Abstract
Much effort has been made to generate human skin organ in the laboratory. Yet, the current models are limited due to the lack of many critical biological and structural features of the skin. Importantly, these in vitro models lack appendages and fail to recapitulate the whole human skin construction. Thus, engineering a human skin with the capacity to generate all components, including appendages, is a major challenge. This review intends to provide an update on the recent efforts underway to regenerate appendage‐bearing skin organs based on scaffold‐free and scaffold‐based bioengineering approaches. Although the mouse skin equivalents containing hair follicles, sebaceous glands, and sweat glands have been established in vitro, there has been limited success in humans. A combination of biofabricated matrices and cell aggregates, such as organoids, can pave the way for generating skin substitutes with human‐like biological, structural, and physical features. Accordingly, the formation of human skin organoids and reconstruction of vascularized skin equipped with immune cells prompt calls for more scientific research. The generation of appendage‐bearing skin substitutes can be applied in practice for wound healing, hair restoration, and scar treatment.
Keywords: 3D culture, bioengineering, regenerative medicine, tissue engineering, wounds
The successful bioengineered skin constructs should reflect the in vivo situation for the formation and growth of all skin layers containing blood vessels and appendages. Yet, there are major challenges ahead to regenerate functional skin. The skin organoids and bioeneering concepts hold promise for reconstructing appendage‐bearing skin organ.
1. Introduction
The skin is a highly complex organ consisting of multiple progenitor cells.[ 1 ] Apart from being an important barrier with sensory and immune functions, resorption and thermoregulation are two paramount physiological mechanisms of skin that reflect the selective penetration of chemicals as well as the stability of core body temperature.[ 2 ]
The skin contains three main layers: the epidermis, the dermis, and the hypodermis. The outermost layer, known as the epidermis, accommodates epidermal stem cells (SCs) that ensure skin homeostasis and regeneration. In contrast, the dermis, a thick layer sandwiched between the epidermis and the subcutaneous fat called the hypodermis, is replete with dermal cells. The dermal layer consists of collagen and elastin proteins that support the skin's mechanical strength and flexibility to stretch, respectively.[ 3 ] In this structure, the subcutaneous fat plays key roles in padding, insulation, and energy storage center.[ 4 ] Furthermore, the skin has sweat glands (SGs), sebaceous glands, and hair follicles (HFs), which are epidermal and dermal‐derived components.[ 5 ]
The in vitro and in vivo models have offered valuable platforms to investigate skin biology and understand the cellular and molecular mechanisms underlying its degenerative diseases.[ 6 ] Yet, the application of such skin models is limited due to the lack of many important biological and structural features of the skin. The biggest barrier in the current in vitro skin models is the lack of skin appendages, such as HFs and SGs.
This review briefly describes skin appendages and the clinical challenges in regenerating appendage‐bearing skin. Also, we summarize the most recent approaches to incorporating the appendages into skin substitutes, along with their potential and limitations.
2. Skin Appendages
Skin appendages, including SGs, sebaceous glands, and HFs, are the important components of the skin. During embryonic development, they are derived from the epidermal buds (Figure 1 ), which continue down to the dermal layer and establish these particular components.[ 7 ]
Figure 1.
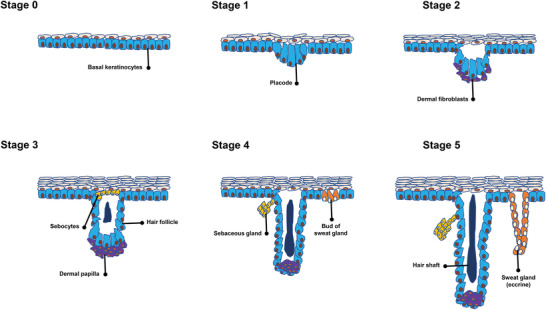
Different stages of skin appendage development. Stage 0 starts with the formation of a single layer of basal keratinocytes, which are multipotent cells. In stage 1, areas of thickening epithelium appear— known as hair placode that will end up hair follicles (HFs). The main trigger behind the formation of placodes is frequent interactions between mesenchymal cells and the epithelium. Stage 2 is associated with the extension of the hair placode downward toward the dermis. At the same time, dermal fibroblasts (Fbs) attract and create an aggregation below the placode condense as a result of an epithelial signal from the placode. During stage 3, a dermal message from the dermal aggregation induces the proliferation of the placode cells, which, in turn, surround the dermal aggregation that ultimately develops into the dermal papilla (DP). In stage 4, subsequent growth and differentiation of the epithelial cells lead to the development of the mature hair shaft. Also, the DP becomes more compact and is fully engulfed by the growing HF cells. While the sebaceous gland is evident alongside the HF, the eccrine gland begins as a basal layer bud. In stage 5, the hair shaft tip invades the hair canal. Furthermore, the swear gland lengthens and arranges in a coil.
2.1. Sweat Glands (SGs)
SGs contribute to sweat secretion, waste excretion, body‐temperature maintenance, and suppression of bacterial growth via producing lactate.[ 8 ] There are two types of SGs, including eccrine SGs and apocrine SGs. The former glands control the body temperature and sweating.[ 9 ] SGs are composed of a coiled secretory portion and a long duct, coursing through the dermis to the epidermis (Figure 2A). Thermal or emotional stimuli activate SGs and induce sweat excretion onto the skin surface.[ 10 ] It has been shown that skin repair in patients with extensive deep burn wounds is associated with the hyperplastic scarring with no SG regeneration, which has serious negative effects on patients' quality of life.[ 11 ] SGs lack a self‐renewal capacity; thus, SG regeneration is of high interest to many tissue engineering and regenerative medicine researchers.[ 12 ]
Figure 2.
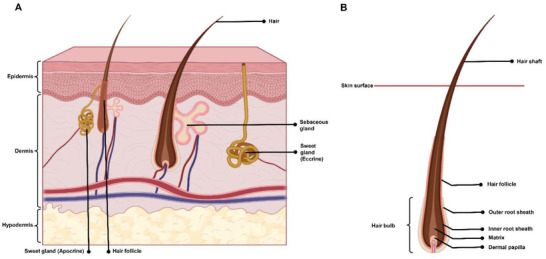
Schematic representation of A) the human skin layers with their appendages as well as B) the hair follicle structure (Created with Biorender.com)
2.2. Sebaceous Glands
Sebaceous glands are in the mid dermis, and are responsible for secreting sebum (i.e., a viscous mixture of lipids) into a duct in the HF junctional zone. The waxy sebum serves to avert water loss from the interfollicular epithelium and contributes to thermoregulation.[ 13 ] The outer layer of the sebaceous glands consists of proliferative cells, while the inner compartment includes sebocytes, specialized keratinocytes (Kcs) responsible for sebum production.[ 13 , 14 ] The sebaceous gland's activity is controlled by a variety of growth factors and hormones.[ 15 ]
2.3. Hair Follicles (HFs)
HFs are responsible for thermoregulation, dispersion of sebum and sweat, and physical protection.[ 16 ] There are two main types of human hair: pigmented terminal hairs on the scalp, which are thicker and longer, and vellus hairs, which are soft, fine, and short.[ 17 ] In the skin, the pilosebaceous unit constitutes HF, the arrector pili muscle, and the sebaceous gland.[ 18 ] Its structure, from deep to superficial, includes the dermal papilla (DP), followed by the basement matrix that acts as the growth front for HF. HFs are self‐renewing structures located in the dermal layer and composed of hair papillae, hair matrix, root sheath, and hair bulges. The papilla is a large structure surrounded by the hair matrix (Figure 2B). It is found at the base and contains the connective tissue and a capillary loop. The root sheath consists of an external and internal root sheath. Lastly, the bulge region, activated upon the follicle growth, is the regenerative center of the follicle.[ 19 ] It resides in the outer root sheath (ORS) at the insertion point of the arrector pili muscle.[ 20 ] HFs reconstruct themselves through a cycle composed of three phases, including anagen (growing phase), catagen (regression phase), and telogen (resting phase), which indicates the self‐renewing capacity of progenitor cells in HFs.[ 21 ]
HFs have three zones: ORS, inner root sheath, and hair shaft (Figure 2B). All HF zones are formed from terminally differentiating Kcs that comprise the matrix cells. The hair shaft is composed of dividing cells in the bulge, which migrate into the base of the shaft—now known as the matrix cells—before stratification into HF cells.[ 7 , 22 ] Melanin, with two distinct classes of pheomelanin (red‐yellow) and eumelanin (brown‐black), is responsible for the hair shaft pigmentation. This pigment is produced from the HF pigmentary units close to the DP. The main cell type in the pigmentary unit is melanocytes, which function as a key local regulator of other skin cell types, including Kcs, lymphocytes, Fbs, mast cells, and endothelial cells.[ 22 ]
2.4. Clinical Challenges to Regeneration of Skin Appendages
Scarring is the endpoint of most wound healing in adult humans. In cutaneous scar tissues, there are no functional appendages, thus the repaired tissue is susceptible to trauma, ultraviolet light exposure, temperature variations, and dry conditions.[ 23 ] As scar formation continues after skin injuries, the surrounding niches for the self‐renewal of the endogenous SCs change in terms of cell number and type and extracellular matrix (ECM) metabolism. Hence, the speed of reparative healing with scar tissues exceeds that of skin appendage regeneration.[ 23d ] The lack of these specialized structures in the repaired skin is problematic and may affect patients' quality of life. Therefore, the partial repair of skin in the scar tissues carries functional, physical, and emotional risks for human and affect their social activities.[ 20 ]
3. Skin Appendages Regeneration Using Stem/Progenitor Cells
The skin substitutes, containing the cultured epidermis and dermal cells, have been widely used to generate skin, but these models often result in the formation of a thin skin‐like structure without any appendages,[ 24 ] thereby being unable to restore the skin organ structure and function. Thus, engineering a human skin with the capacity to generate all skin components, including the appendages, has been a significant challenge in bioengineering and biomedical research fields. Here, we report different stem/progenitor cell‐based strategies to regenerate SGs, sebaceous glands, and HFs.
3.1. Sweat Glands (SGs)
Two primary sources of SCs participate in the generation of skin appendages following an injury: those living in the wound sites[ 25 ] and SCs that migrate to the wound site from other anatomical locations, such as mesenchymal progenitors from the bone marrow.[ 26 ]
To date, limited research has been conducted concerning the regenerative potential of SGs, especially in humans. Mouse studies have shown that adult SGs contain unipotent progenitors, contributing to SG reconstruction upon wounding.[ 27 ] The basal myoepithelial and suprabasal luminal progenitors, which constitute the glandular structure of SGs, have the capacity to regenerate their own lineage. Glandular cells make no contribution to the duct repair, which is retained by its own basal unipotent progenitors. Ductal cells, unlike glandular cells, can reconstruct ductal openings following skin injury. Additionally, they are involved in the restoration of the glabrous epidermis around the SG opening.[ 27a] Furthermore, there are certain progenitors in the acral epithelium of mice that take part in the long‐term retention of SGs, ducts, and inter adnexal epidermis as well as the regeneration of these components after injury.[ 28 ] Further investigations are required to elucidate if human skin also contains similar progenitors.
Mesenchymal progenitors have been used for restoring SG cells. Li et al. cocultured adult human mesenchymal stem cells (MSCs) with heat‐shocked human SG cells in vitro, and then intravenously injected them into full‐thickness skin wounds in rats.[ 29 ] The coculture conditions induced a subset of MSCs underwent differentiation into SG cells, which was strengthened by epidermal growth factor (EGF) and the injured microenvironment, yet was weakened by PD98059, an inhibitor of the mitogen‐activated protein kinase/extracellular signal‐regulated kinases pathway. In the full‐thickness wounds, labeled MSCs were found in HFs, sebaceous glands, blood vessels, and the dermis. Further analyses confirmed the incorporated cells in HFs and sebaceous glands are positive for pan‐cytokeratin, a protein expressed in the ductal and secretory cells of the normal human SGs. After the wound healing, while a proportion of the injected MSCs were kept in the dermis, the rest migrated into the bone marrow. Accordingly, MSCs can harbor at particular sites to support the regeneration of skin appendages and then return to the bone marrow.[ 29 ] Sun et al. employed bone marrow‐derived MSCs and indicated that CRISPR‐mediated activation by targeting the promoter of ectodysplasin can be used to differentiate the MSCs into SG‐like cells.[ 30 ] Xu et al. showed that the culture of umbilical cord‐derived MSCs with the mixing medium (90% of basic SG medium and 10% of conditioned heat‐shock SG medium)[ 31 ] and recombinant human keratinocyte growth factor[ 32 ] could induce the differentiation of MSCs into SG‐like cells. Other relevant studies are listed in Table 1 .
Table 1.
Experimental models for regeneration of sebaceous glands and sweat glands. Abbreviations: fibroblast (Fb); green fluorescent protein (GFP); hair follicles (HFs); keratinocytes (Kcs); mesenchymal stem cells (MSCs); not specified (NS); severe combined immunodeficient (SCID); stem cells (SCs); sweat glands (SGs); tuberous sclerosis complex (TSC)
| Skin appendage | Type and source of cells/tissues | Passage | Culture conditions | Study model | Refs. |
|---|---|---|---|---|---|
| Sweat glands | Autologous SG‐derived progenitors | NS | NS | In vivo; lineage tracing with RosaLacZ or RosaYFP reporter mice | [27a] |
| Adult human bone marrow‐derived MSCs | 3 | Monolayers | In vitro; direct coculture of human SCs with heat‐shocked confluence human SG cells | [29] | |
| SG germ cells | NS | NS | In vivo; transgenic expression of the mouse EDA‐A1 isoform in tabby (EDA‐less) males | [33] | |
| Adult human bone marrow‐derived MSCs | 2–3 | Cocultured with human SG cells |
In vivo; implanting cocultured MSCs in athymic BALB/c nude mice Clinical trial; transplantation of transdifferentiated MSCs |
[34] | |
| Human umbilical cord‐derived MSCs | 3–5 |
Basic SG medium‐plus conditioned heat‐shock SG medium (9:1) Keratinocyte growth factor |
In vitro; culturing human SCs in the media supplemented by the growth factor In vivo; transplanting SG cells differentiated from human SCs into SCID mouse burn model |
[32] | |
| Human bone marrow‐derived MSCs transfected with ectodysplasin | NS | NS | In vivo; transplanting human SCs into the injured areas of burn animal models | [35] | |
| Sebaceous glands | Bulge cells in adolescent hr mice | NS | NS | In vivo; treating hr mice with and without topical dioxin | [36] |
| Adult bulge SCs | NS | Dulbecco's Vogt modified Eagle | In vivo; implanting fragments of bulges from vibrissal follicles of adult mice onto the back of mouse embryos | [19g] | |
| EGFP‐high/α6‐integrin+ cells from Krt1‐15‐EGFP; ROSA26 skin mice | NS | Cell suspension | In vivo; combining the bulge SCs with epithelial and dermal cells and injected subcutaneously or placed in tracheas and then implanted subcutaneously into CB‐17 Icr‐scid/scid mice | [37] | |
| Bulge cells from adult K14‐GFP‐actin mice | NS | Cell suspension | In vivo; combining the bulge cells with epithelial and dermal cells and implanted onto the back of nude mice | [38] | |
| Epidermal cells from Blimp1GFP mice (Blimp1+ sebaceous gland progenitor cells) | NS | Cell suspension | In vivo; combining the bulge cells with epithelial and dermal cells and implanted onto the back of nude mice | [39] | |
| Epidermal cells from transgenic mice (Lrig1+ SCs) | 1 & 5 | Cell suspension | In vivo; combining the SCs with epithelial and dermal cells and implanted onto the back of nude mice | [40] | |
| Epidermal cells from intercrossed mice (Lrg6+ SCs) at the isthmus | NS | NS | In vivo; intercrossing Lgr6‐EGFP‐Ires‐CreERT2 to the Cre reporter R26R‐LacZ mice | [41] | |
| Adult bulge SCs from transgenic mice (Sox9+ SCs) in the upper ORS | NS | NS | In vivo; crossing Sox9‐IRES‐Cre mice to ROSA26Flox‐Stop‐Flox‐βgeo (R26R) mice | [42] | |
| HF progenitor cells from mouse tail epidermis (Lrg6+ SCs) | NS | NS | In vivo; C57Bl/6 mice between E15.5 and P2 were studied using immunostaining | [43] | |
| HF SCs from human facial skin (K15+ cells) | NS | NS | In vivo; grafting human facial skin specimens onto SCID mice | [44] | |
| Human neonatal foreskin Kcs | 3 | TSC2‐null Fb‐like cells incorporated into collagen and overlaid with Kcs | In vivo; grafting composites onto immunodeficient mice | [45] | |
| GFP‐positive bone marrow cells from adult mice | NS | Cell suspension | In vivo; transplanting in a mixture of embryonic mouse epidermal and dermal cells on the backs of nude mice | [46] | |
| Mouse bone marrow‐derived allogeneic mesenchymal stromal cells | 1 | Cell suspension | In vivo; injecting intradermally in the periphery of the excisional wound in NOD mice | [47] | |
| Porcine bone marrow‐derived MSCs | NS | NS | In vivo; engrafting to porcine skin | [48] | |
| MSCs from Wharton's Jelly of the human umbilical cord | NS | Cell suspension | In vivo; (co)transplanting MSCs and/or skin microparticle (autologous) in C57BL/6 mice with a degree III deep burning wound | [49] |
3.2. Sebaceous Glands
Sebaceous glands are essential epidermal appendages and, together with HFs, form the pilosebaceous unit. Many studies have explored the regenerative potential of sebaceous glands, but in rodent models (Table 1). Sebaceous glands contain unipotent progenitors that exclusively contribute to the homeostasis of the sebocyte's pool.[ 50 ] Moreover, as opposed to the SCs present in the DP and dermal sheath, the sebaceous gland's unipotent progenitors exhibit a weaker differentiation potential.[ 51 ] Recently, Wang et al. reported that the transplantation of epidermal SCs from adult human foreskin combined with skin‐derived precursors into the wounds led to the formation of functional sebaceous glands as well as HFs.[ 52 ]
Given the proliferative and multidirectional differentiation capabilities of MSCs, some studies investigated the contribution of MSCs to the regeneration of sebaceous glands in different animal models (Table 1). Fang et al. used porcine skin,[ 48 ] which is a suitable animal model due to the similarity between pig and human skin. Their results demonstrated that MSCs could differentiate into sebaceous duct cells.[ 48 ] In another in vivo study, MSCs from Wharton's Jelly of the human umbilical cord were used and showed abilities to regenerate sebaceous glands.[ 49 ]
3.3. Hair Follicles (HFs)
Historically, a number of significant milestones have driven in vitro, in vivo, and clinical research in the field of HF regeneration (Figure 3 ). The current strategy for the in vitro neogenesis of HF is the combination of inductive mesenchymal and receptive epithelial components from the healthy skin. Such constructs recapitulate the induction phase of fetal skin at 15 weeks of pregnancy.[ 53 ] The replacement of the diseased DP with the DP from healthy donors results in the formation of healthy‐like HFs. Initial investigations by Reynolds et al.[ 54 ] indicated that the HF end bulb could elicit HFs regrowth in the allogeneic transplants. Given the low immunogenicity of allogeneic HFs, they could be used to regenerate HFs individually or alongside long‐term immunosuppression.[ 55 ] Even though the transplantation of the allogeneic microdissected DP and dermal sheath contributes to HF reconstruction, the ideal approach is to use autologous cell sources from healthy individuals to avoid immunosuppressant regimens.
Figure 3.
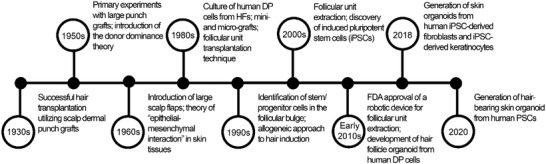
The timeline of the main basic, translational, and clinical advances for hair follicle (HF) regeneration. Abbreviations: dermal papilla (DP); the United States food and drug administration (FDA); induced pluripotent stem cells (iPSC); pluripotent stem cells (PSCs).
To date, only a few studies have used human DP cells for the in vivo regeneration of HF in humans due to the ethical constraints. One report has shown that DP cells from human HFs could survive and develop HFs after transplantation,[ 56 ] and confirmed the limited regenerative capacity of DP from adult individuals ex vivo.
The rodent transplantation models have often been used to investigate the reconstruction of skin appendages. The rodent model studies showed that DP cells could generate HFs following implantation into the recipient site.[ 57 ] DP cells, unlike dermal Fbs, can induce the differentiation of Kcs into a follicular phenotype. Further evidence highlighted the necessity of the DP for HF regeneration in rodents.[ 58 ] An in vivo study revealed the induction of human HFs generation in the skin of nude mice where human DP cells are co‐transplanted with human epidermal cells,[ 59 ] suggesting the critical roles of epithelial‐mesenchymal interactions in the de novo generation of HFs.
To date, great efforts have been made to expand human DP cells in vitro. Nevertheless, the in vitro expansion of DP cells is associated with rapid loss of their hair inductive capacity as well as considerable changes in their gene expression profile.[ 60 ] Unlike dermal Fbs, DP cells can form spheroids during in vitro culture. DP spheroids exhibit the in vivo DP transcription profile and upregulate the expression of DP signature genes that are missing during 2D expansion, such as Actin Alpha 2 (ACTA2), Alkaline Phosphatase (ALPL), and Versican (VCAN).[ 61 ] Thus, multiple 3D models have been developed to expand DP and study HF regeneration. Although 3D systems enable the close interaction between epithelial and DP cells and facilitate spatial organizations,[ 62 ] epithelial cells often produce a cyst‐like spheroidal structure without any follicular‐type differentiation. Accordingly, the functionality of these 3D platforms is limited.
The next generation of 3D skin models aimed to achieve a skin organ containing skin appendages. In one of these methods, DP spheroids were developed on top of Matrigel and then directly cocultured with Kcs.[ 63 ] Another example concerned coating DP cell aggregates with basement membrane molecules, including collagen IV, laminin, and fibronectin, as well as their culture with Kcs and melanocytes.[ 64 ] The main progress by these strategies involves the formation of microfollicles with a suitable topological cellular organization, but these strategies are complicated and often result in unpigmented fiber‐like structures.
The generation of skin appendages can also be achieved by cellular reprogramming, which mainly involves differentiating pluripotent stem cells (PSCs) into adult cells using certain factors. Induced pluripotent stem cells (iPSCs) are reprogrammed by introducing exogenous pluripotency genes into somatic cells. These cells show self‐renewal and differentiation capacities similar to embryonic SCs. In one study, the iPSC‐derived LNGFR+Thy1+ subpopulations were treated with retinoic acid (RA) and differentiated into DP cells.[ 60 ] The DP induction could also be achieved by treating a mixture of iPSC ectodermal precursor cells with a Kc culture medium containing RA and bone morphogenetic protein (BMP).[ 65 ] The HFs could represent suitable sources for producing PSCs, implying that the genetics and microenvironment of HFs enable higher and more efficient re‐differentiation of HFs.[ 66 ] The iPSCs were also established by lentiviral transfection of human DP cells with Oct4, Sox2, Klf4, and c‐Myc. Importantly, culturing conditions like low oxygen content and valproic acid supplementation markedly increased the reprogramming efficacy. Therefore, the DP cells can be used as an alternative source for the generation of iPSCs with a higher reprogramming efficiency and desired induction potentials into the ecto‐ (neuronal differentiation), meso‐ (osteogenic differentiation), and entodermal (hepatic differentiation) directions.[ 67 ] Direct cell reprogramming, also called transdifferentiation, is another approach of producing a specialized cell type via direct conversion and bypassing the SC stage. Zhao et al., reported that human dermal Fbs could be chemically converted into DP cells by applying a combination of fibroblast growth factor (FGF) 2, platelet‐derived growth factor, and 6‐bromoindirubin‐3′‐oxime (BIO).[ 68 ]
Together, the skin appendage‐derived SCs are a rare cell population, and it is challenging to extract, purify, and expand them. Also, these cells might undergo in vitro senescence after isolation.[ 69 ] On the other hand, the reprogramming approaches to the generation of skin progenitors often results in low efficacy, with newly formed appendages showing differences from normal ones.[ 35 ] Other stem/progenitor cells, which have been applied for wound healing could only contribute to the improvement of wound closure with limited enhancement in cosmetic outcomes.[ 70 ] In fact, there is no available data supporting functional skin appendage reconstruction upon therapies based on stem/progenitor cells. Given these, it is speculated that the development of 3D culture systems using bioengineering strategies that mimic the in vivo environment and provide spatiotemporal control over cells can overcome the challenges of skin regeneration containing functional appendages.
4. Cellular Aggregates and Organoids for the Engineering of Skin Appendages
Considering the lack of complexity in the previously mentioned cell culture systems in mirroring skin tissue configuration, the skin organoid models have been developed. Skin organoid refers to a 3D multicellular in vitro tissue construct with the capacity of recapitulating the in vivo skin organ. In general, organoids recapitulate early organ development and have a structural and functional resemblance to the corresponding organ. They can be used to study human developmental processes and tissue homeostasis and to understand the responses to the external stimuli or stress signals in an in vitro system. The term organoid is widely accepted for the description of the constructs obtained from SCs, i.e., embryonic or iPSCs. HF organoids, for example, are established from a mixture of dermal and epidermal components, skin SCs, and PSCs.[ 71 ] In the following sections, the most recent studies are presented considering both rodent and human in vitro models.
4.1. Rodent Models
In 3D cell cultures, the key contributor is the architectural organization of cells in a compact structure that allows a dynamic equilibrium at gene and protein levels, and eventually mimicry of in vivo tissues. Building around the physico‐genetic mechanisms, Lei et al. demonstrated that epidermal and dermal progenitors at a high density could form organoids and undergo tissue‐like organization.[ 6d ] They developed skin aggregates from both adult and newborn skin progenitors. The skin progenitors obtained from newborn mice were able to form hair primordia‐bearing organoids via a stepwise self‐organizing process and generate HFs with normal architecture when transplanted into nude mice. Within 10 d, the dissociated cells developed toward a planar layer of presumptive skin with hair primordia through morphological phase transitions. On the contrary, adult skin progenitors could only form small aggregates, and the development process stalled in vitro. Comparative analyses indicated that the suppression of epidermal differentiation in the adult cells played a key role. The molecular pathways, along with physical cues during organoid morphogenesis and environmental reprogramming, contribute to the restoration of the self‐organizing processes.[ 6d ] To date, the most complete in vitro reconstruction of HF and sebaceous gland‐containing skin involves the use of PSCs. Lee et al. reported the derivation of skin organoids with both epidermal and dermal layers from mouse PSCs.[ 72 ] Initial exposure to a transforming growth factor (TGF)‐β inhibitor and recombinant BMP4, along with subsequent treatment with FGF2 and a BMP inhibitor, produced organoids with a cyst conformation that led to the spontaneous formation of HFs. Yet, these new mouse HFs underwent a catagen‐like degenerative process upon long‐term culture on day 28. Besides HFs, the mouse skin organoids could produce sebaceous glands. Hence, multiple germ layers can be derived from a single SC aggregate with no use of embryonic tissue or undefined media to regenerate hair‐bearing skin organoids.[ 72 ] Interestingly, a 3D integumentary organ system (IOS) created via the self‐assembly of mesenchymal and epidermal SCs from mouse iPSCs led to the regeneration of fully functional HF organoids. The clustering‐dependent embryoid body transplantation method was applied to develop this 3D system. Following implantation into nude mice, the resultant HF organoids could establish proper connections to the surrounding host tissues, like the epidermis, arrector pili muscles, and nerve fibers, with no sign of tumorigenesis at the transplantation sites as well as indicating appropriate hair eruption and repeated hair cycles, through the rearrangement of follicular SCs and their microenvironments. Additionally, the iPSCs‐derived bioengineered IOS models produced other skin appendages, including sebaceous glands, suggesting their considerable potential for skin organ reconstruction therapies.[ 73 ] Together, these studies have confirmed the ability of rodent‐derived progenitor/SCs to develop skin organoids containing HFs and sebaceous glands.
4.2. Human Studies
The differences in skin regeneration and wound healing processes between rodents and humans are evident, and mouse skin models are often poor predictors of human trial outcomes. Thus, it is so important to develop human skin appendage models.
Lindner et al. created human microfollicles in vitro using DP Fbs under low adherent cell culture conditions.[ 64 ] These cells formed DP condensates and accelerated the production of ECM upon the addition of recombinant human ECM proteins (i.e., collagen, fibronectin, and laminin). Once ECM was created around the condensate, well‐defined organoid structures appeared, which were similar to a human vellus HF in terms of composition, properties, and protein expression. The cocultivation of this structure with ORS Kcs and HF melanocytes led to the development of unpigmented hair‐like fibers. The well‐engineered microfollicles approach required only a small quantity of the autologous ex vivo expanded HF‐forming cells before re‐implantation.[ 64 ] Applying a 3D droplet organoid culture, Weber et al. could effectively produce hair peg‐like structures in vitro from fresh fetal scalp dermal progenitor cells associated with cultured neonatal foreskin Kcs through self‐organization.[ 74 ] The highest number of hair peg‐like structures was obtained when an epidermal to dermal cell ratio was 2:3.[ 74 ] Yet, dermal cells were not expanded in culture. Additionally, it is difficult to use this method in case of large‐scale organoids for both research and clinical applications.
Su et al. developed a large number of HF organoids based on the pre‐aggregation of culture‐expanded dermal and epidermal cells in vitro.[ 75 ] Human fetal scalp‐derived dermal progenitors were mixed with human foreskin‐derived epidermal SCs at a 2:1 ratio, resulting in the activation of the Wnt pathway and the formation of pear‐shaped structures, known as type I aggregates, characterized by early HF markers. This platform could support the rapid formation of many HF organoids (type I aggregates) and facilitate studying the crosstalk between epidermal and mesenchymal cells, evaluating the technogenic potential of hair SCs, and screening biomolecules at a large scale for HF regeneration.[ 75 ] In the literature, it remains a formidable challenge to efficiently regenerate HFs in vivo and in vitro from adult dermal cells.[ 75 ] Also, there is almost no available study addressing the scaffold‐free SG and sebaceous gland organoids from human SCs.
5. Biofabrication‐Based Strategies to Establish Skin Appendages
Despite the fact that scaffold‐free 3D platforms outperform conventional 2D cultures, these models cannot mimic biological (multiple cell types), physical (the ECM), and chemical (signaling molecules) features of the skin tissue and its appendages. To overcome these limitations, biofabricated matrices provide proper microenvironmental factors and contribute to the organization and formation of tissues. Hitherto, considerable attempts have been made to establish a niche for stem/progenitor cells. In this regard, the ECM and biochemical factors have the ability to serve as biophysical and biochemical cues. Irrespective of the culturing units, the 3D cultures used for skin appendage regeneration mainly depend on using Matrigel[ 52 , 63 , 76 ] and collagen[ 59 , 77 ] as their supporting matrices (Table 2 ).
Table 2.
A summary of bioengineered constructs used for skin appendage regeneration. Abbreviations: 6‐bromoindirubin‐3′‐oxime (BIO); basic fibroblast growth factor (bFGF); bone morphogenetic proteins (BMP); dermal papilla (DP); epidermal growth factor (EGF); extracellular matrix (ECM); fibroblasts (Fbs); green fluorescent protein (GFP); hair follicles (HFs); human pluripotent stem cells (hPSCs); human umbilical vein endothelial cells (HUVECs); keratinocytes (Kcs); mesenchymal stem cells (MSCs); not reported (NR); poly(ethylene glycol) (PEG); poly(lactic‐co‐glycolic acid) (PLGA); Rho‐associated coiled‐coil kinase (ROCK); severe combined immunodeficient (SCID); skin‐derived precursors (SKPs); sweat glands (SGs); 3D; transforming growth factor (TGF); vascular endothelial growth factor (VEGF)
| Type and source of cells/tissues | Culturing units | Matrix used for in vitro/ vivo models | Morphogens/ biochemical factors | Study model | Regenerative outcome | Refs. |
|---|---|---|---|---|---|---|
| Human hair DP cells | 3D culture | PLGA‐PEG‐PLGA hydrogels | Icariin |
In vitro; culture of the DP cells with the hydrogels In vivo; direct injection of the hydrogels onto the surface of skin full‐thickness wounds in the back of male C57BL/6 mice |
HFs | [78] |
|
DP cells from human scalps Kcs from human middle follicle outer root sheath |
Cell aggregates from a coculture system | Follicular skin composites from ICR mice, including the dermis and epidermis, with cell aggregates being between them | DP activation culture medium containing recombinant BMP2, bFGF, and BIO | In vivo; subcutaneous engraftment of footpad skin composites into BALB/cnu/nu nude mice | Hair | [60] |
| Human DP cells and Kcs | Cell aggregates | Interfacial polyelectrolyte complexation fiber hydrogel | NR | In vivo; implantation of the cell‐laden scaffolds into SCID mice | HFs | [79] |
|
DP cells from human scalps Neonatal dermal Kcs from human foreskin |
Cell spheroids with and without cellular reprogramming of Lef‐1 and Fli‐1 | Human skin constructs including dermal compartment (fb‐populated collagen type I matrix) and DP cells and Kcs on its top | NR |
In vivo; engraftment of human skin constructs onto nude mice In vivo; engraftment of human skin constructs encapsulating GFP‐tagged HUVECs in the dermal compartment to nude mice |
HFs Vascular network |
[59] |
|
DP cells from human scalps Hair germinal matrix cells |
3D spheroids | Matrigel | NR | In vitro; seeding germinal matrix cells and DP spheroids onto Matrigel‐coated plates | HFs | [63] |
|
Epidermal cells (epidermal SCs) from adult human foreskin Dermal cells (SKPs) from adult human foreskin |
SKP spheroids | Matrigel | BMP4 for SKPs treatment | In vivo; implantation of Matrigel containing epidermal and culture‐expanded dermal cells into BALB/c nu/nu mice | HFs | [52] |
| DP cells from human adult scalps | Neopapillae spheroids from expanded and self‐aggregating DP cells | Reconstructed human skin including the epidermis grown on a dermal fb‐populated hydrogel | NR | In vitro; culturing reconstructed human skin with neopapillae spheroids at the air‐liquid interface | HFs | [80] |
| hPSC line | 3D organoids | Matrigel | BMP4, TGF‐β inhibitor; bFGF, and BMP inhibitor | In vivo; grafting pigmented or albino hair‐bearing skin organoids after maturation onto immunodeficient nude mice |
HFs Sebaceous glands Stratified epidermis Fat‐rich dermis |
[76d] |
| Mesenchymal and epithelial cells from mouse embryos | HF‐germ‐like aggregates from 3D coculture | HF‐germ‐like grafts containing printed collagen microgel beads | NR |
In vivo; injection of the grafts into a pocket on the dorsal skin of ICR‐nude mice Transplantation of the grafts into a shallow stan wound on the skin surface of ICR‐nude mice |
HFs | [81] |
| DP cells from vibrissa pads of C57BL/6 mice | 3D spheroid culture | Keratin hydrogel | Exosomes or secretome (high level of miR‐218‐5p) from DP spheroids | In vivo; subcutaneous injection of cells‐hydrogel into C57BL/6 mice | HFs | [82] |
| SKPs from neonatal dorsal skin from C57BL/6 or C57BL/GFP mice & mouse epidermal cells | 3D coculture | Self‐assembling peptide hydrogel (RADA‐PRG) or Matrigel | NR | In vivo; implantation of cells‐hydrogel into BALB/c nu/nu mice | HFs | [76f] |
| DP cells from Wistar rat vibrissa & newborn C57BL/6 epidermal cells | Spheroidal microtissues | Poly(ethylene‐co‐vinyl alcohol) membranes | NR | In vivo; injection of microtissues‐epidermal cells into nude mice |
HFs Sebaceous glands |
[83] |
| DP and epidermal cells from neonatal mice | 3D spheres (spheroids) and single cells | Hydrogel (Extracel) containing crosslinked gelatin and hyaluronic acid | NR | In vivo; transplantation of epidermal cells, freshly isolated DP cells, and cells released from hydrogels in nude mice | HFs | [84] |
| Epithelial and DP cells from adult mouse vibrissa and embryonic skin pelage | 3D organ‐germ culture | Collagen gel | NR | In vivo; transplantation of bioengineered murine HF germ to mice |
HFs Sebaceous glands |
[77d] |
|
DP cells from Wistar rat vibrissae and human scalp Kcs from newborn C57BL/6 mice |
Spheroids | Poly(vinyl alcohol) substratum | NR | In vivo; injection of human and rat DP spheroids and Kcs into nude mice |
Hair HFs |
[85] |
|
DP cells from the vibrissae of neonatal C57BL/6 mice Epidermal Kcs from C57BL/6 mouse skin |
3D preculture system | Collagen‐chitosan scaffold | Supernatant of Wnt1a‐expressing bone marrow MSCs | In vivo; transplantation of the scaffold cells in nude mice | HFs | [77a] |
|
DP cells from C57BL/6J mice vibrissae Epidermal cells from newborn C57BL/6 mice |
Spheroids | Gelatin/alginate nanogel | NR | In vivo; implantation of DP spheroids with epidermal cells onto nude mice | HFs | [86] |
| DP cells from human scalp epidermal cells from newborn C57BL/6 | Spheroids | Decellularized human placenta ECM hydrogel | NR | In vivo; subcutaneous injection of DP spheroids mixed with epidermal cells into nude mice | HFs | [87] |
| DP and epidermal cells from C57BL/6 mouse scalp | Multicellular spheroids | Chitosan/polyvinyl alcohol nanofiber sponge | NR | In vivo; injection of DP microtissues and epidermal cells into nude mice | HFs | [88] |
|
Epidermal (stem/progenitor) cells from C57BL/6J mice Neonatal dermal fbs from C57BL/6J mice |
3C cells (3D cell clusters) | Matrigel | ROCK inhibitor, FGF2, and VEGF‐A | In vivo; injecting a mixture of 3C cells in Matrigel with neonatal dermal fbs into a full‐thickness wound onto BALB/c nude mice | HFs | [76a] |
|
Epidermal cells (epidermal stem cells) from neonatal mice Dermal cells (SKPs) from neonatal mice |
SKP spheroids | Matrigel | BMP4 for SKPs treatment | In vivo; implantation of Matrigel containing epidermal and culture‐expanded dermal cells onto BALB/c nu/nu mice |
Hair HFs |
[52] |
|
Epidermal cells (epidermal stem cells) from neonatal mice or adult human foreskin Dermal cells (SKPs) from neonatal mice Neonatal mouse dermal Fbs |
SKP spheroids | Matrigel | NR |
In vitro; culturing of epidermal cells with a PPARγ agonist and a Wnt signaling inhibitor In vivo; implantation of Matrigel containing epidermal cells with dermal cells (SKPs) or dermal fbs onto BALB/c nu/nu mice |
Sebocytes Sebaceous glands with or without HFs |
[52] |
| Single Blimp1+ cells (sebaceous gland progenitor) from B6.Cg‐Tg(Prdm1‐EYFP)1Mnz/J mice | 3D organoids | Matrigel | EGF, FGF2, BMP inhibitor, and c‐Myc inhibitor | In vitro; culturing of the sebaceous gland organoids | Sebaceous glands | [76c] |
|
SG cells from human skin MSCs from human bone marrow |
Cell suspension (coculture) |
NR NR Scaffold: a sheet of decellularized allogeneic dermal matrix |
NR |
In vitro; Coculture of heat‐shocked SG cells with MSCs In vivo; injection of MSCs transdifferentiated SG cells onto BALB/c nude mice Clinical trial; even spread of transdifferentiated MSCs on the excision wound covered with scaffold and granulated autologous skin grafts |
SG cells SGs |
[34] |
| SG cells, Kcs, & Fbs from human dorsal skin | Organotypic coculture model | Engineered skin construct: human Kcs on top of a Fbs‐embedded collagen‐based matrix | EGF‐containing gelatin microspheres |
In vitro; delivery of SG cells‐microspheres complex into the engineered skin construct In vivo; transplantation of the engineered skin construct onto athymic mice |
SGs | [77b] |
| SG cells from human skin | 3D culture | Matrigel | NR | In vitro; culture of SG cells in Matrigel | SGs | [76, 89] |
| Epithelial stem/progenitor cells from C57/B16 mouse dorsal skin | 3D ECM mimics | Cell‐laden composite hydrogel (bioprinted gelatin/ alginate hydrogel) | EGF, BMP4, and dermal components (dermal homogenates from wild‐type C57/B16 mice plantar skin) | In vivo; transplantation of 3D‐ECM mimics into wild‐type C57/B16 mice | SGs | [90] |
| MSCs from Sprague‐Dawley rat bone marrow | 3D culture | Gene‐activated scaffold based on collagen and chitosan | Plasmid DNA encoding rat EGF |
In vitro; seeding MSCs into the gene‐activated scaffold In vivo; transplantation of a bilayer dermal equivalent consisting of the MSCs/gene‐activated scaffold and silicone membranes in rats |
SGs | [77c] |
| SG cells from mouse thenar skin | 3D organoids | Matrigel | NR |
In vitro; seeding of SG single cells at the ALI culture condition In vivo; transplantation of SG organoids in Matrigel® into C57BL/6 mice |
Epidermis SGs Re‐epidermatization |
[76b] |
| Mammary progenitor cells from C57BL/6 mice | 3D culture | Bioprinted gelatin‐alginate hydrogels | SG‐ECM proteins | In vitro; culturing a mixture of suspended cells and mouse SG‐ECM proteins into the composite hydrogels | SGs | [91] |
Matrigel or murine Engelbreth‐Holm‐Swarm matrix is the most used matrix for the 3D culture of both human and rodent cells. In general, laminin (around 60%) and collagen IV (around 30%) constitute Matrigel,[ 92 ] which imparts structural and biological integrity to the seeded cells. The 3D structure of Matrigel is formed through gelation, mainly resulting from the self‐assembly of laminin as well as the crosslinking of laminin and collagen upon temperature rise.[ 92 ] Miao et al. generated HFs in vitro by using a combination of human DP cells and hair germinal matrix cells.[ 63 ] The DP cells were seeded in a 3D Matrigel culture that provided physical strength for the development of DP spheroids. Moreover, human hair germinal matrix cells in association with human DP spheroids could differentiate into hair‐like fibers in vitro.[ 63 ] By incorporating the biochemical factor (i.e., BMP4) into the culture system with Matrigel, Wang et al. regenerated HFs from epidermal SCs and skin precursors (SKPs).[ 52 ] This method has also been used for (re)generation of sebaceous gland with or without HFs.[ 52 ] Notably, Lee et al. employed Matrigel to establish skin organoids from human pluripotent SCs in the presence of several growth factors (e.g., TGF‐β and basic FGF).[ 76d ] After almost four months, a cyst‐like skin organoid with the stratified epidermis, fat‐rich hypodermis, and pigmented HFs associated with sebaceous glands were obtained (Figure 4 ).[ 76d ]
Figure 4.
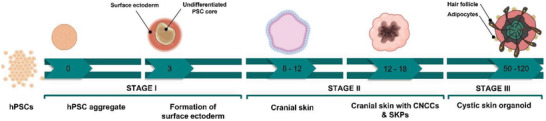
The timing of developmental events during in vitro skin organogenesis using human pluripotent stem cell (hPSCs) derived skin organoid. The human skin organoids are obtained in three main stages. The surface ectoderm and cranial neural crest cells are coinduced in the first stage using hPSCs. As a starting point, these cells form aggregates and then undergo ectodermal induction with critical differentiation factors, including bone morphogenetic protein (BMP) 4, FGF, and a transforming growth factor‐β inhibitor. On day 3, cells erupt outward, leaving the surface ectoderm and undifferentiated hPSC core. When the aggregates are mature (8–12 d), the intermediate layer erupts out, leading to the development of cranial skin. At the end of this stage, skin organoids contain cranial neural crest cells as well as epidermis and dermis precursors. Cystic skin organoids consisting of mesenchymal, neural, and glial progenitor cells surrounded by a sphere of keratinocytes are formed in the second stage. Also, the organoids contain self‐assembled epidermal and dermal layers along with bulb‐like hair follicles. In the third stage, the skin organoids produce a keratinized epidermis with a population of melanocytes, Schwann cells, and sensory neurons. Additionally, hair follicles with sebaceous glands grow outward. Created with Biorender.com.
In another study, Li et al. could successfully restore SGs in vitro from Matrigel‐embedded 3D culture of human SG cells.[ 76e ] Although Matrigel culture systems contribute to the recapitulation of the native skin microenvironment, it is difficult to tune the physicochemical properties of ECM and accordingly regulate their effects. For example, irrespective of their locations, skin tissues are often a lot stiffer (from 0.00109 MPa to 169.1 MPa[ 3 ]) than Matrigel (from 100 Pa to 3 kPa[ 93 ]). Matrigel's viscoelastic behaviors may also confine its handling at room temperature and therapeutic applications, especially at a large scale. Furthermore, Matrigel is an animal‐derived matrix, consisting of indistinct and xenogeneic proteins and other impurities that may not only cause potential antigenic reactivities but also affect cellular behaviors due to interference with the immune system.[ 94 ] Matrigel has demonstrated a great deal of variability in content and properties between batches and even within a single batch.[ 94 ] Therefore, some have raised concerns about the reproducibility of results.[ 95 ]
Collagen (commonly type I) plays a pivotal role in cell adhesions and fibrous tissue formation. Hence, matrices based on collagen type I have been considered a widely used biomimetic and less costly alternative to Matrigel for in vitro and in vivo skin culture. Abaci et al. utilized spheroidal cultures of DP cells and Kcs and subsequently incorporated them into skin constructs containing Fbs and green fluorescent protein‐tagged human umbilical vein endothelial cells in a collagen type I matrix.[ 59 ] The results showed the regeneration of HFs with a vascular network, which, in turn, led to a more native tissue with enhanced functionality and translational capabilities.[ 59 ] Huang et al. adopted a similar approach to restoring SGs in an organotypic coculture model, where SG cells were cultured on gelatin microspheres containing EGF, and the whole complex was then delivered into a bioengineered skin construct with a Fb‐embedded collagen‐based matrix.[ 77b ] This model resulted in an SG‐like structure in vitro.[ 77b ] Nevertheless, since collagen is also often extracted from animal tissues, the final matrices are associated with similar limitations to Matrigel, such as sample variability, poor tunable properties, and antigenicity. Besides these, the collagen microstructure and alignment change with variations in pH and temperature during gelation.[ 96 ] Therefore, there is a possibility that uncontrolled gelation might end up with heterogeneity in the matrix architecture and fibril size across samples, which exert critical effects on cell‐cell and cell‐matrix interactions.[ 97 ]
5.1. Advanced Matrices for Skin Appendage Reconstruction
Several scaffolds have been developed for the 3D culture of cells, spheroids, and organoids, which, in turn, give new insights into a better understanding of wound healing and skin regeneration. The biofabricated matrices used for skin appendage regeneration are highlighted in Table 2.
Keratin is a promising biopolymeric matrix for 3D culture. It contains RGD (Arg‐Gly‐Asp) and LDV (Leu‐Asp‐Val) sequences, supports cell adhesion and growth, and has limited immunogenicity.[ 3 ] In a recent study and using a C57BL/6 mice model, DP spheroids were loaded into a keratin hydrogel to assess HF regeneration.[ 82 ] Keratin improved the overall spheroid engraftment levels and cell survival rate without inducing any acute immune response. Larger HFs and thicker collagen layers were observed in those mice treated with DP spheroid‐loaded keratins.[ 82 ]
Much research has focused on the peptides with self‐assembling features. The peptide‐based materials formed by self‐assembly involve noncovalent interactions, whereby small molecules spontaneously associate into ordered structures, like particles,[ 98 ] fibers,[ 99 ] tubes,[ 100 ] etc., and macroscopically develop hydrogels. For example, RADA16 (Ac‐(RADA)4‐CONH2) is one of the self‐assembling peptides, which contains 16 alternating hydrophobic and hydrophilic amino acids. Wang et al. created a hydrogel scaffold using RADA16 and PRG (which consists of RGD).[ 76f ] RADA16 fibrous hydrogel promoted the proliferation of SKPs and enhanced the expression of hair induction signature genes (i.e., Akp2 and Bmp6). Additionally, RADA‐PRG hydrogel showed higher efficacy for de novo hair genesis than Matrigel in an HF reconstitution model in nude mice.[ 76f ]
The fact that hyaluronic acid (HA) exist in the ECM leaves more room for improvement in skin regeneration. Interestingly, its properties can be enhanced by chemical modifications using adipic hydrazide, tyramide, benzyl ester, glycidyl methacrylate, thiopropionyl hydrazide, or bromoacetate.[ 101 ] Thus far, thiolated HA (Extracel) is a mixture of modified HA and gelatin (1:1 ratio; w/w), where mechanical properties and biodegradation behavior can be tuned.[ 102 ] Not many functional studies have been conducted to elucidate the specific contribution of HA or its composites to the regeneration of skin appendages. Recently, Driskell et al., though, showed that Extracel could provide support to the clonal expansion of DP cells as spheroids, which, in turn, made a contribution toward HF‐inducing activity in vivo.[ 84 ] These proofs of concept studies for the regeneration of HFs in rodent models without the use of Matrigel and collagen type I would speed up the clinical translation of these 3D culture systems.
All matrices discussed herein are hydrogels and have the ability to emulate the ECM and regulate cells dynamically. Furthermore, the encapsulation of stem/progenitor cells within a hydrogel promotes cell viability and eases sustained localized delivery. These cell‐laden hydrogel systems can also carry essential cues for skin and its appendage regeneration. However, hydrogels cannot thoroughly recapitulate the geometry or architecture of tissues, which are critical for controlling cell fate and translating into clinical applications. Alternatively, hybrid hydrogels that incorporate micro/nanopattern (inclusion of micro/nanostructures) or multilayer construct (use of 3D printing/additive manufacturing) can be applied for 3D cell culture.
In a study by Pan et al., the microstructured poly (ethylene glycol) diacrylate (PEGDA) hydrogels were used to recapitulate the in vivo architecture of HFs, which is segregated into the gel compartments for the dermal and epidermal cells.[ 103 ] Likewise, Lim et al. created a 3D fibrous hydrogel to assemble DP and normal human epidermal Kcs utilizing two oppositely charged polyelectrolyte solutions.[ 79 ] This strategy directs cells in two separate domains to interact and thus promotes epithelial‐mesenchymal interactions.
To better reproduce the natural structures of skin appendages, culture systems that include 3D printed structures have received much attention. A recent study developed a highly hair‐inductive tissue graft using the bioprinting technology.[ 81 ] Mesenchymal and epithelia cells were encapsulated into collagen droplets separately. The microgels were spontaneously contracted in the suspension culture as a result of cell traction forces. The in vivo investigations demonstrated that the resultant hair microgels had considerable hair regeneration activity. Nevertheless, the newly formed hair shafts largely remained below the skin. Printing the microgel beads onto aligned suture guides significantly enhanced hair‐shaft sprouting and growth through the skin. Additionally, these suture‐inserted grafts inhibited the formation of epithelial cyst and ingrowth of hair under the skin due to the control over the orientation of the hair microgels.[ 81 ] Another report engineered HF‐like microwells, which were 500 µm in diameter and 4 mm in length. The 3D printed molds facilitated Kcs differentiation into specific hair lineages and enabled human HFs regeneration within human skin constructs.[ 59 ] The 3D printed scaffolds have also been utilized for the regeneration of SGs.[ 104 ]
Huang et al. developed a 3D ECM containing epidermal SCs that allowed the continuous slow release of EGF and BMP4 molecules. Two weeks after transplantation in mice and in the presence of plantar dermal cells, the epidermal cells were successfully differentiated into SG cells.[ 90 ] More recently, Yao et al. developed an alginate/gelatin hydrogel for 3D bioprinting applications. The newly developed hydrogel was able to induce SG differentiation of MSCs and support the regeneration of SG (Figure 5 ). The CTHRC1 and Hmox1 molecules were identified as key biochemical regulators for SG specification.[ 105 ] The current evidence shows its capacity for the regeneration of skin appendages, such as HF[ 59 , 81 ] and SG.[ 90 , 91 ] Indeed, 3D (bio)printing technology provides spatial control over cellular and material orientation. Nanmo et al. reported controlled graft orientation in the skin and considerably enhanced hair outgrowth using the 3D bioprinting technology.[ 81 ] Abaci et al. demonstrated that 3D printing enables the physiological arrangement of cells in HFs, which subsequently contributed to the production of skin constructs with different HF densities and an in vivo‐like DP cell phenotype in the 3D reconstructed dermis.[ 59 ] Applying vertical embedded bioprinting, Lian et al. revealed that extrusion technique by either uniaxial or coaxial nozzles could successfully enhance the efficiency and accuracy of the spatial patterning process for HF regeneration.[ 106 ] Furthermore, bioprinting exhibits a scalable and automated platform for the rapid fabrication of skin tissue with its appendages.[ 81 , 106 ] Therefore, 3D (bio)printing allows for fabricating the appendage‐bearing skin constructs arranged in a multilayer structure.[ 107 ]
Figure 5.
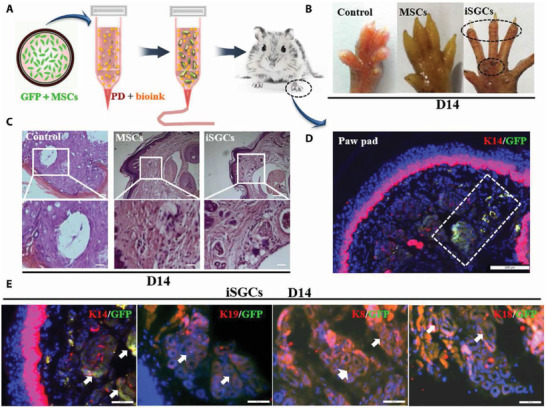
The capacity of induced sweat gland cells (iSGCs) for directed functional regeneration of sweat glands (SGs). A) A schematic representation of the method, where the 3D bioprinted scaffold containing green fluorescent protein (GFP)‐labeled mesenchymal stem cells (MSCs) was transplanted in burned paws of mice. B) Iodine/starch‐based sweat test of mice treated with different cells; the presence of black dots on footpads was indicative of sweating in iSGCs‐treated mice. C) Hematoxylin and eosin staining of a plantar region with and without the cell treatment; histology results revealed SG regeneration in iSGCs‐treated mice (scale bars, 200 µm). D) Participation of GFP‐labeled iSGCs in directed functional regeneration of SG (K14, red; GFP, green; DAPI, blue; scale bar, 200 µm). E) Detection of K14, K19, K8, and K18 as SG‐specific markers in the regenerated SG tissue (arrows); (K14, K19, K8, and K18, red; GFP, green; scale bars, 50 µm). Reproduced with permission.[ 105 ] Copyright 2021, American Association for the Advancement of Science.
6. Conclusions and Outlook
Skin constructs, though, hold promise for wound healing and skin regeneration, suffer from the lack of structural integrity due to the absence of skin appendages, including HFs, sebaceous glands, and SGs. These components play essential roles in the true function of the skin. The current study describes recent methods used to generate complex skin organs bearing the appendages and discusses the potentials and limitations of 3D culture platforms in this regard.
Up to now, many research studies have utilized stem/progenitor cells for the purpose of skin appendage reconstruction. Approaches based on seeding progenitor cells are found to be necessarily supportive in wound healing regardless of cell culture dimension. However, they cannot directly restore the whole skin structure with its appendages largely due to the lack of spatiotemporal organization. Advanced culture systems like organoids and spheroids, which can self‐organize in a particular microenvironment, are promising because they provide cells with a spatial framework to interact with each other. Yet, there is no solid evidence from human resources. In addition, such models still require refinement to match the in vivo cell niche in terms of their biological, chemical, and physical features.
The convergence of animal‐derived matrices and 3D cell aggregates from human PSCs could successfully form cyst‐like skin organoids consisting of the epidermis, dermis, HFs, and sebaceous glands.[ 76d ] Though encouraging, these structures differ from the skin organ in both structure and function. More importantly, the current skin models have no vascular network to protect cells and tissues against hypoxia and lack of growth factors.
Restoration of skin and its physiological function is subject to the inclusion of cells and recapitulation of their microenvironment. All cells that reside in skin tissue neighbor a vascular network that provides not only oxygen and nutrients but also molecular communications to the cells, especially via endothelial cells. The crosstalk between the vasculature and skin appendages has been presented previously.[ 108 ] To achieve a design more rational and closer to skin tissue, it is critical to include a vascular network into the engineered skin. Development of a skin construct with skin appendages and blood vessels are complex and requires considering other components or cells.[ 70 , 109 ]
There are only a few reports that have utilized biofabrication approaches to create vascularized skin constructs containing skin appendages, such as HF,[ 59 ] and SG.[ 110 ] Novel advanced biomanufacturing technologies offer this opportunity to design complex cell niches with specific geometries and architectures that promote the spatiotemporal behavior of stem/progenitor cells and overcome some of the current challenges of regenerating fully functional skin organ.
Biofabrication of skin tissue with its appendages has not been brought to clinical settings yet. We anticipate 3D bioprinting techniques can pave the way to the clinic due to their intrinsic capacity for automation, accuracy, reproducibility, scalability, and personalization of matrices with structural complexity. Future research should consider developing new strategies to integrate multiple cells and biomaterials more effectively in multilayered structures to produce functional skin tissue constructs. Therefore, there is not a long way to go before full translation of a skin construct with its appendages into the clinical practices.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
A.S. acknowledges the support from Herston Biofabrication Institute, and RBWH Foundation, Metro North Hospital and Health Service, Queensland, Australia. A.S. conceptualized the study, and M.H. prepared the original draft. Formal analysis and investigation were carried out by M.H., K.R.K., and A.S.
Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Biography
Abbas Shafiee is a senior research fellow at the Herston Biofabrication Institute (The Royal Brisbane and Women's Hospital, MNHHS), and The University of Queensland. In 2020, he established a research team to develop, implement, and evaluate the applications of 3D printing, cell therapies, and biofabrication technologies for skin wound applications. His team also has found new ways of generating skin from human pluripotent stem cells which could change the way skin diseases are studied and treated. He is also passionate about training the next scientific generation and healthcare professionals and has trained several Ph.D., M.Sc., MD, and undergraduate students.

Hosseini M., Koehler K. R., Shafiee A., Biofabrication of Human Skin with Its Appendages. Adv. Healthcare Mater. 2022, 11, 2201626. 10.1002/adhm.202201626
References
- 1.a) Chen Y. E., Fischbach M. A., Belkaid Y., Nature 2018, 553, 427; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Clevers H., Loh K. M., Nusse R., Science 2014, 346, 1248012. [DOI] [PubMed] [Google Scholar]
- 2.a) Hongbo Z., Maibach H., Dermatotoxicology, CRC Press LCC, Boca Raton, FL: 2004; [Google Scholar]; b) Nielsen J. B., Benfeldt E., Holmgaard R., Curr. Probl. Dermatol. 2016, 49, 103; [DOI] [PubMed] [Google Scholar]; c) Tansey E. A., Johnson C. D., Adv. Physiol. Educ. 2015, 39, 139. [DOI] [PubMed] [Google Scholar]
- 3. Hosseini M., Shafiee A., Small 2021, 17, 2101384. [DOI] [PubMed] [Google Scholar]
- 4.a) Gravitz L., Nature 2018, 563, S83; [DOI] [PubMed] [Google Scholar]; b) Takeo M., Lee W., Ito M., Cold Spring Harbor Perspect. Med. 2015, 5, a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arda O., Goksugur N., Tuzun Y., Clin. Dermatol. 2014, 32, 3. [DOI] [PubMed] [Google Scholar]
- 6.a) Randall M. J., Jungel A., Rimann M., Wuertz‐Kozak K., Front. Bioeng. Biotechnol. 2018, 6, 154; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Heitman N., Saxena N., Rendl M., Curr. Opin. Cell Biol. 2018, 55, 87; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Koehler K. R., Mikosz A. M., Molosh A. I., Patel D., Hashino E., Nature 2013, 500, 217; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lei M., Schumacher L. J., Lai Y.‐C., Juan W.‐T., Yeh C.‐Y., Wu P., Jiang T.‐X., Baker R. E., Widelitz R. B., Yang L., Chuong C.‐M., Proc. Natl. Acad. Sci. USA 2017, 114, E7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Vary J. C., Med. Clin. North Am. 2015, 99, 1195; [DOI] [PubMed] [Google Scholar]; b) Fuchs E., Nature 2007, 445, 834; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hu M. S., Borrelli M. R., Hong W. X., Malhotra S., Cheung A. T. M., Ransom R. C., Rennert R. C., Morrison S. D., Lorenz H. P., Longaker M. T., Organogenesis 2018, 14, 46; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) King A., Balaji S., Keswani S. G., Facial Plast. Surg. Clin. North Am. 2013, 21, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Millar S. E., J. Invest. Dermatol. 2002, 118, 216; [DOI] [PubMed] [Google Scholar]; f) Park B. Y., Saint‐Jeannet J. P., Induction and Segregation of the Vertebrate Cranial Placodes, Morgan & Claypool Life Sciences, San Rafael, CA: 2010. [PubMed] [Google Scholar]
- 8.a) Cheshire W. P., Freeman R., Semin. Neurol. 2003, 23, 399,; [DOI] [PubMed] [Google Scholar]; b) Cui C. Y., Schlessinger D., Exp. Dermatol. 2015, 24, 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shibasaki M., Wilson T. E., Crandall C. G., J. Appl. Physiol. 2006, 100, 1692. [DOI] [PubMed] [Google Scholar]
- 10. Fu X., Qu Z., Sheng Z., J. Surg. Res. 2006, 136, 204. [DOI] [PubMed] [Google Scholar]
- 11. Thompson C. M., Hocking A. M., Honari S., Muffley L. A., Ga M., Gibran N. S., J. Burn Care Res. 2013, 34, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chuong C. M., Randall V. A., Widelitz R. B., Wu P., Jiang T. X., Physiology 2012, 27, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niemann C., Horsley V., Semin. Cell Dev. Biol. 2012, 23, 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Schneider M. R., Paus R., Int. J. Biochem. Cell Biol. 2010, 42, 181; [DOI] [PubMed] [Google Scholar]; b) Zouboulis C. C., Stratakis C. A., Chrousos G. P., Koch C. A., Rev. Endocr. Metab. Disord. 2016, 17, 241. [DOI] [PubMed] [Google Scholar]
- 15. Clayton R. W., Göbel K., Niessen C. M., Paus R., van Steensel M. A. M., Lim X., Br. J. Dermatol. 2019, 181, 677. [DOI] [PubMed] [Google Scholar]
- 16. Grubbs H., Nassereddin A., Morrison M., Embryology, Hair, StatPearls Publishing LLC, Treasure Island, FL: 2021. [PubMed] [Google Scholar]
- 17. Schneider M. R., Schmidt‐Ullrich R., Paus R., Curr. Biol. 2009, 19, R132. [DOI] [PubMed] [Google Scholar]
- 18. Barbieri J. S., Wanat K., Seykora J., in Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms (Eds: McManus L. M., Mitchell R. N.), Academic Press, San Diego, CA: 2014, p. 1140. [Google Scholar]
- 19.a) Cotsarelis G., Sun T. T., Lavker R. M., Cell 1990, 61, 1329; [DOI] [PubMed] [Google Scholar]; b) Lyle S., Christofidou‐Solomidou M., Liu Y., Elder D. E., Albelda S., Cotsarelis G., J. Cell Sci. 1998, 111, 3179; [DOI] [PubMed] [Google Scholar]; c) Morris R. J., Potten C. S., J. Invest. Dermatol. 1999, 112, 470; [DOI] [PubMed] [Google Scholar]; d) Taylor G., Lehrer M. S., Jensen P. J., Sun T. T., Lavker R. M., Cell 2000, 102, 451; [DOI] [PubMed] [Google Scholar]; e) Claudinot S., Nicolas M., Oshima H., Rochat A., Barrandon Y., Proc. Natl. Acad. Sci. USA 2005, 102, 14677; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Ohyama M., Terunuma A., Tock C. L., Radonovich M. F., Pise‐Masison C. A., Hopping S. B., Brady J. N., Udey M. C., Vogel J. C., J. Clin. Invest. 2006, 116, 249; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Oshima H., Rochat A., Kedzia C., Kobayashi K., Barrandon Y., Cell 2001, 104, 233. [DOI] [PubMed] [Google Scholar]
- 20. Weng T., Wu P., Zhang W., Zheng Y., Li Q., Jin R., Chen H., You C., Guo S., Han C., Wang X., J. Transl. Med. 2020, 18, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Fuchs E., Merrill B. J., Jamora C., DasGupta R., Dev. Cell 2001, 1, 13; [DOI] [PubMed] [Google Scholar]; b) Paus R., Cotsarelis G., N. Engl. J. Med. 1999, 341, 491. [DOI] [PubMed] [Google Scholar]
- 22. Tsatmali M., Ancans J., Thody A. J., J. Histochem. Cytochem. 2002, 50, 125. [DOI] [PubMed] [Google Scholar]
- 23.a) Marshall C. D., Hu M. S., Leavitt T., Barnes L. A., Lorenz H. P., Longaker M. T., Adv. Wound Care 2018, 7, 29; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kabashima K., Honda T., Ginhoux F., Egawa G., Nat. Rev. Immunol. 2019, 19, 19; [DOI] [PubMed] [Google Scholar]; c) Sorg H., Tilkorn D. J., Hager S., Hauser J., Mirastschijski U., Eur. Surgical Res. 2017, 58, 81; [DOI] [PubMed] [Google Scholar]; d) Ma K., Tan Z., Zhang C., Fu X., Burns 2016, 42, 492. [DOI] [PubMed] [Google Scholar]
- 24.a) Boyce S. T., Kagan R. J., Yakuboff K. P., Meyer N. A., Rieman M. T., Greenhalgh D. G., Warden G. D., Ann. Surg. 2002, 235, 269; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dearman B. L., Boyce S. T., Greenwood J. E., Front. Surg. 2021, 8, 640879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang R., Liu F., Wang J., Chen X., Xie J., Xiong K., Stem Cell Res. Ther. 2019, 10, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang C., Chen Y., Fu X., Cytotherapy 2015, 17, 526. [DOI] [PubMed] [Google Scholar]
- 27.a) Lu C. P., Polak L., Rocha A. S., Pasolli H. A., Chen S. C., Sharma N., Blanpain C., Fuchs E., Cell 2012, 150, 136; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yao B., Xie J., Liu N., Yan T., Li Z., Liu Y., Huang S., Fu X., Biochem. Biophys. Res. Commun. 2016, 479, 670. [DOI] [PubMed] [Google Scholar]
- 28. Ohe S., Tanaka T., Yanai H., Komai Y., Omachi T., Kanno S., Tanaka K., Ishigaki K., Saiga K., Nakamura N., Ohsugi H., Tokuyama Y., Atsumi N., Hisha H., Yoshida N., Kumano K., Yamazaki F., Okamoto H., Ueno H., Biochem. Biophys. Res. Commun. 2015, 466, 333. [DOI] [PubMed] [Google Scholar]
- 29. Li H., Fu X., Ouyang Y., Cai C., Wang J., Sun T., Cell Tissue Res. 2006, 326, 725. [DOI] [PubMed] [Google Scholar]
- 30. Sun S., Xiao J., Huo J., Geng Z., Ma K., Sun X., Fu X., Stem Cell Res. Ther. 2018, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Y., Huang S., Ma K., Fu X., Han W., Sheng Z., J. Tissue Eng. Regener. Med. 2012, 6, 645. [DOI] [PubMed] [Google Scholar]
- 32. Xu Y., Hong Y., Xu M., Ma K., Fu X., Zhang M., Wang G., Stem Cells Transl. Med. 2016, 5, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srivastava A. K., Durmowicz M. C., Hartung A. J., Hudson J., Ouzts L. V., Donovan D. M., Cui C. Y., Schlessinger D., Hum. Mol. Genet. 2001, 10, 2973. [DOI] [PubMed] [Google Scholar]
- 34. Sheng Z., Fu X., Cai S., Lei Y., Sun T., Bai X., Chen M., Wound Repair Regener. 2009, 17, 427. [DOI] [PubMed] [Google Scholar]
- 35. Cai S., Pan Y., Han B., Sun T.‐z., Sheng Z.‐y., Fu X.‐b., Chin. Med. J. 2011, 124, 2260. [PubMed] [Google Scholar]
- 36. Panteleyev A. A., Rosenbach T., Paus R., Christiano A. M., Arch. Dermatol. Res. 2000, 292, 573. [DOI] [PubMed] [Google Scholar]
- 37. Morris R. J., Liu Y., Marles L., Yang Z., Trempus C., Li S., Lin J. S., Sawicki J. A., Cotsarelis G., Nat. Biotechnol. 2004, 22, 411. [DOI] [PubMed] [Google Scholar]
- 38. Blanpain C., Lowry W. E., Geoghegan A., Polak L., Fuchs E., Cell 2004, 118, 635. [DOI] [PubMed] [Google Scholar]
- 39. Horsley V., O'Carroll D., Tooze R., Ohinata Y., Saitou M., Obukhanych T., Nussenzweig M., Tarakhovsky A., Fuchs E., Cell 2006, 126, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jensen K. B., Collins C. A., Nascimento E., Tan D. W., Frye M., Itami S., Watt F. M., Cell Stem Cell 2009, 4, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Snippert H. J., Haegebarth A., Kasper M., Jaks V., van Es J. H., Barker N., van de Wetering M., van den Born M., Begthel H., Vries R. G., Stange D. E., Toftgard R., Clevers H., Science 2010, 327, 1385. [DOI] [PubMed] [Google Scholar]
- 42. Nowak J. A., Polak L., Pasolli H. A., Fuchs E., Cell Stem Cell 2008, 3, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frances D., Niemann C., Dev. Biol. 2012, 363, 138. [DOI] [PubMed] [Google Scholar]
- 44. Eisinger M., Li W.‐H., Rossetti D. D., Anthonavage M., Seiberg M., J. Invest. Dermatol. 2010, 130, 2131. [DOI] [PubMed] [Google Scholar]
- 45. Li S., Thangapazham R. L., Wang J.‐A., Rajesh S., Kao T.‐C., Sperling L., Moss J., Darling T. N., Nat. Commun. 2011, 2, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kataoka K., Medina R. J., Kageyama T., Miyazaki M., Yoshino T., Makino T., Huh N.‐h., Am. J. Pathol. 2003, 163, 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Mayo T., Conget P., Becerra‐Bayona S., Sossa C. L., Galvis V., Arango‐Rodríguez M. L., PLoS One 2017, 12, e0177533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fang L. J., Fu X. B., Cheng B., Sun T. Z., Li J. F., Cao R., Wang Y. X., Chin. J. Surg. 2004, 42, 1136. [PubMed] [Google Scholar]
- 49. Shi S., Jia S., Liu J., Chen G., Cell Biochem. Biophys. 2015, 71, 951. [DOI] [PubMed] [Google Scholar]
- 50.a) Firth A. L., Yuan J. X., Pulm. Circ. 2012, 2, 84; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Soteriou D., Kostic L., Sedov E., Yosefzon Y., Steller H., Fuchs Y., J. Visualized Exp. 2016, 110, e53931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blanpain C., Fuchs E., Nat. Rev. Mol. Cell Biol. 2009, 10, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X., Wang X., Liu J., Cai T., Guo L., Wang S., Wang J., Cao Y., Ge J., Jiang Y., Tredget E. E., Cao M., Wu Y., Stem Cells Transl. Med. 2016, 5, 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Groot S. C., Ulrich M. M. W., Gho C. G., Huisman M. A., Front. Cell Dev. Biol. 2021, 9, 661787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reynolds A. J., Lawrence C., Cserhalmi‐Friedman P. B., Christiano A. M., Jahoda C. A., Nature 1999, 402, 33. [DOI] [PubMed] [Google Scholar]
- 55. Kim J. P., Hundepool C. A., Friedrich P. F., Moran S. L., Bishop A. T., Shin A. Y., Microsurgery 2018, 38, 66. [DOI] [PubMed] [Google Scholar]
- 56. Messenger A. G., Br. J. Dermatol. 1984, 110, 685. [DOI] [PubMed] [Google Scholar]
- 57.a) Horne K. A., Jahoda C. A., Oliver R. F., J. Embryol. Exp. Morphol. 1986, 97, 111; [PubMed] [Google Scholar]; b) Jahoda C. A., Horne K. A., Oliver R. F., Nature 1984, 311, 560. [DOI] [PubMed] [Google Scholar]
- 58. Rompolas P., Deschene E. R., Zito G., Gonzalez D. G., Saotome I., Haberman A. M., Greco V., Nature 2012, 487, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abaci H. E., Coffman A., Doucet Y., Chen J., Jacków J., Wang E., Guo Z., Shin J. U., Jahoda C. A., Christiano A. M., Nat. Commun. 2018, 9, 5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ohyama M., Kobayashi T., Sasaki T., Shimizu A., Amagai M., J. Cell Sci. 2012, 125, 4114. [DOI] [PubMed] [Google Scholar]
- 61.a) Higgins C. A., Richardson G. D., Ferdinando D., Westgate G. E., Jahoda C. A., Exp. Dermatol. 2010, 19, 546; [DOI] [PubMed] [Google Scholar]; b) Osada A., Iwabuchi T., Kishimoto J., Hamazaki T. S., Okochi H., Tissue Eng. 2007, 13, 975. [DOI] [PubMed] [Google Scholar]
- 62.a) Havlickova B., Bíró T., Mescalchin A., Arenberger P., Paus R., Br. J. Dermatol. 2004, 151, 753; [DOI] [PubMed] [Google Scholar]; b) Havlickova B., Bíró T., Mescalchin A., Tschirschmann M., Mollenkopf H., Bettermann A., Pertile P., Lauster R., Bodó E., Paus R., J. Invest. Dermatol. 2009, 129, 972; [DOI] [PubMed] [Google Scholar]; c) Limat A., Breitkreutz D., Hunziker T., Klein C. E., Noser F., Fusenig N. E., Braathen L. R., Cell Tissue Res. 1994, 275, 169; [DOI] [PubMed] [Google Scholar]; d) Wu J. J., Zhu T. Y., Lu Y. G., Liu R. Q., Mai Y., Cheng B., Lu Z. F., Zhong B. Y., Tang S. Q., Arch. Dermatol. Res. 2006, 298, 183. [DOI] [PubMed] [Google Scholar]
- 63. Miao Y., Sun Y. B., Liu B. C., Jiang J. D., Hu Z. Q., Tissue Eng., Part A 2014, 20, 2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lindner G., Horland R., Wagner I., Ataç B., Lauster R., J. Biotechnol. 2011, 152, 108. [DOI] [PubMed] [Google Scholar]
- 65. Veraitch O., Kobayashi T., Imaizumi Y., Akamatsu W., Sasaki T., Yamanaka S., Amagai M., Okano H., Ohyama M., J. Invest. Dermatol. 2013, 133, 1479. [DOI] [PubMed] [Google Scholar]
- 66. Lim S. J., Ho S. C., Mok P. L., Tan K. L., Ong A. H. K., Gan S. C., PeerJ 2016, 4, e2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Muchkaeva I. A., Dashinimaev E. B., Artyuhov A. S., Myagkova E. P., Vorotelyak E. A., Yegorov Y. Y., Vishnyakova K. S., Kravchenko I. E., Chumakov P. M., Terskikh V. V., Vasiliev A. V., Acta Nat. 2014, 6, 45. [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao Q., Li N., Zhang H., Lei X., Cao Y., Xia G., Duan E., Liu S., Cell Proliferation 2019, 52, e12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.a) Liu S., Liu S., Wang X., Zhou J., Cao Y., Wang F., Duan E., Aging Cell 2011, 10, 661; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xie J., Yao B., Han Y., Huang S., Fu X., Burns Trauma 2016, 4, s41038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.a) Hosseini M., Brown J., Shafiee A., Tissue Eng., Part C 2022, 28, 113; [DOI] [PubMed] [Google Scholar]; b) Hosseini M., Brown J., Khosrotehrani K., Bayat A., Shafiee A., Burns Trauma 2022, 10, tkac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ji S., Zhu Z., Sun X., Fu X., Signal Transduction Targeted Ther. 2021, 6, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee J., Böscke R., Tang P.‐C., Hartman B. H., Heller S., Koehler K. R., Cell Rep. 2018, 22, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Takagi R., Ishimaru J., Sugawara A., Toyoshima K.‐E., Ishida K., Ogawa M., Sakakibara K., Asakawa K., Kashiwakura A., Oshima M., Minamide R., Sato A., Yoshitake T., Takeda A., Egusa H., Tsuji T., Sci. Adv. 2016, 2, e1500887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Weber E. L., Woolley T. E., Yeh C.‐Y., Ou K.‐L., Maini P. K., Chuong C.‐M., Exp. Dermatol. 2019, 28, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Su Y., Wen J., Zhu J., Xie Z., Liu C., Ma C., Zhang Q., Xu X., Wu X., Stem Cell Res. Ther. 2019, 10, 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.a) Chacón‐Martínez C. A., Klose M., Niemann C., Glauche I., Wickström S. A., EMBO J. 2017, 36, 151; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Diao J., Liu J., Wang S., Chang M., Wang X., Guo B., Yu Q., Yan F., Su Y., Wang Y., Cell Death Dis. 2019, 10, 238; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Feldman A., Mukha D., Maor I. I., Sedov E., Koren E., Yosefzon Y., Shlomi T., Fuchs Y., Nat. Commun. 2019, 10, 2348; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lee J., Rabbani C. C., Gao H., Steinhart M. R., Woodruff B. M., Pflum Z. E., Kim A., Heller S., Liu Y., Shipchandler T. Z., Koehler K. R., Nature 2020, 582, 399; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Li H., Chen L., Zhang M., Tang S., Fu X., Cell Tissue Res. 2013, 354, 897; [DOI] [PubMed] [Google Scholar]; f) Wang X., Wang J., Guo L., Wang X., Chen H., Wang X., Liu J., Tredget E. E., Wu Y., Nanomedicine 2016, 12, 2115. [DOI] [PubMed] [Google Scholar]
- 77.a) Dong L., Hao H., Liu J., Tong C., Ti D., Chen D., Chen L., Li M., Liu H., Fu X., Han W., J. Tissue Eng. Regener. Med. 2017, 11, 1479; [DOI] [PubMed] [Google Scholar]; b) Huang S., Xu Y., Wu C., Sha D., Fu X., Biomaterials 2010, 31, 5520; [DOI] [PubMed] [Google Scholar]; c) Kolakshyapati P., Li X., Chen C., Zhang M., Tan W., Ma L., Gao C., Sci. Rep. 2017, 7, 17630; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Toyoshima K.‐e., Asakawa K., Ishibashi N., Toki H., Ogawa M., Hasegawa T., Irié T., Tachikawa T., Sato A., Takeda A., Tsuji T., Nat. Commun. 2012, 3, 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Teng Y.‐Y., Zou M.‐L., Liu S.‐Y., Jia Y., Zhang K.‐W., Yuan Z.‐D., Wu J.‐J., Ye J.‐X., Yu S., Li X., Zhou X.‐J., Yuan F.‐L., Front. Bioeng. Biotechnol. 2022, 10, 902894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lim T. C., Leong M. F., Lu H., Du C., Gao S., Wan A. C., Ying J. Y., Biomaterials 2013, 34, 7064. [DOI] [PubMed] [Google Scholar]
- 80. Vahav I., van den Broek L. J., Thon M., Monsuur H. N., Spiekstra S. W., Atac B., Scheper R. J., Lauster R., Lindner G., Marx U., Gibbs S., J. Tissue Eng. Regener. Med. 2020, 14, 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nanmo A., Yan L., Asaba T., Wan L., Kageyama T., Fukuda J., Acta Biomater. 2022, 3, 43. [DOI] [PubMed] [Google Scholar]
- 82. Hu S., Li Z., Lutz H., Huang K., Su T., Cores J., Dinh P. C., Cheng K., Sci. Adv. 2020, 6, eaba1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Young T. H., Lee C. Y., Chiu H. C., Hsu C. J., Lin S. J., Biomaterials 2008, 29, 3521. [DOI] [PubMed] [Google Scholar]
- 84. Driskell R. R., Juneja V. R., Connelly J. T., Kretzschmar K., Tan D. W. M., Watt F. M., J. Invest. Dermatol. 2012, 132, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huang Y. C., Chan C. C., Lin W. T., Chiu H. Y., Tsai R. Y., Tsai T. H., Chan J. Y., Lin S. J., Biomaterials 2013, 34, 442. [DOI] [PubMed] [Google Scholar]
- 86. Wang J., Miao Y., Huang Y., Lin B., Liu X., Xiao S., Du L., Hu Z., Xing M., Adv. Healthcare Mater. 2018, 7, 1700447. [DOI] [PubMed] [Google Scholar]
- 87. Zhang X., Xiao S., Liu B., Miao Y., Hu Z., Regener. Med. 2019, 14, 741. [DOI] [PubMed] [Google Scholar]
- 88. Zhang K., Bai X., Yuan Z., Cao X., Jiao X., Qin Y., Wen Y., Zhang X., ACS Appl. Mater. Interfaces 2020, 12, 7931. [DOI] [PubMed] [Google Scholar]
- 89. Shu S., Chen L., Li X., Li H., Chin. J. Repar. Reconstr. Surg. 2014, 28, 162. [PubMed] [Google Scholar]
- 90. Huang S., Yao B., Xie J., Fu X., Acta Biomater. 2016, 32, 170. [DOI] [PubMed] [Google Scholar]
- 91. Wang R., Wang Y., Yao B., Hu T., Li Z., Liu Y., Cui X., Cheng L., Song W., Huang S., Fu X., Burns Trauma 2019, 7, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Corning, Corning Matrigel Matrix: Frequently Asked Questions, https://www.corning.com/catalog/cls/documents/faqs/CLS‐DL‐CC‐026.pdf (accessed: April 2022).
- 93.a) Slater K., Partridge J., Nandivada H., Tuning the elastic moduli of Corning Matrigel and collagen I 3D matrices by varying the protein concentration https://www.corning.com/catalog/cls/documents/application‐notes/CLS‐AC‐AN‐449.pdf (accessed: April 2022);; b) Soofi S. S., Last J. A., Liliensiek S. J., Nealey P. F., Murphy C. J., J. Struct. Biol. 2009, 167, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Aisenbrey E. A., Murphy W. L., Nat. Rev. Mater. 2020, 5, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.a) Halme D. G., Kessler D. A., N. Engl. J. Med. 2006, 355, 1730; [DOI] [PubMed] [Google Scholar]; b) Thirumala S., Goebel W. S., Woods E. J., Expert Opin. Biol. Ther. 2013, 13, 673. [DOI] [PubMed] [Google Scholar]
- 96. Hapach L. A., VanderBurgh J. A., Miller J. P., Reinhart‐King C. A., Phys. Biol. 2015, 12, 061002. [DOI] [PubMed] [Google Scholar]
- 97. Velez D. O., Tsui B., Goshia T., Chute C. L., Han A., Carter H., Fraley S. I., Nat. Commun. 2017, 8, 1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.a) Du Z., Li Q., Li J., Su E., Liu X., Wan Z., Yang X., J. Agric. Food Chem. 2019, 67, 11728; [DOI] [PubMed] [Google Scholar]; b) Shuai J., Guan F., He B., Hu J., Li Y., He D., Hu J., J. Agric. Food Chem. 2019, 67, 5720. [DOI] [PubMed] [Google Scholar]
- 99. Zhou S., Hokugo A., McClendon M., Zhang Z., Bakshi R., Wang L., Segovia L. A., Rezzadeh K., Stupp S. I., Jarrahy R., Burns 2019, 45, 1112. [DOI] [PubMed] [Google Scholar]
- 100. Colherinhas G., Fileti E., J. Phys. Chem. B 2014, 118, 12215. [DOI] [PubMed] [Google Scholar]
- 101. Burdick J. A., Prestwich G. D., Adv. Mater. 2011, 23, H41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vanderhooft J. L., Alcoutlabi M., Magda J. J., Prestwich G. D., Macromol. Biosci. 2009, 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pan J., Yung Chan S., Common J. E., Amini S., Miserez A., Birgitte Lane E., Kang L., J. Biomed. Mater. Res., Part A 2013, 101, 3159. [DOI] [PubMed] [Google Scholar]
- 104.a) Office of Research Integrity (ORI) , Mice and rodents, https://www.ori.hhs.gov/education/products/ncstate/rodent.htm (accessed: April 2022);; b) Zomer H. D., Trentin A. G., J. Dermatol. Sci. 2018, 90, 3. [DOI] [PubMed] [Google Scholar]
- 105. Yao B., Wang R., Wang Y., Zhang Y., Hu T., Song W., Li Z., Huang S., Fu X., Sci. Adv. 2020, 6, eaaz1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lian L., Zhou C., Tang G., Xie M., Wang Z., Luo Z., Japo J., Wang D., Zhou J., Wang M., Li W., Maharjan S., Ruelas M., Guo J., Wu X., Zhang Y. S., Adv. Healthcare Mater. 2022, 11, 2102411. [DOI] [PubMed] [Google Scholar]
- 107. Ng W. L., Wang S., Yeong W. Y., Naing M. W., Trends Biotechnol. 2016, 34, 689. [DOI] [PubMed] [Google Scholar]
- 108.a) Song W., Yao B., Zhu D., Zhang Y., Li Z., Huang S., Fu X., Burns Trauma 2022, 10, tkab044; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li K. N., Jain P., He C. H., Eun F. C., Kang S., Tumbar T., eLife 2019, 8, e45977; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Man X. Y., Yang X. H., Cai S. Q., Bu Z. Y., Wu X. J., Lu Z. F., Zheng M., Clin. Exp. Dermatol. 2009, 34, 396; [DOI] [PubMed] [Google Scholar]; d) Yano K., Brown L. F., Detmar M., J. Clin. Invest. 2001, 107, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Huyan Y., Lian Q., Zhao T., Li D., He J., Int. J. Bioprint. 2020, 6, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang M., Li H., Chen L., Fang S., Xie S., Lin C., J. Mol. Histol. 2018, 49, 339. [DOI] [PubMed] [Google Scholar]


