Abstract
Calcium (Ca2+) signaling is fundamental to cellular processes in both eukaryotic and prokaryotic organisms. While the mechanisms underlying eukaryotic Ca2+ transport are well documented, an understanding of prokaryotic transport remains nascent. LMCA1, a Ca2+ adenosine triphosphatase (ATPase) from Listeria monocytogenes, has emerged as a prototype for elucidating structure and dynamics in prokaryotic Ca2+ transport. Here, we used a multidisciplinary approach integrating kinetics, structure, and dynamics to unravel the intricacies of LMCA1 function. A cryo–electron microscopy (cryo-EM) structure of a Ca2+-bound E1 state showed ion coordination by Asp720, Asn716, and Glu292. Time-resolved x-ray solution scattering experiments identified phosphorylation as the rate-determining step. A cryo-EM E2P state structure exhibited remarkable similarities to a SERCA1a E2-P* state, which highlights the essential role of the unique P-A domain interface in enhancing dephosphorylation rates and reconciles earlier proposed mechanisms. Our study underscores the distinctiveness between eukaryotic and prokaryotic Ca2+ ATPase transport systems and positions LMCA1 as a promising drug target for developing antimicrobial strategies.
The structure and dynamics of a bacterial calcium transporter highlight differences in eukaryotic and prokaryotic Ca2+ transport.
INTRODUCTION
Calcium ions (Ca2+) are critical secondary messengers in the eukaryotic cell that regulate a wide variety of physiological processes, such as controlling the heart and skeletal muscle, as well as nerve function. Therefore, dysregulation of calcium is a hallmark of several disease conditions (1). Eukaryotic cells respond to transient increases in nanomolar-to-micromolar levels of Ca2+ concentration resulting from protein-mediated influx from the environment or intracellular compartments. The signaling is then terminated by primary [Ca2+ P-type adenosine triphosphatases (ATPases)] and secondary (H+/Ca2+ or Na+/Ca2+ exchangers) active membrane protein transporters that restore cytosolic Ca2+ concentration to a couple of hundred nanomolar. While the importance and consequences of Ca2+-mediated signaling in eukaryotes are well established, the role of cytosolic Ca2+ concentration in prokaryotes—and the underlying molecular mechanisms for Ca2+ transport—is still emerging.
Bacteria lack membrane-bound organelles, such as the endoplasmic reticulum and mitochondria, which are central in eukaryotic Ca2+ signaling. Nevertheless, bacterial Ca2+-stimulus responses have been observed during environmental stress, exposure to toxicants, and during infection (2). A Ca2+-transporting P-type ATPase in the gram-positive bacterium Listeria monocytogenes (LMCA1) has emerged as a model system to understand prokaryotic Ca2+ efflux during signaling (3–6). Being situated in the cell membrane, LMCA1 transporters enable bacterial survival when engulfed by host cell phagosomes, in which the Ca2+ concentration reaches millimolar levels (7). In addition, LMCA1 is up-regulated at the transcriptional level at high pH (8), which could potentially be an effect of H+/Ca2+ exchangers, which typically regulate cytosolic Ca2+ levels (9), losing their H+ gradient driving force. Listeria can consequently thrive at alkaline pH in phagosomes (10) and pancreatic secretions (11), as well as mild alkaline treatments in the food industry (12). Therefore, LMCA1 is a potential drug target with implications for human health and commercial food processing.
All Ca2+ ATPases share a common fold consisting of the actuator (A), nucleotide (N), and phosphorylation (P) domains in the cytoplasm—and 10 transmembrane helices (TM1–10) in the membrane (M) domain (Fig. 1A) (13). This conserved structural framework enables ATP-fueled transitioning in-between high- (E1) and low-affinity (E2) states that are open to the cytoplasm and extracellular compartment, respectively. The molecular details associated with Ca2+ transport by ATP-dependent P-type ATPases originate to a large extent from eukaryotes, particularly from crystal structures trapping the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) in different reaction states (Fig. 1B) (14). In the E1 state, the binding of Ca2+ leads to the phosphorylation of a conserved aspartic acid in the P domain and subsequent compaction of the cytoplasmic domains to form a phosphorylated E1 (E1P) state. In the next step, the A domain rotates extensively to expose and release adenosine 5′-diphosphate (ADP) from the phosphorylation site while maintaining Ca2+ bound in the M domain. “High-energy” states associated with the Ca2+-bound E1P and E2P states have not been amendable with crystallization but have been observed using time-resolved x-ray solution scattering (TR-XSS) (15), molecular dynamics (MD) simulations (16), and single-molecule fluorescence resonance energy transfer (sm-FRET) (4). A continued A domain rotation puts a conserved TGES loop in the A domain in a position to shield the phosphorylation site in the P domain and thereby prevent the reverse reaction by ADP. Hence, the formation of this so-called ADP-insensitive E2P state ensures the directionality of the transport. In the E2P state, the M domain is opened to the outside, and Ca2+ exchanges for H+, which again closes the M domain. Dephosphorylation forms an E2 state, which, after H+ release to the cytoplasm, reforms the inward-open E1 state.
Fig. 1. Ca2+ P-type ATPase structure topology and reaction cycle.
(A) A schematic showing the 10 TM helices in the M domain and the soluble actuator (A), nucleotide-binding (N), and phosphorylation (P) domains. (B) A reaction cycle showing the principal intermediates states in Ca2+ P-type ATPase transport. The intermediate state structures determined in this study are highlighted in pink.
The mammalian SERCA isoforms show 34 to 39% sequence identity with the bacterial homolog LMCA1 (5). Nevertheless, with increased characterization of LMCA1 structure, dynamics, and kinetics (4–6), it has become clear that evolutionary divergence has resulted in mechanistic differences in eukaryotic and prokaryotic calcium transport. Several such organism-specific differences in calcium transport have emerged by comparing SERCA1a and LMCA1 ATPases: (i) LMCA1 undergoes rapid dephosphorylation and closure of the Ca2+ release pathway (4, 6). Such structural dynamics could potentially reflect the adaptation of prokaryotes to minimize exposure to an environment altered by defense mechanisms. (ii) While SERCA1a binds two Ca2+ ions, only one Ca2+ ion can be accommodated in LMCA1 (5). (iii) The LMCA1 transporter shows higher activity at high pH, which has been attributed to an arginine residue (Arg795) in the M domain (6).
A potentially unique bacterial dephosphorylation mechanism
The LMCA1 activity has been observed to be >4 times higher than SERCA1a in detergent micelles (5), which is a consequence of variations in the reaction mechanism between the two Ca2+ transporters. While, for SERCA1a, the rate-limiting step is the E1P-to-E2P transition (17), the rate-limiting step for LMCA1 is phosphorylation (4). In other words, LMCA1 dephosphorylates more rapidly. These observed differences in rate-limiting states between bacterial and eukaryotic calcium transport can potentially be explained by differences in transitions associated with extracellular Ca2+ release and protonation in the dephosphorylation step. Two detergent-solubilized wild-type LMCA1 structures determined by x-ray crystallography were trapped in states before and during dephosphorylation using phosphate analogs (6). The structures were determined in the presence of BeF3− and AlF4−, which have been shown to trap SERCA1a in a phosphorylated E2 state (E2P) and intermediate state of dephosphorylation (E2-P*), respectively (14). In the corresponding SERCA1a crystal structure of the phosphorylated E2P state, the TGES loop of the A domain was rotated to enable interaction with the phosphorylation site in the P domain to prevent ADP from inducing the reversed reaction. In addition, the M domain in this crystal structure was open toward the extracellular side. Dephosphorylation is associated with the protonation of negatively charged residues in the M domain, which results in the closing of the calcium release path. The SERCA1a E2-P* crystal structure showed a slight further rotation of the A domain to relocate Glu183 in the TGES loop toward the phosphorylation site. In this position, Glu183 can activate a water molecule for nucleophilic attack and hence dephosphorylation (18). Consequently, the M domain in this crystal structure was closed.
In contrast, the E2P and E2-P* LMCA1 crystal structures showed a higher degree of structural similarity, and both corresponded to an E2-P* intermediate state of dephosphorylation in SERCA1a (6) with the corresponding glutamic acid, Glu163, facing the phosphate analogs. In addition, the M domains were closed. These observations could possibly explain why LMCA1 transitions faster through the dephosphorylation steps compared to SERCA1a (4). However, because the LMCA1 wild-type crystal structures were determined at 4.0-Å resolution, such subtle differences in side-chain reorientations are highly uncertain. In addition, it is not clear to what extent crystal packing and the detergent micelle environment might be affecting the adopted structure. Hence, this potential unique LMCA1 dephosphorylation mechanism needs to be further established.
Proposed Ca2+ binding site and consequences for transport regulation
The bacterial LMCA1 transporter has evolved alternative mechanisms for binding Ca2+ and H+, as demonstrated by two orders of magnitude lower Ca2+ binding affinity compared to SERCA1a (5). In addition, LMCA1 crystal structures have shown that the M domain pocket corresponding to Ca2+ binding site II in SERCA is composed of similar amino acid residues and thus likely can accommodate a Ca2+ ion. While site II is universally conserved among Ca2+ ATPases, site I has a higher variability in secretory pathway Ca2+ ATPases (SPCAs) (19) and plasma membrane Ca2+ ATPases (PMCAs) (20)—and as a consequence, these eukaryotic Ca2+ ATPases catalyze transport of a single Ca2+ ion. Similarly, in the prokaryotic LMCA1, site I replaces the ion-binding glutamic acid (Glu908) in SERCA1a with the basic Arg795 and thereby disrupts this ion-binding site (6). An additional substitution in this site introduces an alanine (Ala691) instead of Glu771 in SERCA, thereby accommodating the Arg795 side chain in the LMCA1 M domain pocket. Arg795 has proposed a key role in shifting optimal functionality to alkaline pH levels—with a pH optimum of 8.75 to 9.5 compared to pH 7 for SERCA1a (5).
In addition, no proton transport pathways have yet been identified in the LMCA1 M domain, which can be compared to SERCA1a that have been proposed to host the proton-specific luminal entry pathway (21), as well as a C-terminal cytosolic release pathway (22). In the absence of proton pathways, protons can be expected to follow the Ca2+ route, and Glu292 was assigned as a possible protonation site from pKa calculations based on the existing crystal structures (6). A single protonation site corroborates with a 1 Ca2+:1 H+ transport stoichiometry (5). In summary, while both sites I and II in LMCA1 are closely linked to unique transport properties, they have so far not been characterized in the presence of a Ca2+ ion.
In this work, we present kinetic, structural, and dynamics data of the bacterial LMCA1 Ca2+ transporter. TR-XSS experiments confirm that phosphorylation is the rate-limiting step. A 2.98-Å resolution E2P cryo–electron microscopy (cryo-EM) structure aligns better with SERCA E2-P* with Glu183 facing the BeF3− phosphate analog. This establishes the unique P-A domain interface that was proposed to result in increased rates of dephosphorylation. Moreover, a 3.73-Å cryo-EM structure trapped with AMPPCP showed density in the ion-binding site II with side chains Asp720, Asn716, and Glu292 coordinating the ion. In MD simulations of the ion-bound structure, the Ca2+ binding site remained intact. In site I, Arg795 was facing Glu292, which was shown by the simulations to be positioned at the inner vestibule of a water crevice between TM4 and TM6. The results provide insight into LMCA1 Ca2+ binding, reconcile earlier proposed mechanisms of dephosphorylation, fine-tune our understanding of pH tolerance and H+ counter transport, and thereby contribute to differentiate eukaryotic and prokaryotic Ca2+ ATPase transport—which will be critical to turning LMCA1 into a realistic drug target.
RESULTS
Phosphorylation of LMCA1 is the rate-limiting step in detergent micelles
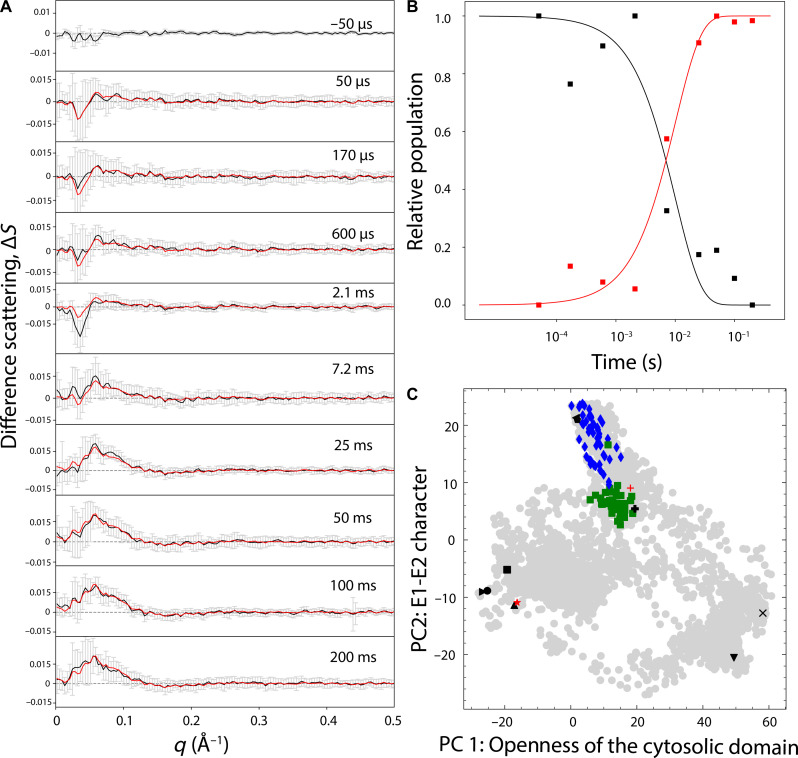
Synchrotron-based TR-XSS (23) was used to characterize the rate-limiting step of wild-type LMCA1. The reaction was initiated by laser-induced release of ATP from a caged compound and the structural rearrangements in the sample were monitored from 50 μs to 200 ms (Fig. 2A). A negative feature at 0.03 Å−1 was present in the early microsecond time points up to 2.1 ms. From 7.2 ms, a broad positive peak centered around 0.05 Å−1 instead became prominent and increased up to 50 ms after which it remained at similar amplitude until the final 200-ms time point. The singular value decomposition of the data showed two major components (fig. S1). Therefore, we applied a two-state kinetic model assuming sequential order to extract the transition time between the two components and their respective time-independent basis spectra (fig. S2), which contain the structural information. The reconstituted data from the two-state kinetic model fitted well with the experimental data (χ2 = 0.16) (Fig. 2A) and showed a transition time of 10.4 ms (Fig. 2B). The decay time for the early state coincided with the expected release of the caged ATP (24), and therefore, we focused structural interpretation on the late state.
Fig. 2. Time-resolved x-ray solution scattering data of the LMCA1 reaction.
(A) Difference scattering data (black lines) and SDs (gray bars) are plotted with the reconstituted data generated by the two-state kinetic model (red lines). (B) Development of the relative population as a function of the experimental time points. (C) Principal components (PC) map showing the LMCA1 models used as start and end points for the steered MD simulations (black symbols). The simulated structures extracted from the resulting MD trajectories are shown as gray circles. The Ca2+-bound E1 (red plus) and E2P (red star) states determined in this study are also shown. The 100-best pre-pulse states are shown as green quadrants and the corresponding excited state structures are shown as blue diamonds.
To translate the one-dimensional (1D) late-state basis spectrum into 3D structural rearrangements, we first generated putative structures by simulating transitions between states of known structures modeled from SERCA1a crystal structures (fig. S3A). Principal components analysis (PCA) of the simulated LMCA1 structures identified two major components, where PC1 described the degree of openness of the cytoplasmic domains and PC2 was associated with E1 versus E2 character (fig. S3B). Plotting the simulated transition structures onto the PC map shows the covered conformational space with respect to states of known structure (fig. S3A). We then calculated theoretical x-ray scattering profiles from all protein conformations. Because the time-resolved data can be considered to consist of one pre-pulse equilibrium state and its corresponding time-dependent excited state, we generated different x-ray scattering profiles by a combination of each possible pair. The correspondence between the difference scattering from each such pair and the late basis spectrum was evaluated by an R-factor (fig. S3C). To consider a structural ensemble in solution, we plotted the 100 best-fitting structural solutions on the PC map and identified common ground (pre-pulse) and excited states (Fig. 2C). The pre-pulse state was best described by the LMCA1 E1 state presented in this study and was also relatively close to a SERCA1a E1 state with an open cytoplasmic pathway to the ion-binding sites [SERCA1a Protein Data Bank (PDB) ID: 4H1W] (Fig. 2C and fig. S3) (25). The excited state structural ensemble was somewhat more scattered but centered around a Ca2+-bound E1 state (PDB ID: 7E7S) (26) and E1P state (PDB ID: 7W7T) (27) in the SERCA2b isoform and a Ca2+-bound E1P SERCA1a state (PDB ID: 3BA6) (28). Hence, the excited LMCA1 state was found in a cluster consisting of both nonphosphorylated and phosphorylated states. Therefore, the rate-limiting state of wild-type LMCA1 in detergent micelles was associated with the phosphorylation step in the reaction cycle.
LMCA1 displays a unique dephosphorylation mechanism in a native-lipid environment
To characterize LMCA1 activity in a native-like environment, we reconstituted the wild-type protein into lipid-containing MSP1D1 nanodiscs (fig. S4). Compared to detergent micelles, the protein showed a higher ATPase activity in nanodiscs with either zwitterionic 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) or anionic 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (POPG) lipids. The POPG lipids resulted in more than twice the ATPase activity compared to POPC lipids, which corroborates with PG being the dominant lipid type in Listeria membranes (29–31). In detergent micelles, the dephosphorylation step in the LMCA1 transport reaction is fast (4), i.e., not rate limiting, and proposed to depend on the E2P state being shifted toward E2-P* (6). A fundamental question, therefore, becomes whether this is true also in native POPG lipids, which sustain a more active protein. To address this question, we determined the structure of a phosphorylated E2P state trapped with BeF3− of LMCA1 in POPG-containing MSP1D1 nanodiscs using cryo–electron microscopy (cryo-EM) at 2.98-Å resolution (fig. S5). The obtained map revealed BeF3− density near Asp334, interacting with a Mg2+ ion (fig. S6). The overall structure showed an arrangement of the soluble domains that was representative of an E2P state (Fig. 3, A to C). Comparison of the cryo-EM structure in nanodiscs with the LMCA1 E2P crystal structure in detergent (PDB ID: 6ZHF) yielded an overall root mean square deviation (RMSD) between the two structures of 1.9 Å. The cytosolic and transmembrane domains are closely aligned, with only a slight shift in the N domain relative to the transmembrane domain (fig. S7). In addition, the LMCA1 E2P structure also aligns well with SERCA1a (PDB ID: 3B9B) and SPCA1 (PDB: 7YAM) trapped in E2P states, with RMSD values of 3.1 and 2.4 Å, respectively (fig. S8).
Fig. 3. The LMCA1 E2P state.
Side view (A) and top view (B) of the cryo-EM 3D map color-coded according to the N (red), P (blue), A (yellow), and M (wheat) domains. A lowpass-filtered density (transparent) shows the contours of the surrounding nanodisc. (C) Atomic model of the LMCA1 E2P state. The TGES loop at the A-P domain interface of the LMCA1 E2P state in this work (blue) superimposed onto (D) the wild-type E2P LMCA1 state in detergent (PDB ID: 6ZHF) (cyan), (E) the G4 E2P LMCA1 state (PDB ID: 6ZHH) (gray), (F) the E2P SERCA1a state (PDB ID: 3B9B) (yellow), and (G) the E2-P* SERCA1a state (PDB ID: 3B9R) (red).
Upon detailed comparison of the wild-type LMCA1 E2P structures, it was clear that while Glu167 in the TGES loop had reoriented to face the P domain in the nanodisc structure, its orientation in the detergent structure was facing away from the P domain (6), as in the corresponding SERCA1a E2P state (Fig. 3D) (28). Hence, the observed orientation of Glu167 in the nanodisc structure was similar to a G4 mutant structure where four glycine residues were introduced into the A-TM1 linker (Fig. 3E) (6). Therefore, the 2.98-Å resolution structure confirms that the Glu167 residue in LMCA1 is facing the P domain phosphorylation site in both E2P and E2-P* states of dephosphorylation, which differs compared to the corresponding SERCA1a states (Fig. 3, F and G) (28). The TM helices in the M domain align better with an E2-P* SERCA1a state (RMSD = 1.2 Å) than an E2P state (RMSD = 2.5 Å), and the M domain is therefore closed. Because the TR-XSS experiments register rate-limiting states, a fast dephosphorylation step is in agreement with the TR-XSS data since no matches are found for this part of the reaction cycle (Fig. 2C).
LMCA1 trapped in a Ca2+-bound E1 state contains a single Ca2+ binding site
To characterize the E1 state in LMCA1, we determined the structure of a Ca2+-bound E1 state in the presence of the ATP analog adenosine 5′-(β,γ-methylene)triphosphate (AMPPCP). Using cryo-EM, we resolved the structure in a POPG-containing MSP1D1 nanodisc at 3.73-Å resolution (fig. S9). The cryo-EM map indicated no density for AMPPCP despite the sample containing 1 mM AMPPCP and including more classes in the heterogeneous refinement process. However, the global arrangement of the soluble domains was in the upright constellation expected for an E1 state (Fig. 4, A to C). By plotting the structure onto the PCA map, the structure was shown to be related to the corresponding Ca2+-bound E1 state in the SERCA2b isoform (PDB ID: 7E7S) (fig. S3A). In addition, the conformation of a Ca2+-free, sarcolipin-stabilized SERCA1a conformation with accessible ion-binding sites (PDB ID: 4H1W) is also similar in structure. The domain arrangement of the LMCA1 E1 structure resembles that of the Ca2+ and ATP-bound SPCA1 E1 structure (PDB ID: 8IWR) and the Ca2+-bound SERCA2b E1 structure (PDB ID: 7E7S) with RMSD values of 2.4 and 3.2 Å, respectively (fig. S10).
Fig. 4. The Ca2+-bound LMCA1 E1 state.
Side view (A) and top view (B) of the cryo-EM 3D map color-coded according to the N (red), P (blue), A (yellow), and M (wheat) domains with a lowpass-filtered density (transparent) showing the contours of the surrounding nanodisc. (C) Atomic model of the LMCA1 E1-Ca2+ state. Comparison of the site II ion-binding site in the (D) E1-Ca2+ and (E) E2P states. Cytosolic view of TM domain and ion-binding site in the (F) LMCA1 E1-Ca2+ state, (G) Ca2+ and ATP-bound SERCA2b state (PDB ID: 6LLE), and (H) Ca2+ and ATP-bound SPCA1 state (PDB ID: 8IWR). The Ca2+ ions are shown as green spheres. Amino acid residues located at the ion-binding sites are shown as sticks.
Additional density was observed between helices TM4 and TM6 in the E1 state (Fig. 4D), which was not present in the E2P state (Fig. 4E). Because this position in the M domain superimposes exactly with a putative ion-binding site, this is highly indicative of the single Ca2+ ion-binding site. The closest amino acid residues are Asp720, Asn716, and Glu292, while the rest of the contacts are nonspecific interactions to backbone carbonyl oxygens (Fig. 4D). These residues change their orientations in between the Ca2+-bound E1 and the E2P states, in particular Asp720, which swings to face away from the binding site in the E2P state. The observed ion-binding site superimposed more or less exactly with site II in SERCA2b (Fig. 4, F and G). The Asn716 (LMCA1)/Asn795 (SERCA2b) and Glu292 (LMCA1)/Glu309 (SERCA2b) side chains showed similar rotameric orientations. However, the Asp720 (LMCA1)/Asp799 (SERCA2b) side chain differed slightly, presumably to enable Asp799 to inter-bridge sites I and II in SERCA2b. In SPCA1 [PDB ID: 8IWR (32)], which also contains a single Ca2+ binding site, the corresponding three ion-binding residues Asp472, Asn378, and Glu308 showed identical Ca2+ coordination as observed in the LMCA1 structure (Fig. 4H).
The overall E1 to E2P conformational transition appears conserved across Ca2+ transporters
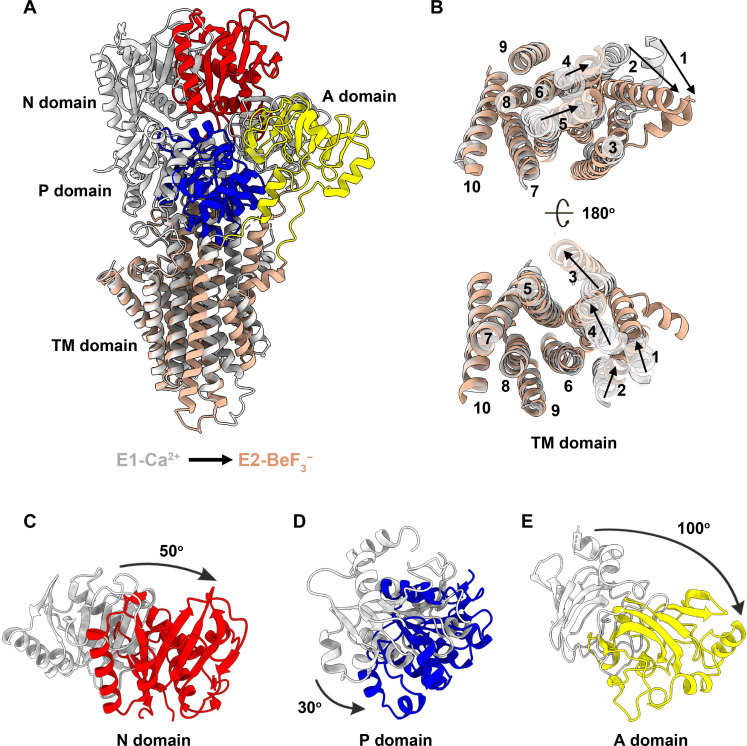
To better understand the conformational transition between the E1 and E2 states in LMCA1, we compared the obtained cryo-EM structures. We found that during the transition from E1-Ca2+ to E2P, the cytosolic domain undergoes substantial conformational changes (Fig. 5). In the E2P state, the N, P, and A domains rotate by 50°, 30°, and 100°, respectively, relative to their positions in the E1-Ca2+ state (Fig. 5, C to E). The rotation of the A domain brings the TGES loop closer to Asp334, shielding the phosphorylation site in the P domain and preventing ADP rebinding. The transition from E1-Ca2+ to E2P also causes a rearrangement in the TM domain (Fig. 5B). The A domain rotation causes TM1 to TM3 to move since they are directly connected to the A domain via linkers. TM4 and TM5 also shifted orientations between the E1-Ca2+ and E2P states. Specifically, TM5 and the cytosolic half of TM4 shift downward, and the bottom half of TM4 moves away from TM6, creating space between them. In contrast, TM7 to TM10 formed a more rigid cluster. Therefore, the rearrangement of the cytosolic domain is closely coupled to the rearrangement of the TM helices, facilitating the release of Ca2+ and the transition from E1-Ca2+ to E2P states. Thus, while LMCA1 displays unique features of kinetics and dynamics, the overall E1-to-E2P domain rearrangements appear conserved in between prokaryotic and eukaryotic Ca2+ transport.
Fig. 5. Conformational transition of LMCA1 from the E1-Ca2+ to E2P states.
(A) Alignment of the E1-Ca2+ (gray) and E2-BeF3− (color) states of LMCA1. (B) Top (cytosolic) and bottom views of the TM domain and relocation of each TM helix during the transition from the E1-Ca2+ (gray) to E2-BeF3− (wheat) states. Relocation of the N (C), P (D), and A (E) domains during the transition from the E1-Ca2+ (gray) to E2-BeF3− (color) state. Rotation angles are shown as black arrows.
Simulations show structural stability also in Site I in a non-hydrated membrane section
While we observed a clear difference in density between TM4 and TM6 in the E1 and E2P states, unambiguous assignment of ion coordination is difficult. We, therefore, decided to characterize ion binding in silico. The Ca2+-bound E1 state LMCA1 structure was inserted into a POPG-containing MSP1D1 nanodisc, and three independent systems were simulated for 1 μs each. For all three simulations, the Ca2+ ion-binding site remained intact. Six residues were found within 3 Å of the Ca2+ ion (fig. S11). Three residues (Ile290, Ala288, and Val287) made backbone interactions with the ion, which differed slightly in frequency in between the three simulations. However, the 3-Å interaction criterium was fulfilled throughout the full trajectories for the polar side chains Asp720, Asn716, and Glu292. The average residue-ion distances were 2.3 (Asp720), 2.2 (Asn716), and 2.3 Å (Glu292) (Fig. 6, A and B and table S1). Water molecules were also observed within a 5-Å sphere from the ion but were not directly engaged in ion coordination (Fig. 6B). Hence, Asp720, Asn716, and Glu292 coordinate the Ca2+ ion in LMCA1.
Fig. 6. Simulated LMCA1, Ca2+, and water dynamics.
(A) Average distances between the Ca2+ ion and the binding residues across the three independent MD trajectories. (B) Detailed view of the ion-binding site with a solid isodensity surface representing Ca2+ positions (orange) and water positions represented by an isodensity surface displayed in red wireframe with a cutoff at 5 Å from the Ca2+ ion. (C) Water solvation in the M domain shown as an isodensity surface (red wireframe) with a cutoff at 15 Å from the Ca2+ ion. The ion-binding residues and Arg795 are displayed as sticks.
The presence of a charged Ca2+ ion could potentially affect the structural organization in site I. In the SERCA2b E1 state, a glutamic acid (Glu907) in site I participates in binding the second Ca2+ ion, while the same residue in SPCA1, which binds a single Ca2+ in site II, is replaced by an aspartic acid (Asp819) (Fig. 4). In the Ca2+-bound LMCA1 E1 state, Arg795 traversed site I and thereby disrupted possibilities of binding a second Ca2+ ion (Fig. 4F). The orientation of Arg795 in the E1 state is very similar to that in the E2P state (fig. S12). Hence, the accommodation of side chains in site I might not undergo notable structural change during the reaction cycle. MD simulations of the Ca2+-bound E1 structure showed that despite the positive charges on Arg795 and the Ca2+ ion present in the M domain, both the Arg795 orientation and the Ca2+ coordination sphere remained intact in three 1-μs trajectories.
We then characterized proton pathways by tracking simulated solvation patterns in the M domain. No extracellular access of water molecules into the M domain was observed, which corroborates with a Ca2+-bound E1 state (Fig. 6C). However, water entered from the cytoplasmic side and formed a C-terminal proton pathway connecting to the ion-coordinating Glu292, which has earlier been assigned putative protonation site (6). No water solvation of Arg795 was detected, which possibly enables stabilization of site I—and also, as a consequence, ion binding in site II.
DISCUSSION
A notable difference between the eukaryotic SERCA1a Ca2+ transporter and the corresponding bacterial LMCA1 transporter is that the kinetic rate-limiting steps differ. In sm-FRET experiments, the rate-limiting step in the LMCA1 transport reaction was shown to be the phosphorylation step (4), whereas in SERCA1a, the E1P-to-E2P transition is the rate-limiting step instead (17). Because sm-FRET experiments track a selected distance between two labels engineered into a mutated variant of the protein, we wanted to register kinetics in the wild-type LMCA1 protein by registering rearrangements in the 3D envelope of the protein. TR-XSS experiments monitor all changes in interatomic distances simultaneously in the native protein in real time (15, 23, 33) and were, therefore, suitable for characterizing LMCA1 kinetics. In the TR-XSS experiments, we monitor the build-up of the state before the rate-limiting state, i.e., the structure that the protein spends the most time in since the transition out of that structure is rate limiting. In TR-XSS experiments of SERCA1a, this structure was observed in the E1-to-E2 transition (15), which means that SERCA1a rapidly becomes phosphorylated and starts the transition to E2P, reaches the stable intermediate, stalls comparatively long, and then overcomes the free energy barrier and rapidly transitions through the E2P and E2 states and back to E1 before again stalling at the E1P-E2P transition. For LMCA1, we observed that the transition originated from a pre-pulse state described by a relatively tight cluster in PC space, which was located proximal to the LMCA1 E1 state presented in this study and to a SERCA1a E1 state (PDB ID: 4H1W), both with an open formation of the soluble domains. The excited state showed higher structural diversity that spanned both nonphosphorylated and phosphorylated states. Hence, the rate-limiting step in the native LMCA1 protein is associated with phosphorylation of the protein. Once phosphorylation is achieved, the rest of the reaction cycle is fast and the protein returns to the E1 states of phosphorylation. In terms of kinetics, the LMCA1 reaction has been observed to be slower than SERCA1a (4). Indeed, while we have observed a SERCA1a intermediate state of phosphorylation after 1.5 ms (15), reaching a similar state in the LMCA1 reaction took 10.4 ms.
Lipid chemistry dictates membrane protein function (34). For type II P-type ATPases, both SERCA1a and the Na+/K+ ATPase have been shown to interact specifically with certain lipid types that affect stabilization and shuttling between E1 and E2 states (35–37). Also, local distortions in protein-interacting lipids were suggested to induce the SERCA1a cytosolic domains to rock back and forth during transport (38) or alternatively to cause membrane deformation (14). Type I Cu+ transporters require additive lipids to show activity in detergent micelles (39), which indicates that specific lipid requirement is a general regulative feature across the P-type ATPase family of transporters. Bacterial membranes are enriched in negatively charged PG lipids as are the Listeria membranes from which LMCA1 originates (29–31). Therefore, the difference in chemistry between a detergent micelle and the native membrane is quite drastic, which calls for structural characterization under native-like lipid conditions. For the LMCA1 in POPG nanodiscs, we observed closure of the extracellular ion-release pathway already in the E2P state, which is open in SERCA1a. This verifies that the proposed unique closing mechanism observed in mutant LMCA1 in detergent (6) is also present in a native-like POPG membrane.
The PC map created to assist structural refinement of the TR-XSS data was also useful for assigning states to the LMCA1 structures presented in this study. The LMCA1 E2P structure determined in this study clustered together with other E2 structures in the PC map (fig. S3A). While it is possible to assign major states that differ in the opening of the cytoplasmic domains (PC 1) and the E1 versus E2 character (PC 2), it is not possible to resolve minor details, such as those separating the E2P and E2-P* states. For example, our LMCA1 E2P structure overlapped with the corresponding E2P SERCA1a state (28) while being slightly shifted away from the detergent LMCA1 E2P structure (6). Therefore, a high-resolution structure is required for proper state assignment. The LMCA1 Ca2+-bound E1 structure was located in between a SERCA1a E1 state with an open cytoplasmic pathway to the ion-binding sites (SERCA1a PDB ID: 4H1W) (22) and a Ca2+-bound E1 state (PDB ID: 7E7S) from SERCA2b (26), which is in agreement with a Ca2+-bound E1 state.
MD simulation of a Ca2+ ion centered in the additional TM4-TM6 density showed a stable ion-binding site coordinated by residues Asp720, Asn716, and Glu292, which corresponds to site II in SERCA1a. Site II is conserved across the Ca2+ ATPases, while site I hosts alternative functionality in SPCAs and PMCAs (6). Arg795 in our LMCA1 structures traverses the entire site I and thereby effectively prevents ion binding. The Arg795 residue has proposed a role in shifting the pH optimum toward 8.75 to 9.5 (5), but the mechanistic role is not clear. One proposed mechanism underlying the alkaline pH optimum is that deprotonation of Arg795 (i.e., achieve electric neutrality) in site I at high pH would enable the accommodation of a Ca2+ ion in site II (6). With this reasoning, a charged Arg795 would destabilize the ion-binding site. In the MD simulations, we observe a stable Ca2+ coordination, which indicates that stability can be achieved even in the presence of two positive charges. Hence, the role of Arg795 as an internal counterion responsible for pH dependency remains to be validated.
To characterize accessibility to the ion-binding site, we tracked water solvation in MD simulations of the Ca2+-bound E1 state. We observed water molecules penetrating the M domain from the cytoplasm reaching up to the carbonyl oxygen of Glu292, which was actively engaged in ion binding by the side chain in all simulations. This Glu292 residue was appointed as a putative candidate for proton shuttling (6). Hence, the water pathway observed in the simulations might constitute a C-terminal proton release pathway similar to those observed in simulations of SERCA1a (40). Such a hydrated pathway is also a likely entry path for the incoming Ca2+ ions as observed for SERCA1a (41, 42). The water was never observed to make direct interaction with Ca2+ ions, which is a prerequisite for an occluded Ca2+-bound E1 state.
In conclusion, the kinetic and structural analyses of the LMCA1 transporter reveal distinct differences in the rate-limiting steps and structural dynamics compared to its eukaryotic counterpart, SERCA1a. These findings provide deeper insights into the conserved and divergent features of P-type ATPase transport, highlighting the intricate relationship between protein structure, lipid interactions, and ion transport kinetics. Future studies should aim to resolve the precise structural details that govern these processes, particularly the role of residues like Arg795 in LMCA1, to further elucidate the molecular mechanisms underlying their function and regulation.
MATERIALS AND METHODS
Materials
Phospholipids POPG and POPC were purchased from Avanti Polar Lipids. Detergent n-dodecyl β-d-maltoside (DDM) was obtained from Thermo Fisher Scientific. Unless indicated otherwise, all other chemicals and reagents were obtained from Sigma-Aldrich.
Time-resolved x-ray solution scattering
The data collection was carried out at the dedicated time-resolved ID09 beamline at the European Synchrotron Radiation Facility Extremely Brilliant Source (43). The reaction was initiated by a laser pulse at 355 nm with fluence of 0.9 mJ/mm2 to release the nitrophenylethyl (NPE)-caged ATP. The sample was probed by an x-ray pulse of 18 keV at a duration of 20 μs. For each image, 100 pulses were recorded, and for each time point, at least 30 images were recorded. Images were captured on a Rayonix MX170-HS detector at a sample-to-detector distance of 380 mm with a helium cone to minimize air scattering. To avoid drift due to experimental conditions, positive time delays were recorded at a predetermined random order with negative time delays in between.
Images from the detector were integrated to obtain the radial intensity curves S(q, Δt). The intensity curves were obtained as a function of the scattering vector q = 4Π sin q/l, with 2θ being the angle of deflection, l being the x-ray wavelength, and Δt being the time delay between the laser pulse and the x-ray pulse. Normalization of the curves was carried out at q between 2 and 2.1 Å−1. To obtain the difference scattering, the dark −50-μs references before and after each time point were subtracted and any curves that deviated more than 3.5 SDs from the average were rejected.
For SVD analysis, the time-resolved data were organized into a m × n matrix with m being the q values and n being the number of time points using the equation
where U(q) is an m × m matrix that spans the orthonormal basis spectra of the ∆S dataset. Sv is an m × n diagonal matrix that contains the contributions of these basis spectra, denoted as singular values. Last, V(∆t) is an n × n matrix that describes the temporal evolution of the basis spectra.
The difference scattering data were decomposed between 0.04 and 0.5 Å−1 using a sequential two-state model. The model assumes that the early state formation occurs with the laser pulse and that the transition is irreversible. The rate constant k1 is indicative of the formation of a transient state
To derive the rate constant and the time-dependent basis spectra, global fitting was applied according to
where U(q) represents the basis spectra, and C(Δt) denotes the time-dependent concentration of the spectral components. The time-dependent concentrations are derived from the following integrated rate equations
To generate candidate structures, the following published SERCA structures were used to generate homology models with SWISS MODEL 3.3.0 (44): 1SU4, 2C9M, 3B9B, 3BA6, 3FGO, 3N5K, 3NAL, 3N8G, and 4H1W. The protein structures were described using the CHARMM36 force field (45) generated by CHARMM-GUI 3.7 (46). The structures were inserted into a lipid bilayer containing 264 POPG lipids with the CHARMM-GUI Membrane Builder (47) and were solvated with TIP3 water and equilibrated in 150 mM KCl. For the simulations, the GROMACS-2019.4 simulation package (48) was used with PLUMED 2.5.4 (49). The energy of each system was minimized until Fmax < 1000 using the steepest descent algorithm. The step-wise equilibration was first 125 ps long with a time step of 1 fs, then increasing to 500 ps with a 2-fs time step for the last three equilibration steps. For the production simulations, the structures were driven toward the target structures during 30 ns with the RMSD collective variable from PLUMED 2.5.4. For the structural refinement, 3612 structures were selected. CRYSOL 2.8.4 (50) was used to calculate the theoretical scattering spectra using a scattering angle <0.6 Å, up to 50 harmonics, and a 17-order Fibonacci grid.
To compare the theoretical and experimental difference spectra, R-factors were calculated as follows
Here, the experimental ΔS is the basis spectrum from the experimental data derived from the kinetic modeling and the theoretical ΔS is the theoretical difference scattering.
For the PCA, the LMCA1 homology models 1SU4, 2C9M, 3B9B, 3BA6, 3FGO, 3N5K, 3NAL, 3NG8, and 4H1W were aligned by the TM domain. The structures were projected onto a map made up of the first two PCs which correspond to the openness of the cytosolic domain and the E1-E2 character of the structure, respectively. For the clustering of the best pairs, an implementation of an agglomerative clustering algorithm was used with a distance threshold of 10.
Protein expression and purification
LMCA1-pET-22b with a 10-histidine tag at the C-terminal was expressed in Escherichia coli C43 (DE3) according to a protocol described previously (5). The cells were lysed on a high-pressure homogenizer, French press, at 172 MPa. Lysed cells were centrifuged at 20,000g for 20 min to remove debris, and membranes were collected by ultracentrifugation at 200,000g for 3 hours. All further steps were carried out at 4°C. The isolated membranes were solubilized with 1% of DDM for 2 hours under gentle stirring. The insoluble material was removed by ultracentrifugation at 200,000g for 45 min. The solubilized membranes were applied to a prepacked HisTrap HP column (GE Healthcare). The protein was eluted with 150 mM imidazole in elution buffer [50 mM tris-HCl (pH 7.6), 200 mM KCl, 20% v/v glycerol, 1 mM MgCl2, 5 mM β-mercaptoethanol (BME), and 0.05% DDM]. The sample was then subjected to size exclusion chromatography (SEC) on a Superose 6 Increase 10/300 GL column (GE Healthcare) equilibrated with SEC buffer [50 mM tris-HCl (pH 7.6), 200 mM KCl, 10% v/v glycerol, 10 mM MgCl2, 1 mM CaCl2, 5 mM BME, and 0.05% DDM]. SEC peak fractions containing LMCA1 were pooled and concentrated with a 100-kDa Amicon centrifugal filter to ~10 mg/ml. Membrane scaffold proteins MSP1D1 were expressed and purified as described previously (51, 52).
Reconstitution of LMCA1 into nanodiscs
LMCA1 nanodiscs were generated with scaffold protein MSP1D1 and either POPG or POPC lipids. The phospholipids were dried overnight and solubilized in 20 mM tris-HCl (pH 8.0) and 5% DDM. LMCA1, MSP1D1, and phospholipids were mixed at a molar ratio of 1:4:200. The mixture was incubated at 4°C for 1 hour. The removal of detergent was initiated by the addition of 100 mM methyl-beta-cyclodextrin (MβCD) solution (53) at the molar ratio of 1.5:1 (MβCD:DDM) followed by incubation at room temperature (RT) for 1 hour. The aggregates were removed by a 0.22-μm filter or precipitated by centrifugation at 50,000 rpm for 20 min at 4°C. The nanodisc sample was subjected to SEC using Superose 6 10/300 GL pre-equilibrated with SEC nanodisc buffer [50 mM tris-HCl (pH 7.6), 200 mM KCl, 10% v/v glycerol, 10 mM MgCl2, 1 mM CaCl2, and 5 mM BME]. SEC peak fractions containing LMCA1 nanodiscs were pooled and concentrated to ~10 mg/ml.
Functional characterization of LMCA1
The purified LMCA1 nanodiscs were subjected to an ATPase activity assay using the Baginski method (54). One microliter of purified LMCA1 at a concentration of 0.1 to 1 mg/ml was added to a final volume of 50 μl in reaction buffer [20 mM tris-HCl (pH 7.6), 200 mM KCl, 20% v/v glycerol, 1 mM MgCl2, 1 mM CaCl2, and 5 mM BME] in a 96-well microtiter plate at RT. Three millimolar ATP was added to start the reaction and then incubated for 1 to 5 min. The reactions were stopped by the addition of 50 μl of ascorbic acid solution [140 mM ascorbic acid, 5 mM ammonium heptamolybdate, 0.1% (w/v) SDS, and 0.4 M HCl]. After incubation for 15 min at RT, 75 μl of sodium bismuth solution (3.5% bismuth citrate, 3.5% sodium citrate, and 1 M HCl) was added to stop color development. Absorbance at 860 nm was measured in a BioTek Synergy microplate reader. Potassium phosphate solutions were used as standards to determine the concentrations of phosphate released upon ATP hydrolysis.
Grid preparation for cryo-EM
For preparation of the E1 state, LMCA1 was incubated with 5 mM CaCl2 and 1 mM AMPPCP for 1 hour. Preparation of the E2-BeF3− state was performed by incubating the sample with 5 mM BeSO4, 10 mM NaF, and 1 mM EGTA for 1 hour. The samples were then loaded into a Superose 6 10/300 GL pre-equilibrated with SEC nanodisc buffer without glycerol for the cryo-EM experiment. For the preparation of cryo-EM grids, Quantifoil holey carbon grid 300 mesh 1.2/1.3 was glow discharged in a PELCO easiGlow. Three microliters of protein was applied to grids using a Vitrobot Mark IV instrument (Thermo Fisher Scientific) at 4°C with a blotting time of 4 s under 100% humidity conditions. Grids were plunge-frozen in liquid ethane. Frozen grids were stored in liquid nitrogen before data collection.
Cryo-EM data collection
Cryo-EM data were collected on a 300-kV FEI Titan Krios equipped with a Falcon 4i direct electron detector (Thermo Fisher Scientific) at the Umeå Core Facility for Electron Microscopy. Movies were collected using the EPU software at a nominal magnification of ×165,000 with a pixel size of 0.704 Å. Defocus values of the images ranged from −0.9 to −2.4 μm. Images were exposed for a total of 2.96 s with a dose rate of 8.34 e−/pixel per second resulting in a total dose of 50 e−/Å2. In total, 6425 movies were collected for the E2 state and 18,526 movies for the E1 state. Data collection parameters are presented in table S2.
Cryo-EM data processing
The data processing workflow for cryo-EM analysis of LMCA1 in the E2-BeF3− and E1-Ca2+ states is presented in figs. S5 and S9, respectively. Cryo-EM data were processed using the software package cryoSPARC (version v4.3) (55). Movie frames were aligned using the patch motion correction and the contrast transfer function (CTF) parameters were estimated from the aligned micrographs using patch CTF estimation in cryoSPARC. Particles with diameters of 80 to 120 Å were picked using the blob picker, and particle extraction was carried out with a box size of 360 pixels. Picked particles were subjected to consecutive rounds of 2D classification to remove junk particles. 2D classes with clear cytosolic domains were selected and used for ab initio 3D reconstruction. One protein-like volume and several junk volumes were used to select protein particles from junk in multiple rounds of heterogeneous refinement. Particles from the best class were then selected for final 3D refinement using nonuniform and local refinements. The resolution was estimated by using the gold standard FSC 0.143 criterion, with an average resolution of 2.98 and 3.73 Å for the LMCA1 E2-BeF3− and the Ca2+-bound E1 LMCA1 state, respectively. The refined map was then subjected to local resolution estimation and filtered using a local filtering function. Postprocessing methods were applied to the final map, including sharpening with cryoSPARC (version v4.3) (55) and density modification with Phenix (56).
Model building and validation
The previous crystal structure of the LMCA1 in detergent (PDB ID: 6ZHF) and a homology model of the SERCA E1-Ca2+ state were used as initial models for the E2-BeF3− and E1-Ca2+ states, respectively. The models were fitted into the refined density maps using UCSF ChimeraX (57) followed by manual model construction in COOT (58). Model refinement was performed using Phenix real-space refinement (59) and validated with Phenix MolProbity (60). The statistics of the 3D reconstruction and model refinement are presented in table S2. All molecular graphics were prepared using UCSF ChimeraX and PyMOL (https://pymol.org).
Unbiased molecular dynamics simulation
The LMCA1 Ca2+-bound E1 structure was inserted into a lipid bilayer consisting of POPC using the CHARMM-GUI (46) membrane builder (47), followed by solvation in TIP3P water and neutralization of charge with 150 mM KCl. Three independent replicas were simulated using the GROMACS-2021 simulation package (48). The protein was described by the charmm36m all-atom force field (61) and the lipids were described by the C36 lipids force field (62). Each system was energy minimized with the steepest descent algorithm until Fmax < 1000 and equilibrated in six steps with gradual release of position restraints. The first three equilibration steps were 125 ps long with a 1-fs time step, and the following three equilibration steps were 500 ps long with a 2-fs time step. Through all equilibration and production simulations, the temperature was kept at 298.15 K using the v-rescale thermostat (63), and the pressure was set to 1 bar with the c-rescale barostat (64) from the third equilibration step onward. The equilibrated systems were simulated for a total of 1 μs each.
Acknowledgments
We are grateful to M. Dyla, M. Kjaergaard, and P. Nissen, Aarhus University, for supplying with the LMCA1 plasmid construct. We acknowledge the European Synchrotron Radiation Facility (ESRF) for the provision of synchrotron radiation facilities at the time-resolved beamline ID09 under proposal numbers LS-3302, LS-3158, and LS-3088. We acknowledge support from the Partnership for Soft Condensed Matter (PSCM) during experiments at the ESRF. Computational resources were provided by the High-Performance Computing Center North (HPC2N) under project hpc2n2023-005. We thank the Chemical Biology Consortium Sweden (CBCS) at Umeå University for access to the Synergy plate reader. We also would like to thank M. Hall and C. Holmlund for assistance with cryo-EM screening and data collection and A. Graça for advice on cryo-EM refinement. The EM data were collected at the Umeå Core Facility for Electron Microscopy, a node of the cryo-EM Swedish National Facility, funded by the Knut and Alice Wallenberg Foundation, the Erling-Persson Foundation, the Kempe Foundation, SciLifeLab, Stockholm University, and Umeå University. The cryo-EM structural refinement was enabled by the supercomputing resource Berzelius provided by the National Supercomputer Centre at Linköping University and the Knut and Alice Wallenberg Foundation.
Funding: This work was supported by the Kempe Foundation (JCK-1918.1 to I.P.) and the Swedish Research Council (2020-03840 to M.A.).
Author contributions: I.P.: Conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization, supervision, and project administration. F.O.: Investigation, writing—review and editing, methodology, validation, and software. K.M.: Methodology, software, validation, formal analysis, investigation, data curation, resources, writing—original draft, writing—review and editing, and visualization. K.P.: Software, validation, and investigation. M.L.: Methodology, investigation, resources, data curation. M.A.: Conceptualization, methodology, software, verification, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration, and funding acquisition.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The E1-Ca2+ state (PDB ID: 9EVC; EMD-19998) and E2-BeF3− state (PDB ID: 9EUQ; EMBD-19980) are available in the Protein Data Bank and Electron Microscopy Data Bank.
Supplementary Materials
This PDF file includes:
Figs. S1 to S14
Tables S1 and S2
REFERENCES AND NOTES
- 1.Chen J., Sitsel A., Benoy V., Sepulveda M. R., Vangheluwe P., Primary active Ca2+ transport systems in health and disease. Cold Spring Harb. Perspect. Biol. 12, a035113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominguez D. C., Guragain M., Patrauchan M., Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium 57, 151–165 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Dyla M., Andersen J. L., Kjaergaard M., Birkedal V., Terry D. S., Altman R. B., Blanchard S. C., Nissen P., Knudsen C. R., Engineering a prototypic P-type ATPase Listeria monocytogenes Ca2+-ATPase 1 for single-molecule FRET studies. Bioconjug. Chem. 27, 2176–2187 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyla M., Terry D. S., Kjaergaard M., Sørensen T. L. M., Lauwring Andersen J., Andersen J. P., Rohde Knudsen C., Altman R. B., Nissen P., Blanchard S. C., Dynamics of P-type ATPase transport revealed by single-molecule FRET. Nature 551, 346–351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faxen K., Andersen J. L., Gourdon P., Fedosova N., Morth J. P., Nissen P., Moller J. V., Characterization of a Listeria monocytogenes Ca2+ Pump: A SERCA-type ATPase with only one Ca2+-binding site. J. Biol. Chem. 286, 1609–1617 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen S. B., Dyla M., Neumann C., Quistgaard E. M. H., Andersen J. L., Kjaergaard M., Nissen P., The crystal structure of the Ca2+-ATPase 1 from Listeria monocytogenes reveals a pump primed for dephosphorylation. J. Mol. Biol. 433, 167015 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Rosch J. W., Sublett J., Gao G., Wang Y. D., Tuomanen E. I., Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol. Microbiol. 70, 435–444 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giotis E. S., Muthaiyan A., Blair I. S., Wilkinson B. J., McDowell D. A., Genomic and proteomic analysis of the alkali-tolerance response (AlTR) in Listeria monocytogenes 10403S. BMC Microbiol. 8, 102 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen B. P., Bacterial calcium transport. Biochim. Biophys. Acta 906, 101–110 (1987). [DOI] [PubMed] [Google Scholar]
- 10.Segal A. W., Geisow M., Garcia R., Harper A., Miller R., The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature 290, 406–409 (1981). [DOI] [PubMed] [Google Scholar]
- 11.Giannella R. A., Broitman S. A., Zamcheck N., Influence of gastric acidity on bacterial and parasitic enteric infections. A perspective. Ann. Intern. Med. 78, 271–276 (1973). [DOI] [PubMed] [Google Scholar]
- 12.Farber J. M., Peterkin P. I., Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55, 476–511 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kühlbrandt W., Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 5, 282–295 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Dyla M., Kjaergaard M., Poulsen H., Nissen P., Structure and mechanism of P-type ATPase ion pumps. Annu. Rev. Biochem. 89, 583–603 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Ravishankar H., Pedersen M. N., Eklund M., Sitsel A., Li C., Duelli A., Levantino M., Wulff M., Barth A., Olesen C., Nissen P., Andersson M., Tracking Ca2+ ATPase intermediates in real time by x-ray solution scattering. Sci. Adv. 6, eaaz0981 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thirman J., Rui H., Roux B., Elusive intermediate state key in the conversion of ATP hydrolysis into useful work driving the Ca2+ pump SERCA. J. Phys. Chem. B 125, 2921–2928 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi S., Shigekawa M., Role of divalent cation bound to phosphoenzyme intermediate of sarcoplasmic reticulum ATPase. J. Biol. Chem. 259, 4427–4436 (1984). [PubMed] [Google Scholar]
- 18.Toyoshima C., Nomura H., Tsuda T., Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432, 361–368 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Chen Z., Watanabe S., Hashida H., Inoue M., Daigaku Y., Kikkawa M., Inaba K., Cryo-EM structures of human SPCA1a reveal the mechanism of Ca2+/Mn2+ transport into the Golgi apparatus. Sci. Adv. 9, eadd9742 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong D., Chi X., Ren K., Huang G., Zhou G., Yan N., Lei J., Zhou Q., Structure of the human plasma membrane Ca2+-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat. Commun. 9, 3623 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karjalainen E. L., Hauser K., Barth A., Proton paths in the sarcoplasmic reticulum Ca2+-ATPase. Biochim. Biophys. Acta 1767, 1310–1318 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Bublitz M., Musgaard M., Poulsen H., Thogersen L., Olesen C., Schiott B., Morth J. P., Moller J. V., Nissen P., Ion pathways in the sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 288, 10759–10765 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orädd F., Andersson M., Tracking membrane protein dynamics in real time. J. Membr. Biol. 254, 51–64 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCray J. A., Herbette L., Kihara T., Trentham D. R., A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc. Natl. Acad. Sci. U.S.A. 77, 7237–7241 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winther A. M., Bublitz M., Karlsen J. L., Moller J. V., Hansen J. B., Nissen P., Buch-Pedersen M. J., The sarcolipin-bound calcium pump stabilizes calcium sites exposed to the cytoplasm. Nature 495, 265–269 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Watanabe S., Tsutsumi A., Kadokura H., Kikkawa M., Inaba K., Cryo-EM analysis provides new mechanistic insight into ATP binding to Ca2+ -ATPase SERCA2b. EMBO J. 40, e108482 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Kobayashi C., Cai X., Watanabe S., Tsutsumi A., Kikkawa M., Sugita Y., Inaba K., Multiple sub-state structures of SERCA2b reveal conformational overlap at transition steps during the catalytic cycle. Cell Rep. 41, 111760 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Olesen C., Picard M., Winther A. M., Gyrup C., Morth J. P., Oxvig C., Moller J. V., Nissen P., The structural basis of calcium transport by the calcium pump. Nature 450, 1036–1042 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Fischer W., Leopold K., Polar lipids of four Listeria species containing L-lysylcardiolipin, a novel lipid structure, and other unique phospholipids. Int. J. Syst. Bacteriol. 49, 653–662 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Mastronicolis S. K., Arvanitis N., Karaliota A., Magiatis P., Heropoulos G., Litos C., Moustaka H., Tsakirakis A., Paramera E., Papastavrou P., Coordinated regulation of cold-induced changes in fatty acids with cardiolipin and phosphatidylglycerol composition among phospholipid species for the food pathogen Listeria monocytogenes. Appl. Environ. Microbiol. 74, 4543–4549 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dare K., Shepherd J., Roy H., Seveau S., Ibba M., LysPGS formation in Listeria monocytogenes has broad roles in maintaining membrane integrity beyond antimicrobial peptide resistance. Virulence 5, 534–546 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu M., Wu C., Song T., Pan K., Wang Y., Liu Z., Structure and transport mechanism of the human calcium pump SPCA1. Cell Res. 33, 533–545 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orädd F., Ravishankar H., Goodman J., Rogne P., Backman L., Duelli A., Nors Pedersen M., Levantino M., Wulff M., Wolf-Watz M., Andersson M., Tracking the ATP-binding response in adenylate kinase in real time. Sci. Adv. 7, eabi5514 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedger G., Sansom M. S. P., Lipid interaction sites on channels, transporters and receptors: Recent insights from molecular dynamics simulations. Biochim. Biophys. Acta 1858, 2390–2400 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habeck M., Kapri-Pardes E., Sharon M., Karlish S. J., Specific phospholipid binding to Na, K-ATPase at two distinct sites. Proc. Natl. Acad. Sci. U. S. A. 114, 2904–2909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonntag Y., Musgaard M., Olesen C., Schiott B., Moller J. V., Nissen P., Thogersen L., Mutual adaptation of a membrane protein and its lipid bilayer during conformational changes. Nat. Commun. 2, 304 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Mahato D. R., Andersson M., Dynamic lipid interactions in the plasma membrane Na+,K+-ATPase. Biochim. Biophys. Acta Mol. Cell. Res. 1870, 119545 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Norimatsu Y., Hasegawa K., Shimizu N., Toyoshima C., Protein-phospholipid interplay revealed with crystals of a calcium pump. Nature 545, 193–198 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Gourdon P., Liu X. Y., Skjorringe T., Morth J. P., Moller L. B., Pedersen B. P., Nissen P., Crystal structure of a copper-transporting PIB-type ATPase. Nature 475, 59–64 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Espinoza-Fonseca L. M., Structural basis for the function of the C-terminal proton release pathway in the calcium pump. Int. J. Mol. Sci. 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musgaard M., Thogersen L., Schiott B., Tajkhorshid E., Tracing cytoplasmic Ca2+ ion and water access points in the Ca2+-ATPase. Biophys. J. 102, 268–277 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bublitz M., Poulsen H., Morth J. P., Nissen P., In and out of the cation pumps: P-type ATPase structure revisited. Curr. Opin. Struct. Biol. 20, 431–439 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Wulff M., A. Plech, L. Eybert, R. Randler, F. Schotte, P. Anfinrud, The realization of sub-nanosecond pump and probe experiments at the ESRF. Faraday Discuss. 122, 13–26 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F. T., de Beer T. A. P., Rempfer C., Bordoli L., Lepore R., Schwede T., SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Best R. B., Zhu X., Shim J., Lopes P. E., Mittal J., Feig M., Mackerell A. D. Jr., Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jo S., Kim T., Iyer V. G., Im W., CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Wu E. L., Cheng X., Jo S., Rui H., Song K. C., Davila-Contreras E. M., Qi Y., Lee J., Monje-Galvan V., Venable R. M., Klauda J. B., Im W., CHARMM-GUI membrane builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham M., Murtola T., Schulz R., Páll S., Smith J., Hess B., Lindahl E., GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015). [Google Scholar]
- 49.Tribello G., Bonomi M., Branduardi D., Camilloni C., Bussi G., PLUMED 2: New feathers for an Old Bird. Comput. Phys. Commun. 185, 604–613 (2014). [Google Scholar]
- 50.Svergun D., Barberato C., Koch M. H. J., CRYSOL–a program to evaluate x-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Cryst. 28, 768–773 (1995). [Google Scholar]
- 51.Ritchie T. K., Grinkova Y. V., Bayburt T. H., Denisov I. G., Zolnerciks J. K., Atkins W. M., Sligar S. G., Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denisov I. G., Grinkova Y. V., Lazarides A. A., Sligar S. G., Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. Am. Chem. Soc. 126, 3477–3487 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Nakanishi H., Hayashida K., Nishizawa T., Oshima A., Abe K., Cryo-EM of the ATP11C flippase reconstituted in nanodiscs shows a distended phospholipid bilayer inner membrane around transmembrane helix 2. J. Biol. Chem. 298, 101498 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baginski E. S., Foa P. P., Zak B., Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin. Chem. 13, 326–332 (1967). [PubMed] [Google Scholar]
- 55.Punjani A., Rubinstein J. L., Fleet D. J., Brubaker M. A., cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Adams P. D., Afonine P. V., Bunkoczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H., PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pettersen E. F., Goddard T. D., Huang C. C., Meng E. C., Couch G. S., Croll T. I., Morris J. H., Ferrin T. E., UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of COOT. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Afonine P. V., Poon B. K., Read R. J., Sobolev O. V., Terwilliger T. C., Urzhumtsev A., Adams P. D., Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams C. J., Headd J. J., Moriarty N. W., Prisant M. G., Videau L. L., Deis L. N., Verma V., Keedy D. A., Hintze B. J., Chen V. B., Jain S., Lewis S. M., Arendall W. B. III, Snoeyink J., Adams P. D., Lovell S. C., Richardson J. S., Richardson D. C., MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang J., Rauscher S., Nawrocki G., Ran T., Feig M., de Groot B. L., Grubmuller H., MacKerell A. D. Jr., CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klauda J. B., Venable R. M., Freites J. A., O’Connor J. W., Tobias D. J., Mondragon-Ramirez C., Vorobyov I., MacKerell A. D. Jr., Pastor R. W., Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bussi G., Donadio D., Parrinello M., Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Bernetti M., Bussi G., Pressure control using stochastic cell rescaling. J. Chem. Phys. 153, 114107 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S14
Tables S1 and S2