Abstract
Angiogenesis plays a critical role in diabetic wound healing. However, no effective strategies have been developed to target endothelial cells (ECs) to facilitate diabetic wound healing. Dapagliflozin (DA) as a sodium‐glucose linked transporter 2 (SGLT2) inhibitor, may promote neovascularization in diabetic mice via HIF‐1α‐mediated enhancement of angiogenesis. Here, the bioinspired nanovesicles (NVs) prepared from induced pluripotent stem cells‐derived ECs through an extrusion approach are reported, which can function as exosome mimetics to achieve targeted deliver of DA. Abundant membrane C‐X‐C motif chemokine receptor 4 conferred the EC‐targeting ability of these NVs and the endothelial homology facilitated the accumulation in ECs. Furthermore, these DA‐loaded induced pluripotent stem cells (iPSC)‐EC NVs can facilitate angiogenesis and diabetic wound healing by HIF‐1α/VEGFA pathway. Taken together, this study indicated that targeting ECs and regulating angiogenesis may be a promising strategy for the treatment of diabetic wound healing.
Keywords: angiogenesis, dapagliflozin, diabetic wound healing, exosome mimetics, HIF‐1α
Bioinspired nanovesicles (NVs) are prepared from human induced pluripotent stem cells‐derived endothelial cells (iPS‐EC) through an extrusion approach, which functions as exosome mimetics to targeted deliver dapagliflozin (DA). These DA‐loaded iPS‐EC NVs facilitate angiogenesis and wound healing by the HIF‐1α/VEGFA pathway, which may be a promising strategy for the treatment of diabetic wound healing.

1. Introduction
With the rapid development of a global aging society, the number of diabetic patients is increasing, and serious complications including diabetic ulcers are attracting growing attention.[ 1 , 2 ] Angiogenesis promotes the transport of nutrients and oxygen to injury sites, thereby playing a vital role in wound healing.[ 3 ] In diabetic patients, hyperglycemia‐induced endothelial dysfunction might impair angiogenesis and further result in delayed wound healing. Therefore, more effective strategies to promote angiogenesis are in great demand for treating diabetic wounds.[ 4 ] As known to all, HIF‐1α could increase angiogenesis and promote wound healing under hypoxic conditions. Whereas, the expression of HIF‐1α is significantly downregulated in diabetic wounds when compared with normal wounds.[ 5 ] It is indicated that hyperglycemia might lead to impaired HIF‐1α protection under hypoxia in diabetic wounds and endothelial dysfunction.[ 6 ] Accordingly, HIF‐1α stabilization is a promising therapeutic strategy for diabetic wound healing.[ 7 ] For example, a previous study showed that VH298 (the stabilizer of HIF‐1α) loaded extracellular vesicles could improve angiogenesis and enhance diabetic wound healing via HIF‐1α/VEGFA signaling pathway.[ 2 ]
Dapagliflozin (DA) as one sodium‐glucose linked transporter 2 (SGLT2) inhibitor is widely utilized to lower blood sugar levels in type‐2 diabetes mellitus (T2DM) patients.[ 8 ] The DECLARE‐TIMI 58 trial suggested that treatment with DA is closely correlated with a significantly reduced risk of major adverse cardiovascular events in T2DM patients.[ 9 ] Moreover, the regulative roles of DA in endothelial dysfunction have also been studied.[ 10 ] Studies in human coronary artery endothelial cells (ECs) suggested that DA neutralized superoxide production and restored NO activity upon stimulation with TNF‐α.[ 11 ] DA could reduce endothelial dysfunction and microvascular damage during cardiac ischemia/reperfusion injury via the XO‐SERCA2‐CaMKII pathway.[ 12 ] Apart from DA, other SGLT2 inhibitors have also been reported to play a vital role in regulating endothelial dysfunction. For instance, canagliflozin could inhibit EC inflammation via the induction of heme oxygenase‐1.[ 13 ] Empagliflozin decreases frailty in diabetic and hypertensive patients by reducing the mitochondrial generation of reactive oxygen species in ECs.[ 14 ] Although these findings are indicative of the broad protective effects of SGLT2 inhibitors on ECs, it remains unclear whether DA would be effective in promoting angiogenesis in diabetic wound healing. One study reported that DA might promote neovascularization in diabetic hindlimb ischemia mice through PHD2/HIF‐1α axis.[ 15 ] Therefore, we hypothesized that DA might promote ECs angiogenesis in diabetic wound healing through HIF‐1α/VEGFA pathway.
Recently, exosomes or extracellular vesicles are membrane‐coated and secreted by various cell types,[ 16 ] which have grown into a promising field of research.[ 17 ] Extracellular vesicles could deliver endogenous molecules or therapeutic agents to recipient cells by endocytosis or direct fusion with cell membranes.[ 18 ] For instance, extracellular vesicles derived from epidermal stem cells promoted wound healing and reduced scar formation by delivering specific small RNAs into lesions.[ 19 ] Whereas, the therapeutic effects of extracellular vesicles are limited by the small number of active agents carried. Accordingly, the preparation of engineered and modified extracellular vesicles to deliver more therapeutic agents has been widely investigated in translational research.[ 20 ] Several studies have shown that the molecular agents delivered by extracellular vesicles to target cells could play a vital role in tissue repair and regeneration.[ 21 , 22 ] Collectively, delivering the angiogenic agents by extracellular vesicles holds great promise in treating diabetic wounds. However, the quantity of exosomes released from cells is relatively low.[ 23 ] The difficult purification, low yield, and high cost have blocked the biomedical applications of exosomes or extracellular vesicles.[ 24 , 25 , 26 ] Owing to the current limitations of exosome production, some studies have introduced an extrusion approach to preparing nanovesicles (NVs) as mimetics for exosomes.[ 23 , 27 ] For example, Cui et al. utilized a classical serial extrusion approach to generate NVs from human induced pluripotent stem cells (iPSCs)‐derived ECs (iPS‐ECs) to treat osteoporosis.[ 28 ] Choo et al. prepared the exosome‐mimetic NVs derived from M1 macrophages to induce macrophage polarization for anti‐cancer treatment.[ 29 ] NVs prepared by the serial extrusion of cells could have cell membranes and sizes similar to exosomes, and are more enriched in proteins and RNAs than exosomes with a higher yield.[ 30 ] Accordingly, NVs are supposed to be more effective in the biomolecule transfer and therapeutic effects in the recipient cells than exosomes.
In this study, it was the first time to use NVs to promote diabetic wound healing. We prepared EC‐targeted bioinspired NVs, which function as exosome mimetics to targeted deliver DA to facilitate diabetic wound healing. A classical serial extrusion approach was utilized to obtain NVs from iPS‐ECs. These iPS‐EC NVs could mimic the source cells with abundant C‐X‐C motif chemokine receptor 4 (CXCR4) on the surfaces. The abundant membrane CXCR4 conferred the EC‐targeting ability of these NVs and the endothelial homology facilitated the accumulation in ECs.[ 28 ] Furthermore, these iPS‐EC NVs were utilized to target deliver DA into ECs and promote angiogenesis and diabetic wound healing by HIF‐1α/VEGFA pathway. Taken together, this study suggested that targeting ECs and regulating angiogenesis could be a feasible approach for the treatment of diabetic wound healing.
2. Results and Discussion
2.1. SGLT2 Inhibitors Enhance High Glucose‐Impaired Endothelial Cell Function
The three common SGLT2 inhibitors, such as dapagliflozin,[ 12 ] canagliflozin,[ 13 ] and empagliflozin,[ 11 ] have been reported to play a vital role in regulating endothelial dysfunction. In our study, we investigated the regulative roles of these three SGLT2 inhibitors in high glucose‐impaired endothelial cell function. CCK‐8 assay showed that these three SGLT2 inhibitors could have a protective effect on ECs (Figure 1A). More importantly, the more significant protective effect was found in high glucose‐impaired ECs treated with dapagliflozin (DA) when compared with canagliflozin (CA) or empagliflozin (EM). As shown in Figure 1B, DA significantly promoted the high glucose‐impaired endothelial cell function in 48 h.
Figure 1.

SGLT2 inhibitors enhance high glucose‐impaired endothelial cell function. A) CCK‐8 assay showed that these three SGLT2 inhibitors could have a protective effect on ECs (**p < 0.01). B) DA significantly promoted the high glucose‐impaired endothelial cell function in 48 h (**p < 0.01). C,D) After being treated with high glucose, HUVEC cells were found with a lower cell death rate in the high glucose+DA group compared with the high glucose group via living‐dead assay (**p < 0.01). C,E) The high glucose+DA group showed higher efficiency in promoting HUVEC tube formation (**p < 0.01). C,F) Transwell assay indicated that the high glucose+DA group showed a higher migration rate (**p < 0.01). G) Compared with normal wounds, the expression of HIF‐1α was markedly decreased in diabetic wounds.
After being treated with high glucose, HUVEC cells were found with a lower cell death rate in the high glucose+DA group compared with the high glucose group via living‐dead assay (Figure 1C,D). Meanwhile, the high glucose+DA group showed higher efficiency in promoting HUVEC tube formation, characterized by more visible tube‐like structures and higher tube numbers (Figure 1C,E). Transwell assay indicated that the high glucose+DA group showed a higher migration rate (Figure 1C,F). Therefore, DA could significantly promote the functions and angiogenesis of HUVEC cells in an environment of high glucose.
Compared with normal wounds, the expression of HIF‐1α was markedly decreased in diabetic wounds (Figure 1G). HIF‐1α/VEGFA has been reported to be important in promoting diabetic wound associated angiogenesis.[ 31 ] Accordingly, DA might promote high glucose‐impaired endothelial cell function through HIF‐1α/VEGFA pathway.
2.2. Preparation and Characterization of DA‐Loaded Exosome Mimetics
iPSCs and their derivatives represent second‐generation stem cells, while multipotent somatic stem cells, such as mesenchymal stem/stromal cells (MSCs) are the first‐generation stem cell types.[ 32 ] The iPS‐ECs as the derivatives of iPSCs could combine the strong proliferation ability of iPSCs and the characteristics of ECs.[ 33 ] In this study, human iPSCs were successfully differentiated into ECs via established protocols (Figure 2A).[ 34 ] The immunofluorescent staining assay further confirmed that the positive rates of EC‐specific markers (CD31 and CD144) were over 90% (Figure 2B). Accordingly, we successfully prepared iPS‐ECs with high purity and powerful proliferative potential.
Figure 2.

Preparation and characterization of DA‐loaded exosome mimetics. A) The image of human iPSCs and iPS‐ECs, which were prepared by established differentiation protocols. B) The immunofluorescent staining assay showed the expression levels of EC‐specific markers (CD31 and CD144). C) Schematic diagram of the extrusion method to obtain NVs to mimic exosomes. D) TEM image of DA@iPS‐EC NVs. E) The characterization of DLS demonstrated that these NVs possessed similar size distributions with a peak diameter of around 200 nm (n.s.: no significance). F) The surface zeta potential of iPS‐EC NV and DA@iPS‐EC NV (n.s.: no significance). G) The behavior of DA released from DA@iPS‐EC NV under an acid environment. H) The behavior of DA released from DA@iPS‐EC NV under high concentrations of glucose. I) SDS‐PAGE was performed to compare the protein compositions of iPS‐ECs and NVs.
CXCR4 functions as the receptor of stromal cell‐derived factor 1 (SDF1), which contribute to the bone marrow homing of peripheral or transplanted CXCR4+ cells.[ 35 ] This targeting effect of the CXCR4‐SDF1 axis has been utilized in damage repair and tumor targeting.[ 36 , 37 ] Most importantly, iPS‐ECs are shown to have a naturally high expression of CXCR4.[ 38 ] Moreover, a classic serial extrusion approach to preparing NVs could reserve the membrane protein constituents and mimic the functions of exosomes.[ 39 , 40 ] Compared with exosomes, NVs from this approach could function as exosome mimetics and exhibit simpler preparation technology and higher yield, which may benefit the translation in the narrow future.
Accordingly, this study utilized the extrusion method to prepare NVs to mimic exosomes (Figure 2C). Transmission electron microscopy (TEM) images of DA@iPS‐EC NVs indicated that these NVs were spherical particles with an intact monolayer membrane structure (Figure 2D). The dynamic light scattering (DLS) suggested that these NVs had similar size distributions with a peak diameter of about 200 nm (Figure 2E). The surface zeta potential (Figure 2F) of DA@iPS‐EC NV was −16.7 ± 3.2 mV, similar to that of iPS‐EC NV (−15.2 ± 4.5 mV), validating the successful preparation of cell membrane‐coated nanovesicles. Furthermore, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) was conducted to show the protein compositions of iPS‐ECs and NVs. The protein brands exhibited highly similar stripes, thereby indicating that the main cellular proteins were preserved in NVs (Figure 2I). Moreover, the NVs during the preparation process was monitored by Fourier transform infrared spectroscopy (FTIR). The infrared spectrum of DA@iPS‐EC NV contained the characteristic peaks of DA and iPS‐EC NV (Figure S1, Supporting Information).
The drug loading efficiency and the drug loading content of DA@iPS‐EC NV were further investigated.[ 30 ] Reacting 0.09 mg DA with 1 mg iPS‐EC NV (DA/iPS‐EC NV = 0.09) was deemed to be the best condition for preparing DA@iPS‐EC NV, considering both loading efficiency and loading content of the drug, with the former being 45% and the latter being 0.28 mg mg−1. It showed that iPS‐EC NV is an effective nanovesicle for the loading and delivery of DA to finally prepare DA@iPS‐EC NV (Figure S2, Supporting Information). The behavior of DA released from DA@iPS‐EC NV was monitored using a UV spectrophotometer. DA released from DA@iPS‐EC NV had a sharp release in 40 h, followed by a slow but steady release level over 240 h. Within the acid solution of pH = 5.5, the release rate of DA@iPS‐EC NV was significantly accelerated when compared with pH = 6.8 or 7.4. Similarly, the release rate of DA@iPS‐EC NV was significantly accelerated in glucose solution. Accordingly, the diabetic wound might accelerate the rapid release of DA@iPS‐EC NV, which is characterized by an acid environment and high concentrations of glucose (Figure 2G,H).
2.3. Exosome Mimetics Target Endothelial Cells and Enhance Endothelial Cell Function
The iPS‐EC NVs were reported to mimic the source cells with abundant CXCR4 on the surfaces. The abundant membrane CXCR4 conferred the EC‐targeting ability of these NVs and the endothelial homology promoted the accumulation in ECs.[ 24 ] In this study, we further explored the EC‐targeting effects of exosome mimetics of iPS‐EC NV and DA@iPS‐EC NV. The membrane of iPS‐EC NV was labeled with DiD (red). It is reported that exosome mimetics could retain their parent membrane proteins and inherit the functions of parent cells. In this study, after incubating with iPS‐EC NV or DA@iPS‐EC NV, we both detected strong fluorescence intensity (red) in both HUVEC cells. However, almost no fluorescence intensity (red) was observed in the low‐CXCR4‐iPS‐EC NV group (Figure 3 ). The results indicated that exosome mimetics could achieve the targeted delivery of DA into HUVEC cells via CXCR4 expressed on the surface.
Figure 3.
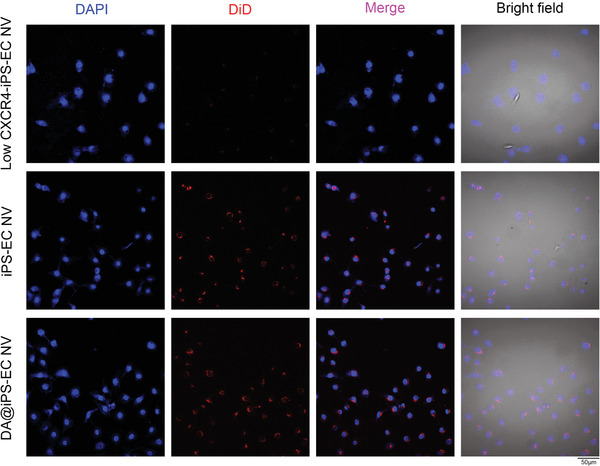
Exosome mimetics target endothelial cells. After incubation with iPS‐EC NV or DA@iPS‐EC NV, a strong fluorescence intensity (red) was observed in HUVEC cells, which indicated that exosome mimetics could achieve the targeted delivery of DA into HUVEC cells.
Neovascularization could transport nutrition and oxygen into wounds and facilitate wound healing.[ 41 ] Accordingly, HUVEC migration and tube formation are essential steps during angiogenesis, which highly affect the curative rate of diabetic wound healing. After being treated with high glucose, the DA@iPS‐EC NV group showed higher efficiency than other groups in promoting HUVEC tube formation, characterized by more visible tube‐like structures and higher tube numbers (Figure 4A,B). Transwell assay indicated that the DA@iPS‐EC NV group showed a higher migration rate than other groups (Figure 4A,C). Meanwhile, HUVEC cells were found with a lower cell death rate in the DA@iPS‐EC NV group compared with other groups via living‐dead assay (Figure 4A,D). Taken together, DA@iPS‐EC NV could significantly promote the functions and angiogenesis of HUVEC cells in an environment of high glucose.
Figure 4.

Exosome mimetics enhance endothelial cell function. A,B) After being treated with high glucose, the DA@iPS‐EC NV group showed higher efficiency than other groups in promoting HUVEC tube formation (**p < 0.01). A,C) Transwell assay indicated that the DA@iPS‐EC NV group showed a higher migration rate than other groups (**p < 0.01). A,D) HUVEC cells were found with a lower cell death rate in the DA@iPS‐EC NV group compared with other groups via living‐dead assay (**p < 0.01).
2.4. Exosome Mimetics Promote Diabetic Wound Healing
The therapeutic strategy in vivo is shown in Figure 5A. The macroscopic images of wounds, wound closure traces, and the corresponding quantitative size of the wound area at different time points were presented in Figure 5B,E. On days 3 and 6, the wound area of the DA@iPS‐EC NV group was remarkably reduced. On day 9, the wound in the DA@iPS‐EC NV group was almost completely healed, with a wound closure ratio of 97%. However, there was still a large wound area in the control, DA, and iPS‐EC NV groups. It suggested that DA@iPS‐EC NV might significantly promote diabetic wound healing.
Figure 5.
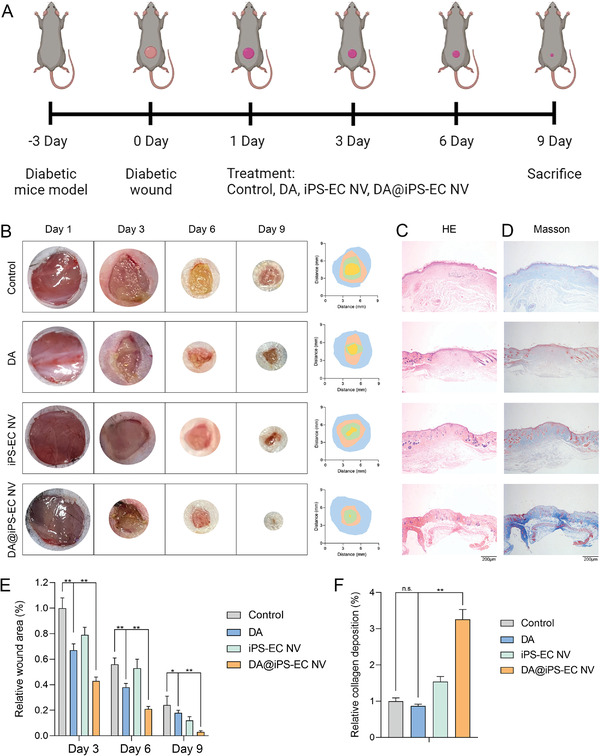
Exosome mimetics promote diabetic wound healing. A) Schematic diagram of the therapeutic strategy in vivo. B,E) The macroscopic images of wounds, wound closure traces, and the corresponding quantitative size of the wound area at different time points (**p < 0.01, *p < 0.05). C) The H&E images showed the formation of epidermis and dermis tissue in diabetic wounds. D,F) The Masson staining images showed the collagen deposition level of diabetic wounds (**p < 0.01, n.s.: no significance).
The histological staining of diabetic wounds was conducted by hematoxylin‐eosin (H&E) and Masson trichrome staining. On day 9, all of the groups had newly formed epidermis, which was more natural and mature in the DA@iPS‐EC NV group than that in the other groups. The H&E images suggested that the diabetic wounds in the DA@iPS‐EC NV group might have satisfactory healing efficiency with the accelerated formation of epidermis and dermis tissue (Figure 5C). Furthermore, the collagen deposition level on day 9 was presented in Figure 5D,F. Larger amounts of collagen were deposited in the DA@iPS‐EC NV group (blue) than in the other groups. The above results suggested that the DA@iPS‐EC NV might promote diabetic wound healing by facilitating epidermis formation, promoting dermal tissue generation, and increasing collagen deposition.
2.5. Exosome Mimetics Promote Angiogenesis In Vivo through HIF‐1α/VEGFA Pathway
Angiogenesis acts as a critical process in the remodeling phase of diabetic wound healing. This study indicated that DA@iPS‐EC NV promoted angiogenesis by accelerating the proliferation, migration, and tube formation of HUVEC cells in vitro. CD31 is one of the most common markers of ECs and plays a vital role in angiogenesis. Double immunofluorescence staining for α‐smooth muscle actin (α‐SMA) and CD31 was performed to investigate the neovascularization. The expression of CD31 and α‐SMA in the DA@iPS‐EC NV group was significantly higher than that in the other groups (Figure 6A–C). Accordingly, DA@iPS‐EC NV had a better ability of vascularization, thereby effectively promoting wound healing. Taken together, DA@iPS‐EC NV might promote diabetic wound healing by advancing epidermis formation, collagen deposition, and neovascularization.
Figure 6.

Exosome mimetics promote angiogenesis in vivo through HIF‐1α/VEGFA pathway. A,B) The expression of CD31 in the DA@iPS‐EC NV group was significantly higher than that in the other groups (**p < 0.01). A,C) The expression of α‐SMA in the DA@iPS‐EC NV group was significantly higher than that in the other groups (**p < 0.01). D) Western blot assay showed that the expressions of HIF‐1α and VEGFA were significantly upregulated in the high glucose+DA group when compared with the high glucose group (**p < 0.01). F,E) Immunohistochemical staining showed that the highest HIF‐1α expression level was observed in the DA@iPS‐EC NV group (**p < 0.01). F,G) Immunohistochemical staining showed that the highest VEGFA expression level was observed in the DA@iPS‐EC NV group (**p < 0.01, *p < 0.05).
Therefore, immunohistochemical staining of HIF‐1α and VEGFA was conducted to evaluate the underlying mechanisms of DA@iPS‐EC NV (Figure 6D–F). The highest HIF‐1α and VEGFA expression levels were observed in the DA@iPS‐EC NV group. The results further validated that DA@iPS‐EC NV could effectively facilitate diabetic wound healing through HIF‐1α/VEGFA pathway (Figure 7 ).
Figure 7.
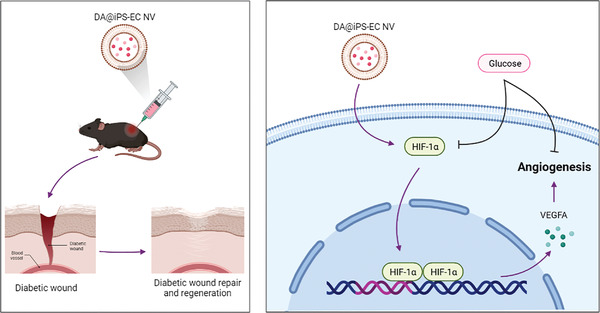
Overview of DA‐loaded exosome mimetics facilitating diabetic wound healing by HIF‐1α‐mediated enhancement of angiogenesis.
3. Conclusion
In this study, we reported bioinspired NVs prepared from human iPS‐ECs through an extrusion approach, which could function as exosome mimetics to targeted deliver DA. Abundant membrane CXCR4 conferred the EC‐targeting ability of these NVs and the endothelial homology facilitated the accumulation in ECs. Furthermore, these DA‐loaded iPS‐EC NVs could promote angiogenesis and diabetic wound healing by HIF‐1α/VEGFA pathway. Taken together, this study demonstrated that targeting ECs and regulating their functions could be a feasible approach for the treatment of diabetic wound healing.
4. Experimental Section
Cell Viability Assay
Cell Counting Kit‐8 (CCK‐8) assay was used to detect cell viability. After treatment, HUVEC cells were cultured in a 96‐well plate. After 12, 24, or 48 h of culturing, the CCK‐8 solution was added to follow the kit protocol. After incubation, the absorbance at 450 nm was tested.
Living‐Dead Assay
HUVEC cells were cultured in 24‐well plates. After 48 h of culturing, the living‐dead assay (Invitrogen, Waltham, MA, USA) was conducted according to the manufacturer's instructions. The images were investigated via a fluorescence microscope.
Tube Formation Assay
HUVEC cells were seeded onto 96‐well plates with Matrigel pre‐coated and treated with different groups. After 6 h, these cells were observed by a light microscope.
Transwell Assay
Treated HUVEC cells were seeded onto the top chamber of Transwell chamber plates (24‐well format with a pore size of 8 µm, BD Biosciences, St Louis, MO) with 100 µl serum‐free medium, and the bottom well was filled with medium containing 20% FBS. After being maintained at 37 °C for 24 h, HUVEC cells migrated to the other side of the pores and were fixed and stained for 30 min. The average number of migratory cells was observed under a light microscope.
Western Blot Assay
Western blot assay was conducted with the standard protocols. Primary antibodies of HIF‐1α, VEGFA, and GAPDH‐actin were utilized. A secondary antibody labeled with horseradish peroxidase (HRP) was utilized to detect the protein bands.
Differentiation of Human iPSCs into iPS‐ECs
Human iPSCs were differentiated into ECs with a classic method.[ 34 ] Then, the marker expressions (CD31 and CD144) of acquired cells were characterized using immunofluorescent staining. Commercial EC medium was utilized to culture iPS‐ECs.
Preparation and Characterization of NVs
The NVs of cells were prepared and purified through an established extrusion approach.[ 28 ] Briefly, cells (1 × 106) were resuspended in PBS and extruded successively through polycarbonate membranes using a mini extruder (Avanti Polar Lipids, Birmingham, Alabama). The extruded samples were centrifuged at 10 000 g for 10 min to remove the cell debris and large vesicles. The NVs were purified and enriched using a centrifugal filter of 100 kDa (Millipore Corp, Bedford, MA) at 1000 g for 15 min. The purified NVs were stored at −80 °C. The DA‐loaded iPS‐EC NVs (DA concentration: 0.04 mg ml−1) were fabricated by extruding 1 ml of DA solution (0.1 mg ml−1) with 1 ml NV solution through 400 and 200 nm polycarbonate membranes for 10 to 20 passes in order. The morphology of NVs was investigated by a TEM (JEM‐1200EX, JEOL Ltd., Tokyo, Japan). The size distribution of NVs was investigated by DLS by a Zetasizer Nano ZS90 (Malvern, Malvern, UK). SDS‐PAGE was performed to compare the protein compositions of iPS‐ECs and NVs. On a Thermo Nicolet iS50 FTIR spectrometer, FTIR was scanned in the 400–4000 cm−1 range. The in vitro experiments were divided into four groups: control (30 mmol l−1 high glucose solution), DA (0.04 mg ml−1 DA solution with high glucose), iPS‐EC NV (NV solution with high glucose), and DA@iPS‐EC NV (DA concentration: 0.04 mg ml−1, with high glucose).
The Drug Loading Behaviors
A solution containing DA (0.01–0.2 mg) and 1 mg of iPS‐EC NV was prepared via the extrusion approach. DA@iPS‐EC NV was collected and washed until the supernatant became color free. The amount of unbound DA in the solution was determined by measuring the absorbance using a UV spectrophotometer. The drug loading efficiency (%) and the drug loading content (mg/mg) were calculated:[ 42 ] a) Loading efficiency (%) = M Encapsulated/M Used × 100%, where M Encapsulated is the net weight (mg) of DA inside NVs, and M Used represents the total weight (mg) of DA used. b) Loading content (mg/mg) = M Agent/M Total, where M Agent is the weight (mg) of DA encapsulated inside the NVs, while the M Total represents the total weight (mg) of agent‐laden nanocarriers, including both the encapsulated DA and the empty NV.
The Drug Release Behaviors
The behavior of DA released from DA@iPS‐EC NV was monitored using a UV spectrophotometer. DA released from DA@iPS‐EC NV was detected within 240 h under an acidic environment (pH = 5.5, 6.8, or 7.4) and high concentrations of glucose (with pH = 7.4).
EC‐Targeting Ability and In Vitro Cellular Uptake
The iPS‐EC cells were transfected with si‐CXCR4 to downregulate the CXCR4 expression on the surface of cells. Then these transfected iPS‐EC cells were extruded to prepare the low‐CXCR4‐iPS‐EC NVs as the control group. The membrane of iPS‐EC NV was labeled with DiD (red) and the nucleus was stained with DAPI (blue). HUVEC cells were seeded in confocal dishes, and cultured for 6 h to permit cell adhesion. Then the cells were incubated with 10 µg ml−1 low‐CXCR4‐iPS‐EC NV, iPS‐EC NV, and DA@iPS‐EC NV for 3 h, respectively. The images were obtained by confocal laser scanning microscopy (CLSM). The Ex/Em parameters were as follows: DiD (Ex/Em: 644/663 nm).
Diabetic Wound Model
In vivo wound healing was investigated with a streptozotocin‐induced diabetic wound model. After the mice were fixed, a 1cm‐diameter circular full‐thickness skin wound was performed on the back of the mice. The diabetic mice were randomly divided into four groups: control (1 ml PBS), DA (1 ml 0.04 mg ml−1 DA solution), iPS‐EC NV (1 ml NV solution), and DA@iPS‐EC NV (DA concentration: 0.04 mg ml−1, 1 ml). The therapeutic agents were injected locally at the edge of the wounds.
Histological Analysis
The skin tissues were fixed immediately after removal from the back of the mice. The samples were then progressively dehydrated and encased in paraffin. Finally, a slicer was utilized to slice the samples for further experiments (H&E staining, Masson trichrome staining, immunohistochemical staining, and immunofluorescent staining).
Statistical Analysis
The results were expressed as mean ± SD and analyzed by GraphPad Prism software. One‐way analysis of variance (ANOVA) and Student's t‐test were utilized to evaluate the statistical significance (p < 0.05).
Ethical Approval
These experimets were approved by the institutional review board and the medical ethics committee of Wuhan Union Hospital, Huazhong University of Science and Technology (2022IEC052).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
W.Y.Z. and H.Y.G.: Conceptualization, investigation, methodology, and writing‐original draft; W.Y.Z. and X.H.: Funding acquisition, writing‐review, and editing; L.T.W., X.H., L.L.C., and H.Y.G.: Software, supervision, writing‐review, and editing. All authors have given final approval to this version of the manuscript to be published.
Supporting information
Supporting Information
Supplemental Table 1
Acknowledgements
This study is supported by the National Natural Science Foundation of China (82203059) and the China Postdoctoral Science Foundation (2021M701335).
Zhang W., Wang L., Guo H., Chen L., Huang X., Dapagliflozin‐Loaded Exosome Mimetics Facilitate Diabetic Wound Healing by HIF‐1α‐Mediated Enhancement of Angiogenesis. Adv. Healthcare Mater. 2023, 12, 2202751. 10.1002/adhm.202202751
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Morton L. M., Phillips T. J., J. Am. Acad. Dermatol. 2016, 74, 589. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y., Cao Z., Wei Q., Ma K., Hu W., Huang Q., Su J., Li H., Zhang C., Fu X., Acta Biomater. 2022, 147, 342. [DOI] [PubMed] [Google Scholar]
- 3. Bakker K., Apelqvist J., Lipsky B. A., Van Netten J. J., Diabetes/Metab. Res. Rev. 2016, 32, 2. [DOI] [PubMed] [Google Scholar]
- 4. Bian X., Ma K., Zhang C., Fu X., Stem Cell Res. Ther. 2019, 10, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catrina S. B., Okamoto K., Pereira T., Brismar K., Poellinger L., Diabetes 2004, 53, 3226. [DOI] [PubMed] [Google Scholar]
- 6. Mace K. A., Yu D. H., Paydar K. Z., Boudreau N., Young D. M., Wound Repair Regener. 2007, 15, 636. [DOI] [PubMed] [Google Scholar]
- 7. Li G., Ko C. N., Li D., Yang C., Wang W., Yang G. J., Di Primo C., Wong V. K. W., Xiang Y., Lin L., Ma D. L., Leung C. H., Nat. Commun. 2021, 12, 3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheen A. J., Nat. Rev. Endocrinol. 2020, 16, 556. [DOI] [PubMed] [Google Scholar]
- 9. Wiviott S. D., Raz I., Bonaca M. P., Mosenzon O., Kato E. T., Cahn A., Silverman M. G., Zelniker T. A., Kuder J. F., Murphy S. A., Bhatt D. L., Leiter L. A., McGuire D. K., Wilding J. P. H., Ruff C. T., Gause‐Nilsson I. A. M., Fredriksson M., Johansson P. A., Langkilde A. M., Sabatine M. S., N. Engl. J. Med. 2019, 380, 347.30415602 [Google Scholar]
- 10. Sposito A. C., Breder I., Soares A. A. S., Kimura‐Medorima S. T., Munhoz D. B., Cintra R. M. R., Bonilha I., Oliveira D. C., Breder J. C., Cavalcante P., Moreira C., Moura F. A., de Lima‐Junior J. C., do Carmo H. R. P., Barreto J., Nadruz W., Carvalho L. S. F., Quinaglia T., Cardiovasc. Diabetol. 2021, 20, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uthman L., Homayr A., Juni R. P., Spin E. L., Kerindongo R., Boomsma M., Hollmann M. W., Preckel B., Koolwijk P., van Hinsbergh V. W. M., Zuurbier C. J., Albrecht M., Weber N. C., Cell Physiol. Biochem. 2019, 53, 865. [DOI] [PubMed] [Google Scholar]
- 12. Ma L., Zou R., Shi W., Zhou N., Chen S., Zhou H., Chen X., Wu Y., Theranostics 2022, 12, 5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peyton K. J., Behnammanesh G., Durante G. L., Durante W., Int. J. Mol. Sci. 2022, 23, 8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mone P., Varzideh F., Jankauskas S. S., Pansini A., Lombardi A., Frullone S., Santulli G., Hypertension 2022, 79, 1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nugrahaningrum D. A., Marcelina O., Liu C., Wu S., Kasim V., Frontiers in Pharmacology 2020, 11, 10.3389/fphar.2020.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lötvall J., Hill A. F., Hochberg F., Buzás E. I., Di Vizio D., Gardiner C., Gho Y. S., Kurochkin I. V., Mathivanan S., Quesenberry P., Sahoo S., Tahara H., Wauben M. H., Witwer K. W., Théry C., J. Extracell. Vesicles 2014, 3, 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Babaei M., Rezaie J., J. Transl. Med. 2021, 19, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roefs M. T., Sluijter J. P. G., Vader P., Trends Cell Biol. 2020, 30, 990. [DOI] [PubMed] [Google Scholar]
- 19. Duan M., Zhang Y., Zhang H., Meng Y., Qian M., Zhang G., Stem Cell Res. Ther. 2020, 11, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu X., Liang Y., Li X., Ouyang K., Wang M., Cao T., Li W., Liu J., Xiong J., Li B., Xia J., Wang D., Duan L., Biomaterials 2021, 269, 120539. [DOI] [PubMed] [Google Scholar]
- 21. Alvarez‐Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M. J., Nat. Biotechnol. 2011, 29, 341. [DOI] [PubMed] [Google Scholar]
- 22. Gudbergsson J. M., Jønsson K., Simonsen J. B., Johnsen K. B., J. Controlled Release 2019, 306, 108. [DOI] [PubMed] [Google Scholar]
- 23. Jang S. C., Kim O. Y., Yoon C. M., Choi D. S., Roh T. Y., Park J., Nilsson J., Lötvall J., Kim Y. K., Gho Y. S., ACS Nano 2013, 7, 7698. [DOI] [PubMed] [Google Scholar]
- 24. Yao Y., Jiang Y., Song J., Wang R., Li Z., Yang L., Wu W., Zhang L., Peng Q., Adv. Healthcare Mater. 2022, 2201989. [DOI] [PubMed] [Google Scholar]
- 25. Yom‐Tov N., Guy R., Offen D., Adv. Drug Delivery Rev. 2022, 190, 114535. [DOI] [PubMed] [Google Scholar]
- 26. Jia Y., Yu L., Ma T., Xu W., Qian H., Sun Y., Shi H., Theranostics 2022, 12, 6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molinaro R., Corbo C., Martinez J. O., Taraballi F., Evangelopoulos M., Minardi S., Yazdi I. K., Zhao P., De Rosa E., Sherman M. B., De Vita A., Toledano Furman N. E., Wang X., Parodi A., Tasciotti E., Nat. Mater. 2016, 15, 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cui Y., Li Z., Guo Y., Qi X., Yang Y., Jia X., Li R., Shi J., Gao W., Ren Z., Liu G., Ye Q., Zhang Z., Fu D., ACS Nano 2022, 16, 11076. [DOI] [PubMed] [Google Scholar]
- 29. Choo Y. W., Kang M., Kim H. Y., Han J., Kang S., Lee J. R., Jeong G. J., Kwon S. P., Song S. Y., Go S., Jung M., Hong J., Kim B. S., ACS Nano 2018, 12, 8977. [DOI] [PubMed] [Google Scholar]
- 30. Li Y. J., Wu J. Y., Liu J., Xu W., Qiu X., Huang S., Hu X. B., Xiang D. X., J. Nanobiotechnol. 2021, 19, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang H., Feng Y., Jin X., Xia R., Cheng Y., Liu X., Zhu N., Zhou X., Yin L., Guo J., Oncotarget 2017, 8, 114251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimbrel E. A., Lanza R., Nat. Rev. Drug Discovery 2020, 19, 463. [DOI] [PubMed] [Google Scholar]
- 33. Gao L., Wang L., Wei Y., Krishnamurthy P., Walcott G. P., Menasché P., Zhang J., Sci. Transl. Med. 2020, 12, aay1318. [DOI] [PubMed] [Google Scholar]
- 34. Patsch C., Challet‐Meylan L., Thoma E. C., Urich E., Heckel T., O'Sullivan J. F., Grainger S. J., Kapp F. G., Sun L., Christensen K., Xia Y., Florido M. H., He W., Pan W., Prummer M., Warren C. R., Jakob‐Roetne R., Certa U., Jagasia R., Freskgård P. O., Adatto I., Kling D., Huang P., Zon L. I., Chaikof E. L., Gerszten R. E., Graf M., Iacone R., Cowan C. A., Nat. Cell Biol. 2015, 17, 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dar A., Goichberg P., Shinder V., Kalinkovich A., Kollet O., Netzer N., Margalit R., Zsak M., Nagler A., Hardan I., Resnick I., Rot A., Lapidot T., Nat. Immunol. 2005, 6, 1038. [DOI] [PubMed] [Google Scholar]
- 36. Sun Y. X., Schneider A., Jung Y., Wang J., Dai J., Wang J., Cook K., Osman N. I., Koh‐Paige A. J., Shim H., Pienta K. J., Keller E. T., McCauley L. K., Taichman R. S., J. Bone Miner. Res. 2005, 20, 318. [DOI] [PubMed] [Google Scholar]
- 37. Hu Y., Li X., Zhang Q., Gu Z., Luo Y., Guo J., Wang X., Jing Y., Chen X., Su J., Bioact. Mater. 2021, 6, 2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho H., Macklin B. L., Lin Y. Y., Zhou L., Lai M. J., Lee G., Gerecht S., Duh E. J., JCI Insight 2020, 5, 131828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan J., Lee C. S., Kim S., Chen C., Aghaloo T., Lee M., ACS Nano 2020, 14, 11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Z., Wang Z., Dinh P. C., Zhu D., Popowski K. D., Lutz H., Hu S., Lewis M. G., Cook A., Andersen H., Greenhouse J., Pessaint L., Lobo L. J., Cheng K., Nat. Nanotechnol. 2021, 16, 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim H. S., Sun X., Lee J. H., Kim H. W., Fu X., Leong K. W., Adv. Drug Delivery Rev. 2019, 146, 209. [DOI] [PubMed] [Google Scholar]
- 42. Wang H., Agarwal P., Zhao S., Yu J., Lu X., He X., Biomaterials 2016, 97, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supplemental Table 1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


