei M., Jafarpour A., Undeland I., Food Chem. 2018, 242, 568. [DOI] [PubMed] [Google Scholar]
- 291. Nalinanon S., Benjakul S., Visessanguan W., Kishimura H., Food Hydrocolloids 2008, 22, 615. [DOI] [PubMed] [Google Scholar]
- 292. Santana J. C. C., Gardim R. B., Almeida P. F., Borini G. B., Quispe A. P. B., Llanos S. A. V., Heredia J. A., Zamuner S., Gamarra F. M. C., Farias T. M. B., Ho L. L., Berssaneti F. T., Polymers 2020, 12, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Chua J. Y., Liu S. Q., Trends Food Sci. Technol. 2019, 91, 24. [Google Scholar]
- 294. Jiang B., Chen C., Liang Z., He S., Kuang Y., Song J., Mi R., Chen G., Jiao M., Hu L., Adv. Funct. Mater. 2020, 30, 1906307. [Google Scholar]
- 295. Jeong H., Baek S., Han S., Jang H., Kim S. H., Lee H. S., Adv. Funct. Mater. 2018, 28, 1704433. [Google Scholar]
- 296. Zhao S., Malfait W. J., Demilecamps A., Zhang Y., Brunner S., Huber L., Tingaut P., Rigacci A., Budtova T., Koebel M. M., Angew. Chem., Int. Ed. 2015, 54, 14282. [DOI] [PubMed] [Google Scholar]
- 297. Ranganathan S., Dutta S., Moses J. A., Anandharamakrishnan C., Heliyon 2020, 6, e04891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Geng Z. C., Sun J. X., Liang S. F., Zhang F. Y., Zhang Y. Y., Xu F., Sun R. C., Int. J. Polym. Anal. Charact. 2006, 11, 209. [Google Scholar]
- 299. Sustainable Polymers from Biomass (Eds: Tang C., Ryu C. Y.), Wiley‐VCH, Weinheim, Germany: 2017. [Google Scholar]
- 300. Dattatraya Saratale G., Bhosale R., Shobana S., Banu J. R., Pugazhendhi A., Mahmoud E., Sirohi R., Kant Bhatia S., Atabani A. E., Mulone V., Yoon J. J., Seung Shin H., Kumar G., Bioresour. Technol. 2020, 314, 123800. [DOI] [PubMed] [Google Scholar]
- 301. Xie Y., Guo X., Ma Z., Gong J., Wang H., Lv Y., Polymers 2020, 12, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Brienzo M., Siqueira A. F., Milagres A. M. F., Biochem. Eng. J. 2009, 46, 199. [Google Scholar]
- 303. Farhat W., Venditti R. A., Hubbe M., Taha M., Becquart F., Ayoub A., ChemSusChem 2017, 10, 305. [DOI] [PubMed] [Google Scholar]
- 304. Hansen N. M. L., Plackett D., Biomacromolecules 2008, 9, 1493. [DOI] [PubMed] [Google Scholar]
- 305. Delcour J. A., Rouseu N., Vanhaesendonck I. P., Cereal Chem. 1999, 76, 1. [Google Scholar]
- 306. Comino P., Shelat K., Collins H., Lahnstein J., Gidley M. J., J. Agric. Food Chem. 2013, 61, 12111. [DOI] [PubMed] [Google Scholar]
- 307. Daus S., Heinze T., Macromol. Biosci. 2010, 10, 211. [DOI] [PubMed] [Google Scholar]
- 308. Melandri D., De Angelis A., Orioli R., Ponzielli G., Lualdi P., Giarratana N., Reiner V., Burns 2006, 32, 964. [DOI] [PubMed] [Google Scholar]
- 309. Bush J. R., Liang H., Dickinson M., Botchwey E. A., Polym. Adv. Technol. 2016, 27, 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Mousavioun P., Doherty W. O. S., Ind. Crops Prod. 2010, 31, 52. [Google Scholar]
- 311. Jia Z., Li M., Wan G., Luo B., Guo C., Wang S., Min D., RSC Adv. 2018, 8, 42269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Nakanishi S. C., Nascimento V. M., Rabelo S. C., Sampaio I. L. M., Junqueira T. L., Rocha G. J. M., Ind. Crops Prod. 2018, 120, 187. [Google Scholar]
- 313. Hassanpour M., Abbasabadi M., Gebbie L., Te'O V. S. J., O'Hara I. M., Zhang Z., ACS Sustainable Chem. Eng. 2020, 8, 10380. [Google Scholar]
- 314. Jonglertjunya W., Juntong T., Pakkang N., Srimarut N., Sakdaronnarong C., LWT ‐ Food Sci. Technol. 2014, 57, 116. [Google Scholar]
- 315. Nascimento V. M., Nakanishi S. C., Rocha G. J. M., Rabelo S. C., Pimenta M. T. B., Rossell C. E. V., ACS Sustainable Chem. Eng. 2016, 4, 3609. [Google Scholar]
- 316. Österberg M., Sipponen M. H., Mattos B. D., Rojas O. J., Green Chem. 2020, 22, 2712. [Google Scholar]
- 317. Ago M., Huan S., Borghei M., Raula J., Kauppinen E. I., Rojas O. J., ACS Appl. Mater. Interfaces 2016, 8, 23302. [DOI] [PubMed] [Google Scholar]
- 318. Lourencon T. V., Greca L. G., Tarasov D., Borrega M., Tamminen T., Rojas O. J., Balakshin M. Y., ACS Sustainable Chem. Eng. 2020, 8, 1230. [Google Scholar]
- 319. Bertolo M. R. V., Paiva L. B. B., Nascimento V. M., Gandin C. A., Neto M. O., Driemeier C. E., Rabelo S. C., Ind. Crops Prod. 2019, 140, 111591. [Google Scholar]
- 320. Arapitsas P., Food Chem. 2012, 135, 1708. [DOI] [PubMed] [Google Scholar]
- 321. Widsten P., Cruz C. D., Fletcher G. C., Pajak M. A., McGhie T. K., J. Agric. Food Chem. 2014, 62, 11146. [DOI] [PubMed] [Google Scholar]
- 322. Russell A., Chem. Rev. 1935, 17, 155. [Google Scholar]
- 323. Low J. H., Rahman W. A. W. A., Jamaluddin J., J. Cleaner Prod. 2015, 101, 222. [Google Scholar]
- 324. Pizzi A., Rev. Adhes. Adhes. 2013, 1, 88. [Google Scholar]
- 325. Ping L., Pizzi A., Guo Z. D., Brosse N., Ind. Crops Prod. 2012, 40, 13. [Google Scholar]
- 326. Liu C., Zhang Y., Li X., Luo J., Gao Q., Li J., RSC Adv. 2017, 7, 21226. [Google Scholar]
- 327. Kämäräinen T., Ago M., Greca L. G., Tardy B. L., Müllner M., Johansson L.‐S., Rojas O. J., ACS Sustainable Chem. Eng. 2019, 7, 16985. [Google Scholar]
- 328. Mülhaupt R., Macromol. Chem. Phys. 2013, 214, 159. [Google Scholar]
- 329. Miles M. J., Morris V. J., Orford P. D., Ring S. G., Carbohydr. Res. 1985, 135, 271. [Google Scholar]
- 330. Nakthong N., Wongsagonsup R., Amornsakchai T., Ind. Crops Prod. 2017, 105, 74. [Google Scholar]
- 331. Park S. H., Bean S. R., Wilson J. D., Schober T. J., Cereal Chem. J. 2006, 83, 611. [Google Scholar]
- 332. Waliszewski K. N., Aparicio M. A., Bello L. A., Monroy J. A., Carbohydr. Polym. 2003, 52, 237. [Google Scholar]
- 333. Li Z., Guo K., Lin L., He W., Zhang L., Wei C., Molecules 2018, 23, 2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Saengchan K., Nopharatana A., Songkasiri W., Powder Technol. 2019, 346, 1. [Google Scholar]
- 335. Jiang T., Duan Q., Zhu J., Liu H., Yu L., Adv. Ind. Eng. Polym. Res. 2020, 3, 8. [Google Scholar]
- 336. Müller‐Maatsch J., Bencivenni M., Caligiani A., Tedeschi T., Bruggeman G., Bosch M., Petrusan J., Van Droogenbroeck B., Elst K., Sforza S., Food Chem. 2016, 201, 37. [DOI] [PubMed] [Google Scholar]
- 337. Waldron K. W., Faulds C. B., in Comprehensive Glycoscience: From Chemistry to Systems Biology, (Ed: Kamerling H.), Elsevier, Amsterdam: 2007, Ch. 1.05. [Google Scholar]
- 338. Voragen A. G. J., Coenen G. J., Verhoef R. P., Schols H. A., Struct. Chem. 2009, 20, 263. [Google Scholar]
- 339. Byun C., Zheng Y., Pierce A., Wagner W. L., Scheller H. V., Mohnen D., Ackermann M., Mentzer S. J., Molecules 2019, 25, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Jarvis M. C., Planta 1982, 154, 344. [DOI] [PubMed] [Google Scholar]
- 341. Guo X., Han D., Xi H., Rao L., Liao X., Hu X., Wu J., Carbohydr. Polym. 2012, 88, 441. [Google Scholar]
- 342. Xu J., Zhao X., Wang Z., Xu H., Hu J., Ma J., Liu Y., Small 2019, 15, 1803970. [DOI] [PubMed] [Google Scholar]
- 343. Groult S., Budtova T., Eur. Polym. J. 2018, 108, 250. [DOI] [PubMed] [Google Scholar]
- 344. Goa K. L., Benfield P., Drugs 1994, 47, 536. [DOI] [PubMed] [Google Scholar]
- 345. Chong B. F., Blank L. M., Mclaughlin R., Nielsen L. K., Appl. Microbiol. Biotechnol. 2005, 66, 341. [DOI] [PubMed] [Google Scholar]
- 346. Sumogod A., Nacional L., Nillos M. G., Yap E. E., Asian Fish. Sci. 2020, 33, 1. [Google Scholar]
- 347. Salas C., Thompson Z., Bhattarai N., in Electrospun Nanofibers (Ed: Afshari M.), Elsevier, Amsterdam: 2017, Ch. 15. [Google Scholar]
- 348. Sivashankari P. R., Prabaharan M., in Chitosan Based Biomaterials, Vol. 1, (Eds: Jennings J. A., Bumgardner J. D.), Elsevier, Amsterdam: 2017, Ch. 5. [Google Scholar]
- 349. Rizzi V., Gubitosa J., Fini P., Romita R., Nuzzo S., Cosma P., Materials 2019, 12, 3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Yadav M., Goswami P., Paritosh K., Kumar M., Pareek N., Vivekanand V., Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar]
- 351. Song Y. S., Kim M. W., Moon C., Seo D. J., Han Y. S., Jo Y. H., Noh M. Y., Park Y. K., Kim S. A., Kim Y. W., Jung W. J., Entomol. Res. 2018, 48, 227. [Google Scholar]
- 352. Ruan J., Wang X., Yu Z., Wang Z., Xie Q., Zhang D., Huang Y., Zhou H., Bi X., Xiao C., Gu P., Fan X., Adv. Funct. Mater. 2016, 26, 1085. [Google Scholar]
- 353. Yang Y., Wang X., Yang F., Shen H., Wu D., Adv. Mater. 2016, 28, 7178. [DOI] [PubMed] [Google Scholar]
- 354. Guerrero‐Alburquerque N., Zhao S., Adilien N., Koebel M. M., Lattuada M., Malfait W. J., ACS Appl. Mater. Interfaces 2020, 12, 22037. [DOI] [PubMed] [Google Scholar]
- 355. Takeshita S., Yoda S., Chem. Mater. 2015, 27, 7569. [Google Scholar]
- 356. Diener M., Adamcik J., Sánchez‐Ferrer A., Jaedig F., Schefer L., Mezzenga R., Biomacromolecules 2019, 20, 1731. [DOI] [PubMed] [Google Scholar]
- 357. Li Z., Zhang S., Chen Y., Ling H., Zhao L., Luo G., Wang X., Hartel M. C., Liu H., Xue Y., Haghniaz R., Lee K., Sun W., Kim H., Lee J., Zhao Y., Zhao Y., Emaminejad S., Ahadian S., Ashammakhi N., Dokmeci M. R., Jiang Z., Khademhosseini A., Adv. Funct. Mater. 2020, 30, 2003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Kim J. R., Netravali A. N., Adv. Funct. Mater. 2016, 26, 4786. [Google Scholar]
- 359. Silvipriya K. S., Kumar K. K., Bhat A. R., Kumar B B. D., John A., Lakshmanan P., J. Appl. Pharm. Sci. 2015, 5, 123. [Google Scholar]
- 360. Alfaro A. T., Balbinot E., Weber C. I., Tonial I. B., Machado‐Lunkes A., Food Eng. Rev. 2015, 7, 33. [Google Scholar]
- 361. Market Analysis Report , Gelatin Market Size, Analysis, Industry Trends Report, 2020–2027, https://www.grandviewresearch.com/industry-analysis/gelatin-market-analysis (accessed: June 2021).
- 362. Harrington W. F., Von Hippel P. H., Advances in Protein Chemistry 1962, 16, 1. [DOI] [PubMed] [Google Scholar]
- 363. Courts A., Biochem. J. 1960, 74, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Gorgieva S., Kokol V., Biomaterials Applications for Nanomedicine, InTechOpen, London, 2011. [Google Scholar]
- 365. Kittiphattanabawon P., Benjakul S., Visessanguan W., Shahidi F., Eur. Food Res. Technol. 2010, 230, 475. [Google Scholar]
- 366. Wang S., Jiang D., Zhang Z., Chen Y., Yang Z., Zhang J., Shi J., Wang X., Yu J., Adv. Mater. 2019, 31, 1904341. [DOI] [PubMed] [Google Scholar]
- 367. Raeis‐Hosseini N., Park Y., Lee J.‐S., Adv. Funct. Mater. 2018, 28, 1800553. [Google Scholar]
- 368. Ying G., Jiang N., Parra‐Cantu C., Tang G., Zhang J., Wang H., Chen S., Huang N., Xie J., Zhang Y. S., Adv. Funct. Mater. 2020, 30, 2003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Swain S. N., Biswal S. M., Nanda P. K., Nayak P. L., J. Polym. Environ. 2004, 12, 35. [Google Scholar]
- 370. Tian H., Guo G., Fu X., Yao Y., Yuan L., Xiang A., Int. J. Biol. Macromol. 2018, 120, 475. [DOI] [PubMed] [Google Scholar]
- 371. Lindsay M. J., Walker T. W., Dumesic J. A., Rankin S. A., Huber G. W., Green Chem. 2018, 20, 1824. [Google Scholar]
- 372. Heck J. X., Hertz P. F., Ayub M. A. Z., Braz. J. Microbiol. 2002, 33, 213. [Google Scholar]
- 373. Martins E. H., Vilela A. P., Mendes R. F., Mendes L. M., Vaz L. E. V. S. B., Guimarães Junior J. B., Ciênc. Agrotecnol. 2018, 42, 186. [Google Scholar]
- 374. Moure A., Domínguez H., Parajó J. C., J. Agric. Food Chem. 2005, 53, 7600. [DOI] [PubMed] [Google Scholar]
- 375. Thrane M., Paulsen P. V., Orcutt M. W., Krieger T. M., in Sustainable Protein Sources (Eds: Nadathur S. R., Wanasundara J. P. D., Scanlin L.), Elsevier, Amsterdam: 2017, Ch. 2. [Google Scholar]
- 376. Wang L., Wu Z., Zhao B., Liu W., Gao Y., J. Food Eng. 2013, 119, 377. [Google Scholar]
- 377. Astley B. M., Greek yogurt waste ‘acid whey’ a concern for USDA: Jones Laffin, https://www.dairyreporter.com/Article/2014/01/30/Greek-yogurt-waste-acid-whey-a-concern-for-USDA-Jones-Laffin (accessed: June 2021).
- 378. Schmid M., Held J., Hammann F., Schlemmer D., Noller K., Packag. Technol. Sci. 2015, 28, 883. [Google Scholar]
- 379. Farooq M. A., Aquib M., Ghayas S., Bushra R., Haleem Khan D., Parveen A., Wang B., Polym. Adv. Technol. 2019, 30, 2183. [Google Scholar]
- 380. Ahn S., Chantre C. O., Gannon A. R., Lind J. U., Campbell P. H., Grevesse T., O'Connor B. B., Parker K. K., Adv. Healthcare Mater. 2018, 7, 1701175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Elzoghby A. O., Elgohary M. M., Kamel N. M., in Protein and Peptide Nanoparticles for Drug Delivery (Ed: Donev R.), Elsevier, Amsterdam: 2015, Ch. 6. [Google Scholar]
- 382. Anderson T. J., Lamsal B. P., Cereal Chem. J. 2011, 88, 159. [Google Scholar]
- 383.EU Horizon 2020, Industrial Feather Waste Valorisation for Sustainable Keratin based Materials, https://cordis.europa.eu/project/id/723268 (accessed: June 2021).
- 384. Mu B., Li W., Xu H., Xu L., Yang Y., J. Mater. Chem. A 2018, 6, 10320. [Google Scholar]
- 385. Mu B., Hassan F., Wu Q., Yang Y., Compos. Sci. Technol. 2020, 200, 108462. [Google Scholar]
- 386. Fagbemi O. D., Sithole B., Tesfaye T., Sustainable Chem. Pharm. 2020, 17, 100267. [Google Scholar]
- 387. Karan H., Funk C., Grabert M., Oey M., Hankamer B., Trends Plant Sci. 2019, 24, 237. [DOI] [PubMed] [Google Scholar]
- 388. Gandini A., Lacerda T. M., Prog. Polym. Sci. 2015, 48, 1. [Google Scholar]
- 389. Monica F. D., Kleij A. W., Polym. Chem. 2020, 11, 5109. [Google Scholar]
- 390. Davidowski S., DiMarco B., PerkinElmer Application Note 2009, 1, https://www.perkinelmer.com/PDFs/downloads/APP_Limonene_In_Citrus_Rinds_By_GCMS.pdf (accessed June 2021). [Google Scholar]
- 391. Gerchman D., Bones B., Pereira M. B., Takimi A. S., Prog. Org. Coat. 2019, 129, 133. [Google Scholar]
- 392. Arachchilage I. H. A. M., Patel M. K., Basi J., Harmon J. P., Polym. Eng. Sci. 2020, 60, 607. [Google Scholar]
- 393. Heredia‐Guerrero J. A., Heredia A., Domínguez E., Cingolani R., Bayer I. S., Athanassiou A., Benítez J. J., J. Exp. Bot. 2017, 68, 5401. [DOI] [PubMed] [Google Scholar]
- 394. Osman S. F., Irwin P., Fett W. F., Connor J. V. O., Parris N., J. Agric. Food Chem. 1999, 47, 799. [DOI] [PubMed] [Google Scholar]
- 395. Gómez‐Patiño M. B., Gutiérrez‐Salgado D. Y., García‐Hernández E., Mendez‐Mendez J. V., Andraca Adame J. A., Campos‐Terán J., Arrieta‐Baez D., Front. Mater. 2015, 2, 67. [Google Scholar]
- 396. Benítez J. J., Heredia‐Guerrero J. A., Guzmán‐Puyol S., Barthel M. J., Domínguez E., Heredia A., Front. Mater. 2015, 2, 59. [Google Scholar]
- 397. Heredia‐Guerrero J. A., Heredia A., García‐Segura R., Benítez J. J., Polymer 2009, 50, 5633. [Google Scholar]
- 398. Gandini A., Pascoal Neto C., Silvestre A. J. D., Prog. Polym. Sci. 2006, 31, 878. [Google Scholar]
- 399. Sousa A. F., Gandini A., Silvestre A. J. D., Pascoal Neto C., ChemSusChem 2008, 1, 1020. [DOI] [PubMed] [Google Scholar]
- 400. Olsson A., Lindström M., Iversen T., Biomacromolecules 2007, 8, 757. [DOI] [PubMed] [Google Scholar]
- 401. Matos M., Barreiro M. F., Gandini A., Ind. Crops Prod. 2010, 32, 7. [Google Scholar]
- 402. Pavier C., Gandini A., Ind. Crops Prod. 2000, 12, 1. [Google Scholar]
- 403. Pavier C., Gandini A., Carbohydr. Polym. 2000, 42, 13. [Google Scholar]
- 404. Pavier C., Gandini A., Eur. Polym. J. 2000, 36, 1653. [Google Scholar]
- 405. Lin C. S. K., Pfaltzgraff L. A., Herrero‐Davila L., Mubofu E. B., Abderrahim S., Clark J. H., Koutinas A. A., Kopsahelis N., Stamatelatou K., Dickson F., Thankappan S., Mohamed Z., Brocklesby R., Luque R., Energy Environ. Sci. 2013, 6, 426. [Google Scholar]
- 406. Bhunia H. P., Nando G. B., Chaki T. K., Basak A., Lenka S., Nayak P. L., Eur. Polym. J. 1999, 35, 1381. [Google Scholar]
- 407. Philip J. Y. N., Buchweishaija J., Mkayula L. L., Ye L., J. Agric. Food Chem. 2007, 55, 8870. [DOI] [PubMed] [Google Scholar]
- 408. Poland S. J., Darensbourg D. J., Green Chem. 2017, 19, 4990. [Google Scholar]
- 409. Thomsett M. R., Stockman R. A., Moore J. C., Buchard A., Howdle S. M., Green Chem. 2019, 21, 149. [Google Scholar]
- 410. Grignard B., Gennen S., Jérôme C., Kleij A. W., Detrembleur C., Chem. Soc. Rev. 2019, 48, 4466. [DOI] [PubMed] [Google Scholar]
- 411. Rosatella A. A., Simeonov S. P., Frade R. F. M., Afonso C. A. M., Green Chem. 2011, 13, 754. [Google Scholar]
- 412. Sousa A. F., Vilela C., Fonseca A. C., Matos M., Freire C. S. R., Gruter G.‐J. M., Coelho J. F. J., Silvestre A. J. D., Polym. Chem. 2015, 6, 5961. [Google Scholar]
- 413. Van de Vyver S., Román‐Leshkov Y., Catal. Sci. Technol. 2013, 3, 1465. [Google Scholar]
- 414. Tachibana Y., Masuda T., Funabashi M., Kunioka M., Biomacromolecules 2010, 11, 2760. [DOI] [PubMed] [Google Scholar]
- 415. Gallezot P., Chem. Soc. Rev. 2012, 41, 1538. [DOI] [PubMed] [Google Scholar]
- 416. Seachrist D. D., Bonk K. W., Ho S.‐M., Prins G. S., Soto A. M., Keri R. A., Reprod. Toxicol. 2016, 59, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Fenouillot F., Rousseau A., Colomines G., Saint‐Loup R., Pascault J.‐P., Prog. Polym. Sci. 2010, 35, 578. [Google Scholar]
- 418. Skoog E., Shin J. H., Saez‐Jimenez V., Mapelli V., Olsson L., Biotechnol. Adv. 2018, 36, 2248. [DOI] [PubMed] [Google Scholar]
- 419. Vardon D. R., Franden M. A., Johnson C. W., Karp E. M., Guarnieri M. T., Linger J. G., Salm M. J., Strathmann T. J., Beckham G. T., Energy Environ. Sci. 2015, 8, 617. [Google Scholar]
- 420. Wang Y., Tashiro Y., Sonomoto K., J. Biosci. Bioeng. 2015, 119, 10. [DOI] [PubMed] [Google Scholar]
- 421. Ahmad A., Banat F., Taher H., Environ. Technol. Innovation 2020, 20, 101138. [Google Scholar]
- 422. Abdulkhani A., Amiri E., Sharifzadeh A., Hedjazi S., Alizadeh P., J. Environ. Manage. 2019, 231, 819. [DOI] [PubMed] [Google Scholar]
- 423. Chopda R., Ferreira J. A., Taherzadeh M. J., Processes 2020, 8, 435. [Google Scholar]
- 424. Hortsch R., Corvo P., Chem. Ing. Tech. 2020, 92, 1803. [Google Scholar]
- 425. Morschbacker A., Polym. Rev. 2009, 49, 79. [Google Scholar]
- 426. Mendieta C. M., Vallejos M. E., Felissia F. E., Chinga‐Carrasco G., Area M. C., J. Polym. Environ. 2020, 28, 1. [Google Scholar]
- 427.Braskem, The life cycle assessment of its green plastic, http://plasticoverde.braskem.com.br/Portal/Principal/Arquivos/Download/Upload/GreenPE-LCASummary2017-CarbonTrust_v.2_230.pdf (accessed: June 2021).
- 428. Klemm D., Schumann D., Kramer F., Heßler N., Hornung M., Schmauder H. P., Marsch S., in Polysaccharides II (Ed.: Klemm D.), Springer, Amsterdam: 2006, Ch. 2. [Google Scholar]
- 429. Czaja W., Romanovicz D., malcolm Brown R., Cellulose 2004, 11, 403. [Google Scholar]
- 430. Gupta A., Briffa S. M., Swingler S., Gibson H., Kannappan V., Adamus G., Kowalczuk M., Martin C., Radecka I., Biomacromolecules 2020, 21, 1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Melo P. T. S., Otoni C. G., Barud H. S., Aouada F. A., Moura M. R., ACS Appl. Mater. Interfaces 2020, 12, 46661. [DOI] [PubMed] [Google Scholar]
- 432. Lehtonen J., Hassinen J., Kumar A. A., Johansson L. S., Mäenpää R., Pahimanolis N., Pradeep T., Ikkala O., Rojas O. J., Cellulose 2020, 27, 10719. [Google Scholar]
- 433. Wang S., Jiang F., Xu X., Kuang Y., Fu K., Hitz E., Hu L., Adv. Mater. 2017, 29, 1702498. [DOI] [PubMed] [Google Scholar]
- 434. Wang S., Li T., Chen C., Kong W., Zhu S., Dai J., Diaz A. J., Hitz E., Solares S. D., Li T., Hu L., Adv. Funct. Mater. 2018, 28, 1707491. [Google Scholar]
- 435. Greca L. G., Lehtonen J., Tardy B. L., Guo J., Rojas O. J., Mater. Horiz. 2018, 5, 408. [Google Scholar]
- 436. Greca L. G., Rafiee M., Karakoç A., Lehtonen J., Mattos B. D., Tardy B. L., Rojas O. J., ACS Nano 2020, 14, 12929. [DOI] [PubMed] [Google Scholar]
- 437. Schaffner M., Rühs P. A., Coulter F., Kilcher S., Studart A. R., Sci. Adv. 2017, 3, eaao6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Shin S., Kwak H., Shin D., Hyun J., Nat. Commun. 2019, 10, 4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Yang J., Wang L., Zhang W., Sun Z., Li Y., Yang M., Zeng D., Peng B., Zheng W., Jiang X., Yang G., Small 2018, 14, 1702582. [DOI] [PubMed] [Google Scholar]
- 440. Rühs P. A., Malollari K. G., Binelli M. R., Crockett R., Balkenende D. W. R., Studart A. R., Messersmith P. B., ACS Nano 2020, 14, 3885. [DOI] [PubMed] [Google Scholar]
- 441. Hussain Z., Sajjad W., Khan T., Wahid F., Cellulose 2019, 26, 2895. [DOI] [PubMed] [Google Scholar]
- 442. Andriani D., Apriyana A. Y., Karina M., Cellulose 2020, 27, 6747. [Google Scholar]
- 443. Molina‐Ramírez C., Castro C., Zuluaga R., Gañán P., J. Polym. Environ. 2018, 26, 830. [Google Scholar]
- 444. Żywicka A., Junka A. F., Szymczyk P., Chodaczek G., Grzesiak J., Sedghizadeh P. P., Fijałkowski K., Carbohydr. Polym. 2018, 199, 294. [DOI] [PubMed] [Google Scholar]
- 445. Wu M., Chen W., Hu J., Tian D., Shen F., Zeng Y., Yang G., Zhang Y., Deng S., Sci. Total Environ. 2019, 695, 133898. [DOI] [PubMed] [Google Scholar]
- 446. Wang Q., Tian D., Hu J., Huang M., Shen F., Zeng Y., Yang G., Zhang Y., He J., ACS Sustainable Chem. Eng. 2020, 8, 13400. [Google Scholar]
- 447. Molina‐Ramírez C., Cañas‐Gutiérrez A., Castro C., Zuluaga R., Gañán P., Carbohydr. Polym. 2020, 240, 116341. [DOI] [PubMed] [Google Scholar]
- 448. Lin D., Lopez‐Sanchez P., Li R., Li Z., Bioresour. Technol. 2014, 151, 113. [DOI] [PubMed] [Google Scholar]
- 449. Fan X., Gao Y., He W., Hu H., Tian M., Wang K., Pan S., Carbohydr. Polym. 2016, 151, 1068. [DOI] [PubMed] [Google Scholar]
- 450. Güzel M., Akpınar Ö., Waste Biomass Valorization 2019, 10, 2165. [Google Scholar]
- 451. Tsouko E., Maina S., Ladakis D., Kookos I. K., Koutinas A., Renewable Energy 2020, 160, 944. [Google Scholar]
- 452. Costa A. F. S., Almeida F. C. G., Vinhas G. M., Sarubbo L. A., Front. Microbiol. 2017, 8, 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Nawawi W. M. F. B. W., Jones M., Murphy R. J., Lee K. Y., Kontturi E., Bismarck A., Biomacromolecules 2020, 21, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Jones M., Gandia A., John S., Bismarck A., Nat. Sustain. 2020, 4, 9. [Google Scholar]
- 455. Nguyen P. Q., Courchesne N. M. D., Duraj‐Thatte A., Praveschotinunt P., Joshi N. S., Adv. Mater. 2018, 30, 1704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Jones M., Mautner A., Luenco S., Bismarck A., John S., Mater. Des. 2020, 187, 108397. [Google Scholar]
- 457. Haneef M., Ceseracciu L., Canale C., Bayer I. S., Heredia‐Guerrero J. A., Athanassiou A., Sci. Rep. 2017, 7, 41292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Nawawi W. M. F. W., Jones M. P., Kontturi E., Mautner A., Bismarck A., Compos. Sci. Technol. 2020, 198, 108327. [Google Scholar]
- 459. Janesch J., Jones M., Bacher M., Kontturi E., Bismarck A., Mautner A., React. Funct. Polym. 2020, 146, 104428. [Google Scholar]
- 460. Jones M., Weiland K., Kujundzic M., Theiner J., Kählig H., Kontturi E., John S., Bismarck A., Mautner A., Biomacromolecules 2019, 20, 3513. [DOI] [PubMed] [Google Scholar]
- 461. Jones M., Bhat T., Huynh T., Kandare E., Yuen R., Wang C. H., John S., Fire Mater. 2018, 42, 816. [Google Scholar]
- 462. Jones M., Bhat T., Kandare E., Thomas A., Joseph P., Dekiwadia C., Yuen R., John S., Ma J., Wang C. H., Sci. Rep. 2018, 8, 17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Joshi K., Meher M. K., Poluri K. M., ACS Appl. Bio Mater. 2020, 3, 1884. [DOI] [PubMed] [Google Scholar]
- 464. Ongpeng J. M. C., Inciong E., Sendo V., Soliman C., Siggaoat A., Appl. Sci. 2020, 10, 5303. [Google Scholar]
- 465. Khoo S. C., Peng W. X., Yang Y., Ge S. B., Soon C. F., Ma N. L., Sonne C., J. Hazard. Mater. 2020, 400, 123296. [DOI] [PubMed] [Google Scholar]
- 466. Jones M. P., Lawrie A. C., Huynh T. T., Morrison P. D., Mautner A., Bismarck A., John S., Process Biochem. 2019, 80, 95. [Google Scholar]
- 467. Hasanin M. S., Hashem A. H., J. Microbiol. Methods 2020, 168, 105802. [DOI] [PubMed] [Google Scholar]
- 468. Chae H. J., Ahn J. H., Int. Biodeterior. Biodegrad. 2013, 80, 66. [Google Scholar]
- 469. El‐malek F. A., Khairy H., Farag A., Omar S., Int. J. Biol. Macromol. 2020, 157, 319. [DOI] [PubMed] [Google Scholar]
- 470. Amaro T. M. M. M., Rosa D., Comi G., Iacumin L., Front. Microbiol. 2019, 10, 992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Nielsen C., Rahman A., Rehman A. U., Walsh M. K., Miller C. D., Microb. Biotechnol. 2017, 10, 1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Ravindran R., Jaiswal A. K., Trends Biotechnol. 2016, 34, P58. [DOI] [PubMed] [Google Scholar]
- 473. Chen M., Zhang X., Liu C., Sun R., Lu F., ACS Sustainable Chem. Eng. 2014, 2, 1164. [Google Scholar]
- 474. Bayer I. S., Guzman‐Puyol S., Heredia‐Guerrero J. A., Ceseracciu L., Pignatelli F., Ruffilli R., Cingolani R., Athanassiou A., Macromolecules 2014, 47, 5135. [Google Scholar]
- 475. Kiper A. G., Özyuguran A., Yaman S., J. Mater. Cycles Waste Manage. 2020, 22, 1999. [Google Scholar]
- 476. Zhao H., Holladay J. E., Kwak J. H., Zhang Z. C., J. Phys. Chem. B 2007, 111, 5295. [DOI] [PubMed] [Google Scholar]
- 477. Kun D., Pukánszky B., Eur. Polym. J. 2017, 93, 618. [Google Scholar]
- 478. Kai D., Tan M. J., Chee P. L., Chua Y. K., Yap Y. L., Loh X. J., Green Chem. 2016, 18, 1175. [Google Scholar]
- 479. Jin X., Hu Z., Wu S., Song T., Yue F., Xiang Z., Carbohydr. Polym. 2019, 215, 235. [DOI] [PubMed] [Google Scholar]
- 480. Li Y., He T., Liang R., Luo Z., Zhu Y., Yang C., Int. J. Biol. Macromol. 2018, 106, 1279. [DOI] [PubMed] [Google Scholar]
- 481. Eriksen M. K., Christiansen J. D., Daugaard A. E., Astrup T. F., Waste Manage. 2019, 96, 75. [DOI] [PubMed] [Google Scholar]
- 482. Andrade R. M. S., Ferreira M. S. L., Gonçalves É. C. B. A., J. Food Sci. 2016, 81, E412. [DOI] [PubMed] [Google Scholar]
- 483. Otoni C. G., Lodi B. D., Lorevice M. V., Leitão R. C., Ferreira M. D., Moura M. R., Mattoso L. H. C., Ind. Crops Prod. 2018, 121, 66. [Google Scholar]
- 484. Wu H., Lei Y., Zhu R., Zhao M., Lu J., Xiao D., Jiao C., Zhang Z., Shen G., Li S., Food Hydrocolloids 2019, 90, 41. [Google Scholar]
- 485. Perotto G., Ceseracciu L., Simonutti R., Paul U. C., Guzman‐Puyol S., Tran T.‐N. N., Bayer I. S., Athanassiou A., Green Chem. 2018, 20, 894. [Google Scholar]
- 486. Fai A. E. C., Souza M. R. A., Barros S. T., Bruno N. V., Ferreira M. S. L., Gonçalves É. C. B. A., Postharvest Biol. Technol. 2016, 112, 194. [Google Scholar]
- 487. Famá L., Gerschenson L., Goyanes S., Carbohydr. Polym. 2009, 75, 230. [Google Scholar]
- 488.Tridge, Market intelligence, https://www.tridge.com/ (accessed: June 2021).
- 489. Kang H. J., Min S. C., LWT ‐ Food Sci. Technol. 2010, 43, 903. [Google Scholar]
- 490. Liu M., Arshadi M., Javi F., Lawrence P., Davachi S. M., Abbaspourrad A., J. Cleaner Prod. 2020, 276, 123353. [Google Scholar]
- 491. Mohammadi Nafchi A., Moradpour M., Saeidi M., Alias A. K., Starch/Stärke 2013, 65, 61. [Google Scholar]
- 492. Paggiola G., Van Stempvoort S., Bustamante J., Barbero J. M. V., Hunt A. J., Clark J. H., Biofuels, Bioprod. Biorefin. 2016, 10, 686. [Google Scholar]
- 493. Mangalara S. C. H., Varughese S., ACS Sustainable Chem. Eng. 2016, 4, 6095. [Google Scholar]
- 494. Shin C., Chase G. G., Polym. Bull. 2005, 55, 209. [Google Scholar]
- 495. Miranda K. W. E., Mattoso L. H. C., Bresolin J. D., Hubinger S. Z., Medeiros E. S., Oliveira J. E., J. Appl. Polym. Sci. 2019, 136, 47337. [Google Scholar]
- 496. Ververi M., Goula A. M., Chem. Eng. Process. ‐ Process Intensif. 2019, 138, 86. [Google Scholar]
- 497. Lu Z., Ye F., Zhou G., Gao R., Qin D., Zhao G., Food Chem. 2020, 330, 127325. [DOI] [PubMed] [Google Scholar]
- 498. Aliakbarian B., Paini M., Adami R., Perego P., Reverchon E., Innovative Food Sci. Emerging Technol. 2017, 40, 2. [Google Scholar]
- 499. Riaz A., Lagnika C., Abdin M., Hashim M. M., Ahmed W., J. Polym. Environ. 2020, 28, 411. [Google Scholar]
- 500. Jiang Y., Zhu Y., Li F., Du J., Huang Q., Sun‐Waterhouse D., Li D., Int. J. Biol. Macromol. 2020, 151, 193. [DOI] [PubMed] [Google Scholar]
- 501. Kamada A., Levin A., Toprakcioglu Z., Shen Y., Lutz‐Bueno V., Baumann K. N., Mohammadi P., Linder M. B., Mezzenga R., Knowles T. P. J., Small 2020, 16, 1904190. [DOI] [PubMed] [Google Scholar]
- 502. Otoni C. G., Avena‐Bustillos R. J., Azeredo H. M. C., Lorevice M. V., Moura M. R., Mattoso L. H. C., McHugh T. H., Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151. [DOI] [PubMed] [Google Scholar]
- 503. Reyes G., Borghei M., King A. W. T., Lahti J., Rojas O. J., Biomacromolecules 2019, 20, 502. [DOI] [PubMed] [Google Scholar]
- 504. Khakalo A., Tanaka A., Korpela A., Hauru L. K. J., Orelma H., ACS Sustainable Chem. Eng. 2019, 7, 3195. [Google Scholar]
- 505. Tardy B. L., Richardson J. J., Greca L. G., Guo J., Ejima H., Rojas O. J., Adv. Mater. 2020, 32, 1906886. [DOI] [PubMed] [Google Scholar]
- 506. Mekonnen T., Mussone P., Khalil H., Bressler D., J. Mater. Chem. A 2013, 1, 13379. [Google Scholar]
- 507. Santos D. M., Correa D. S., Medeiros E. S., Oliveira J. E., Mattoso L. H. C., ACS Appl. Mater. Interfaces 2020, 12, 45673. [DOI] [PubMed] [Google Scholar]
- 508. Kuan C., Yuen K., Liong M., Br. Food J. 2012, 114, 853. [Google Scholar]
- 509. Fung W.‐Y., Yuen K.‐H., Liong M.‐T., J. Agric. Food Chem. 2011, 59, 8140. [DOI] [PubMed] [Google Scholar]
- 510. Kuan C.‐Y., Yuen K.‐H., Bhat R., Liong M.‐T., Food Res. Int. 2011, 44, 2822. [Google Scholar]
- 511. Figueroa‐Lopez K. J., Torres‐Giner S., Enescu D., Cabedo L., Cerqueira M. A., Pastrana L. M., Lagaron J. M., Nanomaterials 2020, 10, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Martínez‐Sanz M., Lopez‐Rubio A., Villano M., Oliveira C. S. S., Majone M., Reis M., Lagarón J. M., J. Appl. Polym. Sci. 2016, 133, 42486. [Google Scholar]
- 513. Mercante L. A., Scagion V. P., Migliorini F. L., Mattoso L. H. C., Correa D. S., TrAC, Trends Anal. Chem. 2017, 91, 91. [Google Scholar]
- 514. Yang T., Zhan L., Huang C. Z., TrAC, Trends Anal. Chem. 2020, 124, 115813. [Google Scholar]
- 515. Mane P. P., Ambekar R. S., Kandasubramanian B., Int. J. Pharm. 2020, 583, 119364. [DOI] [PubMed] [Google Scholar]
- 516. Rahmati M., Mills D. K., Urbanska A. M., Saeb M. R., Venugopal J. R., Ramakrishna S., Mozafari M., Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar]
- 517. Jeevahan J. J., Chandrasekaran M., Venkatesan S. P., Sriram V., Britto Joseph G., Mageshwaran G., Durairaj R. B., Trends Food Sci. Technol. 2020, 100, 210. [Google Scholar]
- 518. Parisotto E. I. B., Teleken J. T., Laurindo J. B., Carciofi B. A. M., Drying Technol. 2020, 38, 1024. [Google Scholar]
- 519. Munhoz D. R., Moreira F. K. V., Bresolin J. D., Bernardo M. P., Sousa C. P., Mattoso L. H. C., ACS Sustainable Chem. Eng. 2018, 6, 9883. [Google Scholar]
- 520. Piccinno F., Hischier R., Seeger S., Som C., ACS Sustainable Chem. Eng. 2015, 3, 1047. [Google Scholar]
- 521. Claro P. I. C., Corrêa A. C., Campos A., Rodrigues V. B., Luchesi B. R., Silva L. E., Mattoso L. H. C., Marconcini J. M., Carbohydr. Polym. 2018, 181, 1093. [DOI] [PubMed] [Google Scholar]
- 522. Kade J. C., Dalton P. D., Adv. Healthcare Mater. 2021, 10, 2001232. [Google Scholar]
- 523. Bahçegül E. G., Bahçegül E., Özkan N., ACS Appl. Polym. Mater. 2020, 2, 2622. [Google Scholar]
- 524. Ferreira F. V., Otoni C. G., De France K. J., Barud H. S., Lona L. M. F., Cranston E. D., Rojas O. J., Mater. Today 2020, 37, 126. [Google Scholar]
- 525. Lavoine N., Bergström L., J. Mater. Chem. A 2017, 5, 16105. [Google Scholar]
- 526. Cancelliere R., Zurlo F., Micheli L., Melino S., Talanta 2021, 223, 121671. [DOI] [PubMed] [Google Scholar]
- 527. Lee J., Jung H., Park N., Park S.‐H., Ju J. H., Sci. Rep. 2019, 9, 20194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Koeppel A., Holland C., ACS Biomater. Sci. Eng. 2017, 3, 226. [DOI] [PubMed] [Google Scholar]
- 529. Irfan M., Rabel S., Bukhtar Q., Qadir M. I., Jabeen F., Khan A., Saudi Pharm. J. 2016, 24, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Costantini M., Colosi C., Święszkowski W., Barbetta A., Biofabrication 2018, 11, 012001. [DOI] [PubMed] [Google Scholar]
- 531. Moro G., Bottari F., Van Loon J., Du Bois E., De Wael K., Moretto L. M., Biosens. Bioelectron. 2019, 146, 111758. [DOI] [PubMed] [Google Scholar]
- 532. Cataldi P., Condurache O., Spirito D., Krahne R., Bayer I. S., Athanassiou A., Perotto G., ACS Sustainable Chem. Eng. 2019, 7, 12544. [Google Scholar]
- 533. Irimia‐Vladu M., Chem. Soc. Rev. 2014, 43, 588. [DOI] [PubMed] [Google Scholar]
- 534. Hwang S. W., Song J. K., Huang X., Cheng H., Kang S. K., Kim B. H., Kim J. H., Yu S., Huang Y., Rogers J. A., Adv. Mater. 2014, 26, 3905. [DOI] [PubMed] [Google Scholar]
- 535. Hwang S. W., Tao H., Kim D. H., Cheng H., Song J. K., Rill E., Brenckle M. A., Panilaitis B., Won S. M., Kim Y. S., Song Y. M., Yu K. J., Ameen A. A., Li R., Su Y., Yang M., Kaplan D. L., Zakin M. R., Slepian M. J., Huang Y., Omenetto F. G., Rogers J. A., Science 2012, 337, 1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Han W. B., Lee J. H., Shin J., Hwang S., Adv. Mater. 2020, 2002211. [DOI] [PubMed] [Google Scholar]
- 537. Güder F., Ainla A., Redston J., Mosadegh B., Glavan A., Martin T. J., Whitesides G. M., Angew. Chem., Int. Ed. 2016, 55, 5727. [DOI] [PubMed] [Google Scholar]
- 538. Tortorich R. P., Shamkhalichenar H., Choi J. W., Appl. Sci. 2018, 8, 288. [Google Scholar]
- 539. Singh A. T., Lantigua D., Meka A., Taing S., Pandher M., Camci‐Unal G., Sensors 2018, 18, 2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. López‐Marzo A. M., Merkoçi A., Lab Chip 2016, 16, 3150. [DOI] [PubMed] [Google Scholar]
- 541. Cinti S., Moscone D., Arduini F., Nat. Protoc. 2019, 14, 2437. [DOI] [PubMed] [Google Scholar]
- 542. Migliorini F. L., Santos D. M., Soares A. C., Mattoso L. H. C., Oliveira O. N. Jr, Correa D. S., Chemosensors 2020, 8, 87. [Google Scholar]
- 543. Tao H., Brenckle M. A., Yang M., Zhang J., Liu M., Siebert S. M., Averitt R. D., Mannoor M. S., McAlpine M. C., Rogers J. A., Kaplan D. L., Omenetto F. G., Adv. Mater. 2012, 24, 1067. [DOI] [PubMed] [Google Scholar]
- 544. Kang S. K., Koo J., Lee Y. K., Rogers J. A., Acc. Chem. Res. 2018, 51, 988. [DOI] [PubMed] [Google Scholar]
- 545. Kang S. K., Murphy R. K. J., Hwang S. W., Lee S. M., Harburg D. V., Krueger N. A., Shin J., Gamble P., Cheng H., Yu S., Liu Z., McCall J. G., Stephen M., Ying H., Kim J., Park G., Webb R. C., Lee C. H., Chung S., Wie D. S., Gujar A. D., Vemulapalli B., Kim A. H., Lee K. M., Cheng J., Huang Y., Lee S. H., Braun P. V., Ray W. Z., Rogers J. A., Nature 2016, 530, 71. [DOI] [PubMed] [Google Scholar]
- 546. Park J., Sempionatto J. R., Kim J., Jeong Y., Gu J., Wang J., Park I., ACS Sens. 2020, 5, 1363. [DOI] [PubMed] [Google Scholar]
- 547. Zhai Q., Yap L. W., Wang R., Gong S., Guo Z., Liu Y., Lyu Q., Wang J., Simon G. P., Cheng W., Anal. Chem. 2020, 92, 4647. [DOI] [PubMed] [Google Scholar]
- 548. Kim J., Campbell A. S., Wang J., Talanta 2018, 177, 163. [DOI] [PubMed] [Google Scholar]
- 549. Yano H., Sugiyama J., Nakagaito A. N., Nogi M., Matsuura T., Hikita M., Handa K., Adv. Mater. 2005, 17, 153. [Google Scholar]
- 550. Fresta E., Fernández‐Luna V., Coto P. B., Costa R. D., Adv. Funct. Mater. 2018, 28, 1707011. [Google Scholar]
- 551. Navarro J. R. G., Wennmalm S., Godfrey J., Breitholtz M., Edlund U., Biomacromolecules 2016, 17, 1101. [DOI] [PubMed] [Google Scholar]
- 552. Hubbe M. A., Rojas O. J., Lucia L. A., Sain M., Int. J. Interact. Mob. Technol. 2018, 12, 929. [Google Scholar]
- 553. Saxena S., Devnani H., Verma S. K., Satsangee S. P., in 2016 IEEE Region 10 Humanitarian Technology Conf., IEEE, Piscataway, NJ: 2016, pp. 1–6. [Google Scholar]
- 554. Noremberg B. S., Silva R. M., Paniz O. G., Alano J. H., Gonçalves M. R. F., Wolke S. I., Labidi J., Valentini A., Carreño N. L. V, Sens. Actuators, B 2017, 240, 459. [Google Scholar]
- 555. Andre R. S., Santos D. M., Mercante L. A., Facure M. H. M., Campana‐Filho S. P., Mattoso L. H. C., Correa D. S., J. Environ. Chem. Eng. 2020, 8, 104163. [Google Scholar]
- 556. Akhavan O., Bijanzad K., Mirsepah A., RSC Adv. 2014, 4, 20441. [Google Scholar]
- 557. Ruan G., Sun Z., Peng Z., Tour J. M., ACS Nano 2011, 5, 7601. [DOI] [PubMed] [Google Scholar]
- 558. Luong D. X., Bets K. V, Algozeeb W. A., Stanford M. G., Kittrell C., Chen W., Salvatierra R. V, Ren M., McHugh E. A., Advincula P. A., Wang Z., Bhatt M., Guo H., Mancevski V., Shahsavari R., Yakobson B. I., Tour J. M., Nature 2020, 577, 647. [DOI] [PubMed] [Google Scholar]
- 559. Panahi‐Kalamuei M., Amiri O., Salavati‐Niasari M., J. Mater. Res. Technol. 2020, 9, 2679. [Google Scholar]
- 560. Faraco T. A., Silva H. O. X., Barud H. S., Maciel I. O., Silva R. R., Quirino W. G., Fragneaud B., Ribeiro C. A., Dias D. S., Pandoli O. G., Cremona M., Legnani C., ACS Appl. Mater. Interfaces 2019, 11, 42420. [DOI] [PubMed] [Google Scholar]
- 561. Xu L., Yang X., Ding H., Li S., Li M., Wang D., Xia J., Mater. Sci. Eng. C 2019, 102, 917. [DOI] [PubMed] [Google Scholar]
- 562. Bastos‐Arrieta J., Florido A., Pérez‐Ràfols C., Serrano N., Fiol N., Poch J., Villaescusa I., Nanomaterials 2018, 8, 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563. Silva R. R., Raymundo‐Pereira P. A., Campos A. M., Wilson D., Otoni C. G., Barud H. S., Costa C. A. R., Domeneguetti R. R., Balogh D. T., Ribeiro S. J. L., Oliveira O. N. Jr, Talanta 2020, 218, 121153. [DOI] [PubMed] [Google Scholar]
- 564. Eynaki H., Kiani M. A., Golmohammadi H., Nanoscale 2020, 12, 18409. [DOI] [PubMed] [Google Scholar]
- 565. Gomes N. O., Carrilho E., Machado S. A. S., Sgobbi L. F., Electrochim. Acta 2020, 349, 136341. [Google Scholar]
- 566. Wang X., Xu W., Chatterjee P., Lv C., Popovich J., Song Z., Dai L., Kalani M. Y. S., Haydel S. E., Jiang H., Adv. Mater. Technol. 2016, 1, 1600059. [Google Scholar]
- 567. Xiong R., Singh A., Yu S., Zhang S., Lee H., Yingling Y. G., Nepal D., Bunning T. J., Tsukruk V. V., ACS Appl. Mater. Interfaces 2020, 12, 35345. [DOI] [PubMed] [Google Scholar]
- 568. Matharu A. S., Melo E. M., Remón J., Wang S., Abdulina A., Kontturi E., ChemSusChem 2018, 11, 1344. [DOI] [PubMed] [Google Scholar]
- 569. Noori A., El‐Kady M. F., Rahmanifar M. S., Kaner R. B., Mousavi M. F., Chem. Soc. Rev. 2019, 48, 1272. [DOI] [PubMed] [Google Scholar]
- 570. Chen D., Jiang K., Huang T., Shen G., Adv. Mater. 2020, 32, e1901806. [DOI] [PubMed] [Google Scholar]
- 571. Lin L., Lei W., Zhang S., Liu Y., Wallace G. G., Chen J., Energy Storage Mater. 2019, 19, 408. [Google Scholar]
- 572. Wu Y., Cao J. P., Zhao X. Y., Zhuang Q. Q., Zhou Z., Zhao M., Cui X., Zhao Y. P., Wei X. Y., Energy Technol. 2020, 8, 1901263. [Google Scholar]
- 573. Zhao J., Wu W., Jia X., Xia T., Li Q., Zhang J., Wang Q., Zhang W., Lu C., J. Mater. Chem. A 2020, 8, 18198. [Google Scholar]
- 574. Talekar S., Patti A. F., Vijayraghavan R., Arora A., ACS Sustainable Chem. Eng. 2018, 6, 16363. [Google Scholar]
- 575. Ahmed I., Lin H., Zou L., Li Z., Brody A. L., Qazi I. M., Lv L., Pavase T. R., Khan M. U., Khan S., Sun L., Packag. Technol. Sci. 2018, 31, 449. [Google Scholar]
- 576. Mustafa F., Andreescu S., Foods 2018, 7, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577. Lakshmipathy R., Palakshi Reddy B., Sarada N. C., Chidambaram K., Khadeer Pasha S., Appl. Nanosci. 2015, 5, 223. [Google Scholar]
- 578. Sivakumar P., Sivakumar P., Anbarasu K., Pandian K., Renganathan S., Ind. Eng. Chem. Res. 2013, 52, 17676. [Google Scholar]
- 579. Xu X., Su X., Bai B., Wang H., Suo Y., RSC Adv. 2016, 6, 103199. [Google Scholar]
- 580. Meng Y., Liu X., Li C., Liu H., Cheng Y., Lu J., Zhang K., Wang H., Int. J. Biol. Macromol. 2019, 135, 815. [DOI] [PubMed] [Google Scholar]
- 581. Schneider Teixeira A., Deladino L., Zaritzky N., ACS Sustainable Chem. Eng. 2016, 4, 2449. [Google Scholar]


