Abstract
The chinchilla crista ampullaris was studied in 10 samples, each containing 32 consecutive ultrathin sections of the entire neuroepithelium. Dissector methods were used to estimate the incidence of various synaptic features, and results were confirmed in completely reconstructed hair cells. There are large regional variations in cellular and synaptic architecture. Type I and type II hair cells are shorter, broader, and less densely packed in the central zone than in the intermediate and peripheral zones. Complex calyx endings are most common centrally. On average, there are 15–20 ribbon synapses and 25–30 calyceal invaginations in each type I hair cell. Synapses and invaginations are most numerous centrally. Central type II hair cells receive considerably fewer afferent boutons than do peripheral type II hair cells, but have similar numbers of ribbon synapses. The numbers are similar because central type II hair cells make more synapses with the outer faces of calyx endings and with individual afferent boutons. Most afferent boutons get one ribbon synapse. Boutons without ribbon synapses were only found peripherally, and boutons getting multiple synapses were most frequent centrally. Throughout the neuroepithelium, there is an average of three to four efferent boutons on each type II hair cell and calyx ending. Reciprocal synapses are rare. Most synaptic ribbons in type I hair cells are spherules; those in type II hair cells can be spherical or elongated and are particularly heterogeneous centrally. Consistent with the proposal that the crista is concentrically organized, the intermediate and peripheral zones are each similar in their cellular and synaptic architecture near the base and near the planum. An especially differentiated subzone may exist in the middle of the central zone.
Indexing terms: vestibular labyrinth, hair cells, afferent, efferent, calyx
The mammalian crista has been divided into central, intermediate, and peripheral zones that differ in their cellular architecture (Lindeman, 1969; Fernández et al., 1995), afferent innervation (Lorente de Nó, 1926; Poljak, 1927; Fernández et al., 1988, 1995), and afferent physiology (Baird et al., 1988; Lysakowski et al., 1995). Are there parallel differences in synaptic organization? Past ultra-structural studies (for review, see Wersäll and Bagger-Sjöbäck, 1974), while clarifying the morphology of hair cells and of their afferent and efferent innervation, have not considered this possibility, nor have they provided numeric estimates of the various synaptic structures related to type I and type II hair cells and their innervating nerve fibers. Structures of interest are illustrated in Figure 1 and include ribbon synapses, calyceal invaginations, afferent and efferent boutons, outer-face calyx synapses, and reciprocal synapses.
Fig. 1.
Diagram of sensory cells and synaptic elements in the vestibular neuroepithelium. I, type I hair cell. II, type II hair cell. Calyx (Cal), the afferent ending surrounding the type I hair cell, can invaginate into the hair cell, forming a calyceal invagination (CI). Ribbon synapses (RS) are made between the type I hair cell and the calyx ending, between the type II hair cell and its afferent bouton (Aff), and between the type II hair cell and the outer surface of the calyx ending. The latter is called an outer-face synapse (OF). Afferent endings (Cal and Aff) arise from primary afferent neurons with cell bodies in the vestibular ganglion. Efferent boutons (Eff), highly vesiculated endings of neurons originating in the brainstem, contact calyx endings and other afferent processes at synapses marked by presynaptic and postsynaptic densities (PsD). Efferent contacts with type II hair cells are characterized by a subsurface cistern (SC). Reciprocal synapses (Rec) are single endings with neighboring zones resembling afferent and efferent synapses.
To illustrate the need for numeric estimates, we can consider ribbon synapses, which are thought to mediate conventional (chemical) synaptic transmission between hair cells and afferent terminals (Smith and Sjöstrand, 1961; Heuser and Reese, 1977; Roberts et al., 1990; Parsons et al., 1994). In a ribbon synapse, an electron-dense core is surrounded by a halo of vesicles and is attached to the presynaptic membrane of the hair cell by a density, which is thicker than the corresponding postsynaptic density (Smith and Sjöstrand, 1961; Wersäll and Bagger-Sjöbäck, 1974). Although exact numbers are lacking, it is clear that each type II hair cell receives many afferent boutons and makes ribbon synapses with most of these endings (Wersäll and Bagger-Sjöbäck, 1974; Bagger-Sjöbäck and Gulley, 1979). Ribbon synapses are also found between type I hair cells and calyx endings (Spoendlin, 1966), but the number of such synapses has remained controversial. Hamilton (1968) observed as many as 10 synapses in individual type I hair cells. In contrast, Favre and Sans (1979) emphasized that there were considerably fewer synapses in type I hair cells of the adult cat, compared with newborn kittens and Gulley and Bagger-Sjöbäck (1979) found few synapses in type I hair cells from the cristae of adult guinea pigs. Other studies (Goldberg et al., 1990; Yamashita and Ohmori, 1991; Lysakowski, 1996) indicate that ribbon synapses are plentiful in mature type I hair cells.
The unusual structure of the type I hair cell and its calyx ending has suggested that they are involved in novel modes of intercellular communication (Gulley and Bagger-Sjöbäck, 1979; Goldberg, 1996). Possible sites of novel transmission are the close appositions that occur where the calyx ending invaginates into the type I hair cell (Spoendlin, 1966; Hamilton, 1968; Gulley and Bagger-Sjöbäck, 1979). In a typical invagination, the intercellular cleft narrows from 20–30 to 6–7 nm. Because of the narrowing, invaginations were once thought to be electrical synapses (Spoendlin, 1966; Hamilton, 1968). This conjecture seems unlikely because gap junctions are not found in the invaginations or elsewhere in the type I hair cell (Gulley and Bagger-Sjöbäck, 1979; Ginzberg, 1984). It has also been suggested that invaginations may be involved in chemical transmission, as they are sometimes seen in proximity to ribbon synapses (Engström et al., 1972).
Another source of synaptic input to calyx endings comes from type II hair cells, which can form ribbon synapses with calyx outer faces (Engström, 1970; Bagger-Sjöbäck and Gulley, 1979; Ross, 1985, 1997). Many afferents give rise to both calyx and bouton endings and thereby receive a mixed input from type I and type II hair cells (Fernández et al., 1988, 1990, 1995). Outer-face synapses are of interest because their presence implies that individual calyx endings can also get a mixed input.
The vestibular neuroepithelium also receives an efferent innervation arising from the brainstem (Gacek and Lyon, 1974; Warr, 1975; Goldberg and Fernández, 1980; Lysakowski, 1996) and terminating as highly vesiculated boutons on type II hair cells, on calyx endings and on other afferent nerve processes (Hilding and Wersäll, 1962; Smith and Rasmussen, 1968; Wersäll, 1968; Iurato et al., 1972). Subsynaptic cisterns are found in type II hair cells opposite efferent boutons. Efferent synapses onto afferent processes do not have subsynaptic cisterns; rather, there is a thin condensation of dense material on both sides of the synapse (Wersäll, 1968; Iurato et al., 1972). Different suggestions have been made concerning regional differences in efferent innervation. Nomura et al. (1965) observed that efferent fibers, selectively stained by acetylcholinesterase histochemistry, were most heavily concentrated in the peripheral zone of the crista. Goldberg and Fernández (1980), in contrast, found that responses to electrical stimulation of efferent pathways were largest in afferents that were shown by later studies to be located in the central zone (Baird et al., 1988).
Mixed or reciprocal synapses may also be present. These involve individual bouton endings that form two kinds of contacts with hair cells. The hair cell makes a ribbon synapse with the bouton, which in turn gives rise to an efferent-like synapse, characterized by an accumulation of vesicles in the bouton and a subsynaptic cistern in the hair cell. Reciprocal synapses have been observed in vestibular (Engström et al., 1972; Dunn, 1980) and auditory organs (Tanaka and Smith, 1978; Nadol, 1983; Francis and Nadol, 1993). Based on work in the organ of Corti, Pujol and Carlier (1982) suggested that the boutons containing reciprocal synapses were of afferent origin and might result from the degeneration of neighboring efferent boutons. The frequency of reciprocal synapses in vestibular organs is unknown.
Our interest in the regional distribution of synaptic elements was stimulated when we combined hair-cell counts and afferent innervation patterns to calculate a so-called afferent reconstruction (Fernández et al., 1988, 1995). The reconstruction predicted that type II hair cells in the peripheral zone were contacted by many more afferent boutons than those in the central zone. A search of the ultrastructural literature failed to provide evidence concerning the prediction. Even more remarkably, in only one paper from this substantial literature was there any indication as to the regions of the neuroepithelium from which material was selected (Lindeman et al., 1981). To explore the possibility that there were regional variations in synaptic organization, a preliminary ultrastructural study was done on material cut in the transverse plane from one crista (Lysakowski and Goldberg, 1989; Goldberg et al., 1990). The neuroepithelium was divided into central, intermediate, and peripheral zones of equal areas (Lindeman, 1969; Fernández et al., 1988, 1995) and the dissector method (Gundersen, 1986) was used to count the number of synaptic structures related to type I and type II hair cells in each zone. There were substantial regional variations, particularly in the numbers of afferent boutons innervating individual type II hair cells and in the numbers of outer-face ribbon synapses.
The present study is intended to describe regional variations in the cellular and synaptic architecture of the chinchilla cristae. The chinchilla was chosen because this animal was used in our past work on afferent branching patterns (Fernández et al., 1988) and their relationship to afferent physiology (Baird et al., 1988). Several considerations influenced the experimental design. (1) The aforementioned preliminary study was done on a superior crista. To assess whether the results were representative, we took material from three additional organs, including samples of the posterior and horizontal cristae. (2) Some of the organs were cut in the transverse plane; others in the longitudinal plane. This allowed us to compare the intermediate and peripheral zones near the base of the organ (transverse material) and near the planum semilunatum (longitudinal material; Fig. 2). The comparison was of interest because our current model of crista organization, originally proposed by Lindeman (1969), assumes that the structure of the organ is similar near the base and near the planum. (3) Several hair cells were completely reconstructed. The reconstructions were used to check the accuracy of the dissector results and to provide information about the spatial distribution of synaptic elements within individual hair cells.
Fig. 2.

The crista of a chinchilla with the boundaries of the central (C), intermediate (I), and peripheral (P) zones indicated by arrowheads. Boundaries were determined from the relative distances between the midpoint of the neuroepithelium and its edges (see Materials and Methods, Zones of the crista). A: Transverse section. B: Longitudinal section. The planum semilunatum is indicated on the right by an asterisk. Semithin (2-μm) sections, methylene blue, and azure II. Scale bar = 100 μm in A,B.
MATERIALS AND METHODS
Tissue preparation
Four adult chinchillas were anesthetized with 10% 5,5-diallylbarbituric acid, 40% urethane, and 40% mono-ethyl urea (0.3 ml/kg, i.p.) and perfused transcardially with 100–200 ml of a warm 0.1 M sodium cacodylate-0.9% NaCl buffer (pH 7.4), followed by 1,000 ml of a warm trialdehyde fixative, consisting of 3% glutaraldehyde, 2% paraformaldehyde, 1% acrolein, and 5% sucrose in 0.08 M cacodylate buffer (DeGroot et al., 1987). Individual organs were dissected, postfixed for 1 hour in either 1% OsO4 or 1% OsO4/1.5% K4Fe(CN)6 · 3H2O in 0.1 M cacodylate buffer, dehydrated in graded alcohols and propylene oxide, and embedded in Araldite. Experiments were approved by the University of Chicago Institutional Animal Care and Use Committee.
Material came from four cristae, each from a different animal. Two of the organs were sectioned transversely, and the other two were sectioned longitudinally. Serial sections, 5-μm-thick, were cut with a glass knife through the whole organ and mounted on glass slides. Outlines of the neuroepithelium were drawn with a camera lucida from every 10th section. Distances, measured along the top surface of the neuroepithelium in each drawing, were combined to provide a flattened reconstruction of the sensory surface (Fig. 3). The only criterion used in selecting samples for ultrastructural analysis was that each section pass through all three zones of the crista (see below for a delineation of zones). This criterion necessitated that the sections be chosen near the middle of the organ and away from the corners of the epithelium. As a result, none of the selected samples passed through the corners of the neuroepithelium. Sections were remounted on prepared Araldite blocks and were serially sectioned with a diamond knife. Thirty-two consecutive ultrathin (75-nm) sections, starting at a randomly selected point in the original semithin section, constituted a sample. Ten samples were studied; three came from each transversely cut organ and two from each longitudinally cut organ. One of the transversely cut organs (Fig. 3A) was used previously (Goldberg et al., 1990).
Fig. 3.
Locations of 32-section samples are indicated by parallel lines on surface reconstructions of four cristae after transfer to a standard drawing. Each crista was taken from a different animal. A: Left superior crista, three samples. B: Right horizontal crista, three samples. C: Right posterior crista, two samples. D: Right horizontal crista, two samples; the peripheral zone at both ends of the organ was destroyed (stippled areas). Central zone: apex (CA), slope (CS), inner (CI), and outer (CO). Intermediate zone: near base (IB) and near planum (IP). Peripheral zone: near base (PB) and near planum (PP).
Sections were collected on formvar-coated 2-mm slot grids, stained with uranyl acetate and lead citrate (Reynolds, 1963) and examined in a Phillips 201 electron microscope. Low-power (×2,000) photomicrographs were taken of every fourth ultrathin section (≈ 0.3 μm apart), and a photomontage (×5,000 final magnification) was made of the entire neuroepithelium contained in the section. All hair-cell and supporting-cell nuclei in the 32-section sample were identified. Ribbon synapses were counted by direct serial examination in the electron microscope. Individual afferent boutons, calyceal invaginations, and efferent boutons were identified and counted from the montages.
Zones of the crista
The neuroepithelium was divided into central (C), intermediate (I), and peripheral (P) zones of equal areas (Fernández et al., 1988; Figs. 2, 3, present paper). To determine boundaries, we adopted a two-dimensional rectangular coordinate system whose coordinates were normalized to the distances between the midpoint and the edges of the neuroepithelium along the longitudinal and transverse axes of the organ. The corners of the C, I, and P zones occur at (±1/√3, ±1/√3), (± √2/√3, ± √2/√3), and (±1, ±1), respectively. Distances in the section plane were measured directly; those in the orthogonal direction were estimated from cumulative section thickness. To determine whether the cellular and synaptic architecture was uniform throughout the central zone, we divided it into two subzones of equal area. For transverse material, the subzones occupied the apex (CA) and slopes (CS; Fig. 3A). The division for longitudinal material was into inner (CI) and outer (CO) subzones (Fig. 3C). Somewhat different parts of the central zone, as well as of the intermediate and peripheral zones, were sampled in the longitudinal and transverse material. The two sets of material allowed us to compare the crista near the planum semilunatum (longitudinal sections) and near the base of the crista (transverse sections; Figs. 2, 3). We designate the various subzones as IP, IB, PP, and PB. The first letter refers to the zone (I or P) and the second letter to the planum (P) or base (B; Fig. 3A,C).
Analysis of dissector samples
Number of cells, cell densities, and cell ratios
The equivalent numbers of type I and type II hair cells and of supporting cells were estimated in each 32-section sample by counting the corresponding nuclei with the dissector technique (Gundersen, 1986). The three kinds of cells were easily distinguished. Supporting-cell nuclei had much denser heterochromatin than did hair-cell nuclei. Also, type I (but not type II) hair cells were surrounded by calyx axoplasm. Every type I, type II, or supporting-cell nucleus present in the first (“reference”) section of the 32-section sample, but not in the last (“lookup”) section was counted. As nuclear size is larger than the sample thickness of 2.3 μm, no nucleus fell entirely within the sample. For this reason, reversing the order of the reference and lookup sections provided a second, independent set of counts. The two counts were averaged to give Q−, an estimate of the equivalent number of hair cells or supporting cells contained in the sample.1
Hair-cell and supporting-cell densities, determined for each zone of the crista, were estimated as the ratio between Q− and the surface area of the neuroepithelium. The latter was obtained by measuring the distance along the top of the neuroepithelium and multiplying it by the sample thickness. Hair-cell densities and the ratio of type I to type II hair cells were determined for both section planes; supporting-cell densities and the ratio of supporting cells to hair cells were only obtained for the transverse plane.
Hair-cell dimensions
Various dimensions were measured from the montages, including the width (diameter) and length of hair cells and the width of calyx endings. Hair-cell and calyx widths were determined by drawing a line through the midpoint of the hair-cell nucleus parallel to the top surface of the epithelium. Calyx width was taken as the distance between the ending’s inner and outer surfaces. A so-called neck width of type I hair cells was determined above the cell body, where the width reached a local minimum. Apical widths were measured along straight lines connecting the two edges of the hair cell at the top of the neuroepithelium. Lengths were measured along a curved line through the center of the cell from its apical surface to its base. Measurements were only kept in the analysis if they reached a maximum in the 32-section sample.
Number of synaptic elements per hair cell
Double counting of synaptic structures was avoided by not counting any element found in the lookup section. The number of elements per hair cell was obtained as a ratio between the number of elements and Q− in each dissector sample. Ratios were obtained for each synaptic structure listed in Tables 4 and 5, and were separately compiled for type I and type II hair cells in the various zones of the crista.
TABLE 4.
Synaptic Structures per Type I Hair Cell, Chinchilla Crista1
| Zone | Samples | Hair cells | Inner-face ribbon synapses | Calyceal invaginations | Efferent boutons | Outer-face ribbon synapses |
|---|---|---|---|---|---|---|
| Central | 10 | 50 | 22 ± 4 | 53 ± 8 | 2.9 ± 0.5 | 2.9 ± 0.6 |
| Intermediate | 10 | 40 | 17 ± 2 | 17 ± 2 | 3.2 ± 0.5 | 1.0 ± 0.4 |
| Peripheral | 8 | 23 | 10 ± 1 | 10 ± 2 | 3.3 ± 0.4 | 0.1 ± 0.1 |
| All | 28 | 113 | 17 ± 2 | 28 ± 5 | 3.1± 0.3 | 1.4 ± 0.3 |
Samples, number of 31-section dissectors. Hair cells, equivalent number of type I hair cells in the samples. All other values, mean ± SE, number of structures per type I hair cell. Results of an ANOVA with categorical variables as in Table 1. Zone effects: Inner-face ribbon synapses: overall (P < 0.01); C vs. I (P < 0.10); C vs. P (P < 0.005). Calyceal invaginations: overall (P < 0.001); C vs. I and C vs. P (P < 0.001). Efferent boutons: overall (P > 0.8). Outer-face ribbon synapses: overall (P < 0.001); C vs. I and C vs. P (P < 0.001); I vs. P (P < 0.1). Axis effects: Calyceal invaginations: CL > CT (P < 0.001); Outer-face ribbon synapses: CL > CT (P < 0.001); IP > IB (P < 0.02).
TABLE 5.
Synaptic Structures per Type II Hair Cell, Chinchilla Crista1
| Zone | Samples | Hair cells | Ribbon synapses | Afferent boutons | Efferent boutons | Outer-face ribbon synapses | Ribbon synapses per afferent bouton |
|---|---|---|---|---|---|---|---|
| Central | 10 | 58 | 15 ± 1 | 9 ± 1 | 3.5 ± 0.4 | 2.5 ± 0.5 | 1.8 ± 0.4 |
| Intermediate | 10 | 30 | 22 ± 2 | 22 ± 3 | 4.2 ± 0.4 | 1.5 ± 0.6 | 1.0 ± 0.1 |
| Peripheral | 8 | 30 | 17 ± 2 | 22 ± 2 | 2.5 ± 0.4 | 0.1 ± 0.1 | 0.8 ± 0.1 |
| All | 28 | 118 | 18 ± 1 | 17 ± 2 | 3.5 ± 0.3 | 1.5 ± 0.3 | 1.2 ± 0.2 |
Samples, number of 31-section dissectors. Hair cells, equivalent number of type II hair cells in the samples. All other values, mean ± SE, number of structures per type II hair cell. The last column was obtained by taking a ratio in each sample between the number of ribbon synapses not making outer-face synapses and the number of afferent boutons. Results of an ANOVA with categorical variables as in Table 1. Zone effects: Ribbon synapses: overall (P < 0.01); C vs. I (P < 0.005); I vs. P (P < 0.02). Afferent boutons: overall (P < 0.001); C vs. I (P < 0.001); C vs. P (P < 0.005). Efferent boutons: overall (P < 0.05); I vs. P (P < 0.02). Outer-face ribbon synapses: overall (P < 0.002); C vs. P (P < 0.001); I vs. P (P < 0.02). Ribbon synapses per afferent bouton: overall (P < 0.05); C vs. I and C vs. P (P < 0.05). Axis effects: Efferent boutons: CL > CT (P < 0.02). Outer-face ribbon synapses: CL > CT (P < 0.10); IP > IB (P < 0.001).
Statistical procedures
Values were entered into 2 × 2 analyses of variance (ANOVAs) in SYSTAT for the Macintosh (Wilkinson et al., 1992). In some cases, the logarithms of the variables were used to minimize differences between intragroup variances. For cell densities and cell ratios (Table 1) and for the numbers of synaptic elements per hair cell (Tables 4, 5), values for each of the 10 dissector samples were used. Cell dimensions (Tables 2, 3) were entered for individual hair cells. Unless stated otherwise, class variables were the zones (central [C], intermediate [I], peripheral [P]) and the section axes (longitudinal [L], transverse [T]); an interaction term (zones × axes) was included and the algorithm was adjusted for an unbalanced design. Probabilities of main and interaction effects were calculated from F tests and were considered significant if P < 0.05. Fisher’s LSD tests were used to evaluate planned comparisons between individual means (C vs. I, C vs. P, and I vs. P) and between corresponding zones along the two section axes (CL vs. CT, IP vs. IB, and PP vs. PB). The first three comparisons were tested only if the zone effect was significant (P < 0.05); the last three comparisons required that the axis or the interaction effect was significant. In Tables 1–5, entries are means ± SE based on the original (untransformed) values. Probability values for individual comparisons are included in the tables only if P < 0.1.
TABLE 1.
Hair-Cell and Supporting Cell Densities and Ratios, Chinchilla Crista1
| Zone | Density (cells/100 μm2)
|
Ratio
|
||
|---|---|---|---|---|
| Hair cell | Supporting cell | Type I/type II | Supporting cell/hair cell | |
| Central | 1.69 ± 0.09 | 1.93 ± 0.05 | 0.90 ± 0.06 | 1.08 ± 0.05 |
| Intermediate | 2.48 ± 0.10 | 2.34 ± 0.12 | 1.33 ± 0.13 | 0.98 ± 0.07 |
| Peripheral | 3.31 ± 0.14 | 3.42 ± 0.16 | 0.79 ± 0.03 | 1.09 ± 0.06 |
| All | 2.44 ± 0.14 | 2.56 ± 0.17 | 1.02 ± 0.07 | 1.05 ± 0.04 |
Entries, mean ± SE based on ten 31-section dissector samples (central and intermediate zones) and eight such samples (peripheral zone). Hair-cell densities and type I/type II ratios were entered into an analysis of variance (ANOVA) with zone (central [C], intermediate [I], peripheral [P]) and section axis (longitudinal [L], transverse [T]) as categorical variables; an interaction term (zone × axis) was included. For axis effects, the sign of the difference is indicated. Supporting-cell densities and supporting cell/hair cell ratios were analyzed by an ANOVA with the zone as the categorical variable, transverse only. For other details, see text. Zone effects: Hair-cell densities: overall (P < 0.001); C vs. I, C vs. P, and I vs. P (P < 0.001). Supporting-cell densities: overall (P < 0.001); C vs. I (P < 0.02); C vs. P and I vs. P (P < 0.001). Type I/type II ratio: overall (P < 0.001); C vs. I and I vs. P (P < 0.001). Supporting cell/hair cell ratio: overall (P < 0.1). Axis effects: Hair-cell densities: CL < CT (P < 0.05); PP > PB (P < 0.1). Type I/type II ratio: CL < CT (P < 0.1); IL > IT (P < 0.05).
TABLE 2.
Dimensions, Type I Hair Cells, Chinchilla Crista1
| Zone | Cell width (μm)
|
Cell height (μm)
|
Neck width (μm)
|
Apical width (μm)
|
Calyx width (μm)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SE | N | Mean ± SE | N | Mean ± SE | N | Mean ± SE | N | Mean ± SE | |
| Central | 75 | 8.20 ± 0.13 | 64 | 22.4 ± 0.3 | 69 | 2.11 ± 0.08 | 56 | 5.35 ± 0.11 | 47 | 0.75 ± 0.05 |
| Intermediate | 37 | 7.15 ± 0.12 | 31 | 22.9 ± 0.6 | 30 | 1.39 ± 0.08 | 28 | 4.54 ± 0.14 | 37 | 0.56 ± 0.03 |
| Peripheral | 28 | 6.56 ± 0.19 | 36 | 22.5 ± 0.6 | 36 | 1.11 ± 0.04 | 30 | 3.74 ± 0.17 | 28 | 0.49 ± 0.03 |
| All | 140 | 7.59 ± 0.10 | 131 | 22.6 ± 0.3 | 135 | 1.68 ± 0.06 | 114 | 4.72 ± 0.10 | 112 | 0.62 ± 0.03 |
N, number of hair cells. Values were entered into a 2 × 2 ANOVA with categorical variables as in Table 1. Zone effects: Cell width: overall (P < 0.001); C vs. I and C vs. P (P < 0.001). Cell height: overall (P > 0.1). Neck width: overall (P < 0.001); C vs. I and C vs. P (P < 0.001); I vs. P (P < 0.02). Apical width: overall (P < 0.001); C vs. I and C vs. P (P < 0.001); I vs. P (P < 0.01). Calyx width: overall (P < 0.001); C vs. I (P < 0.002); C vs. P (P < 0.001). Axis effects: Cell width: PP < PB (P < 0.001). Cell height: IP > IB and PP > PB (P < 0.001). Neck width: CL > CT (P < 0.02). Apical width: CL < CT (P < 0.001); IP < IB (P < 0.1); PP < PB (P < 0.001).
TABLE 3.
Dimensions, Type II Hair Cells, Chinchilla Crista1
| Zone | Cell width (μm)
|
Cell height (μm)
|
||
|---|---|---|---|---|
| N | Mean ± SE | N | Mean ± SE | |
| Central | 57 | 7.85 ± 0.16 | 67 | 21.9 ± 0.4 |
| Intermediate | 36 | 7.29 ± 0.13 | 26 | 23.5 ± 0.8 |
| Peripheral | 22 | 6.69 ± 0.13 | 37 | 25.3 ± 0.7 |
| All | 115 | 7.45 ± 0.10 | 130 | 23.2 ± 0.4 |
N, number of hair cells. Values were entered into a 2 × 2 ANOVA with categorical variables as in Table 1. Zone effects: Cell width: overall (P < 0.001); C vs. I (P < 0.02); C vs. P (P < 0.001); I vs. P (P < 0.1). Cell height: overall (P < 0.001); C vs. I (P < 0.05); C vs. P (P < 0.001); I vs. P (P < 0.05). Axis effects: Cell height: CL < CT (P < 0.05); PP > PB (P < 0.001).
Reconstruction of individual hair cells
Several completely sectioned type I and type II hair cells were serially photographed at ×11,250. The material came from a set of 300 consecutive sections, which included the three samples of Fig. 3A. For each type I hair cell, we determined, among other things, the numbers and locations of inner- and outer-face ribbon synapses, of calyceal invaginations, and of efferent contacts on the calyx ending. The list for type II hair cells included the numbers and locations of ribbon synapses (including those contacting the outer faces of neighboring calyx endings), of afferent and efferent boutons, and of reciprocal synapses.
RESULTS
Material consisted of ten 32-section samples taken from one superior, one posterior, and two horizontal cristae (Fig. 3). Results from the 10 samples were combined for statistical purposes. Two of the cristae were cut in the transverse plane (Fig. 3A,B); the other two were sectioned longitudinally (Fig. 3C,D). In one of the longitudinal cases the peripheral zone was destroyed (Fig. 3D). All other samples included the entire cross-section of the neuroepithelium.
There were 601 type I and 580 type II hair-cell nuclei identified in the 10 samples. Dissector calculations provided estimates of 113 type I and 118 type II hair-cell equivalents. Seven type I and 10 type II hair cells were serially photographed at ×11,250. Another type I and five other type II hair cells were serially examined, but not photographed.
Cytoarchitecture of the crista ampullaris
Central, intermediate, and peripheral zones
The cytoarchitecture of the crista ampullaris was described by Lindeman (1969) from surface preparations and by Fernández et al. (1995) in semithin sections. We have examined the cytoarchitecture at an ultrastructural level. Figure 4 is a longitudinal section through the central zone, which can be compared with a longitudinal section from the peripheral zone near the planum semilunatum (PP; Fig. 5A) and a transverse section, also from the peripheral zone, but at the base of the organ (PB; Fig. 5B).
Fig. 4.

Electron photomicrograph, longitudinal section, central zone of posterior crista (cf. Fig. 3C). Three type II hair cells (cells 67, 72, and 73) and five type I hair cells (66R, 68, 70R, 71L, and 71R). Cells 71L and 71R are innervated by a complex calyx ending whose parent axon is seen entering the neuroepithelium. Nuclei of supporting cells, located basally, have multiple nucleoli and denser heterochromatin than the hair-cell nuclei. Scale bar = 10 μm.
Fig. 5.

Electron photomicrographs, peripheral zone. A: Longitudinal section near planum semilunatum (PP subzone), posterior crista (cf. Fig. 3C) includes four type I (136, 138, 140R, and 145) and four type II (137, 139, 142, and 146) hair cells. B: Transverse section near base (PB subzone), superior crista (cf. Fig. 3A) includes three type I (47bL, 47bR, and 47c) and four type II (45a, 48a, 51a, and 52) hair cells. Scale bar = 10 μm in B (applies to A, B).
Several features are similar in all regions: (1) Nuclei are arranged in layers, with supporting-cell nuclei located basally in the neuroepithelium, type I hair-cell nuclei midway, and type II hair-cell nuclei more apically (Figs. 4, 5). (2) Type I and type II hair cells are found side by side throughout the neuroepithelium. The ratio between the numbers of type I and type II hair cells has a value near unity when averaged over the entire end organ; the ratio is highest in the intermediate zone and lowest in the peripheral zone (Table 1). (3) There are approximately equal numbers of supporting cells and hair cells in each of the three zones (Table 1). (4) The height of the neuroepithelium is ≈ 30 μm (Figs. 2A, 4, 5B), except near the planum, where it approaches 40 μm (Figs. 2B, 5A). (5) Throughout most of the crista, hair cells of either type have lengths of 20–25 μm (Fig. 7C,D). Near the planum, type II hair cells approach 30 μm.
Fig. 7.

Cell diameters (A,B), cell lengths (C,D), neck width (E), and calyx-wall thickness (F) are plotted against a normalized distance from the center (0) to the edge (1) of the neuroepithelium along its transverse (filled circles) and longitudinal (open circles) axes. Type I (B,D–F) and type II (A,C) hair cells. Circles, means; bars, SE. Zonal abbreviations as in Figure 3.
Obvious differences exist between the central (Fig. 4) and peripheral zones (Fig. 5). Several of the differences are summarized in Tables 1–3. (1) Both hair cells and supporting cells are more densely packed in the peripheral zone (Table 1). (2) Calyx endings can be described as simple (enclosing a single hair cell) or complex (enclosing two or more hair cells). Complex endings are most common in the central zone. Based on the entire set of 10 samples, the proportion of type I hair cells with complex endings is 19 of 50 (38%) in the central zone and 6 of 60 (10%) in the intermediate and peripheral zones. (3) Hair cells in the central and peripheral zones differ in their sizes and shapes. As summarized in Table 2, central type I hair cells are thicker and have thicker calyx endings. Type II hair cells also differ regionally. Similar to type I hair cells, type II hair cells are thickest in the central zone (Table 3). Most central type II hair cells are cylindrically shaped (Fig. 4, cells 67 and 73; Fig. 8A, cells 24, 24a, 27 and 30b). In the periphery, many type II hair cells are goblet- or dumbbell-shaped (Fig. 5A, cells 139 and 142; Fig. 5B, cells 45a and 48a; Fig. 8B, cells 43 and 46), shapes complementary to those of neighboring type I hair cells and their calyx endings. In addition, peripheral type II hair cells extend deeper into the epithelium than do their type I neighbors, whereas the two kinds of hair cells have similar heights in the central zone (cf. Tables 2, 3; Fig. 8A,B). One gains the impression that the peripheral zone is so tightly packed that type II hair cells are squeezed between the enlarged perinuclear volumes of type I hair cells. The central zone is more loosely arranged and accommodates the synaptic poles of type I and type II hair cells at the same level of the neuroepithelium.
Fig. 8.

Reconstruction of central zone (A) and peripheral zone (B) from a superior crista, transverse sections (cf. Fig. 3A). Hair cells (pale yellow), supporting cell nuclei (SC, gray), afferent boutons (yellow), efferent boutons (E, green), calyx endings, and thick processes (orange). Shapes of synaptic ribbons and efferent synaptic specializations, see key in A. PSD, postsynaptic density; CA and CS, central subzones (apex and slope) with an arrow indicating the boundary between them. See Figure 9A for a photomicrograph of cells 24, 25L, and 25R. A photomicrograph of cells 24a and 25L is seen in Figure 11. Scale bar = 5 μm (applies to A,B).
Transverse and longitudinal material
To examine the cytoarchitecture of the central zone in more detail, we divided it into apical (CA) and slope (CS) subzones in the transverse material and into inner (CI) and outer (CO) subzones in the longitudinal material (Fig. 3). Results are summarized in Figures 6 and 7. Compared with the other three subzones, CS has a higher hair-cell density (Fig. 6A), its type I hair cells are narrower (Fig. 7B,E), and proportionately fewer of them are innervated by complex endings (1.5 of 13 or 11% in CS vs. 17.5 of 36.5 or 48% in the other three subzones). Type II hair cells in CS are distinctively thin (Fig. 7A) and long (Fig. 7C). In all of these respects, the cytoarchitecture of CS resembles that of the intermediate and peripheral zones. An ANOVA, done on all four subzones, suggests that the dimensional differences among type I cells are related to the epithelial location, rather than to the type of calyx ending (simple or complex).
Fig. 6.

Hair-cell density (A), ratio between the numbers of type I and type II hair cells (B), and epithelial height (C) are plotted against a normalized distance from the center (0) to the edge (1) of the neuroepithelium along its transverse (filled circles) and longitudinal (open circles) axes. Circles, means; bars, SE. Zonal abbreviations as in Figure 3.
The peripheral subzones near the planum (PP) and near the base of the crista (PB) were compared (Fig. 5). Hair-cell densities are higher in PP (Fig. 6A); type I hair cells are narrower (Fig. 7B); and type I and type II hair cells (Fig. 7C,D) and the neuroepithelium (Fig. 6C) are taller. From these results, it would appear that the cytoarchitectural features that distinguish the peripheral from the central zone are particularly prominent in the vicinity of the planum. A similar comparison for the intermediate zone (IP vs. IB) indicates that the type I/type II ratio is higher in IP (Fig. 6B) and type I hair cells are taller there (Fig. 7D).
Locations and numbers of afferent synaptic elements
There are differences in the synaptic organization of the central and peripheral zones. These will be described in serially photographed hair cells. Information from several of these is summarized in Figure 8. Quantitative data based on dissector calculations are presented in Tables 4 and 5.
Type I hair cells, central zone
Figure 9A includes two type I hair cells (25L and 25R) enclosed in a complex calyx. Cell 25L had 20 ribbon synapses and 25R had 15; two examples are seen at higher magnification in the insets (Fig. 9A, bottom left and bottom right). A third serially photographed type I hair cell had 23 synapses. From dissector counts, individual central type I hair cells contain 20–25 ribbon synapses (Table 4). As indicated by the arrows in Figure 9A, ribbons are distributed in a seemingly random fashion about the long axis of the hair cell. Most ribbons are located at subnuclear levels (Figs. 8A, 9A). Fewer are found at the level of the nucleus and still fewer at supranuclear levels. None are seen in the hair-cell neck.
Fig. 9.

A: Electron photomicrograph of two type I hair cells (25L and 25R) enclosed in a complex calyx ending. Central zone, superior crista (cf. Fig. 3A). The ending was part of an afferent fiber whose termination included at least one other calyx ending, which was simple, and several boutons on a collateral. Type II hair cells 24 and 27a (24 and 27a) and cells 24a and 27 (not shown) contribute outer-face ribbon synapses to the calyx ending. Ribbon synapses in the section are indicated (thick arrows); locations of ribbon synapses in other sections are projected onto the section (thin arrows). This convention also holds for Figures 10–12, 14. Invaginations appearing in section are numbered. Two efferent boutons, both labeled E2, contact the calyx ending. Insets: Upper left: a ribbon synapse, forming part of a cluster, is made by cell 24 on the calyx ending surrounding cell 25L. Lower left and lower right: inner-face ribbon synapses made by cells 25L and 25R, respectively. B,C: Two of the numbered invaginations in A are seen at higher power. Although the calyx axoplasm in C appears to be “pinched-off,” it can be traced in other sections to the parent ending. Small arrows and arrowheads point, respectively, to the intercellular space inside and outside of the invaginations. D: The efferent bouton contacting the calyx ending surrounding cell 25L also synapses with cell 24. The postsynaptic cistern in cell 24 is studded with ribosomes (arrowheads) and is continuous with the smooth endoplasmic reticulum (ser). Scale bars = 5 μm in A, 100 nm in insets, 0.5 μm in B–D.
In addition to the synaptic inputs they receive on their inner face from type I hair cells, calyx endings in the central zone are commonly contacted on their outer face by ribbon synapses from type II hair cells. There are a total of 10 outer-face synapses on the calyx ending surrounding cells 25L and 25R (Figs. 8A, 9A). The synapses occur at relatively long appositions between the ending and neighboring type II hair cells. An example can be seen in the upper left hand inset of Figure 9A; here, a type II hair cell (cell 24) makes a 3.5-μm apposition with the complex calyx adjacent to cell 25L and provides three ribbon synapses, of which one is seen in the micrograph. Three others come from another type II hair cell (cell 24a, Fig. 8A). The ending, where it surrounds cell 25R, receives two outer-face synapses from cell 27A (Fig. 9A) and another two from a second type II hair cell (cell 27, Fig. 8A). According to Table 4, 10–15% of the ribbon synapses on central calyx endings are of the outer-face variety.
Calyx endings in the central zone make a large number of invaginations into type I hair cells. Cell 25L has 55 invaginations and 25R has 110 invaginations. Two of those from cell 25R (Fig. 9A) can be seen at higher magnification in Figures 9B and C. On average, there are about 50 calyceal invaginations per central type I hair cell, more than twice the number of ribbon synapses (Table 4). As is the case for ribbon synapses, invaginations are common at the base of the hair cell and never present in the neck.
Type I hair cells, peripheral zone
A peripheral type I hair cell (cell 5a), innervated by a simple calyx ending, is illustrated in Figure 10. There are 15 ribbon synapses made with the inner face of the calyx, and all of them are located below the nucleus. One of them is seen in the inset to Figure 10. In three other serially photographed hair cells, the number of ribbons ranged from 7 to 11. Appositions between peripheral calyx endings and neighboring type II hair cells are short. In Figure 9, for example, a type II hair cell (2b) contacts the calyx of cell 5a over a distance of <1 μm and no outer-face synapses are found there or elsewhere on the ending. Cell 5a has a total of 11 invaginations; none are seen in Figure 10.
Fig. 10.
Electron photomicrograph of a type I hair cell (5a) from the peripheral zone, superior crista (cf. Fig. 3A). Locations of 15 inner-face ribbon synapses in other sections are projected onto this section (thin arrows). Although the calyx ending makes a short appositional contact with type II cell 2b, no outer-face ribbons are found. The ending receives three efferent boutons, of which two (E1 and E2) are seen here. Arrow conventions as in Fig. 9. Inset: an inner-face ribbon synapse is made onto the ending in a nearby section. Scale bars = 5 μm in figure, 0.25 μm in inset.
Cell 5a is typical of peripheral type I hair cells (Table 4 and Fig. 8B). In comparison to their central counterparts, there are fewer ribbon synapses and considerably fewer calyceal invaginations. Outer-face calyx synapses are found only rarely. As indicated by Table 4, statistics for the intermediate zone fall between those for the central and peripheral zones. The locations of ribbon synapses in type I hair cells were determined for the three zones. In a survey of 691 synapses from 41 type I hair cells, 67% were subnuclear, 24% nuclear, and 9% supranuclear. Supranuclear synapses were more common in the central zone (51 of 350 = 15%), than in the peripheral zone (0 of 126).
Type II hair cells, central zone
Cell 24a (Fig. 11) illustrates typical features of central type II hair cells, which include a relatively small number of afferent boutons and a larger number of ribbon synapses. Three afferent boutons contact cell 24a. Of the nine synapses, three supply one bouton, two another bouton, and one the third bouton (Fig. 11, left inset). In addition, three synapses contact the outer face of a neighboring calyx ending, of which one is shown in Figure 11 (right inset). All of the synapses in cell 24a are below the nucleus.
Fig. 11.
Electron photomicrograph of a type II hair cell (24a) from the central zone, superior crista (cf. Fig. 3A). The cell is contacted by three afferent boutons and six efferent boutons. All three afferent boutons (1, 2, and 3) and one efferent bouton (E2) appear in the section. Arrow conventions as in Figure 9. The ribbon contacting bouton 2 is a plate (left inset), whereas the ribbon contacting the calyx innervating cell 25L is a spherule (right inset). In addition, two ribbons contact bouton 1, three contact bouton 3, and two contact the calyx ending of cell 25L. Scale bar = 5 μm in figure, 0.5 μm in insets.
Central type II hair cells have, on average, about 10 afferent boutons and 15 ribbon synapses (Table 5). About 15–20% of the synapses are made with calyx outer faces. Synaptic contacts were counted in 23 completely sectioned afferent boutons. All of the endings had at least one ribbon synapse. Eleven boutons (48%) had one synapse, four (17%) had two synapses, three (13%) had three synapses, two (9%) had four synapses, and three (13%) had seven synapses. The data give a mean (± SE) of 2.5 ± 0.4 synapses per bouton, slightly higher than the dissector count of 1.8 ± 0.4 (Table 5).
Type II hair cells, peripheral zone
Peripheral type II hair cells are contacted by large numbers of afferent boutons. For example, the dumbbell-shaped cell 45a (Fig. 12) is innervated by 36 afferent boutons located well below its nucleus. Most of the boutons receive a single ribbon synapse from the hair cell (Fig. 12, inset). Short appositions (<1 μm) are made by cell 45a with two neighboring type I hair cells (44a and 47bL), but no outer-face calyx synapses are found.
Fig. 12.
Electron photomicrograph of a dumbbell-shaped type II hair cell (45a) squeezed between two type I hair cells (44a and 47bL), peripheral zone, superior crista (cf. Fig. 3A). The type II cell receives 35 afferent and 6 efferent boutons (including E1 and E2). A synapse from a near-adjacent section onto bouton 17 is shown at higher magnification (inset). No synapses are made with the calyx endings of the neighboring type I hair cells or with the large collateral (LC1) contacting cell 45a at its base. Arrow conventions as in Figure 9. Scale bar = 5 μm in figure, 0.5 μm in inset.
Dissector counts indicate that, on average, peripheral type II hair cells have ≈15 ribbon synapses and contact 20–25 afferent boutons (Table 5). Counts were higher in four serially photographed peripheral cells. Each had more than 20 ribbons and about 30 afferent boutons. The number of synapses on individual fully reconstructed boutons was studied in the same four cells. There were a total of 119 boutons. Forty (34%) had no synapses, 68 (57%) had one synapse, nine (8%) had two synapses, one (1%) had three synapses, and one (1%) had four synapses. The mean (± SE) is 0.78 ± 0.06 synapses per bouton, virtually identical to the value obtained from dissector counts (0.78 ± 0.05; Table 5).
Not all synapses from type II hair cells are made onto boutons. Thick processes, either parent axons or collaterals, course through the neuroepithelium above the level of supporting-cell nuclei. Thick processes terminate as calyx endings (Fernández et al., 1988). In addition, there are thin processes, ≈0.2 μm in diameter, emerging from parent axons, from thick collaterals or from calyx endings. Bouton endings are exclusively found along and at the ends of the thin processes. In a survey of four dissector samples, including all three zones, 24 of 715 or 3.4% of the ribbon synapses from type II hair cells were found on thick processes, rather than on afferent boutons. Such contacts were more common in the central than in the peripheral zone (12 vs. 0.9%). Ribbon synapses were not found on the thin processes between the bouton endings.
Outer-face contacts account for only a small fraction (6 of 453 or 1.3%) of the ribbon synapses in the dissector samples of peripheral type II hair cells, compared with 135 of 784 (17%) for central type II hair cells. Two further points can be made from Table 5. First, the numbers of ribbon synapses and of boutons are highest in the intermediate zone. Second, synapse counts are similar for type II hair cells in the central and peripheral zones. This is so even though central cells have considerably fewer boutons. The numbers are similar because central type II hair cells make more ribbon synapses with calyx endings and with individual afferent boutons than do peripheral type II hair cells.
A common feature of type II hair cells, regardless of zone, is that ≈95% of their ribbons are located at sub-nuclear levels (Fig. 8). In the peripheral zone, type II hair cells are longer than type I hair cells (Tables 2, 3) and have more basally located synapses (Fig. 8B). In contrast, the heights of central type I and type II hair cells are similar and the synapses made by the two kinds of hair cells are found at approximately the same level of the neuroepithelium (Fig. 8A).
Transverse and longitudinal material
The two sets of material were used to compare the numbers of synaptic elements in the four central subzones (Fig. 3). Results are summarized in Figure 13. Regional differences among central type II hair cells are small and statistically insignificant. In contrast, there are large variations for type I hair cells and these depend on both epithelial location and the type of calyx ending. The CI subzone is distinctive: especially large numbers of ribbon synapses (Fig. 13A) and of invaginations (Fig. 13B) are found in CI hair cells innervated by complex endings. Outer-face ribbons are particularly numerous on simple CI calyx endings, whether the results for complex endings are expressed per hair cell (Fig. 13C) or per ending (not shown). To discern trends in the other three subzones, an ANOVA was confined to them. No regional differences remained. Even with CI removed, there were differences related to calyx type. When compared with type I hair cells having simple endings, those with complex endings had a larger number of invaginations (mean ± SE, 61.4 ± 6.5 vs. 36.7 ± 6.5, P < 0.02) and more inner-face ribbons (27.6 ± 3.7 vs. 17.6 ± 3.7, P < 0.1). On the other hand, the numbers of outer-face ribbons per type I hair cell were similar (simple: 2.5 ± 0.7; complex: 1.8 ± 0.7, P > 0.4).
Fig. 13.

A–E: Synaptic structures for type I and type II hair cells located in the various central subzones. Type I hair cells are distinguished by their being contacted by simple or complex endings (see key in B). The numbers of synaptic elements per hair cell were estimated by dissector methods. Central zone subdivisions: transverse axis, apex (CA), slope (CS); longitudinal axis, inner (CI), outer (CO). Histogram columns, means; error bars, SE.
The analysis was extended to type I and type II hair cells in the intermediate and peripheral zones. Here, we were interested in comparing IP with IB and PP with PB. Outer-face ribbon synapses were more common in IP than in IB. No other statistically significant difference was found.
Locations and numbers of efferent synaptic elements
Efferent boutons are highly vesiculated and make synapses with type II hair cells and with afferent processes, including calyces, boutons, and dendrites (Smith and Rasmussen, 1968; Wersäll, 1968; Iurato et al., 1972). The contacts on type II hair cells are marked by a postsynaptic cistern (Fig. 14A, thick solid arrow). There is an accumulation of vesicles, although this need not occur directly opposite the cistern (Smith and Rasmussen, 1968). Efferent synapses with calyces and other afferent processes are characterized by a dense accumulation of vesicles and by slight presynaptic and postsynaptic thickenings (Fig. 14A, thick open arrows, 14C). Individual efferent boutons can make contacts with both afferent processes and type II hair cells (Fig. 9D). It was relatively easy to distinguish afferent and efferent boutons in our material. This is illustrated in Figure 14, which includes two efferent boutons from a single parent axon (EFF), as well as an afferent bouton (AFF). In addition to an accumulation of vesicles and the presence of other synaptic specializations, efferent boutons and their processes are distinctive in having a more uniform size, smaller mitochondria and a darker, more filamentous cytoplasm than do afferent boutons. The latter contain a small number of vesicles, but these are less homogeneous in size than their efferent counterparts.
Fig. 14.
A: An afferent fiber (AFF) and an efferent fiber (EFF) penetrate the supporting-cell (SC) layer to innervate two type II (II) hair cells in the central zone of a posterior crista (cf. Fig. 3C). The afferent forms a bouton at the base of the two hair cells, and receives two ribbon synapses from the hair cell on the left in near-adjacent sections (locations indicated by thin arrows). Two boutons are made by the efferent, one with the hair cell and the other with the afferent. Both efferent boutons have smaller mitochondria and a darker, more filamentous cytoplasm than the afferent bouton. There are a few vesicles in the afferent, which are more heterogeneous in size than those in the efferent. The efferent bouton on the hair cell makes a single synapse (filled arrow) marked by a postsynaptic cistern at some distance from an accumulation of synaptic vesicles. Two synapses are made by the other efferent bouton (open arrows) and have a clustering of vesicles and thin pre- and postsynaptic densities. Higher magnification of the postsynaptic cistern (B) and the upper of the two synapses on the afferent (C). Scale bar = 2.5 μm in A, 0.5 μm in C (applies to B,C).
The efferent innervation of afferent calyces is similar in all three zones (Table 4). By taking into account the prevalence of complex endings in the three zones, the results can also be expressed per calyx ending. An extracellular labeling study indicates that there are, on average, 1.3 type I hair cells per calyx ending in the central zone compared with 1.05 in the peripheral zone (Fernández et al., 1988). Applying these conversion factors gives values of 4.0 and 3.8 boutons per calyx ending in the central and peripheral zones, respectively. There are 2.5–4 efferent boutons per type II hair cell (Table 5). The efferent innervation of type II hair cells is slightly heavier in the intermediate (and possibly the central) zone than in the peripheral zone and slightly heavier in CL than in CT (mean ± SE, 4.7 ± 0.6 vs. 2.7 ± 0.1 boutons per hair cell). No attempt was made to count the number of efferent contacts on afferent processes other than calyces. In serially sectioned material, we have observed as many as eight efferent boutons contacting a type II hair cell and as many as six on a calyx. As illustrated in Figure 8, most of the efferent boutons are located near the bases of type II hair cells and of calyces.
There were a total of 798 efferent boutons in the dissector samples, compared with 2,053 afferent boutons. Efferent boutons thus make up 25–30% of all the bouton endings in the neuroepithelium. The ratio of afferent to efferent boutons ranges from 1.8:1 in the central zone to 4.4:1 in the peripheral zone. Only 12 (0.4%) of the 2,851 boutons in the dissector samples contained reciprocal synapses, and only one instance (0.6%) was found in a sample of 161 serially photographed boutons.
Synaptic ultrastructure
In this section, we describe the electron-dense cores of ribbon synapses for both type I and type II hair cells. We refer to the cores as ribbons even though many of them are spherules. Calyceal invaginations into type I hair cells and their association with ribbon synapses will also be considered.
Type I hair cells
Ribbons in type I hair cells are spherules (Fig. 15A,B,D–H), rods (Fig. 15I,J) or, rarely, plates (Fig. 15C). Spherules have diameters of ≈90 nm. Rods are usually 200–300 nm tall and appear in one to three serial sections. The synaptic vesicles surrounding either kind of ribbon are always round, clear, and 25–50 nm in diameter. Plates will be described below.
Fig. 15.
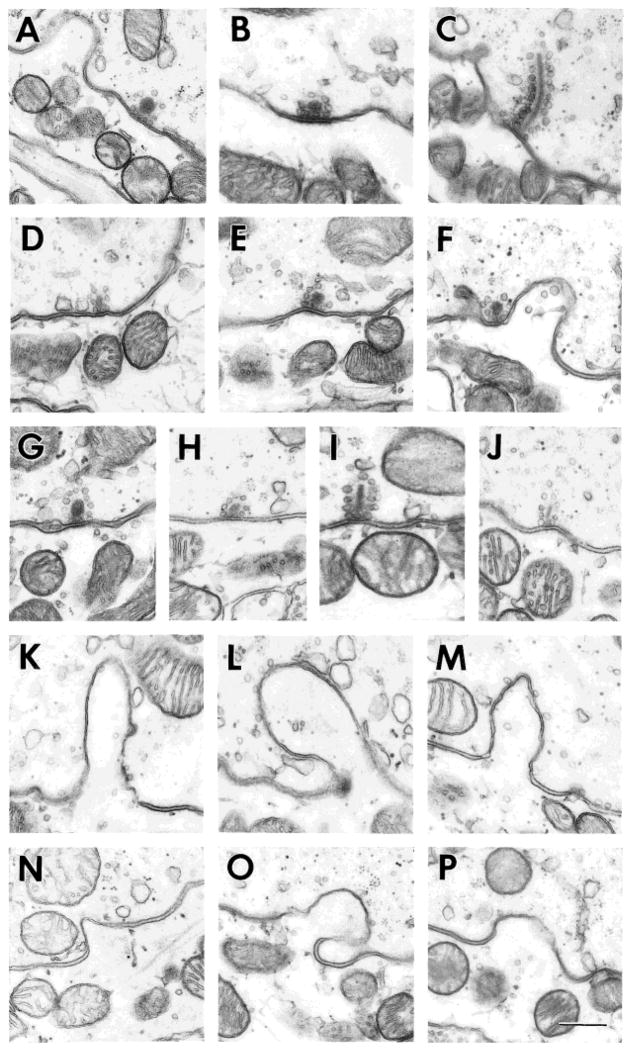
Synaptic ribbons in type I hair cells in the central (A–C), intermediate (D–F), and peripheral zones (G–J). Most ribbons in type I hair cells are spherical (A,B,D–H). Elongated ribbons are almost always rods and are common only in the peripheral zone near the planum semilunatum (I,J). A rare plate-like ribbon is seen in a central type I hair cell (C). The ribbon synapse in F is in contact with a calyceal invagination. Calyceal invaginations into type I hair cells in the central (K–M) and peripheral zones (N–P). The narrowing of the intercellular space is particularly evident in K and N–P. Invaginations are deeper in the central than in the peripheral zone. Scale bar = 0.25 μm in P (applies to A–P).
The relative frequency of spherules and rods was evaluated in six dissector samples, three from a transverse series (Fig. 3A), another two from one longitudinal series (Fig. 3C), and one from the other longitudinal series (Fig. 3D). A total of 1,452 ribbons in type I hair cells were surveyed. Rods were infrequent in the transverse series, making up only 26 of 469 or 6% of the sample. In the longitudinal material, rods were only seen in 40 of 577 or 7% of the synapses in the central zone, but became more frequent in the intermediate (52 of 280 or 19%) and peripheral (79 of 126 or 63%) zones. Apparently, there is a difference in ribbon morphology for type I hair cells near the base and near the planum. Although the sizes of ribbons from the various zones overlapped, those in the central zone (Fig. 15A–C) were somewhat larger than those in the peripheral zone (Fig. 15G–J).
It has already been noted that invaginations are more numerous in central, compared with peripheral, type I hair cells. In addition, central invaginations (Fig. 15K–M) are deeper than those found peripherally (Fig. 15N–P). On average, the depths of invaginations measured 0.75 ± 0.04 μm (mean ± SE, n = 54) in the central zone and 0.48 ± 0.02 μm (n = 54) in the peripheral zone. We estimated the percentage of the hair-cell plasmalemma below the level of the nucleus devoted to calyceal invaginations. Measurements were made on each of the seven serially photographed type I hair cells, including three from the central zone and four from the peripheral zone. Mean percentages were 20.2 ± 6.3% (central zone) and 10.4 ± 4.7% (peripheral zone).
Engström et al. (1972) observed that ribbon synapses and calyceal invaginations could be near each other. To evaluate the spatial relations between these structures, we counted the number of synapses in contact with invaginations in the seven serially photographed type I hair cells. Only subnuclear synapses were considered. Slightly over 25% (26 of 98) of the synapses touched invaginations, and percentages were similar for the two zones (central: 16 of 57 = 28%; peripheral: 10 of 41 = 24%). Another seven synapses were within 300 nm of an invagination. Were there a random association between synapses and invaginations, the percentage of contacting synapses should be similar to the percentage of plasmalemma devoted to invaginations. This is the case for the central (but not for the peripheral) zone. The results suggest a nonrandom relationship possibly confined to the peripheral zone. At the same time, nearly two-thirds of the synapses are neither in contact with nor near an invagination, which suggests, at most, a loose association.
Type II hair cells
Ribbons in type II hair cells can be spherical (Fig. 16C–F,K,L,O–Q) or elongated (Fig. 16A,B,G–J,M,N). The latter can be rods (Fig. 16G,M,N), plates (Fig. 16H,I,J), or barrels (Fig. 16A,B). Spherules and rods resemble the comparable structures seen in type I hair cells. A few elongated ribbons were plates that remain attached to the presynaptic plasmalemma through 7–10 sections. Plates can be quite tall, extending away from the plasmalemma for up to 600 nm (Fig. 16I). Barrels are distinctively thicker than other elongated ribbons and most of them have a hollow (electron-lucent) region, usually located near the end of the ribbon facing away from the presynaptic plasmalemma (Fig. 16A,B). Hollow regions are less often seen in spherules (Fig. 16E) or plates (Fig. 16J). Spherules occasionally occur in clusters (Fig. 16D–F), with adjacent spherules sometimes sharing synaptic vesicles (Fig. 16E).
Fig. 16.
Synaptic ribbons from type II hair cells in the central (A–I), intermediate (J–L), and the peripheral zones (M–Q). Examples include synapses on afferent boutons (B,D,E,G–P) and on calyx outer faces (A,C,F,Q). Ribbons are spherical (C–F,K,L,O–Q) or elongated (A,B,G–J,M,N). Most spherules occur singly. Less frequently, they form clusters (D–F) and may appear to share vesicles (E). Elongated ribbons can be rods (G,M,N), barrels (A,B) or, much less frequently, plates (H,I,J). Hollow (electron-lucent) regions are seen in barrels (A,B) and, less often, in spherules (E) or plates (J). Scale bar = 0.25 μm in Q (applies to A–Q).
In a survey of 1,483 ribbons from type II hair cells, spherical and elongated ribbons occurred in almost equal numbers in all zones. This finding was in contrast to type I hair cells, in which spherules predominated except in the peripheral zone near the planum. Both kinds of ribbons were observed in individual type II hair cells (Fig. 8). Rods and spherules were the only ribbons found in peripheral type II hair cells. The spherules found there never occurred in clusters and never had hollow cores. Plates were only found in the central zone. Barrels and spherule clusters were more frequently encountered in the central than in the intermediate zone. A large fraction of the barrels (5 of 21 = 24%) and clusters (8 of 21 = 38%) were associated with outer-face synapses. Ribbons were somewhat larger in the central (Fig. 16A–I), compared with the peripheral zone (Fig. 16M–Q).
DISCUSSION
Previous studies had established that there were regional differences in the cellular architecture of the crista (Lindeman, 1969; Fernández et al., 1995), in its afferent innervation patterns (Lorente de Nó, 1926; Poljak, 1927; Wersäll, 1956), and in its afferent physiology (Baird et al., 1988). The present ultrastructural study confirms that there are regional differences in cytoarchitecture and establishes the presence of parallel differences in synaptic architecture. As such, the study bears on the zonal organization of the crista and on its afferent and efferent innervation. In the following sections, some of the structural implications of the findings are considered. Functional implications have been discussed elsewhere (Lysakowski et al., 1995; Goldberg, 1996).
Zones of the cristae ampullares
Early workers (Lorente de Nó, 1926; Poljak, 1927) described differences in the afferent innervation of the apex, slopes, and base of the crista. Wersäll (1956), besides confirming some of the differences in afferent innervation, suggested that type I hair cells predominated at the apex and that type II hair cells predominated at the base of the guinea-pig crista. More recent studies have shown that there are roughly equal numbers of type I and type II hair cells throughout the guinea-pig (Lindeman, 1969; Lindeman et al., 1981; Watanuki and Meyer zum Gottesberge, 1971) and chinchilla cristae (Fernández et al., 1995). A somewhat different situation occurs in the monkey crista, where type I hair cells outnumber type II hair cells by a 5:1 ratio in the central zone, but only by a 2:1 ratio in the peripheral zone (Fernández et al., 1995).
Much of the earlier work apparently relied on transverse sections confined to the middle of the crista. Lindeman (1969), by using surface preparations of the entire organ, was able to consider regions near the planum semilunatum. He concluded that the neuroepithelium of the guinea-pig crista was concentrically organized into central, intermediate, and peripheral zones. Included in the intermediate and peripheral zones were parts of the neuroepithelium near the planum, as well as near the base of the crista. The three zones were described as differing in the size and density of their hair cells and supporting cells. Similar differences were observed in a previous light-microscopic study of the chinchilla crista (Fernández et al., 1995) and in the present ultrastructural study.
The proposal that the crista is concentrically organized implies that the peripheral zone should be similar near the planum (PP subzone) and near the crista base (PB sub-zone). The two peripheral subzones resemble each other in terms of their afferent innervation (Fernández et al., 1988, 1995) and their afferent discharge properties (Baird et al., 1988). In the present study, the two subzones were statistically indistinguishable in all of the synaptic features listed in Tables 4 and 5. A quantitative analysis of the cytoarchitecture (Figs. 6, 7) disclosed some differences between PP and PB, all of which were consistent with the conclusion that those features distinguishing the peripheral from the central zone were more pronounced in PP. There was also a difference in synaptic ultrastructure: synapses with elongated ribbons were far more common in type I hair cells located in PP. A similar analysis of the intermediate zone indicated that the IP and IB subzones were similar in most respects.
More substantial differences were observed within the central zone. In terms of cytoarchitecture, the CS subzone resembled the rest of the neuroepithelium more than it did the CA subzone. Complex calyx endings were more common in CA, an observation that may be related to the observation that calyx units are concentrated there (Fernández et al., 1988; Desmadryl and Dechesne, 1992). Central type I hair cells located in the middle of the crista (CI subzone) had many more ribbon synapses and calyceal invaginations than did those located nearer the planum (CO subzone). The results suggest that there may be an especially differentiated region within the central zone, but our sampling was inadequate to define its boundaries.
Lindeman’s (1969) tripartite division of the crista has provided a useful scheme to describe regional differences in morphology and physiology. The basic similarity of the intermediate and peripheral zones near the base and near the planum is consistent with the scheme. Large differences were observed in our longitudinal and transverse material from the central zone, and it may be necessary to divide it into two or more subzones.
Regional variations in afferent innervation
The three zones can also be distinguished by the complement of afferent nerve fibers they receive (Fernández et al., 1988, 1995). Three kinds of afferents are found in the neuroepithelium. Calyx fibers supply type I hair cells; bouton fibers innervate type II hair cells; and dimorphic fibers provide a mixed innervation of both hair-cell types. The central zone is innervated by calyx and dimorphic fibers. Dimorphic fibers make up almost the entire afferent innervation of the intermediate zone except near the apex, which also has a small number of calyx fibers. The peripheral zone is supplied with dimorphic and bouton fibers. The regional pattern of afferent innervation is similar in the chinchilla (Fernández et al., 1988) and the squirrel monkey (Fernández et al., 1995).
An important contribution of the present paper is the finding that there are regional variations in the afferent innervation of individual hair cells. (1) Type I hair cells in the central zone, compared with those in the other two zones, are more likely to be innervated by complex calyx endings. This finding may reflect the regional distribution of calyx fibers, because these afferents are more likely than are dimorphic fibers to terminate as complex endings (Fernández et al., 1988, 1995). (2) As was predicted from the results of an extracellular labeling study (Fernández et al., 1988), type II hair cells in the peripheral zone are supplied with many more afferent boutons than are those in the central zone. A similar regional difference is present in other rodents (Lysakowski, 1996). (3) Despite the almost 3:1 ratio in the number of afferent boutons they receive, peripheral and central type II hair cells in the chinchilla contain similar numbers of ribbon synapses. The discrepancy between the numbers of synapses and endings can be explained, in part, by the observations that boutons lacking synaptic input were only common peripherally, whereas boutons making multiple synaptic contacts were more frequent centrally. (4) A second reason as to why the number of synapses is not reduced in central type II hair cells relates to the presence of outer-face synapses, which are rare in the peripheral zone, but common in the central zone. (5) There are twice as many ribbon synapses and five times as many calyceal invaginations in central, compared with peripheral, type I hair cells.
Parallel studies have now been done in the squirrel monkey (Lysakowski and Goldberg, 1993; Fernández et al., 1995). It is instructive to compare the results for the two species because not all of the trends seen in the chinchilla were confirmed in the monkey. (1) As in the chinchilla, complex calyces are concentrated in the central zone of the monkey crista and are more common in calyx than in dimorphic fibers. (2) There is a central-to-peripheral gradient in the number of afferent boutons innervating type II hair cells that is even more striking than that seen in the chinchilla crista. (3) Unlike the situation in the chinchilla, the number of ribbon synapses in monkey type II hair cells increases from the central to the peripheral zone, more or less in parallel with the number of afferent boutons. Dissector counts suggest that peripheral boutons lacking synaptic input are common in both species, whereas multiple synapses onto central boutons are less frequent in the monkey. (4) Reflecting the relatively small number of type II hair cells, very few outer-face ribbon synapses are found in the monkey central zone. As in the chinchilla, outer-face ribbon synapses are rare in the intermediate and peripheral zones. (5) A central-to-peripheral gradient in the number of calyceal invaginations is seen in monkey type I hair cells, without an accompanying gradient in the number of ribbon synapses.
Afferent innervation
Type I hair cells
Despite reports to the contrary (Favre and Sans, 1979; Gulley and Bagger-Sjöbäck, 1979), large numbers of ribbon synapses are found in type I hair cells. In the present study of the chinchilla cristae, dissector counts provided an estimated average of 15–20 such synapses per type I hair cell and individual, serially photographed hair cells had as many 25 synapses. Smaller, but appreciable numbers of ribbon synapses have been observed in type I hair cells from other species (Yamashita and Ohmori, 1991; Lysakowski and Goldberg, 1993; Lysakowski, 1996).
The presence of ribbon synapses argues that there is conventional (quantal) synaptic transmission between type I hair cells and their calyx endings. Physiological evidence for such transmission was obtained in recordings from calyx endings in a lizard horizontal crista (Schessel et al., 1991). Outer-face ribbon synapses made by neighboring type II hair cells are another potential source of chemical synapses to calyx endings. Outer-face ribbons have been described in the vestibular neuroepithelium of mammals (Engström, 1970; Bagger-Sjöbäck and Gulley, 1979; Ross, 1985, 1997), as well as turtles and birds (Lysakowski, 1996). In the present study, outer-face ribbons are common in the central zone of the chinchilla crista, but rare in the peripheral zone. A similar arrangement occurs in other rodents (Lysakowski, 1996). Outer-face ribbons are so ubiquitous in the chinchilla central zone that most calyx afferents, even though they lack bouton endings, could receive synaptic inputs from type II hair cells. In contrast, outer-face synapses are infrequent even in the central zone of monkeys (Lysakowski and Goldberg, 1993; Lysakowski, 1996) and most calyx afferents should get their input only from type I hair cells. As the discharge properties of calyx afferents are similar in the chinchilla (Baird et al., 1988) and the squirrel monkey (Lysakowski et al., 1995), it would appear that the presence of outer-face synapses does not qualitatively alter the physiology of these afferents. This finding may be related to the observation that, even in the chinchilla central zone, inner-face ribbons outnumber outer-face ribbons by a 7:1 ratio.
Calyceal invaginations are a peculiar feature of type I hair cells and their calyx endings. These specializations outnumber ribbon synapses especially in the central zone, where they make up >20% of the hair-cell plasmalemma at subnuclear levels. Despite their peculiar structure and their ubiquity, the function of the invaginations is unclear. Freeze-fracture studies are not particularly illuminating since they indicate that the invaginations are almost devoid of intramembranous particles (Gulley and Bagger-Sjöbäck, 1979). Three possibilities can be considered. (1) Engström et al. (1972) observed that invaginations and ribbon synapses could be located close to each other. Our results imply that the two structures are only loosely associated with less than one-third of synaptic ribbons contacting or being close to invaginations. Such a loose association would suggest that invaginations are not involved in vesicle release. It would be consistent with their being involved in vesicle recycling, as the retrieval of vesicle membrane from the plasmalemma can take place at some distance from the active zone (Heuser and Reese, 1973; Schaeffer and Raviola, 1978; Siegel and Brownell, 1986). (2) Gulley and Bagger-Sjöbäck (1979) suggested that calyceal invaginations might be involved in ephaptic transmission from the hair cell to the ending. A theoretical analysis indicates that such transmission would result in a postsynaptic depolarization too small to make more than a minor contribution to sensory coding (Goldberg, 1996). Furthermore, it is unclear how invaginations would facilitate such transmission. (3) Invaginations may contribute to the adhesion between the type I hair cell and the calyx ending (Goldberg, 1996).
Type II hair cells
The afferent innervation of vestibular organs, originally described in silver-stained material (Lorente de Nó, 1926; Poljak, 1927), has been reexamined with extracellular labeling techniques (Fernández et al., 1988, 1990, 1995). Bouton endings were found on dimorphic and bouton fibers along and at the ends of thin processes. Thicker processes terminated as calyx endings. Based on previous ultrastructural studies (Wersäll, 1956; Wersäll and Bagger-Sjöbäck, 1974), it was suggested that the boutons provided the major, if not exclusive, source of afferent innervation to type II hair cells. Because calyx endings are the exclusive afferent innervation of type I hair cells, the possibility arose that the proportion of type I and type II inputs received by an afferent could be deduced from the relative numbers of its calyx and bouton endings.
Several difficulties with this interpretation were noted. For one thing, transmission from type II hair cells could take place in the absence of bouton endings since the hair cells could synapse with the outer faces of calyx endings, with the thick processes leading to the same endings, or with the thin branches interconnecting bouton endings. The present study clarifies matters. About 15–20% of the synapses of central type II hair cells are made with calyx outer faces, but this is so for only 1% of peripheral type II synapses. A small fraction of type II synapses (≈10% in the central zone and ≈1% in the peripheral zone) are made with thick intraepithelial branches and few, if any, are found on thin intraepithelial branches.
Another difficulty concerns the relationship between afferent boutons and synaptic ribbons. Multiple synapses are more common in central than in peripheral boutons. All of the serially examined central boutons in the present study were contacted by at least one ribbon synapse. In contrast, about one-third of the peripheral boutons lacked a ribbon synapse or any other obvious synaptic specialization. One interpretation of the apparent lack of synaptic input is that we misidentified enlargements contacting type II hair cells as boutons. Although this is a real possibility in dissector samples, where we only had micrographs of every fourth section, we consider the explanation less likely in our serially photographed material. In the latter, an enlargement was considered a bouton only if it was completely sectioned and only if it could be traced to a thin process at one end. Most of the enlargements had thin processes at both ends, consistent with the interpretation that they were boutons en passant. Some afferent boutons apparently lack a synaptic input. A similar situation occurs in the organ of Corti (Nadol, 1983; Hashimoto and Kimura, 1988; Liberman et al., 1990; Francis and Nadol, 1993). Studies in retinal photoreceptors (Wagner, 1973; Case and Plummer, 1993), in pinealocytes (Vollrath and Seidel, 1989; McNulty and Fox, 1992) and in hair cells (Ross, 1994; Henry and Mulroy, 1995) suggest that ribbon synapses are dynamic structures and can rapidly turn over under physiological conditions. Possibly, boutons lacking synaptic specializations are in the process of such turnover.
Synaptic-ribbon morphology
Ribbon synapses are specialized presynaptic structures that are found, among other places, in vertebrate photoreceptors (Sjöstrand, 1958) and hair cells (Smith and Sjöstrand, 1961). These sensory cells are characterized by a continually high rate of vesicular release (Parsons et al., 1994). One thought is that the electron-dense ribbon serves to maintain a large number of release-ready vesicles in the immediate vicinity of the active zone (Heuser and Reese, 1977; Parsons et al., 1994). Were this the case, the size and shape of the ribbons might be an important determinant of their function.
Variations in the size and shape of ribbons have been noted in retinal photoreceptors (Vollrath et al., 1989), in pinealocytes (Khaledpour and Vollrath, 1987), and in hair cells of the organ of Corti (Liberman et al., 1990). Ribbon morphology in vestibular hair cells varies across the vertebrate scale. In fish and amphibians, which only have type II hair cells, ribbons are large spherules with a diameter of 200–500 nm (Wersäll and Bagger-Sjöbäck, 1974; Lysakowski, 1996). Large spherules are found in both type I and type II hair cells of turtle, whereas smaller spherules (≈100 nm, diameter) are seen in birds (Lysakowski, 1996). In the present study of the chinchilla crista, ribbon morphology varied with hair-cell type and zone. Most type I hair cells had small, spherical ribbons, except near the planum where more of them were elongated. Roughly equal numbers of round and elongated ribbons were found in type II hair cells. Ribbon structure was particularly heterogeneous in central type II hair cells. Differences in the ribbon morphology of type I and type II hair cells had previously been observed by Ross (1994) in the utricular macula and have now been seen in the cristae of several mammals (Lysakowski, 1996).
The size and shapes of ribbons would appear related to the number of vesicles associated with the synapse. For example, ≈500 vesicles surround the large spherules in the frog sacculus (Parsons et al., 1994). Fewer vesicles are found around the smaller ribbons in the chinchilla: 20–30 vesicles for small spherules, 40–80 for the larger rods, 100–150 for the still larger barrels, and 150–300 for plates (A. Lysakowski, unpublished observations). A comparison of these counts with ribbon dimensions suggests that the number of vesicles is at least roughly proportional to ribbon surface area.
Efferent innervation
There are relatively few parent efferent axons (Gacek and Lyon, 1974; Goldberg and Fernández, 1980; Warr, 1975) compared with the number of afferents innervating the vestibular organs (Gacek and Rasmussen, 1961; Honrubia et al., 1987). The ratio of afferent to efferent axons approaches 20:1. Despite their small numbers, the efferents provide a major innervation. In the present study, for example, it was estimated that afferent boutons outnumbered highly vesiculated, presumed efferent boutons by only a 2–3:1 ratio. The disparity between the two ratios reflects the highly divergent nature of the efferent innervation (Smith and Rasmussen, 1968; Iurato et al., 1972; Purcell and Perachio, 1996).
Efferent endings are more or less uniformly distributed throughout the chinchilla crista. On average, three to four efferent boutons contact type II hair cells in all three zones and a similar number contact each calyx ending. Our results are expressed per hair cell or per calyx ending. Were the density expressed on an areal basis, it would parallel the density of hair cells and calyx endings and would be more than twice as high in the peripheral as compared with the central zone. The conclusion is in accord with results from an acetylcholinesterase histochemical study (Nomura et al., 1965). In contrast to the morphological results, physiological studies indicate that the responses of vestibular-nerve afferents to electrical stimulation of efferent pathways are far from uniform (Goldberg and Fernández, 1980). The mechanisms responsible for the regional variations in efferent action are unclear (for discussion, see Goldberg et al., 1997).
Not all vesiculated synapses need be of efferent origin. An example is provided by reciprocal synapses, which consist of a single bouton ending with an efferent-like synapse in proximity to a typical afferent synapse. In our material, the incidence of reciprocal synapses was low, being found in <1% of our completely reconstructed bouton endings. Another potential source of vesiculated boutons has been considered by Ross (1985, 1997) in the utricular and saccular maculi of rats. In the maculae, most of the afferent innervation of type II hair cells is derived from thin collaterals of dimorphic fibers (see also Fernández et al., 1990). Some of these collaterals were considered “efferent-type” because they had a beaded appearance, a large number of mitochondria and variously sized vesicles. Efferent-type collaterals were found to terminate on type II hair cells as vesiculated boutons, which contained fewer vesicles and were more electron-lucent than were efferent boutons of extramacular origin. Whether these collaterals differ in their physiology from the other collaterals of dimorphic afferents is undetermined. Here, two comments can be made. (1) Only a very small fraction of the boutons in our material were intermediate in appearance between afferent and efferent boutons and, so, might be considered “efferent-type.” Some of these turned out to contain reciprocal synapses. Most “efferent-type” boutons made ribbon synapses with type II hair cells, but lacked typical efferent synapses. For this reason, they were classified as afferent boutons. (2) There is strong evidence that the great majority of highly vesiculated boutons originate in the brain. Almost all such boutons degenerate after central lesions (Smith and Rasmussen, 1968) and the vast majority of them stain positively for acetylcholinesterase (Hilding and Wersäll, 1962; Iurato et al., 1972; Lysakowski, unpublished observations), a marker for brainstem efferent neurons, but not for afferent nerve fibers (Gacek and Lyon, 1974; Warr, 1975).
Acknowledgments
We thank Ginger Tsien for the preparation of histological materials, Maureen Cummings for electron microscopic assistance, and Steven D. Price for excellent photographic assistance. A previous version of the manuscript was read by Drs. R.A. Eatock, G.D. Pappas, and J.H. Siegel. This work was funded by National Institute of Deafness and Communication Disorders grants DC 00070 to J.M.G., DC 01474 to A.L., DC 02058 to J.M.G., and DC 02521 to A.L., and National Aeronautics and Space Administration grant NAG 2-148 to J.M.G. A.L. was a NASA Research Associate. J.M.G. was a Pepper Neuroscience Investigator of the NIDCD.
Footnotes
The choice of 32-section samples was based on three considerations: (1) In order that each sample provide two independent estimates of the number of equivalent hair cells or supporting cells (Q−), sample thickness (T) must be smaller than the minimum diameter (minD) of the structures being counted. In our case these were hair-cell and supporting-cell nuclei with a minD ≈ 4 μm. (2) It is essential that none of the sections in the sample are lost. (3) The number of profiles contributing to Q− increases with T. The third consideration implies that the sample be as thick as possible consistent with the first two considerations. A sample thickness, T = 31 × 0.075 = 2.3 μm, was taken as a reasonable compromise. Note that the lookup section is not included in the sample and, so, does not contribute to T.
LITERATURE CITED
- Bagger-Sjöbäck D, Gulley RL. Synaptic structures in the type II hair cell in the vestibular system of the guinea pig. A freeze-fracture and TEM study. Acta Otolaryngol. 1979;88:401–411. doi: 10.3109/00016487909137185. [DOI] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Case CP, Plummer CJ. Changing the light intensity of the visual environment results in large differences in numbers of synapses and in photoreceptor size in the retina of the young adult rat. Neuroscience. 1993;55:653–666. doi: 10.1016/0306-4522(93)90431-e. [DOI] [PubMed] [Google Scholar]
- de Groot JC, Veldman JE, Huizing EH. An improved fixation method for guinea pig cochlear tissues. Acta Otolaryngol. 1987 Sep-Oct;104(3-4):234–42. doi: 10.3109/00016488709107323. [DOI] [PubMed] [Google Scholar]
- Desmadryl G, Dechesne CJ. Calretinin immunoreactivity in chinchilla and guinea pig vestibular end organs characterizes the calyx unit sub population. Exp Brain Res. 1992;89:105–108. doi: 10.1007/BF00229006. [DOI] [PubMed] [Google Scholar]
- Dunn RF. Reciprocal synapses between hair cells and first-order afferent dendrites in the crista ampullaris of the bullfrog. J Comp Neurol. 1980;193:255–264. doi: 10.1002/cne.901930117. [DOI] [PubMed] [Google Scholar]
- Engström H. The first-order vestibular neuron. Fourth Symposium on the Role of the Vestibular Organs in Space Exploration; Washington, DC: US Govt. Printing Office (NASA SP-187); 1970. pp. 123–134. [Google Scholar]
- Engström H, Bergström HB, Ades HW. Macula utriculi and macula sacculi in the squirrel monkey. Acta Otolaryngol Suppl. 1972;301:75–126. doi: 10.3109/00016487209122691. [DOI] [PubMed] [Google Scholar]
- Favre D, Sans A. Morphological changes in afferent vestibular hair cell synapses during the postnatal development of the cat. J Neurocytol. 1979;8:765–775. doi: 10.1007/BF01206675. [DOI] [PubMed] [Google Scholar]
- Fernández C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla: I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol. 1988;60:167–181. doi: 10.1152/jn.1988.60.1.167. [DOI] [PubMed] [Google Scholar]
- Fernández C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla: III. Peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990;63:767–780. doi: 10.1152/jn.1990.63.4.767. [DOI] [PubMed] [Google Scholar]
- Fernández C, Lysakowski A, Goldberg JM. Hair-cell counts and afferent innervation patterns in the cristae ampullares of the squirrel monkey with a comparison to the chinchilla. J Neurophysiol. 1995;73:1253–1281. doi: 10.1152/jn.1995.73.3.1253. [DOI] [PubMed] [Google Scholar]
- Francis HW, Nadol JB., Jr Patterns of innervation of outer hair cells in a chimpanzee: I. Afferent and reciprocal synapses. Hear Res. 1993;64:184–190. doi: 10.1016/0378-5955(93)90004-k. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Lyon M. The localization of vestibular efferent neurons in the kitten with horseradish peroxidase. Acta Otolaryngol. 1974;77:92–101. doi: 10.3109/00016487409124603. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Rasmussen GL. Fiber analysis of statoacoustic nerve of guinea pig, cat and monkey. Anat Rec. 1961;139:455–463. doi: 10.1002/ar.1091390402. [DOI] [PubMed] [Google Scholar]
- Ginzberg RD. Freeze-fracture morphology of the vestibular hair cell-primary afferent synapse in the chick. J Neurocytol. 1984;13:393–405. doi: 10.1007/BF01148330. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. Theoretical analysis of intercellular communication between the vestibular type I hair cell and its calyx ending. J Neurophysiol. 1996;76:1942–1957. doi: 10.1152/jn.1996.76.3.1942. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernández C. Efferent vestibular system in the squirrel monkey: Anatomical location and influence on afferent activity. J Neurophysiol. 1980;43:986–1025. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Lysakowski A, Fernández C. Morphophysiological and ultrastructural studies in the mammalian cristae ampullares. Hear Res. 1990;49:89–102. doi: 10.1016/0378-5955(90)90097-9. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brichta AM, Wackym PA. Efferent vestibular system: Anatomy, physiology and neurochemistry. In: Beitz AJ, Anderson JH, editors. Neurochemistry of the Vestibular System. Boca Raton, FL: CRC Press; 2000. pp. 61–94. [Google Scholar]
- Gulley RL, Bagger-Sjöbäck D. Freeze fracture studies on the synapse between the type I hair cell and the calyceal terminal in the guinea pig vestibular system. J Neurocytol. 1979;8:591–603. doi: 10.1007/BF01208511. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG. Stereology of arbitrary particles: A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Hamilton DW. The calyceal synapse of type I vestibular hair cells. J Ultrastruct Res. 1968;23:98–114. doi: 10.1016/s0022-5320(68)80034-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Kimura RS. Computer-aided three-dimensional reconstruction and morphometry of the outer hair cells of the guinea pig cochlea. Acta Otolaryngol. 1988;105:64–74. doi: 10.3109/00016488809119447. [DOI] [PubMed] [Google Scholar]
- Henry WR, Mulroy MJ. Afferent synaptic changes in auditory hair cells during noise-induced temporary threshold shift. Hear Res. 1995;84:81–90. doi: 10.1016/0378-5955(95)00014-u. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Structure of the synapse. In: Kandel ER, editor. Handbook of Physiology, Section 1: The Nervous System, Vol I. Cellular Biology of Neurons. Baltimore: Williams and Wilkins; 1977. pp. 261–294. [Google Scholar]
- Hilding D, Wersäll J. Cholinesterase and its relation to the nerve endings in the inner ear. Acta Otolaryngol. 1962;55:205–217. doi: 10.3109/00016486209127354. [DOI] [PubMed] [Google Scholar]
- Honrubia V, Kuruvilla A, Mamikunian D, Eichel JE. Morphological aspects of the vestibular nerve of the squirrel monkey. Laryngoscope. 1987;97:228–238. doi: 10.1288/00005537-198702000-00018. [DOI] [PubMed] [Google Scholar]
- Iurato S, Luciano L, Pannese E, Reale E. Efferent vestibular fibers in mammals: Morphological and histochemical aspects. Prog Brain Res. 1972;37:429–443. doi: 10.1016/s0079-6123(08)63917-5. [DOI] [PubMed] [Google Scholar]
- Khaledpour C, Vollrath L. Evidence for the presence of two 24-h rhythms 180 degrees out of phase in the pineal gland of male Pirbright-White guinea pigs as monitored by counting “synaptic” ribbons and spherules. Exp Brain Res. 1987;66:185–190. doi: 10.1007/BF00236214. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: Quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Lindeman HH. Studies on the morphology of the sensory regions of the vestibular apparatus. Ergeb Anat Entwicklungsgesch. 1969;42:1–113. [PubMed] [Google Scholar]
- Lindeman HH, Reith A, Winther FØ. The distribution of type I and type II cells in the cristae ampullares of the guinea pig. Acta Otolaryngol. 1981;92:315–321. doi: 10.3109/00016488109133267. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. Études sur l’anatomie et la physiologie du labyrinthe de l’oreille et du VIIIe nerf: II. Quelque données au sujet de l’anatomie des organes sensoriels du labyrinthe. Trav Lab Rech Biol Univ Madrid. 1926;24:53–153. [Google Scholar]
- Lysakowski A. Synaptic organization of the crista ampullaris in vertebrates. Ann N Y Acad Sci. 1996;781:164–182. doi: 10.1111/j.1749-6632.1996.tb15700.x. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Regional variations in synaptic innervation of the chinchilla crista. Soc Neurosci Abstr. 1989;15:502. [Google Scholar]
- Lysakowski A, Goldberg JM. Regional variations in synaptic innervation of the squirrel monkey crista. Soc Neurosci Abstr. 1993;19:1578. [Google Scholar]
- Lysakowski A, Minor LB, Fernández C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol. 1995;73:1270–1281. doi: 10.1152/jn.1995.73.3.1270. [DOI] [PubMed] [Google Scholar]
- McNulty JA, Fox LM. Pinealocyte synaptic ribbons and neuroendocrine function. Microsc Res Tech. 1992 May 1;21(3):175–87. doi: 10.1002/jemt.1070210302. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr Serial section reconstruction of the neural poles of hair cells in the human organ of Corti: II. Outer hair cells. Laryngoscope. 1983;93:780–791. doi: 10.1288/00005537-198306000-00015. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Gacek RR, Balogh K., Jr Efferent innervation of vestibular labyrinth. Arch Otolaryngol. 1965;81:335–339. doi: 10.1001/archotol.1965.00750050346004. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: Capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Poljak S. Uber die Nervenendigungen in den vestibulären Sinnesendstellen bei den Säugetieren. Z Anat Entwicklungsgesch. 1927;84:131–144. [Google Scholar]
- Pujol R, Carlier E. Cochlear synaptogenesis after sectioning the efferent bundle. Brain Res. 1982;255:151–154. doi: 10.1016/0165-3806(82)90084-0. [DOI] [PubMed] [Google Scholar]
- Purcell IM, Perachio AA. Regional distributions of efferent neurons in the semicircular canals in the gerbil. Ann N Y Acad Sci. 1996;781:680–682. doi: 10.1111/j.1749-6632.1996.tb15758.x. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MD. Anatomic evidence for peripheral neural processing in mammalian graviceptors. Aviat Space Environ Med. 1985;56:338–343. [PubMed] [Google Scholar]
- Ross MD. A spaceflight study of synaptic plasticity in adult rat vestibular maculas. Acta Otolaryngol Suppl. 1994;516:1–14. [PubMed] [Google Scholar]
- Ross MD. Morphological evidence for local microcircuits in the rat vestibular maculae. J Comp Neurol. 1997;379:333–346. doi: 10.1002/(sici)1096-9861(19970317)379:3<333::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schaeffer SF, Raviola E. Membrane recycling in the cone cell endings of the turtle retina. J Cell Biol. 1978;79:802–825. doi: 10.1083/jcb.79.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schessel DA, Ginzberg R, Highstein SM. Morphophysiology of synaptic transmission between type I hair cells and vestibular primary afferents: An intracellular study employing horseradish peroxidase in the lizard, Calotes versicolor. Brain Res. 1991;544:1–16. doi: 10.1016/0006-8993(91)90879-z. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Brownell WE. Synaptic and Golgi membrane recycling in cochlear hair cells. J Neurocytol. 1986;15:311–328. doi: 10.1007/BF01611434. [DOI] [PubMed] [Google Scholar]
- Sjöstrand FS. Ultrastructure of retinal rod synapses as revealed by three-dimensional reconstructions from serial sections. J Ultrastruct Res. 1958;2:122–170. doi: 10.1016/s0022-5320(58)90050-9. [DOI] [PubMed] [Google Scholar]
- Smith CA, Rasmussen GL. Nerve ending in the maculae and cristae of the chinchilla vestibule, with a special reference to the efferents. Third Symposium on the Role of the Vestibular Organs in Space Exploration; Washington DC: US Govt. Printing Office (NASA SP-152); 1968. pp. 183–201. [Google Scholar]
- Smith CA, Sjöstrand FS. Structure of the nerve endings on the external hair cells of the guinea pig cochlea as studied by serial sections. J Ultrastruct Res. 1961 Dec;5:523–56. doi: 10.1016/s0022-5320(61)80025-7. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Some morphofunctional and pathological aspects of the vestibular sensory epithelium. Second Symposium on the Role of the Vestibular Organs in Space Exploration; Washington, DC: US Govt. Printing Office (NASA SP-115); 1966. pp. 99–116. [Google Scholar]
- Tanaka K, Smith CA. Structure of the chicken’s inner ear: SEM and TEM study. Am J Anat. 1978;153:251–261. doi: 10.1002/aja.1001530206. [DOI] [PubMed] [Google Scholar]
- Vollrath L, Meyer A, Buschmann F. Ribbon synapses of the mammalian retina contain two types of synaptic bodies—ribbons and spheres. J Neurocytol. 1989;18:115–120. doi: 10.1007/BF01188430. [DOI] [PubMed] [Google Scholar]
- Vollrath L, Seidel A. The plasticity of synaptic ribbons. Microsc Res Tech. 1989;21:175–187. [Google Scholar]
- Wagner HJ. Darkness-induced reduction of the number of synaptic ribbons in fish retina. Nat New Biol. 1973 Nov 14;246(150):53–5. doi: 10.1038/newbio246053a0. [DOI] [PubMed] [Google Scholar]
- Warr WB. Olivocochlear and vestibular efferent neurons of the feline brainstem: Their location, morphology and number determined by retrograde axonal transport and acetylcholinesterase histochemistry. J Comp Neurol. 1975;161:159–182. doi: 10.1002/cne.901610203. [DOI] [PubMed] [Google Scholar]
- Watanuki K, Meyer zum Gottesberge A. Light microscopic observations of the sensory epithelium of the crista ampullaris in the guinea pig. Ann Otol Rhinol Laryngol. 1971 Jun;80(3):450–4. doi: 10.1177/000348947108000325. [DOI] [PubMed] [Google Scholar]
- Wersäll J. Studies on the structure and innervation of the sensory epithelium of the cristae ampullaris in the guinea pig: A light and electron microscopic investigation. Acta Otolaryngol Suppl. 1956;126:1–85. [PubMed] [Google Scholar]
- Wersäll J. Efferent innervation of the inner ear. In: von Euler C, Skoglund C, Söderberg U, editors. Structure and Function of Inhibitory Neuronal Mechanisms. Oxford: Pergamon; 1968. pp. 123–139. [Google Scholar]
- Wersäll J, Bagger-Sjöbäck D. Morphology of the vestibular sense organ. In: Kornhuber HH, editor. Handbook of Sensory Physiology, Vol VI. Vestibular System, Part 1: Basic Mechanisms. Berlin: Springer-Verlag; 1974. pp. 123–170. [Google Scholar]
- Wilkinson L, Hill MA, Vang E. SYSTAT: Statistics, Version 5.2 Edition. Evanston, IL: SYSTAT Inc; 1992. [Google Scholar]
- Yamashita N, Ohmori H. Synaptic bodies and vesicles in calix type synapse of chicken semicircular canal ampullae. Neurosci Lett. 1991;129:43–46. doi: 10.1016/0304-3940(91)90716-7. [DOI] [PubMed] [Google Scholar]