Abstract
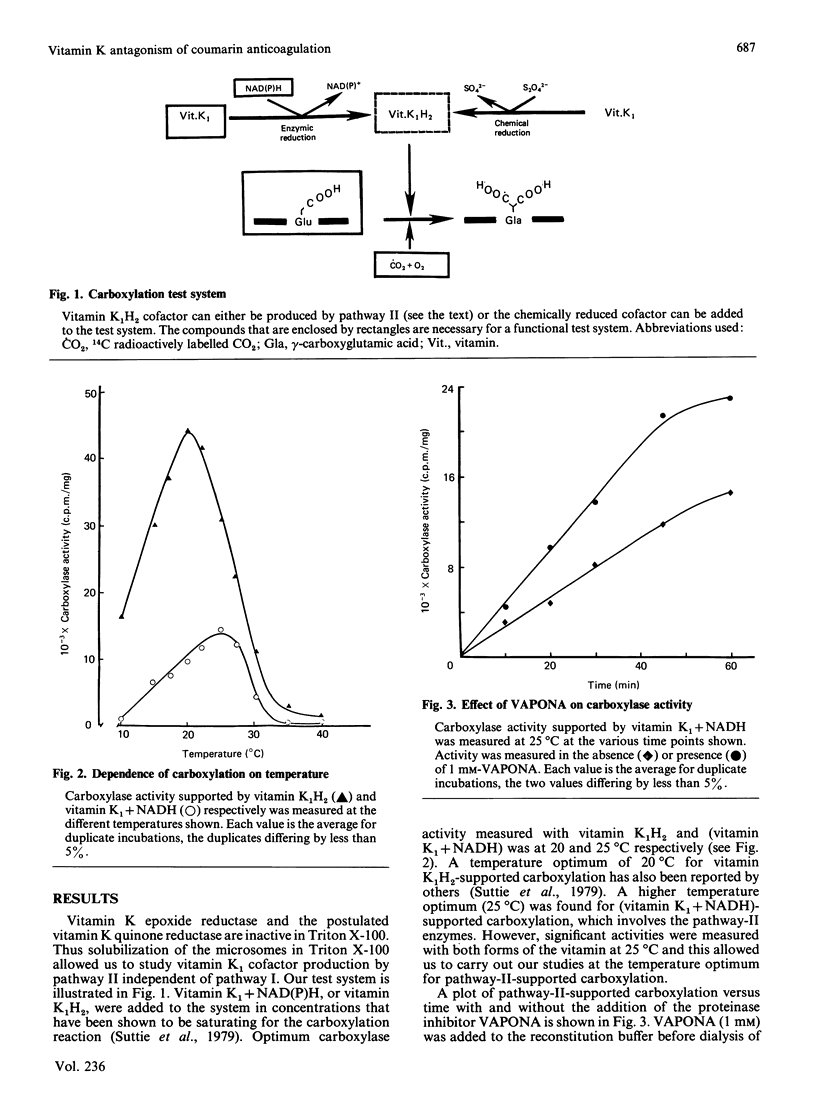
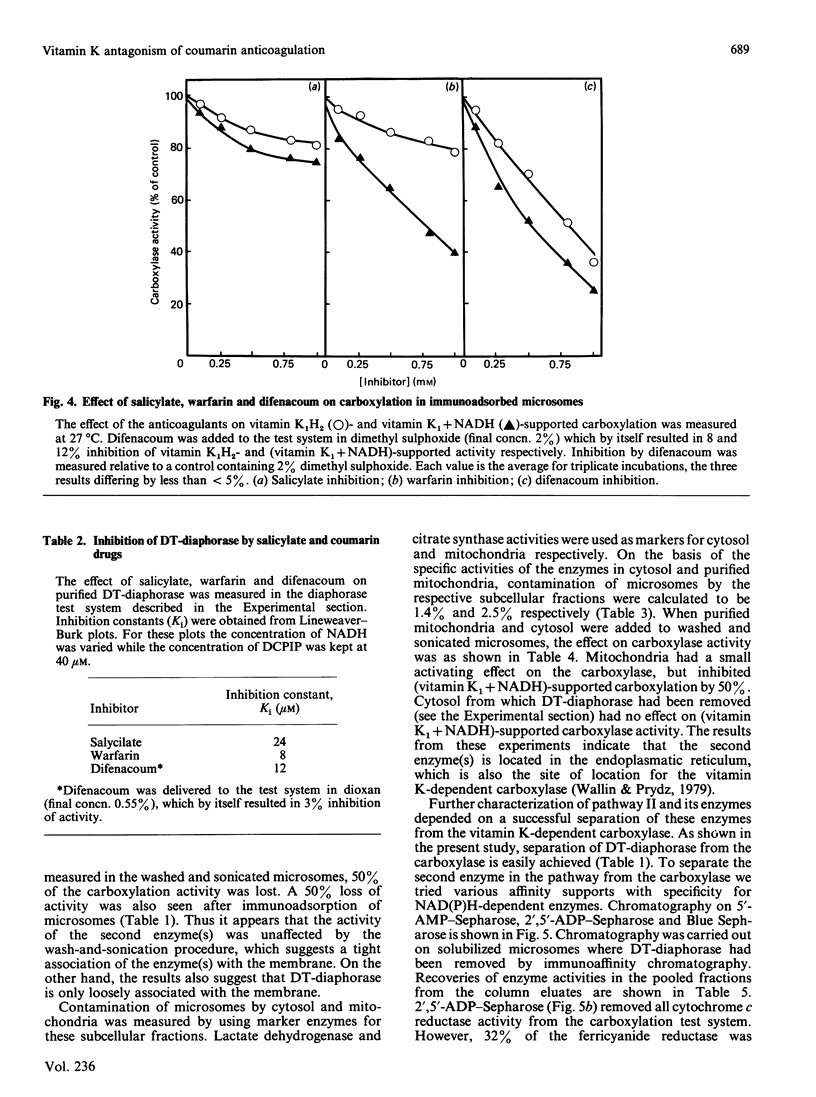
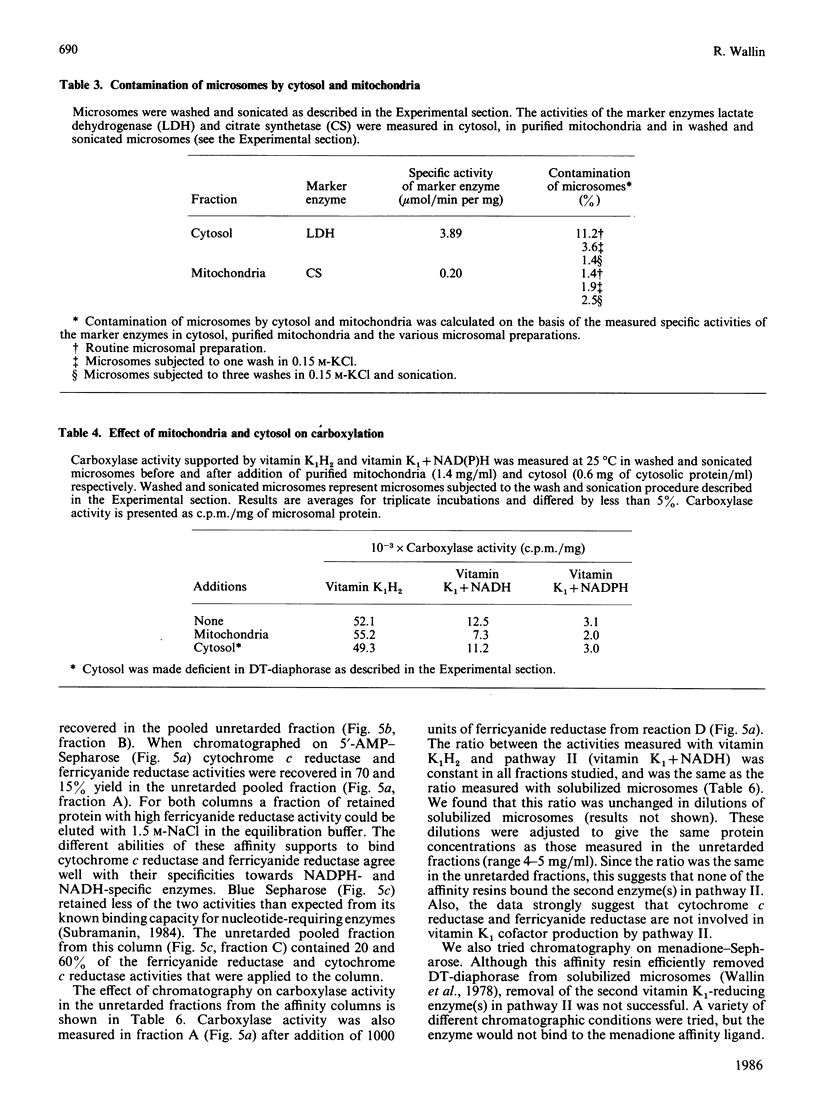
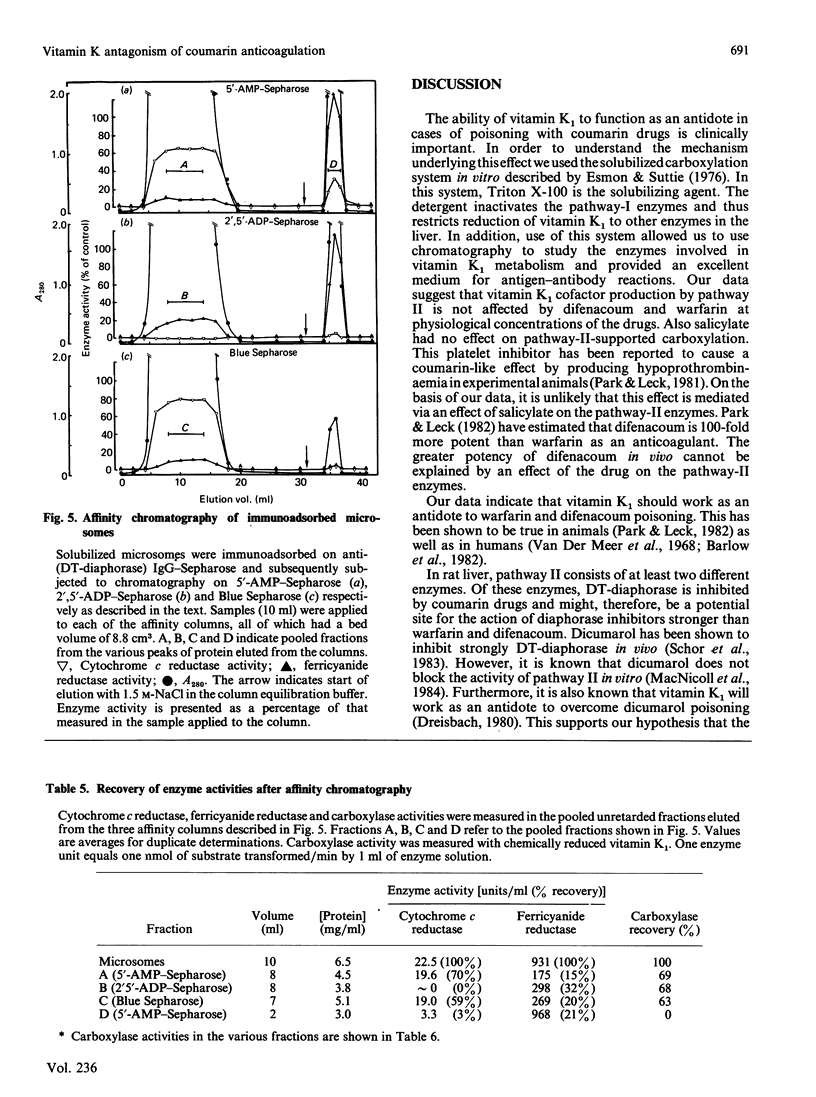
In the liver, it appears that there are two different pathways for vitamin K reduction. One pathway is irreversibly inhibited by coumarin anticoagulant drugs. The other pathway has been shown in the present study to be composed of enzymes that are not effected by physiological 'in vivo' concentrations of these drugs. This pathway appears to be responsible for the antidotal effect of vitamin K in overcoming coumarin poisoning. In rat liver the pathway has been shown to be composed of DT-diaphorase (EC.1.6.99.2) and a microsomal dehydrogenase(s). The activity of the microsomal dehydrogenase(s) was 3.6-fold higher with NADH than with NADPH present in the test system. It appears that this enzyme is the physiologically important enzyme in the pathway. In contrast with DT-diaphorase, this enzyme(s) is shown to be tightly associated with the mirosomal membrane. The enzyme(s) is not identical with either of the quinone-reducing enzymes cytochrome P-450 reductase or cytochrome-b5 reductase. Our data thus postulate the existence of an as-yet-unidentified microsomal dehydrogenase that appears to have an important function in the pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann K. A., Sullivan T. J. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27(5):281–288. doi: 10.1159/000137881. [DOI] [PubMed] [Google Scholar]
- Barlow A. M., Gay A. L., Park B. K. Difenacoum (Neosorexa) poisoning. Br Med J (Clin Res Ed) 1982 Aug 21;285(6341):541–541. doi: 10.1136/bmj.285.6341.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. G., Sadowski J. A., Matschiner J. T. Mechanism of action of warfarin. Warfarin and metabolism of vitamin K 1 . Biochemistry. 1972 May 9;11(10):1959–1961. doi: 10.1021/bi00760a034. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Hunkeler M. J., Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L., Lind C., Rase B. A study of the DT-diaphorase activity of warfarin-resistant rats. Eur J Biochem. 1972 Jan 31;25(1):198–206. doi: 10.1111/j.1432-1033.1972.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Suttie J. W. Vitamin K-dependent carboxylase. Solubilization and properties. J Biol Chem. 1976 Oct 25;251(20):6238–6243. [PubMed] [Google Scholar]
- Fasco M. J., Hildebrandt E. F., Suttie J. W. Evidence that warfarin anticoagulant action involves two distinct reductase activities. J Biol Chem. 1982 Oct 10;257(19):11210–11212. [PubMed] [Google Scholar]
- Fasco M. J., Principe L. M. R- and S-Warfarin inhibition of vitamin K and vitamin K 2,3-epoxide reductase activities in the rat. J Biol Chem. 1982 May 10;257(9):4894–4901. [PubMed] [Google Scholar]
- Fasco M. J., Principe L. M. Vitamin K1 hydroquinone formation catalyzed by DT-diaphorase. Biochem Biophys Res Commun. 1982 Jan 15;104(1):187–192. doi: 10.1016/0006-291x(82)91957-x. [DOI] [PubMed] [Google Scholar]
- Hart J. A., Haynes B. P., Park B. K. A study of factors which determine the pharmacological response to vitamin K in coumarin anticoagulated rabbits. Biochem Pharmacol. 1984 Oct 1;33(19):3013–3019. doi: 10.1016/0006-2952(84)90602-6. [DOI] [PubMed] [Google Scholar]
- Herrera E., Freinkel N. Internal standards in the estimation of acetyl-CoA in liver extracts. J Lipid Res. 1967 Sep;8(5):515–518. [PubMed] [Google Scholar]
- Hildebrandt E. F., Suttie J. W. Mechanism of coumarin action: sensitivity of vitamin K metabolizing enzymes of normal and warfarin-resistant rat liver. Biochemistry. 1982 May 11;21(10):2406–2411. doi: 10.1021/bi00539a020. [DOI] [PubMed] [Google Scholar]
- Hollander P. M., Ernster L. Studies on the reaction mechanism of DT diaphorase. Action of dead-end inhibitors and effects of phospholipids. Arch Biochem Biophys. 1975 Aug;169(2):560–567. doi: 10.1016/0003-9861(75)90200-3. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Fasco M. J. Metabolism of vitamin K and vitamin K 2,3-epoxide via interaction with a common disulfide. Biochemistry. 1984 May 8;23(10):2246–2252. doi: 10.1021/bi00305a024. [DOI] [PubMed] [Google Scholar]
- Lind C., Höjeberg B. Biospecific adsorption of hepatic DT-diaphorase on immobilized dicoumarol. II. Purification of mitochondrial and microsomal DT-diaphorase from 3-methylcholanthrene-treated rats. Arch Biochem Biophys. 1981 Mar;207(1):217–224. doi: 10.1016/0003-9861(81)90027-8. [DOI] [PubMed] [Google Scholar]
- Liptay-Reuter I., Dose K., Guenthner T., Wörner W., Oesch F. Vitamin K epoxide reductase activity in the metabolism of epoxides. Biochem Pharmacol. 1985 Aug 1;34(15):2617–2620. doi: 10.1016/0006-2952(85)90557-x. [DOI] [PubMed] [Google Scholar]
- MacNicoll A. D., Nadian A. K., Townsend M. G. Inhibition by warfarin of liver microsomal vitamin K-reductase in warfarin-resistant and susceptible rats. Biochem Pharmacol. 1984 Apr 15;33(8):1331–1336. doi: 10.1016/0006-2952(84)90188-6. [DOI] [PubMed] [Google Scholar]
- Oram J. F., Wenger J. I., Neely J. R. Regulation of long chain fatty acid activation in heart muscle. J Biol Chem. 1975 Jan 10;250(1):73–78. [PubMed] [Google Scholar]
- Park B. K., Leck J. B. A comparison of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol. 1982 Nov 15;31(22):3635–3639. doi: 10.1016/0006-2952(82)90587-1. [DOI] [PubMed] [Google Scholar]
- Park B. K., Leck J. B. On the mechanism of salicylate-induced hypothrombinaemia. J Pharm Pharmacol. 1981 Jan;33(1):25–28. doi: 10.1111/j.2042-7158.1981.tb13695.x. [DOI] [PubMed] [Google Scholar]
- SCHELLENBERG K. A., HELLERMAN L. Oxidation of reduced diphosphopyridine nucleotide. J Biol Chem. 1958 Mar;231(1):547–556. [PubMed] [Google Scholar]
- Sadowski J. A., Esmon C. T., Suttie J. W. Vitamin K-dependent carboxylase. Requirements of the rat liver microsomal enzyme system. J Biol Chem. 1976 May 10;251(9):2770–2776. [PubMed] [Google Scholar]
- Sadowski J. A., Schnoes H. K., Suttie J. W. Vitamin K epoxidase: properties and relationship to prothrombin synthesis. Biochemistry. 1977 Aug 23;16(17):3856–3863. doi: 10.1021/bi00636a022. [DOI] [PubMed] [Google Scholar]
- Schor N. A., Huddleson R. L., Kane G. M., Lee G. Effects of the administration of anticoagulants on the activity of the enzyme-reduced NAD(P)H dehydrogenase in rat livers, hepatomas and precarcinomatous rat liver lesions. Enzyme. 1983;30(4):244–251. doi: 10.1159/000469584. [DOI] [PubMed] [Google Scholar]
- Subramanian S. Dye-ligand affinity chromatography: the interaction of Cibacron Blue F3GA with proteins and enzymes. CRC Crit Rev Biochem. 1984;16(2):169–205. doi: 10.3109/10409238409102302. [DOI] [PubMed] [Google Scholar]
- Suttie J. W., Hageman J. M. Vitamin K-dependent carboxylase. Development of a peptide substrate. J Biol Chem. 1976 Sep 25;251(18):5827–5830. [PubMed] [Google Scholar]
- Suttie J. W., Lehrman S. R., Geweke L. O., Hageman J. M., Rich D. H. Vitamin K-dependent carboxylase: requirements for carboxylation of soluble peptide and substrate specificity. Biochem Biophys Res Commun. 1979 Feb 14;86(3):500–507. doi: 10.1016/0006-291x(79)91742-x. [DOI] [PubMed] [Google Scholar]
- Suttie J. W. Mechanism of action of vitamin K: synthesis of gamma-carboxyglutamic acid. CRC Crit Rev Biochem. 1980;8(2):191–223. doi: 10.3109/10409238009105469. [DOI] [PubMed] [Google Scholar]
- Talalay P., Benson A. M. Elevation of quinone reductase activity by anticarcinogenic antioxidants. Adv Enzyme Regul. 1982;20:287–300. doi: 10.1016/0065-2571(82)90021-8. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Wallin R., Gebhardt O., Prydz H. NAD(P)H dehydrogenase and its role in the vitamin K (2-methyl-3-phytyl-1,4-naphthaquinone)-dependent carboxylation reaction. Biochem J. 1978 Jan 1;169(1):95–101. doi: 10.1042/bj1690095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Little C. NAD(P)H dehydrogenase from rabbit and rat liver: purification and some properties. Int J Biochem. 1984;16(11):1099–1106. doi: 10.1016/0020-711x(84)90001-6. [DOI] [PubMed] [Google Scholar]
- Wallin R., Martin L. F. Vitamin K-dependent carboxylation and vitamin K metabolism in liver. Effects of warfarin. J Clin Invest. 1985 Nov;76(5):1879–1884. doi: 10.1172/JCI112182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Prydz H. Studies on a subcellular system for vitamin K-dependent carboxylation. Thromb Haemost. 1979 May 25;41(3):529–536. [PubMed] [Google Scholar]
- Wallin R. Some molecular properties of NAD(P)H dehydrogenase from rat liver. Biochem J. 1979 Jul 1;181(1):127–135. doi: 10.1042/bj1810127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Suttie J. W. Vitamin K-dependent carboxylase: possible artifact of analysis due to a pyridine nucleotide-dependent carboxylation. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1374–1380. doi: 10.1016/0006-291x(80)90571-9. [DOI] [PubMed] [Google Scholar]
- Whitlon D. S., Sadowski J. A., Suttie J. W. Mechanism of coumarin action: significance of vitamin K epoxide reductase inhibition. Biochemistry. 1978 Apr 18;17(8):1371–1377. doi: 10.1021/bi00601a003. [DOI] [PubMed] [Google Scholar]
- Zimmermann A., Matschiner J. T. Biochemical basis of hereditary resistance to warfarin in the rat. Biochem Pharmacol. 1974 Mar 15;23(6):1033–1040. doi: 10.1016/0006-2952(74)90002-1. [DOI] [PubMed] [Google Scholar]


