Abstract
The human T-cell leukemia virus type 1 (HTLV-1) Tax protein activates the HTLV-1 long terminal repeat and key regulatory proteins involved in inflammation, activation, and proliferation and may induce cell transformation. Tax is also the immunodominant target antigen for cytotoxic T cells in HTLV-1 infection. We found that Tax bound to assembled nuclear proteasomes, but Tax could not be detected in the cytoplasm. Confocal microscopy revealed a partial colocalization of Tax with nuclear proteasomes. As Tax translocated into the nucleus very quickly after synthesis, this process probably takes place prior to and independent of proteasome association. Tax mutants revealed that both the Tax N and C termini play a role in proteasome binding. We also found that proteasomes from Tax-transfected cells had enhanced proteolytic activity on prototypic peptide substrates. This effect was not due to the induction of the LMP2 and LMP7 proteasome subunits. Furthermore, Tax appeared to be a long-lived protein, with a half-life of around 15 h. These data suggest that the association of Tax with the proteasome and the enhanced proteolytic activity do not target Tax for rapid degradation and may not determine its immunodominance.
Human T-cell leukemia virus type 1 (HTLV-1) is a complex human retrovirus associated with the neurological inflammatory disease tropical spastic paraparesis/HTLV-1 associated myelopathy (TSP/HAM) (19) and adult T-cell leukemia (ATL) (52). Tax is a 40-kDa phosphoprotein encoded by the pX region in the HTLV-1 genome. It activates transcription from the HTLV-1 long terminal repeat (LTR) and is essential for viral replication (50). Tax also induces an array of cellular genes involved in T-cell activation and proliferation. Additionally, Tax has transformation properties which are likely related to ATL (58).
TSP/HAM patients (2 to 3% of infected individuals) as well as healthy carriers have a strong chronically activated cytotoxic T-lymphocyte (CTL) response against the virus, which is mainly directed against the Tax protein (12). In addition, several distinct peptide epitopes processed from the Tax protein may be recognized simultaneously by CTLs in an individual (34). This vigorous immune control determines the HTLV-1 viral load in vivo, which is the main determinant of disease progression in TSP/HAM (25).
Tax can translocate to the nucleus via an unconventional nuclear localization signal domain (48). Tax is not itself a DNA binding protein and exerts its transactivation properties by modulating the function of various host transcription factors. It activates transcription from the viral LTR by binding to members of the CREB/ATF family, thereby enhancing their dimerization and DNA binding, and by recruiting the coactivator CREB binding protein (30, 35, 55). Tax also activates transcription of other viral and cellular genes (e.g., interleukin-2, interleukin-2 receptor α, human immunodeficiency virus type 1 [HIV-1]) via the NF-κB pathway (46, 47) and upregulates activation genes c-fos, erg-1, and erg-2 by interaction with serum response factor p65SRF (16). In HTLV-1-transformed cells, Tax is present in distinct nuclear structures (nuclear bodies) containing splicing factors, NF-κB, p300, the largest subunit of RNA polymerase II, and the cyclin-dependent kinase CDK8 (7).
The NF-κB/Rel family of transcription factors activate transcription by forming dimers and binding to κB enhancer sequences in the promoters of genes (54). In a resting cell, NF-κB dimers are sequestered in the cytoplasm by their interaction with members of a family of inhibitory proteins, most notably IκBα, which mask their nuclear localization signals (3, 24). Upon induction by a variety of signals, IκBα is phosphorylated on specific serine residues by a large (700 to 900 kDa) cytoplasmic IκB kinase (IKK) complex (9, 27). This phosphorylation marks it out for polyubiquitination and subsequent degradation by the proteasome. IκBα degradation leads to release of NF-κB, which then translocates to the nucleus to activate transcription (33).
HTLV-1-infected and Tax-expressing cells demonstrate constitutive nuclear expression of NF-κB (10, 57). Tax appears to act at multiple levels to initiate and maintain NF-κB activation. Probably most importantly, Tax induces increased IκBα phosphorylation and degradation. Tax can be recruited to the IKK complex by its physical association with IKKγ/NEMO, an essential regulatory component of the IKK complex (11, 22, 26). This recruitment of Tax leads to activation of the IKKα and IKKβ kinases, probably with the involvement of upstream kinases MEKK1 and NIK (18, 53, 57).
IκBα can translocate to the nucleus where it can bind to NF-κB factors, inhibit their DNA binding, and relocate NF-κB to the cytoplasm (1, 2). Tax has been shown to bind directly to the ankyrin domain of IκBα, thereby preventing its interaction with NF-κB factors (51).
Interestingly, Tax was also reported to enhance the constitutive biosynthetic turnover of IκBα by tethering it directly to the proteasome for phosphorylation- and ubiquitin-independent degradation. It was not determined in which cellular compartment this took place (29, 37). In addition, Tax was reported to enhance the processing of p105 into p50 by enhancing the binding of p105 to the proteasome (44). Although several other interactions of Tax with proteins of the NF-κB family have been described, the functional significance of these is not clear.
The proteasome is a multicatalytic proteinase complex implicated in the degradation of most cellular proteins (41). The catalytic core of the proteasome is formed by the 20S proteasome, which has a cylindrical structure composed of four rings, with the outer two each containing seven structural α-subunits and the inner two each containing seven β-subunits, of which only three are proteolytically active (21). Proteasomes have three important regulatory functions: the removal of abnormal proteins, the recognition and degradation of proteins involved in transcription regulation and cell cycle and signal transduction processes, and the proteolytic processing of proteins for presentation by the major histocompatibility complex (MHC) class I pathway (40). In mammalian cells, proteasomes are localized in both the nucleus and the cytoplasm (38).
Tax has been shown to associate with subunits HC9 (α3) and HsN3 (β7) of the proteasome (5, 37, 44). These interactions were observed following cotransfection of cells with plasmids expressing Tax and individual proteasome subunits. The physiological relevance of this is uncertain, because the subunits may be present in proteasome subcomplexes or even independent of assembled proteasomes.
To better understand the physical association of Tax with proteasomal subunits and the consequences of this interaction for cellular processes, we have investigated the ability of Tax to bind to intact cellular proteasomes and studied the resulting proteolytic activity of the complexes.
MATERIALS AND METHODS
Plasmids, mutants, and transfections.
Tax-encoding plasmid pJFE-Tax has been described (31). Mutant plasmids were generated using a site-directed mutagenesis kit (Promega) according to the manufacturer's instructions. All new constructs were completely sequenced before use in transfections. The Tax mutants generated carried the following mutations (also see reference 49): M7, 29Cys30Pro→Ala-Ser; M9, 41His42Arg→Ala-Ser; M12, 51Glu52His→Ala-Ser; M47, 319Leu320Leu→Arg-Ser; M9M47, contains the M9 and M47 mutations; M12M47, contains the M12 and M47 mutations.
293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 2 mM l-glutamine, 10% fetal calf serum, and antibiotics. Transfections were carried out using Easyfector reagent (EquiBio) and either pJFE-Tax, pJFE-Tax-mutant, or pJFE-L (empty control vector).
Immunoprecipitation, protein analysis, and antibodies.
Transfected cells were harvested into buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 0.5% NP-40, 0.5% Triton X-100, 1× protease inhibitor cocktail [Boehringer]) and allowed to lyse on ice for 30 min. When nuclear and cytoplasmic fractions were prepared, cells were suspended first in a solution containing 15 mM Tris-HCl (pH 7.5), 60 mM NaCl, 1 mM EDTA, and 14 mM β-mercaptoethanol (TNEβ buffer) with 7.5% polyethylene glycol 6000 plus 0.05% NP-40 on ice for 30 min. Nuclei were pelleted and the cytosolic supernatant fraction was transferred. Washed nuclei were subsequently lysed in TNEβ buffer with 1% sodium dodecyl sulfate (SDS). Lysates were precleared with fixed Staphylococcus aureus organisms at 4°C for at least 4 h and tumbled with antibody at 4°C overnight in the presence of 1% bovine serum albumin. Antibody complexes were captured on protein A-Sepharose (Bioprocessing Ltd.). For Western blotting, reduced proteins resolved by SDS-polyacrylamide gel electrophoresis (PAGE) were transferred to Hybond-C membranes (Amersham). Blots were visualized by chemiluminescence using ECL (Amersham).
For metabolic labeling of proteins, cells were starved in methionine- and cysteine-free medium for 1 h, after which 20 μCi of [35S]methionine-cysteine (Amersham; SJQ0079; >1,000Ci/mmol) per ml was added, and cells were incubated for 15 min, 1 h, or 2.5 h as indicated. Labeling mix was removed and chased with Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Immunoprecipitations were performed as described above. Following SDS-PAGE, gels were fixed, stained with Coomassie brilliant blue, dried, and exposed to X-ray film.
MCP21 antibody recognizes the HC3 subunit of the human 20S proteasome and has been previously described (23). Polyclonal rabbit anti-Tax antisera BR-76 and 1135TB were raised against the C-terminal Tax peptide MISPGGLERPSEKHFRETEV. Y8 is a mouse antibody recognizing an uncharacterized epitope of Tax. MAb104 is specific for a phospho-epitope in members of the serine- and arginine-rich (SR) family of nuclear pre-mRNA splicing factors (43). W6/32 recognizes a conformation-specific epitope in the human class I α chain. LMP2 and LMP7 antisera have previously been described (4).
Immunocytochemistry and confocal microscopy.
293T cells were grown on coverslips and either not infected or infected with SFV6007 for expression of the Tax protein fused to the hemagglutinin (HA) epitope. After 18 h the cells were fixed with 4% paraformaldehyde for 10 min and permeabilized with a solution of 0.05% Triton X-100 in phosphate-buffered saline (PBS) for 10 min at room temperature. The samples were saturated with PBS containing 0.5% gelatin and 0.25% bovine serum albumin for 1 h and stained for 1 h with a 1/1,000 dilution of a rabbit polyclonal serum directed against HA (Y11 from Santa Cruz Biotechnology) (Tax staining) and 10 μg of MCP21 monoclonal antibody (proteasome staining) per ml in the same saturation solution. The samples were then washed three times with PBS containing 0.25% gelatin and incubated for 1 h with a 1/100 dilution of the following secondary antibodies: goat anti-rabbit immunoglobulin G conjugated to lissamine rhodamine sulfchloride (red color for Tax) and goat anti-mouse immunoglobulin G conjugated to fluorescein isothiocyanate (green color for MCP21) (Jackson Immunoresearch). The samples were washed three times in PBS with 0.25% gelatin and mounted for analysis on a Zeiss LSM510 laser scanning confocal microscope.
Peptide cleavage assays.
Peptidase activity was determined using synthetic substrates N-succinyl-LLVY-mca and N-cbz-GGR-mca (Sigma) dissolved in dimethyl sulfoxide and diluted to 100 μM in 20 mM Tris-HCl (pH 7.5). Proteasome inhibitors lactacystin and calpain inhibitor I (LLnL) were included at 25 and 200 μM final concentrations, respectively, where indicated. Proteasomes were immunoprecipitated with MCP21 or W6/32 (negative control) and added to a total of 250 μl of reaction mix. After a 1- to 2-h incubation at 37°C, 50-μl samples were taken and fluorescent measurements of duplicate samples were taken at an excitation wavelength of 370 nm and an emission wavelength of 460 nm on a spectrofluorometer. Values of the negative controls were subtracted and activity was quantified relative to the amount of proteasome in the immunoprecipitated proteasome preparation. This was determined by densitometry of SDS-PAGE Western blots with MCP21.
RESULTS
Tax rapidly translocates to the nucleus after synthesis.
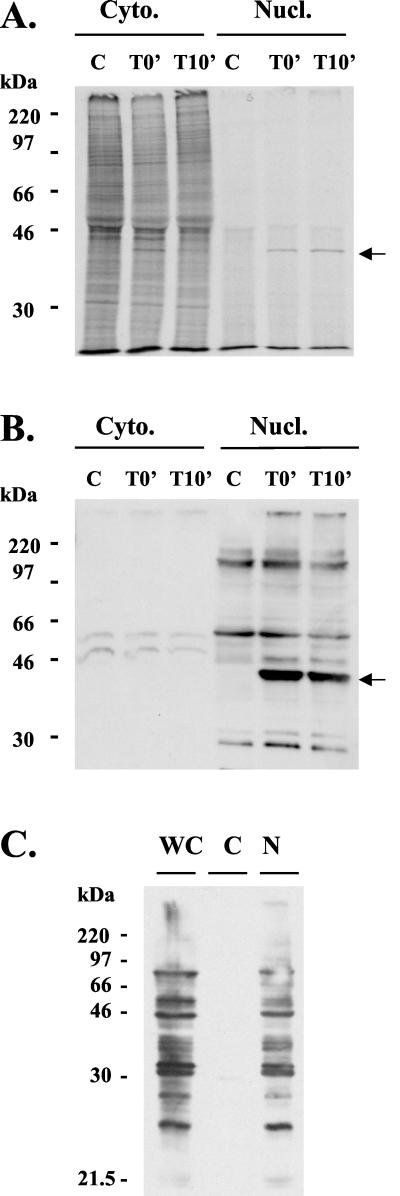
Tax is a 40-kDa phosphoprotein which is localized predominantly in the nucleus of infected cells (7). Tax does not contain a highly basic nuclear localization signal, but instead its N-terminal 48 amino acids comprise a functional nuclear localization domain (48). In transiently transfected human 293T cells, Tax translocated to the nucleus within less than 15 min of synthesis (Fig. 1A). Analysis of steady-state levels of Tax in both fractions by immunoblotting revealed abundant Tax in the nuclear fraction, whereas Tax was undetectable in the cytoplasmic fraction (Fig. 1B). Tax was further shown to form characteristic nuclear bodies in the nucleus (see Fig. 3) (7). Nuclear and cytoplasmic fractionation was confirmed by Western blotting of lysate fractions using a monoclonal antibody against nuclear SR pre-mRNA splicing factors (Fig. 1C). Together, these results show that Tax rapidly translocates to the nucleus after synthesis and that at a steady state Tax is almost exclusively localized in the nucleus.
FIG. 1.
Tax rapidly translocates into the nucleus. (A) Tax-transfected 293T cells were metabolically pulse-labeled for 15 min and either harvested immediately (T0′) or chased for 10 min (T10′). Control transfected cells were labeled for 15 min and harvested (C). Tax-specific immunoprecipitates (BR-76 antiserum) from nuclear (Nucl.) and cytoplasmic (Cyto.) fractions from each time point were resolved by SDS–10% PAGE, and the gel was dried and exposed to X-ray film. The arrow on the right indicates the Tax band. (B) Equal amounts of protein of the cell fractions from the samples of panel A were separated by SDS–10% PAGE and blotted onto nitrocellulose, and Tax protein was detected with Y8 anti-Tax antibody. The arrow on the right indicates the Tax band. (C) Whole-cell lysate (WC) and cytoplasmic (C) and nuclear (N) fractions of Tax-transfected 293T cells were separated by SDS–10% PAGE, blotted onto nitrocellulose, and probed with the monoclonal antibody MAb104 specific for members of the nuclear SR family of pre-mRNA splicing factors. This shows that there is no contamination of the cytoplasmic fraction with the nuclear fraction.
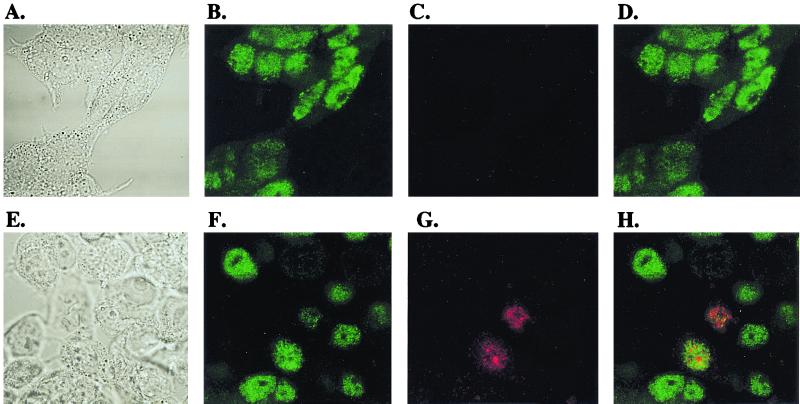
FIG. 3.
Tax partially colocalizes with nuclear proteasomes outside Tax-induced nuclear bodies. Confocal microscopy was performed as described in Materials and Methods. (A to D) Uninfected 293T cells. (E to H) SFV-Tax-infected 293T cells, 18 h postinfection. (A, E) Differential interference contrast. (B, F) Proteasome staining with MCP21 antibody. (C, G) Tax staining with anti-HA antibody. (D, H) Superimposition of panels B and C and panels F and G, respectively. The yellow color in panel H indicates the overlap of MCP21 and anti-HA staining.
Tax physically associates with nuclear proteasomes.
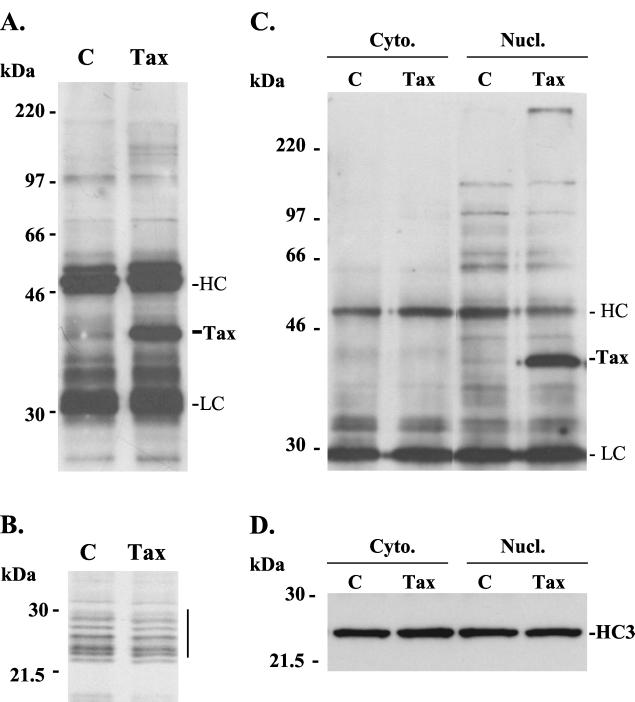
Previous studies had shown that Tax binds to the proteasomal α-subunit HC9 (α3) and (noncatalytic) β-subunit HsN3 (β7) (5, 44). These studies were performed by ectopically expressing these subunits in the cytosol of COS7 cells. It was unclear from these studies whether the expressed subunits were incorporated into mature 20S proteasomes and whether Tax binding to assembled proteasomes occurred. To investigate this, we immunoprecipitated proteasomes from Tax-transfected or control-transfected 293T cell lysates using the monoclonal HC3(α2)-subunit-specific antibody MCP21. The immunoprecipitated proteasomes were electrophoresed and immunoblotted with a Tax-specific antibody. Tax protein was clearly detected in proteasome immunoprecipitations from whole-cell lysates of Tax-transfected cells (Fig. 2A). Immunoprecipitation of intact proteasomes by MCP21 antibody was confirmed in metabolically labeled cell lysates by the presence of a characteristic stack of proteins of 22 to 32 kDa which is typical of the 20S proteasome (36) and indicated an equivalent efficiency of proteasome recovery from Tax-transfected and control cells (Fig. 2B).
FIG. 2.
Tax coprecipitates with nuclear proteasomes. (A) Lysates from control (C) and Tax-transfected (Tax) 293T cells were subjected to immunoprecipitation with antiproteasome (HC3 subunit specific) antibody MCP21 and the immunoprecipitates were resolved by SDS–10% PAGE and analyzed by Western blotting with anti-Tax antibody Y8. Coimmunoprecipitated Tax is indicated. The secondary antibody used in the Western blot also recognizes the heavy chain (HC) and light chain (LC) of the MCP21 antibody, as indicated. (B) Control or Tax-transfected 293T cells were metabolically labeled for 2.5 h, the lysates were subjected to immunoprecipitation with MCP21 antibody, and the precipitates were resolved by SDS–12% PAGE. Autoradiography showed a characteristic stack of proteasome subunits spanning 22 to 32 kDa in size and of similar intensity in each preparation (bar). (C) Cytoplasmic (Cyto.) and nuclear (Nucl.) fractions of control and Tax-transfected 293T cells were subjected to immunoprecipitation with MCP21 antibody, and the precipitates were resolved by SDS–10% PAGE and analyzed by Western blotting with anti-Tax antibody Y8. Tax and the heavy chain (HC) and light chain (LC) of MCP21 are indicated on the right. (D) Immunoprecipitates from panel C were probed with MCP21 antibody to detect the HC3 subunit. This shows that equal amounts of proteasome were immunoprecipitated from all fractions.
As Tax is predominantly a nuclear protein, we prepared nuclear and cytoplasmic fractions from cell lysates and repeated the proteasome immunoprecipitations on fractionated material. Immunodetection of these preparations with Tax-specific antibody showed that Tax was restricted to proteasomes from the nuclear fraction (Fig. 2C). Western blotting of the immunoprecipitates with MCP21 confirmed that proteasomes from both fractions were immunoprecipitated (Fig. 2D).
Reciprocal experiments on whole-cell lysates revealed that proteasome proteins were not detected in Tax immunoprecipitates (data not shown). As the Tax-specific antibody was raised to a C-terminal peptide in Tax, this may suggest that Tax binds the proteasome via a C-terminal domain. Alternatively, it may indicate that only a small proportion of the total amount of Tax in the nucleus is bound to proteasomes. A calculated comparison of the amount of Tax detected in proteasome immunoprecipitates with the total amount detected in whole nuclear fractions supports this suggestion. Similar results were obtained using a Tax antibody (Y8) recognizing a distinct but uncharacterized epitope in Tax (data not shown).
If Tax and proteasomes interact in the nucleus, then at least partial colocalization of the two components in the nucleus would be expected. To address this, we performed immunofluorescence confocal microscopy experiments. We detected proteasomes in both the cytoplasm and the nucleus (Fig. 3B), with especially strong detection of proteasomes in the nuclear compartment, as has previously been observed for cultured cells (39). Tax expression did not obviously alter the distribution pattern of proteasomes (Fig. 3F). Tax was localized in the nucleus, both in defined nuclear bodies and as more diffuse staining (Fig. 3G). Superimposition of the two staining patterns revealed a partial colocalization of Tax with nuclear proteasomes outside the nuclear bodies (Fig. 3H).
Together, these data indicate that Tax associates with assembled nuclear proteasomes. This interaction may be transient, with only a limited amount of Tax associated with proteasomes at any one time.
The N and C termini of Tax play a role in proteasome binding.
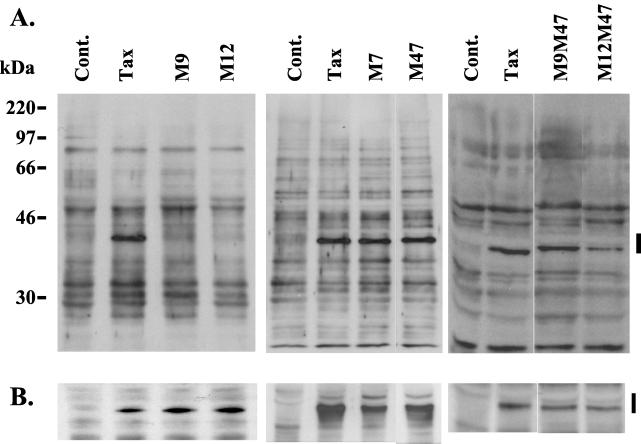
To determine which regions of the Tax protein are important for proteasome binding, we generated several Tax mutants that have been previously described (49). All mutants were tested for their ability to coimmunoprecipitate with proteasomes from whole-cell lysates (Fig. 4). M9 (41H42R→AS) and M12 (51E52H→AS) mutants were clearly deficient in proteasome binding, implicating the Tax N terminus in proteasome binding. Maintenance of proteasome binding capacity by M7 (29C30P→AS), a mutant localized in the cytoplasm, indicated that nuclear localization is not required for proteasome binding. The C-terminal mutant M47 (319L320L→RS) not only maintained proteasome binding capacity as a single mutant, it was also able to compensate in cis for the abrogation of proteasome binding by the M9 and M12 mutations, as shown by the double mutants M9M47 and M12M47. Together, these data indicate that both the N and C termini of Tax play a role in proteasome binding. Nuclear localization appears not to be a prerequisite for proteasome binding.
FIG. 4.
Tax mutants reveal regions important for proteasome binding. (A) Coimmunoprecipitation of Tax mutants with 20S proteasome. Lysates from control (Cont.), Tax-transfected, and mutant Tax-transfected 293T cells were subjected to immunoprecipitation with antiproteasome antibody MCP21 and the immunoprecipitates were resolved by SDS–10% PAGE. Tax was detected by Western blotting with a polyclonal anti-Tax serum (1135TB). Tax is indicated by the bar on the right. Tax mutants were as follows: M7, 29C30P→AS; M9, 41H42R→AS; M12, 51E52H→AS; M47, 319L320L→RS; M9M47, contains the M9 and M47 mutations; M12M47, contains the M12 and M47 mutations. (B) Expression of Tax mutants. Equal amounts of total cell lysate were separated by SDS–10% PAGE and blotted onto nitrocellulose, and Tax protein was detected with a polyclonal rabbit anti-Tax serum (1135TB). Lanes correspond to the lanes of panel A. Tax is indicated by the bar on the right.
Tax expression stimulates proteolytic activities of the 20S proteasome.
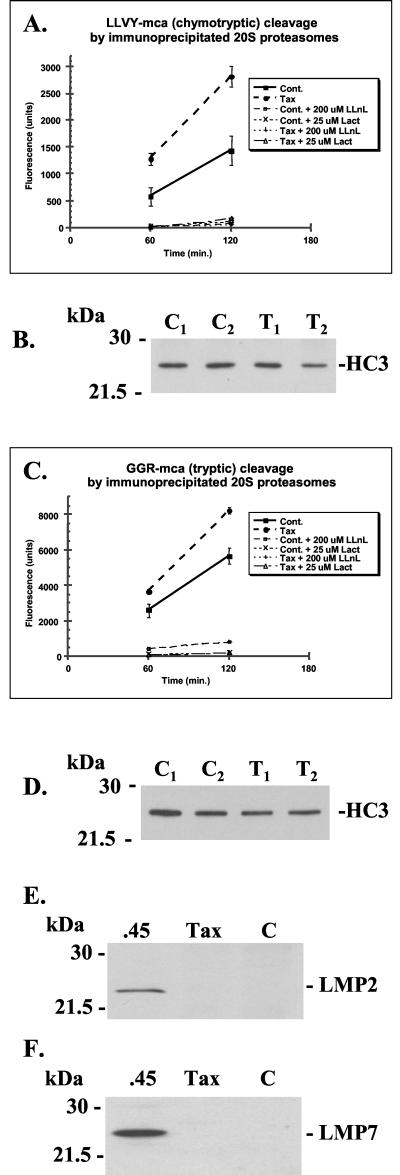
The proteolytic properties of the 20S proteasome are modulated by incorporation of the gamma interferon-inducible subunits LMP2, LMP7, and MECL-1 and attachment of the PA28 activator to the end(s) of the 20S proteasome complex (14, 17, 20). Although up to five different activities have been described for proteasomes, the chymotrypsin-like (cleavage after hydrophobic residues), trypsin-like (cleavage after basic residues), and caspase-like (cleavage after acidic residues) hydrolyzing activities represent the three main types (13). The chymotrypsin-like activity is thought to determine the rate of protein breakdown by the proteasome (28). Given the strong association of Tax with nuclear proteasomes, we wished to establish if this association altered their proteolytic activity. Prototypic fluorogenic peptide substrates N-succinyl-LLVY-mca and N-cbz-GGR-mca were used to assay chymotryptic and tryptic activity, respectively, in immunoprecipitated proteasome preparations from whole-cell lysates. Under conditions which allowed coprecipitation of Tax with proteasomes, increased activity was seen with both substrates by proteasomes purified from Tax-transfected cells compared with control cells (Fig. 5A and C). The relative amounts of proteasome in the different samples were determined by separating the immunoprecipitates by SDS-PAGE after the cleavage assay and immunoblotting with MCP21 antibody to detect the HC3 subunit. The bands were quantified and used to normalize the fluorescence measured in the cleavage assay (Fig. 5B and D). Cleavage of substrates was abrogated by the addition to assays of the proteasome inhibitors lactacystin or calpain inhibitor I (LLnL), indicating that the proteolytic activity we measured was indeed proteasomal (Fig. 5A and C). Because the stoichiometry of the Tax-proteasome interaction is not known, no quantitative inferences can be made from the cleavage experiments. However, given that our previous data suggested that only a fraction of proteasomes were bound to Tax at one time (see Fig. 2 and 3 and text), detection of any enhancement of cleavage by immunoprecipitated proteasomes is significant.
FIG. 5.
Immunoprecipitated proteasomes from Tax-transfected cells show enhanced proteolytic activity. (A, C) Immunoprecipitated 20S proteasomes from control or Tax-transfected 293T cells were used to cleave synthetic fluorogenic peptide substrates as a measure of proteolytic activity in three separate experiments. Activity is measured in arbitrary fluorescent units relative to a background reading using W6/32 class I immunoprecipitates. Fluorescent activity was normalized for the relative total amount of proteasome protein recovered in immunoprecipitates and quantified by densitometry (see panels B and D). Panel A shows cleavage of substrate N-succinyl-LLVY-mca (chymotrypsin-like activity), and panel C shows cleavage of substrate N-cbz-GGR-mca (trypsin-like activity). Analysis was done after 1- or 2-h incubations at 37°C. Squares, Tax-transfected cells; circles, control cells. Proteasome inhibitors lactacystin (Lact) and calpain inhibitor I (LLnL) were included at 25 and 200 μM final concentrations, respectively, where indicated. Data points show means ± standard errors of duplicate samples for one representative experiment. (B, D) The amounts of 20S proteasome present in the immunoprecipitates used for the activity measurement (see panels A and C) were determined by separation by SDS-PAGE followed by Western blotting with MCP21 antibody to detect the HC3 subunit. Amounts were quantified by densitometry and used to normalize the fluorescence measured in the cleavage assay. C, control cells; T, Tax transfectant. (E, F) Total cell lysates were resolved by SDS-PAGE followed by Western blot analysis using rabbit polyclonal antibodies to LMP2 (E) or LMP7 (F). Human .45 cells (.45) expressing LMP2 and LMP7 were used as positive controls for expression of these subunits. Tax, Tax-transfected 293T cells; C, control transfected 293T cells. Tax does not induce expression of LMP2 and LMP7.
We also attempted to determine the cleavage activities of proteasomes immunoprecipitated from nuclear and cytoplasmic fractions. However, reproducibility of these data from fractionated material was poor and we were unable to draw conclusions about the comparative cleavage activities. This may reflect the different conditions used during lysis and preparation of nuclear and cytoplasmic fractions (data not shown).
The Tax-specific enhancement of the chymotryptic and tryptic activities we observed is similar to that reported for proteasomes containing LMP2 and LMP7 subunits (14, 17). Tax-induced expression and substitution of these subunits in cellular proteasomes could explain our observations. However, LMP2 and LMP7 proteins were not detected by Western blot analysis of lysates from Tax-transfected or control 293T cells (Fig. 5E and F), indicating that this was not the case. Similarly, Tax did not induce expression of the MECL-1 subunit (data not shown).
Tax is a long-lived protein.
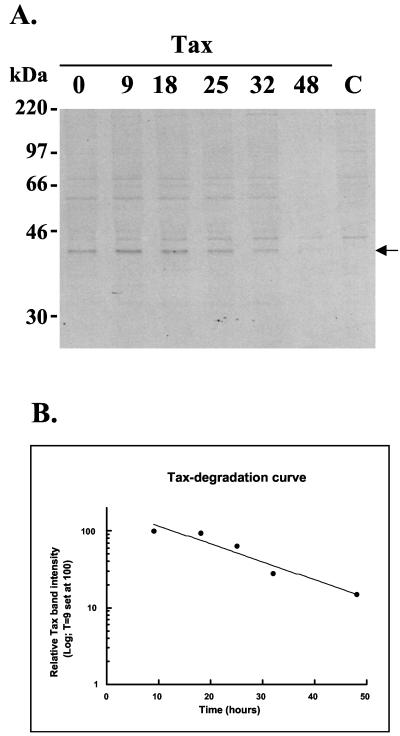
The observed association of Tax with nuclear proteasomes and the stimulation of their catalytic activity suggested that Tax may affect nuclear proteasomal proteolysis. Tax is strikingly immunodominant as a cytotoxic T-cell target (12), and peptides generated for presentation by MHC class I molecules are typically derived from proteasomal processing of intracellular proteins (41). Therefore, one might expect that Tax is destined for rapid degradation by nuclear proteasomes. However, we found that in transfected 293T cells Tax is a stable, long-lived protein with a metabolic half-life of 15 h (Fig. 6). No significant change was observed in the presence of the proteasome inhibitor calpain inhibitor 1 (data not shown). A similar stability of Tax was seen in the HTLV-1-transformed cell line MT2 (data not shown). This suggests that the association of Tax with the proteasome does not target it for rapid degradation and may not determine its immunodominance.
FIG. 6.
Tax is a long-lived protein. (A) Tax-transfected 293T cells were metabolically pulse-labeled for 1 h and chased for 0 to 48 h. Tax was immunoprecipitated from lysates with anti-Tax serum BR-76. Following resolution by SDS–10% PAGE, the gel was dried and exposed to X-ray film. The Tax band is indicated. (B) Tax was quantified by densitometry at each time point. Timepoint zero was omitted from the analysis, as the sample had not been completely loaded on the gel. The decay curve predicted a half-life of approximately 15 h.
DISCUSSION
In this study, we observed that the HTLV-1 transactivator protein Tax translocated into the nucleus very rapidly after synthesis. At steady state, nuclear Tax accounted for almost all of the Tax expressed in the cell, although we cannot exclude a minor fraction of Tax present in the cytosol, where it is synthesized. In the nucleus it was strongly associated with assembled nuclear 20S proteasomes and we could not detect Tax bound to cytoplasmic proteasomes.
The mechanism of proteasome transport into the nucleus is incompletely understood. Several of the α-subunit components contain highly conserved short nuclear localization sequences, and nuclear pore complexes appear able to translocate large protein complexes like proteasomes (15, 32, 38). However, subunits might also be transported individually or in subcomplexes and may assemble intranuclearly (39).
It has been shown that proteasomes diffuse rapidly throughout the nucleus and cytoplasm and throughout the cell during cell division. Proteasomes are contained in the nucleus upon nuclear membrane reassembly after cell division and they are transported over the nuclear membrane very slowly and unidirectionally from the cytoplasm to the nucleus (38). As Tax is efficiently translocated to the nucleus after synthesis, it is unlikely that it binds to preassembled proteasomes in the cytosol and the whole complex is shuttled into the nucleus. In addition, the fact that a cytoplasmic Tax mutant (M7) was still able to bind to the proteasome indicated that proteasome binding is not sufficient for nuclear translocation. It also shows that nuclear translocation is not necessary for proteasome binding, rendering it unlikely that a nuclear cofactor is necessary for proteasome binding. It remains possible that Tax binds to one or more independent proteasome components prior to nuclear translocation and facilitates this process for subunits lacking a nuclear localization signal sequence. However, the predominance of proteasome-Tax complexes in the nuclear compartment probably reflects the efficient translocation of Tax, independent of proteasome, into the nucleus following synthesis.
Two mutations in the Tax N terminus (M9 and M12) abrogated the ability of Tax to bind the proteasome. A second mutation in the C terminus (M47) was able to restore the proteasome binding lost by the M9 or M12 mutation. The M47 mutation on its own was reported to result in a twofold enhancement of binding of Tax to the HsN3 and HC9 subunits in a two-hybrid assay (44). We did not see an enhancement of coimmunoprecipitation of M47 with proteasomes (Fig. 4), which might indicate that our assay is not sensitive enough to detect such a difference or that Tax binding to the assembled proteasome is different from that observed with single subunits (see below).
However, it is possible that both the N and C termini of Tax bind to the proteasome and that enhanced binding affinity of the C-terminal domain caused by the M47 mutation can overcome the loss of binding affinity of the N-terminal domain caused by the M9 and M12 mutations. Alternatively, the M47 mutation may affect the folding of the N-terminal domain and therefore affect proteasome binding through an indirect effect on the N-terminal domain, rather than by a direct interaction with the proteasome.
It also remains a possibility that the level of phosphorylation of Tax plays a role in determining the strength of the association (8).
The stoichiometry of the Tax-proteasome interaction is unknown. It remains possible that Tax binds to other subunits in addition to (or instead of) the two noncatalytic subunits mentioned earlier. In addition, Tax can form dimers which could increase the number of Tax molecules bound to the proteasome at any one time (35). Our immunoprecipitation and confocal microscopy data indicate that a relatively small proportion of Tax molecules is associated with proteasomes at any one time.
The fact that two different proteolytic activities (the chymotryptic and tryptic activities) within the proteasome were enhanced by Tax suggests that the effect is on the whole proteasomal complex rather than on single subunits. The intimate contacts seen between adjacent subunits in the 20S proteasome crystal structure (21) are consistent with a Tax effect being transferred to the whole structure, perhaps by inducing an altered conformation. Activation might be achieved through changes in the catalytic sites or by improving the entry or translocation of substrates in the proteasome. The proteasome regulator PA28 modifies the activity of the proteasome in a similar way by inducing a conformational change of the 20S proteasome which results in increased activity (56).
Although the function of the Tax-proteasome complex is unclear, a likely possibility is a regulatory role in transcriptional control. IκBα can translocate to the nucleus to remove NFκB from the DNA, thereby inhibiting transcriptional activation (1, 2). In a Tax-expressing cell the cytoplasmic pool of IκBα is quickly degraded by IKK complex-mediated phosphorylation of IκBα (6, 11, 53, 57). The nuclear pool of IκBα is, however, protected from this induced signaling pathway, due to the cytoplasmic localization of the IKK complex (42). Tax was reported to increase the constitutive phosphorylation- and ubiquitination-independent turnover of IκBα by enhancing the binding of IκBα to proteasome subunits (37). It was not determined in which cellular compartment this took place. We propose that Tax mediates enhancement of constitutive phosphorylation- and ubiquitin-independent degradation of IκBα by tethering nuclear IκBα to nuclear proteasomes and stimulating proteasomal proteolytic activity. This could deplete the nucleus of IκBα and contribute to the constitutive activation of NFκB seen in Tax-expressing cells. This in turn could lead to cellular activation and proliferation, as seen in HTLV-1 infection, and could ultimately contribute to cell transformation and the development of ATL (58).
The CTL response against HTLV-1 determines the equilibrium viral load, which is the main determinant of the risk of developing TSP/HAM (25). Tax is the immunodominant antigen in the CTL response against HTLV-1 and multiple epitopes may be recognized simultaneously within an individual (12, 34). The proteasome has been implicated in the generation of the majority of peptides for presentation via the MHC class I pathway (40). After our findings that Tax associates with nuclear proteasomes and stimulates proteasomal proteolytic activity, it was surprising to find that Tax was such a stable protein (half-life of ≈15 h). Its proximity to the proteasome apparently doesn't target it for rapid degradation and may not determine its immunodominance. Recently it was reported that a large proportion of newly synthesized proteins (called defective ribosomal products) is rapidly degraded by the ubiquitin-proteasome pathway and that the degradation products can be presented by class I molecules. Specifically, the HIV Gag protein is a long-lived protein, but a large proportion of newly synthesized Gag proteins was subject to ubiquitin-proteasomal degradation (45). Therefore, Tax peptide epitopes for presentation via the MHC class I pathway may also be mainly derived from Tax defective ribosomal products.
ACKNOWLEDGMENTS
We thank G. Screaton for the gift of MAb104 antibody, J. Trowsdale for the gift of LMP2- and LMP7-specific antisera, and E. Corey for the supply of lactacystin. We are grateful to Veronique Braud and Simon Davis for helpful discussions.
This work was supported by the Medical Research Council (J.H., V.C., and A.M.), the Wellcome Trust (S.D.), a Marshall Scholarship (B.B.), and the Fèdèration Belge contre le Cancer and Fonds National de la Recherche Scientifique (F.B.).
REFERENCES
- 1.Arenzana-Seisdedos F, Thompson J, Rodriguez M S, Bachelerie F, Thomas D, Hay R T. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA-binding and transcriptional activities of NF-kappa B. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenzana-Seisdedos F, Turpin P, Rodriguez M, Thomas D, Hay R T, Virelizier J L, Dargemont C. Nuclear localization of I kappa B alpha promotes active transport of NF-kappa B from the nucleus to the cytoplasm. J Cell Sci. 1997;110:369–378. doi: 10.1242/jcs.110.3.369. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 4.Belich M P, Glynne R J, Senger G, Sheer D, Trowsdale J. Proteasome components with reciprocal expression to that of the MHC-encoded LMP proteins. Curr Biol. 1994;4:769–776. doi: 10.1016/s0960-9822(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 5.Beraud C, Greene W C. Interaction of HTLV-I Tax with the human proteasome: implications for NF-kappa B induction. J AIDS Hum Retrovir. 1996;13:S76–S84. doi: 10.1097/00042560-199600001-00014. [DOI] [PubMed] [Google Scholar]
- 6.Bex F, Gaynor R B. Regulation of gene expression by HTLV-I Tax protein. Methods. 1998;16:83–94. doi: 10.1006/meth.1998.0646. [DOI] [PubMed] [Google Scholar]
- 7.Bex F, McDowall A, Burny A, Gaynor R. The human T-cell leukemia virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-kappaB proteins. J Virol. 1997;71:3484–3497. doi: 10.1128/jvi.71.5.3484-3497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bex F, Murphy K, Wattiez R, Burny A, Gaynor R B. Phosphorylation of the human T-cell leukemia virus type 1 transactivator Tax on adjacent serine residues is critical for Tax activation. J Virol. 1999;73:738–745. doi: 10.1128/jvi.73.1.738-745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 10.Chu Z L, DiDonato J A, Hawiger J, Ballard D W. The Tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IkappaB kinases containing IKKalpha and IKKbeta. J Biol Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 11.Chu Z L, Shin Y A, Yang J M, DiDonato J A, Ballard D W. IKKgamma mediates the interaction of cellular IkappaB kinases with the Tax transforming protein of human T cell leukemia virus type 1. J Biol Chem. 1999;274:15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- 12.Daenke S, Kermode A G, Hall S E, Taylor G, Weber J, Nightingale S, Bangham C R. High activated and memory cytotoxic T-cell responses to HTLV-1 in healthy carriers and patients with tropical spastic paraparesis. Virology. 1996;217:139–146. doi: 10.1006/viro.1996.0101. [DOI] [PubMed] [Google Scholar]
- 13.Dick T P, Nussbaum A K, Deeg M, Heinemeyer W, Groll M, Schirle M, Keilholz W, Stevanovic S, Wolf D H, Huber R, Rammensee H G, Schild H. Contribution of proteasomal beta-subunits to the cleavage of peptide substrates analyzed with yeast mutants. J Biol Chem. 1998;273:25637–25646. doi: 10.1074/jbc.273.40.25637. [DOI] [PubMed] [Google Scholar]
- 14.Driscoll J, Brown M G, Finley D, Monaco J J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993;365:262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- 15.Feldherr C M, Kallenbach E, Schultz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984;99:2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 17.Gaczynska M, Rock K L, Goldberg A L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993;365:264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- 18.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Jr, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-kappaB involves activation of the IkappaB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 20.Groettrup M, Ruppert T, Kuehn L, Seeger M, Standera S, Koszinowski U, Kloetzel P M. The interferon-gamma-inducible 11S regulator (PA28) and the LMP2/LMP7 subunits govern the peptide production by the 20S proteasome in vitro. J Biol Chem. 1995;270:23808–23815. doi: 10.1074/jbc.270.40.23808. [DOI] [PubMed] [Google Scholar]
- 21.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik H D, Huber R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 22.Harhaj E W, Sun S C. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 23.Hendil K B, Kristensen P, Uerkvitz W. Human proteasomes analysed with monoclonal antibodies. Biochem J. 1995;305:245–252. doi: 10.1042/bj3050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs M D, Harrison S C. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery K J, Usuku K, Hall S E, Matsumoto W, Taylor G P, Procter J, Bunce M, Ogg G S, Welsh K I, Weber J N, Lloyd A L, Nowak M A, Nagai M, Kodama D, Izumo S, Osame M, Bangham C R. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin D Y, Giordano V, Kibler K V, Nakano H, Jeang K T. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J Biol Chem. 1999;274:17402–17405. doi: 10.1074/jbc.274.25.17402. [DOI] [PubMed] [Google Scholar]
- 27.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 28.Kisselev A F, Akopian T N, Castillo V, Goldberg A L. Proteasome active sites allosterically regulate each other, suggesting a cyclical bite-chew mechanism for protein breakdown. Mol Cell. 1999;4:395–402. doi: 10.1016/s1097-2765(00)80341-x. [DOI] [PubMed] [Google Scholar]
- 29.Krappmann D, Wulczyn F G, Scheidereit C. Different mechanisms control signal-induced degradation and basal turnover of the NF-kappaB inhibitor IkappaB alpha in vivo. EMBO J. 1996;15:6716–6726. [PMC free article] [PubMed] [Google Scholar]
- 30.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 31.Niewiesk S, Daenke S, Parker C E, Taylor G, Weber J, Nightingale S, Bangham C R. Naturally occurring variants of human T-cell leukemia virus type I Tax protein impair its recognition by cytotoxic T lymphocytes and the transactivation function of Tax. J Virol. 1995;69:2649–2653. doi: 10.1128/jvi.69.4.2649-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno M, Fornerod M, Mattaj I W. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- 33.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 34.Parker C E, Nightingale S, Taylor G P, Weber J, Bangham C R. Circulating anti-Tax cytotoxic T lymphocytes from human T-cell leukemia virus type I-infected people, with and without tropical spastic paraparesis, recognize multiple epitopes simultaneously. J Virol. 1994;68:2860–2868. doi: 10.1128/jvi.68.5.2860-2868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perini G, Wagner S, Green M R. Recognition of bZIP proteins by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- 36.Peters J M. Proteasomes: protein degradation machines of the cell. Trends Biochem Sci. 1994;19:377–382. doi: 10.1016/0968-0004(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 37.Petropoulos L, Hiscott J. Association between HTLV-1 Tax and I kappa B alpha is dependent on the I kappa B alpha phosphorylation state. Virology. 1998;252:189–199. doi: 10.1006/viro.1998.9430. [DOI] [PubMed] [Google Scholar]
- 38.Reits E A J, Benham A M, Plougastel B, Neefjes J, Trowsdale J. Dynamics of proteasome distribution in living cells. EMBO J. 1997;16:6087–6094. doi: 10.1093/emboj/16.20.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivett A J. Intracellular distribution of proteasomes. Curr Opin Immunol. 1998;10:110–114. doi: 10.1016/s0952-7915(98)80040-x. [DOI] [PubMed] [Google Scholar]
- 40.Rock K L, Goldberg A L. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–779. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 41.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez M S, Thompson J, Hay R T, Dargemont C. Nuclear retention of IkappaBalpha protects it from signal-induced degradation and inhibits nuclear factor kappaB transcriptional activation. J Biol Chem. 1999;274:9108–9115. doi: 10.1074/jbc.274.13.9108. [DOI] [PubMed] [Google Scholar]
- 43.Roth M B, Zahler A M, Stolk J A. A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J Cell Biol. 1991;115:587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rousset R, Desbois C, Bantignies F, Jalinot P. Effects on NF-kappa B1/p105 processing of the interaction between the HTLV-1 transactivator Tax and the proteasome. Nature. 1996;381:328–331. doi: 10.1038/381328a0. [DOI] [PubMed] [Google Scholar]
- 45.Schubert U, Anton L C, Gibbs J, Norbury C C, Yewdell J W, Bennink J R. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 46.Siekevitz M, Feinberg M B, Holbrook N, Wong-Staal F, Greene W C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci USA. 1987;84:5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siekevitz M, Josephs S F, Dukovich M, Peffer N, Wong-Staal F, Greene W C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987;238:1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- 48.Smith M R, Greene W C. Characterization of a novel nuclear localization signal in the HTLV-I tax transactivator protein. Virology. 1992;187:316–320. doi: 10.1016/0042-6822(92)90320-o. [DOI] [PubMed] [Google Scholar]
- 49.Smith M R, Greene W C. Identification of HTLV-I Tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. . (Erratum, 9:2324, 1995.) [DOI] [PubMed] [Google Scholar]
- 50.Sodroski J, Rosen C, Goh W C, Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985;228:1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki T, Hirai H, Murakami T, Yoshida M. Tax protein of HTLV-1 destabilizes the complexes of NF-kappa B and I kappa B-alpha and induces nuclear translocation of NF-kappa B for transcriptional activation. Oncogene. 1995;10:1199–1207. [PubMed] [Google Scholar]
- 52.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 53.Uhlik M, Good L, Xiao G, Harhaj E W, Zandi E, Karin M, Sun S C. NF-kappaB-inducing kinase and IkappaB kinase participate in human T-cell leukemia virus I Tax-mediated NF-kappaB activation. J Biol Chem. 1998;273:21132–21136. doi: 10.1074/jbc.273.33.21132. [DOI] [PubMed] [Google Scholar]
- 54.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 55.Wagner S, Green M R. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 56.Whitby F G, Masters E I, Kramer L, Knowlton J R, Yao Y, Wang C C, Hill C P. Structural basis for the activation of 20S proteasomes by 11S regulators. Nature. 2000;408:115–120. doi: 10.1038/35040607. [DOI] [PubMed] [Google Scholar]
- 57.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IkappaB kinase activity and NF-kappaB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida M. Multiple viral strategies of htlv-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19:475–496. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]