Abstract
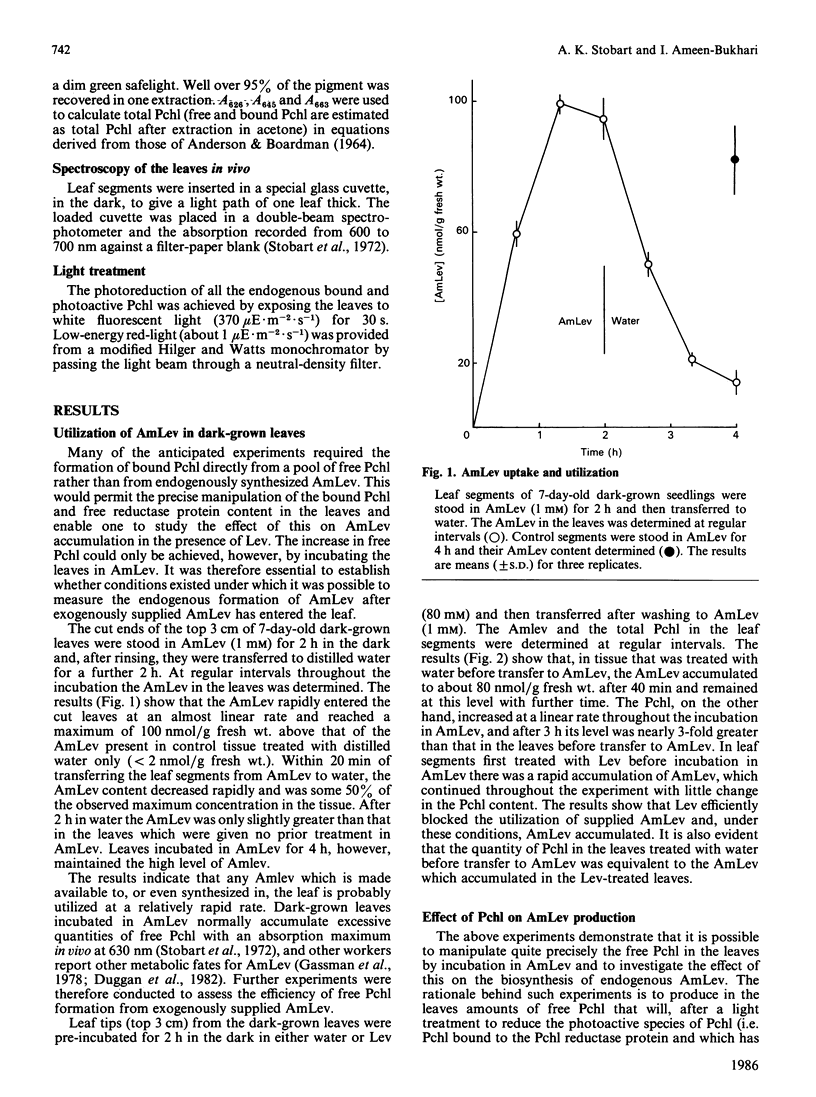
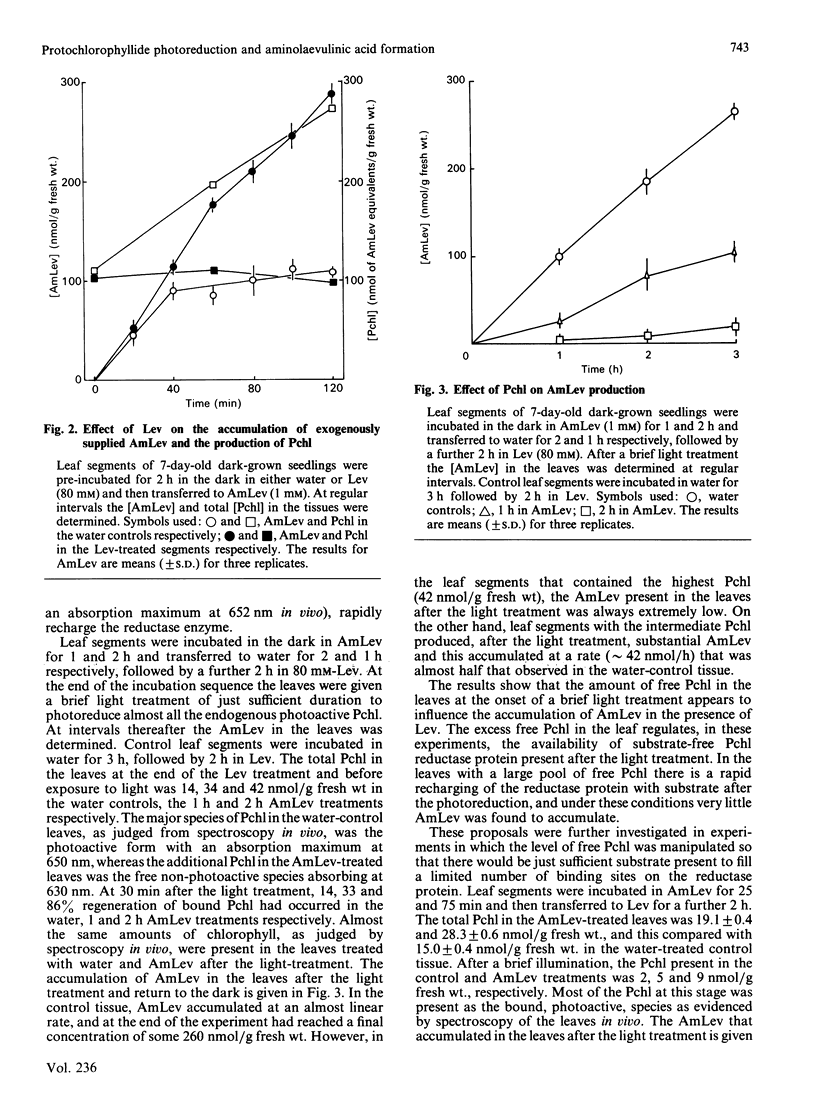
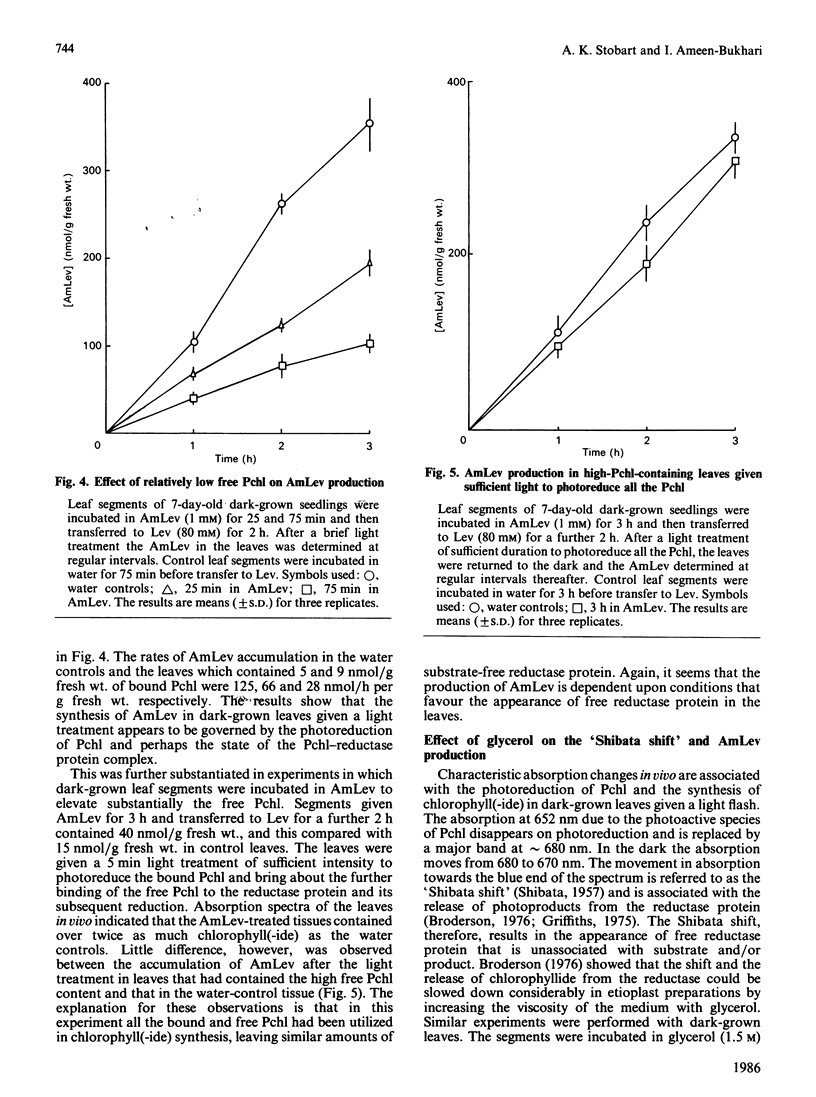
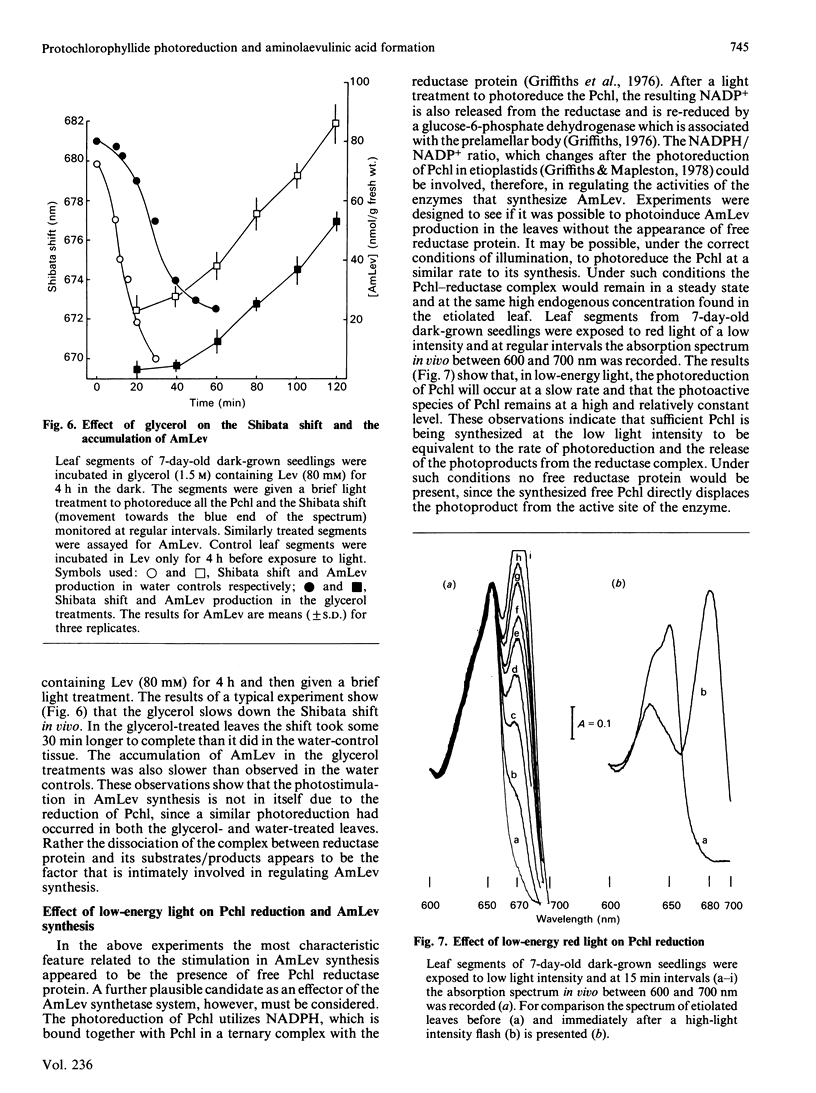
The photoreduction of protochlorophyllide (Pchl) in dark-grown leaves of barley (Hordeum vulgare) brings about the synthesis of delta-aminolaevulinic acid (AmLev). Manipulation of the Pchl level in the leaves by incubation in AmLev indicated that the production of AmLev was intimately related to the state of the Pchl reductase ternary complex. Free Pchl reductase that is unassociated with substrate/product appeared at first to be essential for the photoinduction of AmLev synthesis. Experiments on the photoreduction of Pchl in dark-grown leaves exposed to low-energy red-light, however, showed that photoreduction and AmLev synthesis would occur when the Pchl reductase, together with substrate, was maintained at relatively high endogenous concentration. Under such conditions the availability of free reductase protein would be negligible. An alternative scheme is presented, therefore, that can explain many, if not all, of the observations on AMLev synthesis and its close relationship to Pchl reduction, and which is based on a common supply of NADPH for the reduction of glutamate to AmLev and the synthesis of chorophyll(-ide).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duggan J. X., Meller E., Gassman M. L. Catabolism of 5-Aminolevulinic Acid to CO(2) by Etiolated Barley Leaves. Plant Physiol. 1982 Jan;69(1):19–22. doi: 10.1104/pp.69.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Harel E., Klein S., Meller E. Control of delta-Aminolevulinic Acid and Chlorophyll Accumulation in Greening Maize Leaves upon Light-Dark Transitions. Plant Physiol. 1975 Oct;56(4):497–501. doi: 10.1104/pp.56.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths W. T. Characterization of the terminal stages of chlorophyll (ide) synthesis in etioplast membrane preparations. Biochem J. 1975 Dec;152(3):623–635. doi: 10.1042/bj1520623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel E., Klein S. Light dependent formation of -aminolevulinic acid in etiolated leaves of higher plants. Biochem Biophys Res Commun. 1972 Oct 17;49(2):364–370. doi: 10.1016/0006-291x(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Klein S., Katz E., Neeman E. Induction of delta-Aminolevulinic Acid Formation in Etiolated Maize Leaves Controlled by Two Light Systems. Plant Physiol. 1977 Sep;60(3):335–338. doi: 10.1104/pp.60.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart A. K., Ameen-Bukhari I. Regulation of delta-aminolaevulinic acid synthesis and protochlorophyllide regeneration in the leaves of dark-grown barley (Hordeum vulgare) seedlings. Biochem J. 1984 Sep 1;222(2):419–426. doi: 10.1042/bj2220419. [DOI] [PMC free article] [PubMed] [Google Scholar]


